Sulforaphane Regulates eNOS Activation and NO Production via Src-Mediated PI3K/Akt Signaling in Human Endothelial EA.hy926 Cells
Abstract
1. Introduction
2. Results
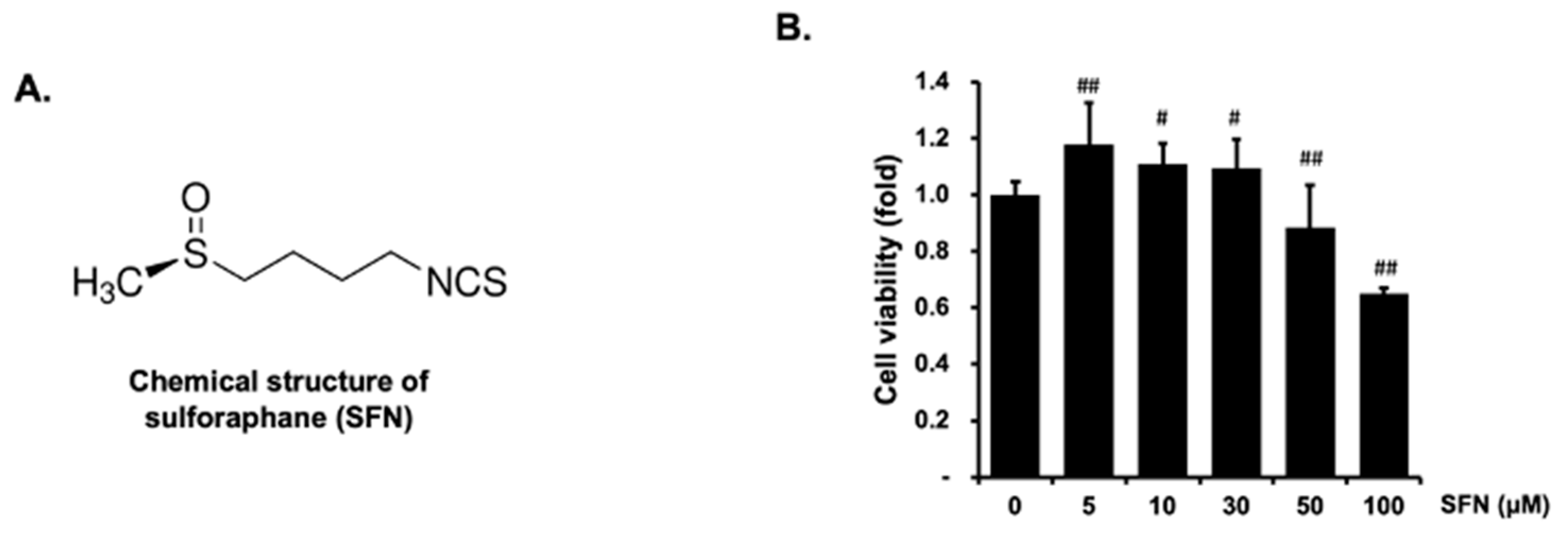
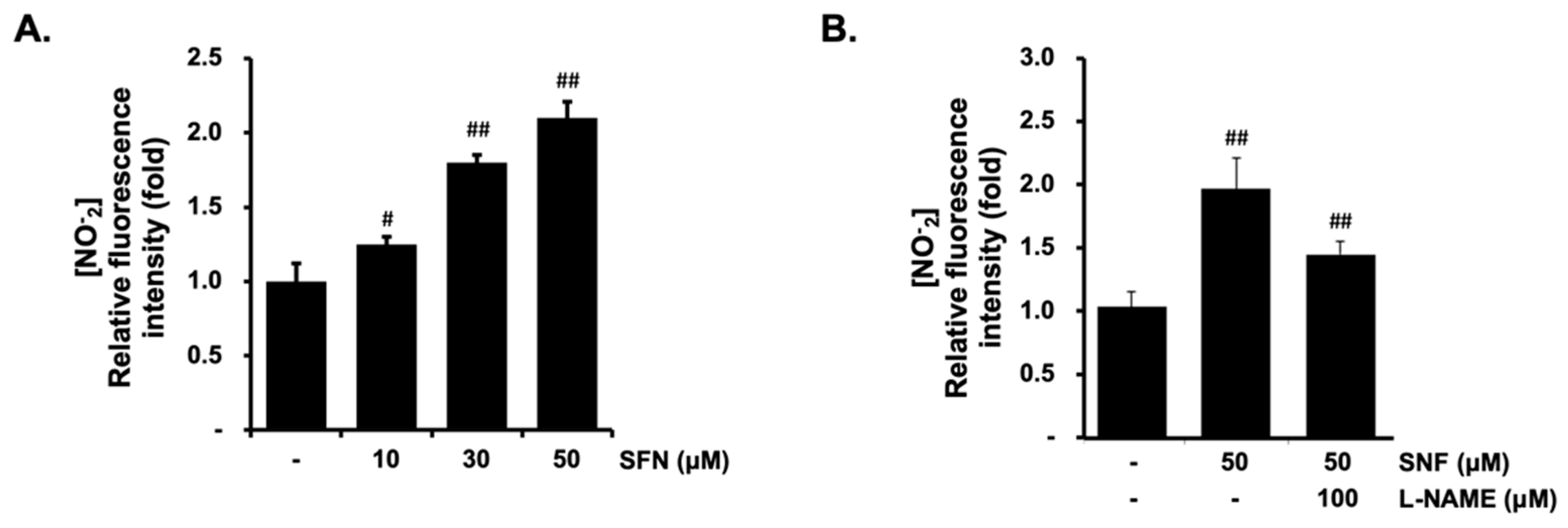
2.1. Effect of SFN on the Cell Viability and NO Production in EA.hy926 Cells
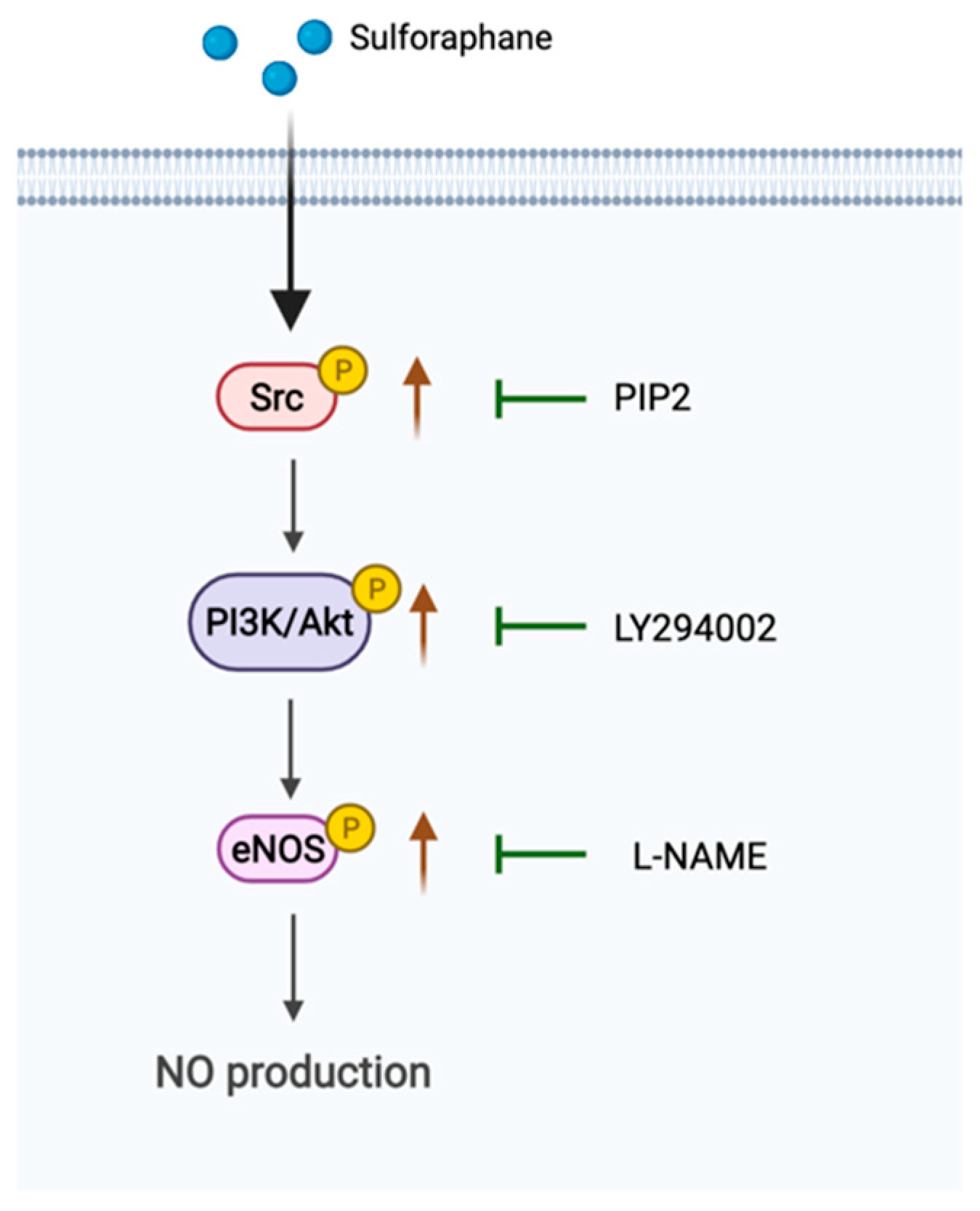
2.2. Effect of SFN on the Phosphorylation of eNOS in EA.hy926 Cells
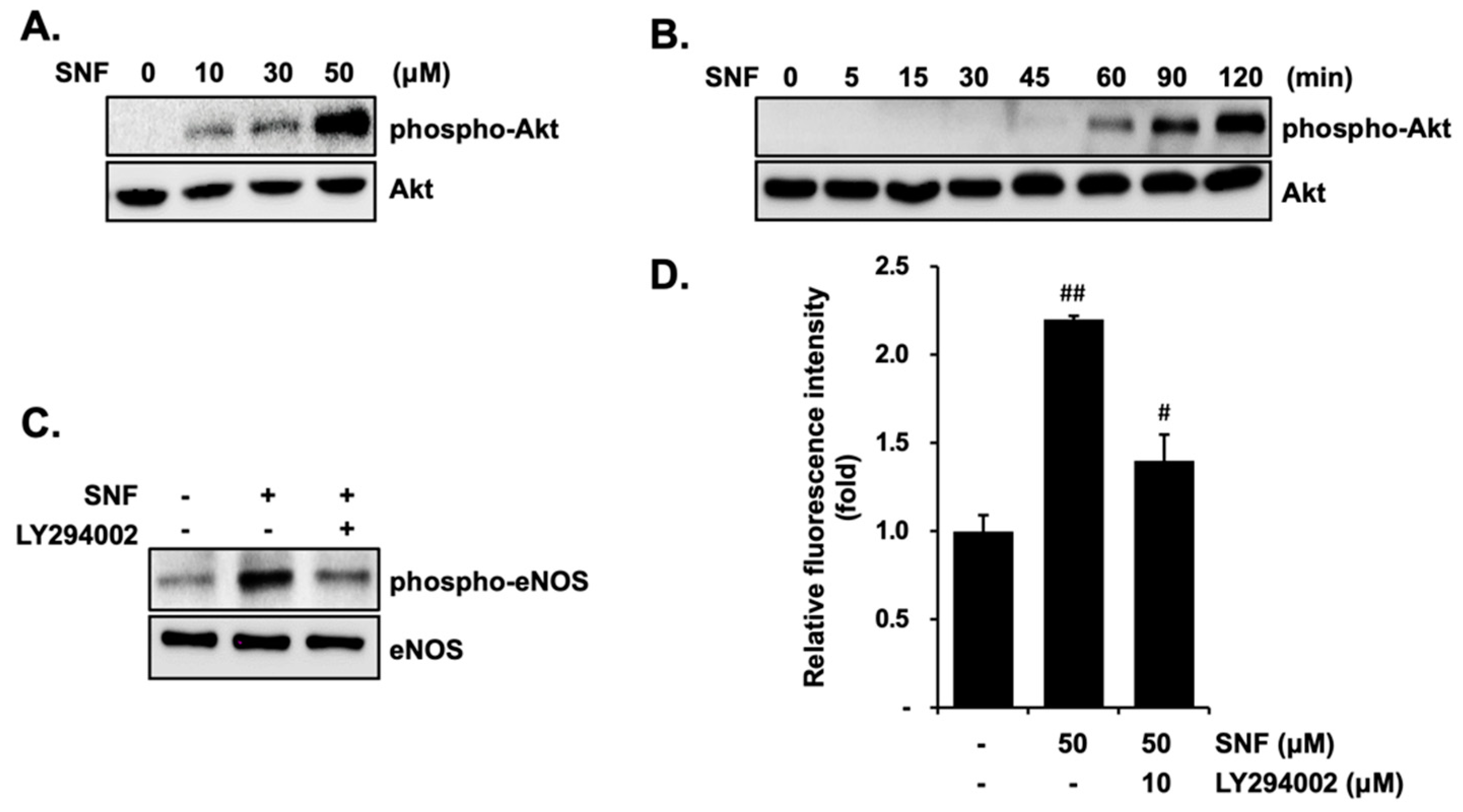
2.3. Involvement of PI3K/Akt in SFN-Induced eNOS Phosphorylation
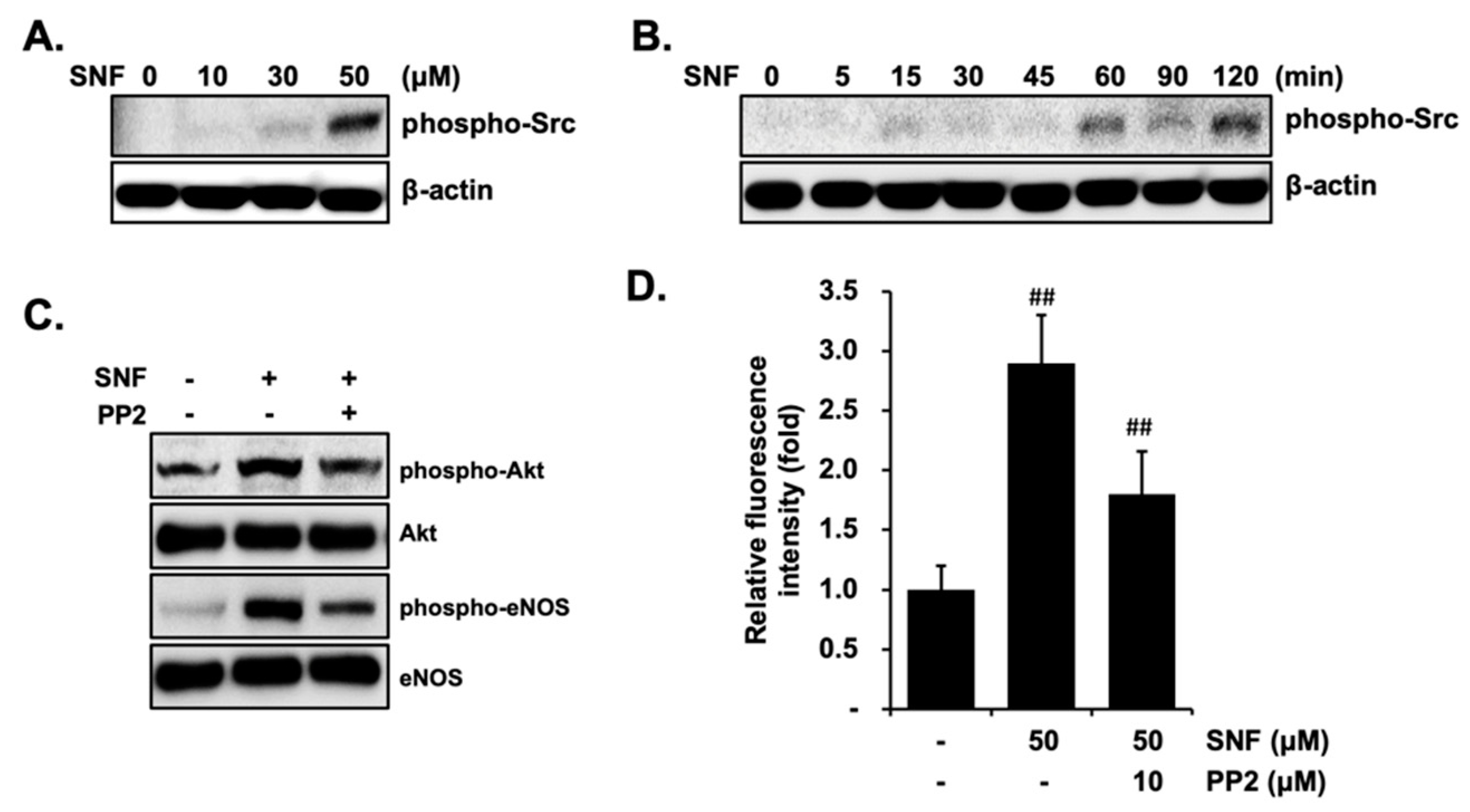
2.4. Involvement of Src Kinases in SFN-Induced eNOS Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability
4.4. Measurement of NO Production
4.5. Western Blot Analysis
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fedak, P.M.W.; Rao, V.; Verma, S.; Ramzy, D.; Tumiati, L.; Miriuka, S.; Boylen, P.; Weisel, R.D.; Feindel, C.M. Combined endothelial and myocardial protection by endothelin antagonism enhances transplant allograft preservation. J. Thorac. Cardiovasc. Surg. 2005, 129, 407–415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sellke, F.W.; Boyle, E.B.; Boyle, E.M., Jr.; Verrier, E.D. Endothelial cell injury in cardiovascular surgery: The pathophysiology of vasomotor dysfunction. Ann. Thorac. Surg. 1996, 62, 1222–1228. [Google Scholar] [CrossRef]
- Sellke, F.W.; Shafique, T.; Schoen, F.J.; Weintraub, R.M. Impaired endothelium-dependent coronary microvascular relaxation after cold potassium cardioplegia and reperfusion. J. Thorac. Cardiovasc. Surg. 1993, 105, 52–58. [Google Scholar] [CrossRef]
- Verma, S.; Buchanan, M.R.; Anderson, T.J. Endothelial function testing as a biomarker of vascular disease. Circulation 2003, 108, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Shukla, P.C.; Quan, A.; Teoh, H.; Szmitko, P.E.; Peterson, M.D.; Gupta, M.; Al-Omran, M.; Verma, S. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: Translational implications for atherosclerosis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1440–E1449. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 1991, 88, 4651–4655. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Rudic, R.D.; Shesely, E.G.; Maeda, N.; Smithies, O.; Segal, S.S.; Sessa, W.C. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Investig. 1998, 101, 731–736. [Google Scholar] [CrossRef]
- Fernandes, T.; Gomes-Gatto, C.V.; Pereira, N.P.; Alayafi, Y.R.; das Neves, V.J.; Oliveira, E.M. NO Signaling in the Cardiovascular System and Exercise. Adv. Exp. Med. Biol. 2017, 1000, 211–245. [Google Scholar]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Chen, Z.P.; Mitchelhill, K.I.; Michell, B.J.; Stapleton, D.; Rodriguez-Crespo, I.; Witters, L.A.; Power, D.A.; de Montellano, P.R.O.; Kemp, B.E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999, 443, 285–289. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Xu, Q.; Gao, M.; Yamori, Y. Phosphorylated endothelial NOS Ser1177 via the PI3K/Akt pathway is depressed in the brain of stroke-prone spontaneously hypertensive rat. J. Stroke Cereb. Dis. Off. J. Natl. Stroke Assoc. 2011, 20, 406–412. [Google Scholar] [CrossRef]
- Chen, R.; Kim, O.; Yang, J.; Sato, K.; Eisenmann, K.M.; McCarthy, J.; Chen, H.; Qiu, Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 2001, 276, 31858–31862. [Google Scholar] [CrossRef] [PubMed]
- Haynes, M.P.; Li, L.; Sinha, D.; Russell, K.S.; Hisamoto, K.; Baron, R.; Collinge, M.; Sessa, W.C.; Bender, J.R. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J. Biol. Chem. 2003, 278, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prieto, C.F.; Pulido-Olmo, H.; Ruiz-Hurtado, G.; Gil-Ortega, M.; Aranguez, I.; Rubio, M.A.; Ruiz-Gayo, M.; Somoza, B.; Fernandez-Alfonso, M.S. Mild caloric restriction reduces blood pressure and activates endothelial AMPK-PI3K-Akt-eNOS pathway in obese Zucker rats. Vasc. Pharmacol. 2015, 65–66, 3–12. [Google Scholar] [CrossRef]
- Meng, X.; Li, X.; Xu, X.; Li, P.; Chen, Y.; Fu, X.; Xu, X. Elevated luteinizing hormone contributes to atherosclerosis formation by inhibiting nitric oxide synthesis via PI3K/Akt pathway. Vasc. Pharmacol. 2019, 121, 106582. [Google Scholar] [CrossRef]
- Banquet, S.; Delannoy, E.; Agouni, A.; Dessy, C.; Lacomme, S.; Hubert, F.; Richard, V.; Muller, B.; Leblais, V. Role of G(i/o)-Src kinase-PI3K/Akt pathway and caveolin-1 in beta(2)-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell. Signalling. 2011, 23, 1136–1143. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Pharmacological prevention of eNOS uncoupling. Curr. Pharm. Design 2014, 20, 3595–3606. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef]
- Xia, Y.; Kang, T.W.; Jung, Y.D.; Zhang, C.; Lian, S. Sulforaphane Inhibits Nonmuscle Invasive Bladder Cancer Cells Proliferation through Suppression of HIF-1alpha-Mediated Glycolysis in Hypoxia. J. Agric. Food Chem. 2019, 67, 7844–7854. [Google Scholar] [CrossRef]
- Klomparens, E.A.; Ding, Y. The neuroprotective mechanisms and effects of sulforaphane. Brain Circ. 2019, 5, 74–83. [Google Scholar] [PubMed]
- Shehatou, G.S.; Suddek, G.M. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp. Biol. Med. 2016, 241, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Casino, P.R.; Kilcoyne, C.M.; Quyyumi, A.A. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation 1993, 87, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Endothelial dysfunction in hypertension. Journal of hypertension. Suppl. Off. J. Int. Soc. Hypertens. 1996, 14, S83–S93. [Google Scholar]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxidative Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef]
- Sotokawauchi, A.; Ishibashi, Y.; Matsui, T.; Yamagishi, S.I. Aqueous Extract of Glucoraphanin-Rich Broccoli Sprouts Inhibits Formation of Advanced Glycation End Products and Attenuates Inflammatory Reactions in Endothelial Cells. Evid. -Based Complementary Altern. Med. 2018, 2018, 9823141. [Google Scholar] [CrossRef]
- Ning, W.H.; Zhao, K. Propionyl-L-carnitine induces eNOS activation and nitric oxide synthesis in endothelial cells via PI3 and Akt kinases. Vasc. Pharmacol. 2013, 59, 76–82. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Sinha, S.; Majumder, S.; Muley, A.; Siamwala, J.H.; Gupta, R.; Chatterjee, S. Shear stress promotes nitric oxide production in endothelial cells by sub-cellular delocalization of eNOS: A basis for shear stress mediated angiogenesis. Nitric Oxide Biol. Chem. 2010, 22, 304–315. [Google Scholar] [CrossRef]
- Khoi, P.N.; Park, J.S.; Kim, N.H.; Jung, Y.D. Nicotine stimulates urokinase-type plasminogen activator receptor expression and cell invasiveness through mitogen-activated protein kinase and reactive oxygen species signaling in ECV304 endothelial cells. Toxicol. Appl. Pharmacol. 2012, 259, 248–256. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Khoi, P.N.; Cai, B.; Sah, D.K.; Jung, Y.-D. Sulforaphane Regulates eNOS Activation and NO Production via Src-Mediated PI3K/Akt Signaling in Human Endothelial EA.hy926 Cells. Molecules 2022, 27, 5422. https://doi.org/10.3390/molecules27175422
Zhang Y, Khoi PN, Cai B, Sah DK, Jung Y-D. Sulforaphane Regulates eNOS Activation and NO Production via Src-Mediated PI3K/Akt Signaling in Human Endothelial EA.hy926 Cells. Molecules. 2022; 27(17):5422. https://doi.org/10.3390/molecules27175422
Chicago/Turabian StyleZhang, Ying, Pham Ngoc Khoi, Bangrong Cai, Dhiraj Kumar Sah, and Young-Do Jung. 2022. "Sulforaphane Regulates eNOS Activation and NO Production via Src-Mediated PI3K/Akt Signaling in Human Endothelial EA.hy926 Cells" Molecules 27, no. 17: 5422. https://doi.org/10.3390/molecules27175422
APA StyleZhang, Y., Khoi, P. N., Cai, B., Sah, D. K., & Jung, Y.-D. (2022). Sulforaphane Regulates eNOS Activation and NO Production via Src-Mediated PI3K/Akt Signaling in Human Endothelial EA.hy926 Cells. Molecules, 27(17), 5422. https://doi.org/10.3390/molecules27175422






