Bridging a Century-Old Problem: The Pathophysiology and Molecular Mechanisms of HA Filler-Induced Vascular Occlusion (FIVO)—Implications for Therapeutic Interventions
Abstract
1. Introduction
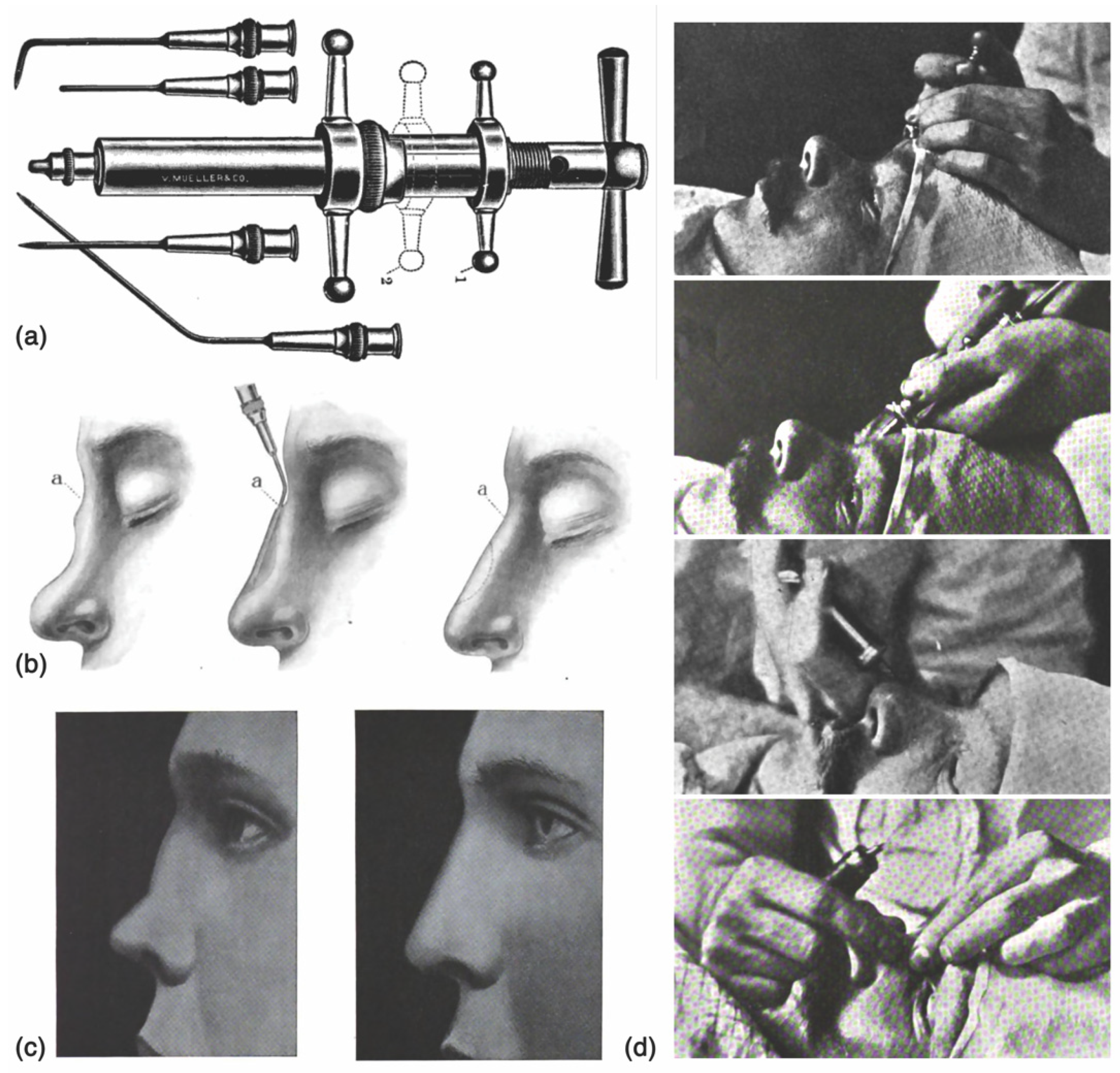
1.1. Historical Perspective
1.2. Modern Parallels
1.3. Clinical Scope of the Problem
2. Hyaluronan Biophysiology and Aesthetic Applications
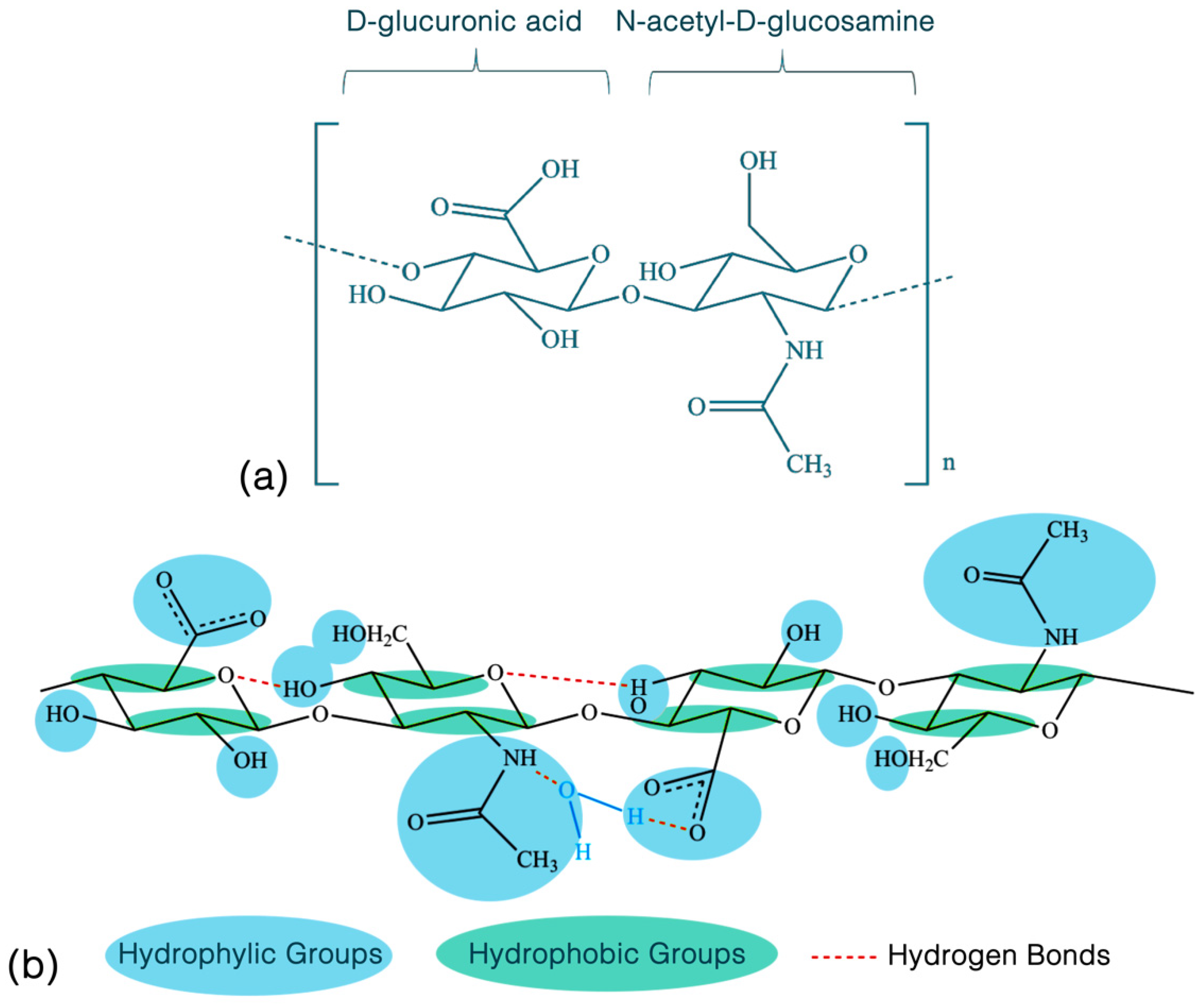
2.1. Basic Biochemical Properties and Function
2.2. Physiological HA Biosynthesis and Degradation
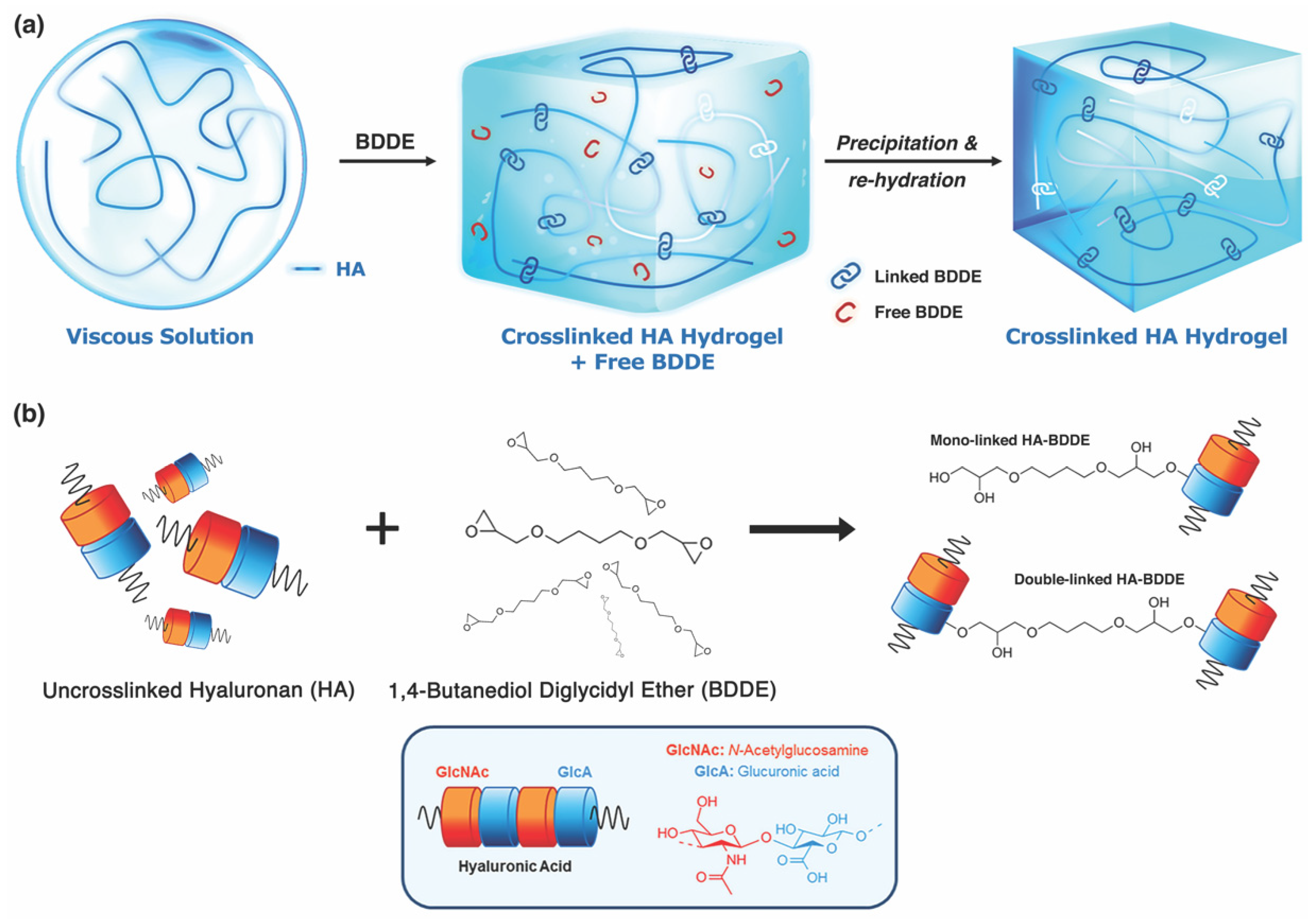
2.3. Commercial HA Synthesis and Reversal Agents
2.4. Rheological Properties and Particle Size
3. Pathophysiology of HA-Mediated Vascular Occlusion
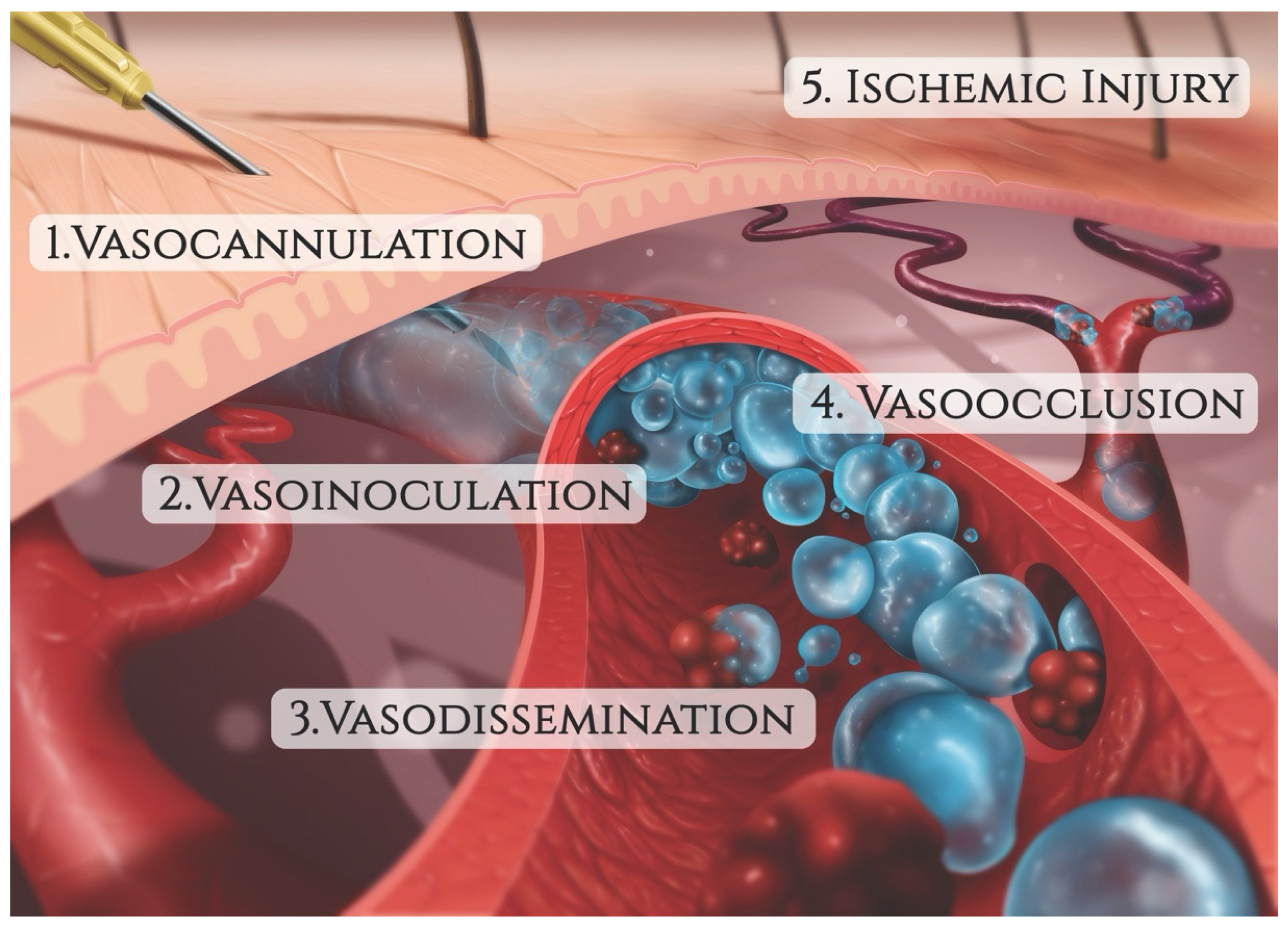
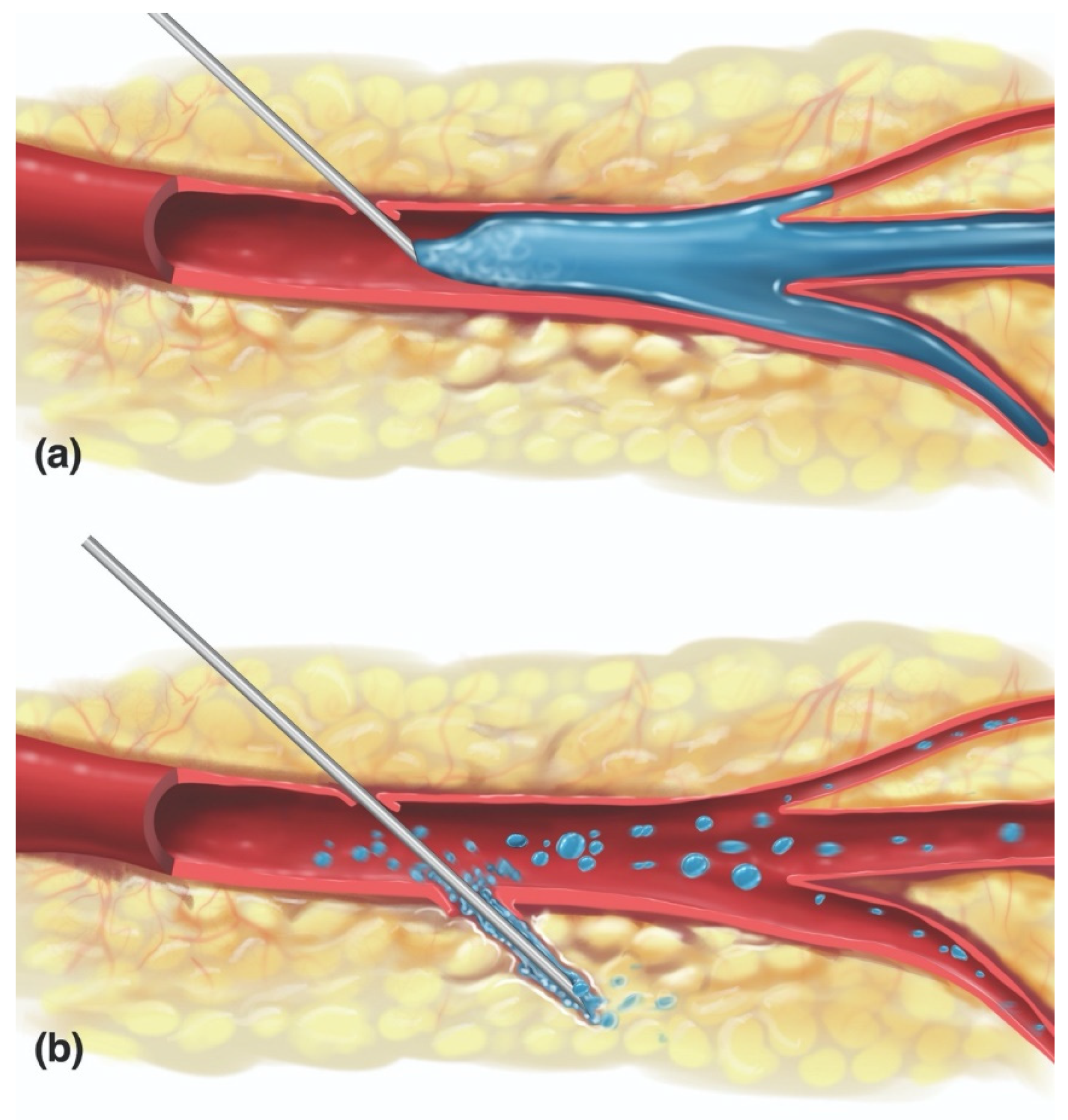
3.1. Vaso-Cannulation
3.2. Vaso-Inoculation
3.3. Vaso-Dissemination
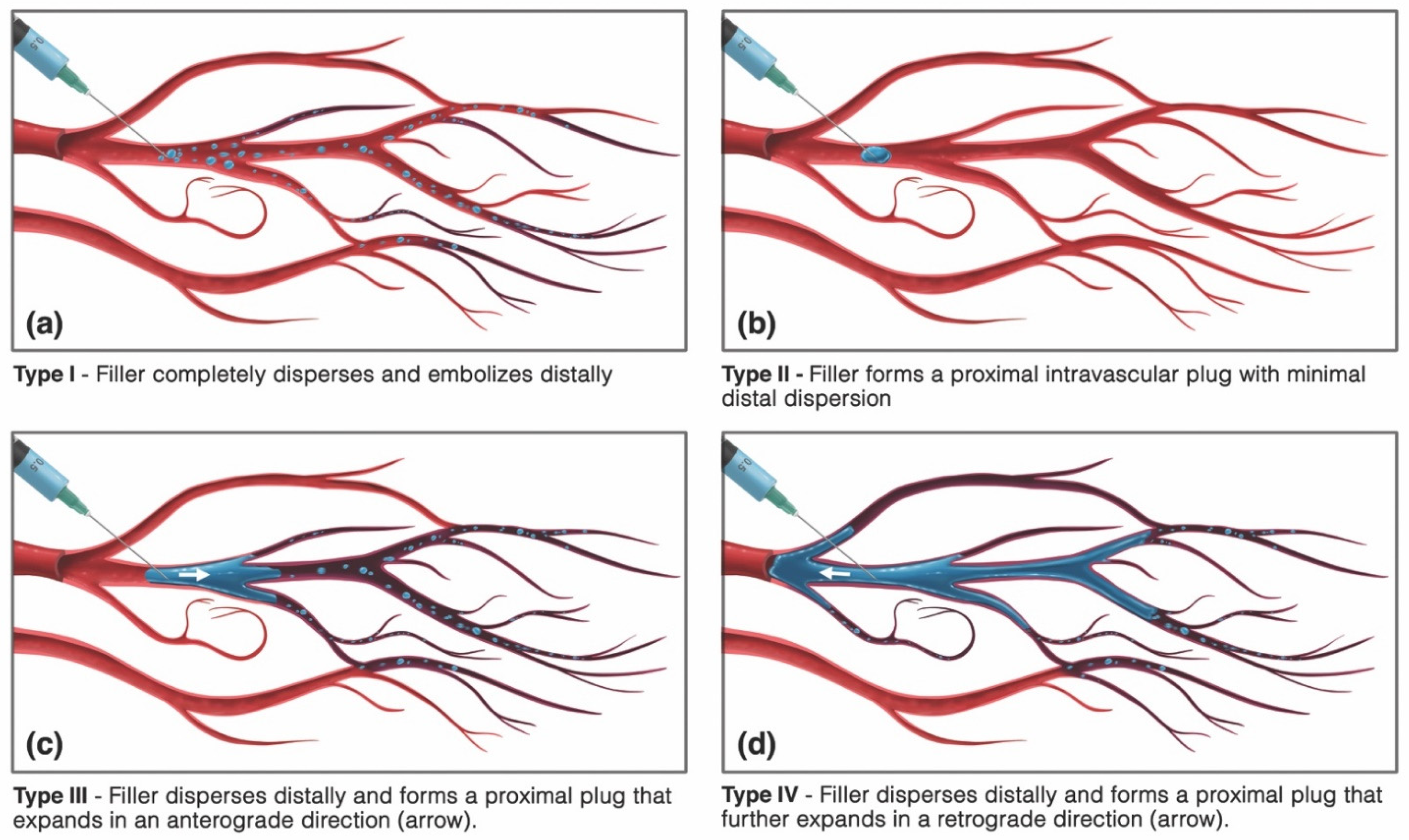
3.4. Vaso-Occlusion
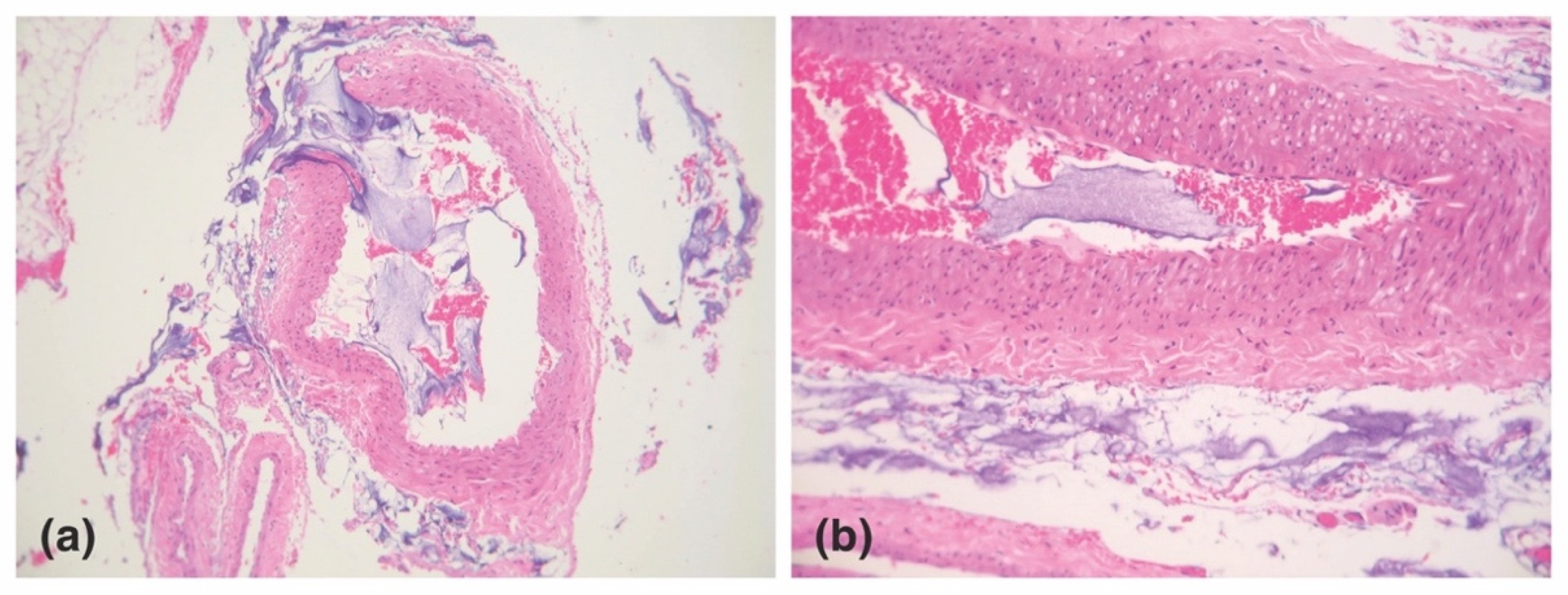
3.4.1. HA Bolus-Mediated Occlusion
3.4.2. Thrombus-Mediated Occlusion
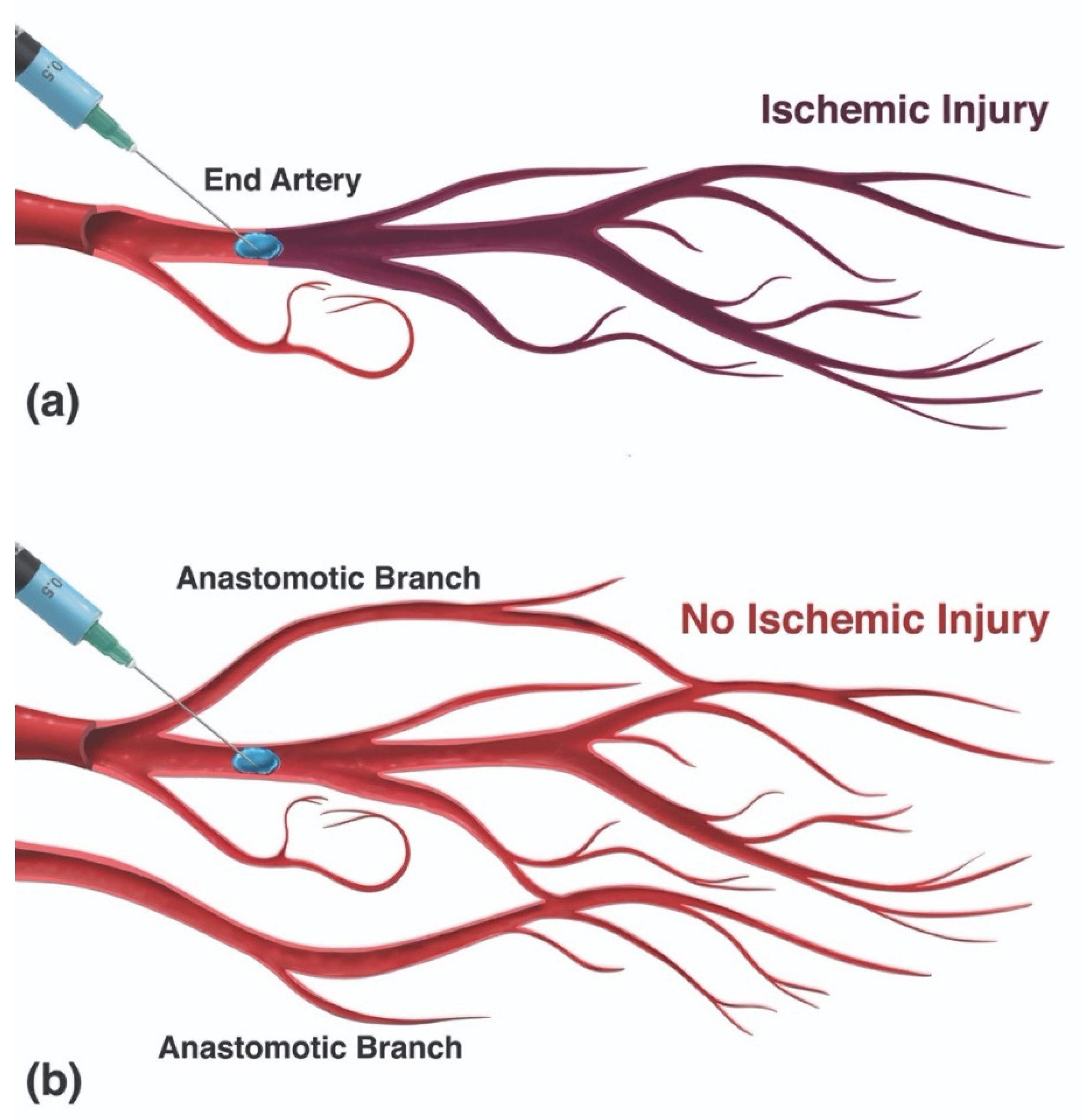
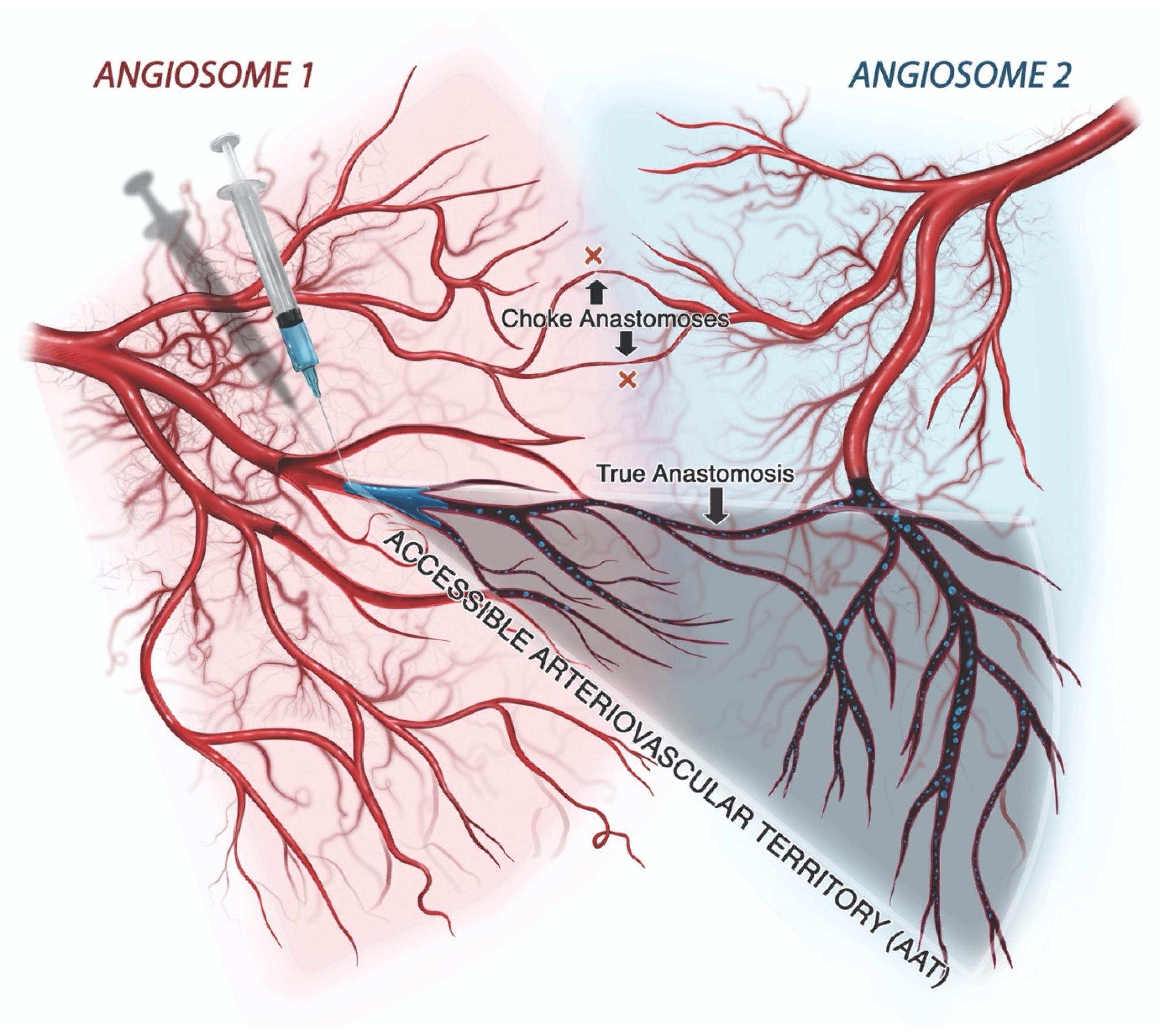
3.5. Tissue Ischemia
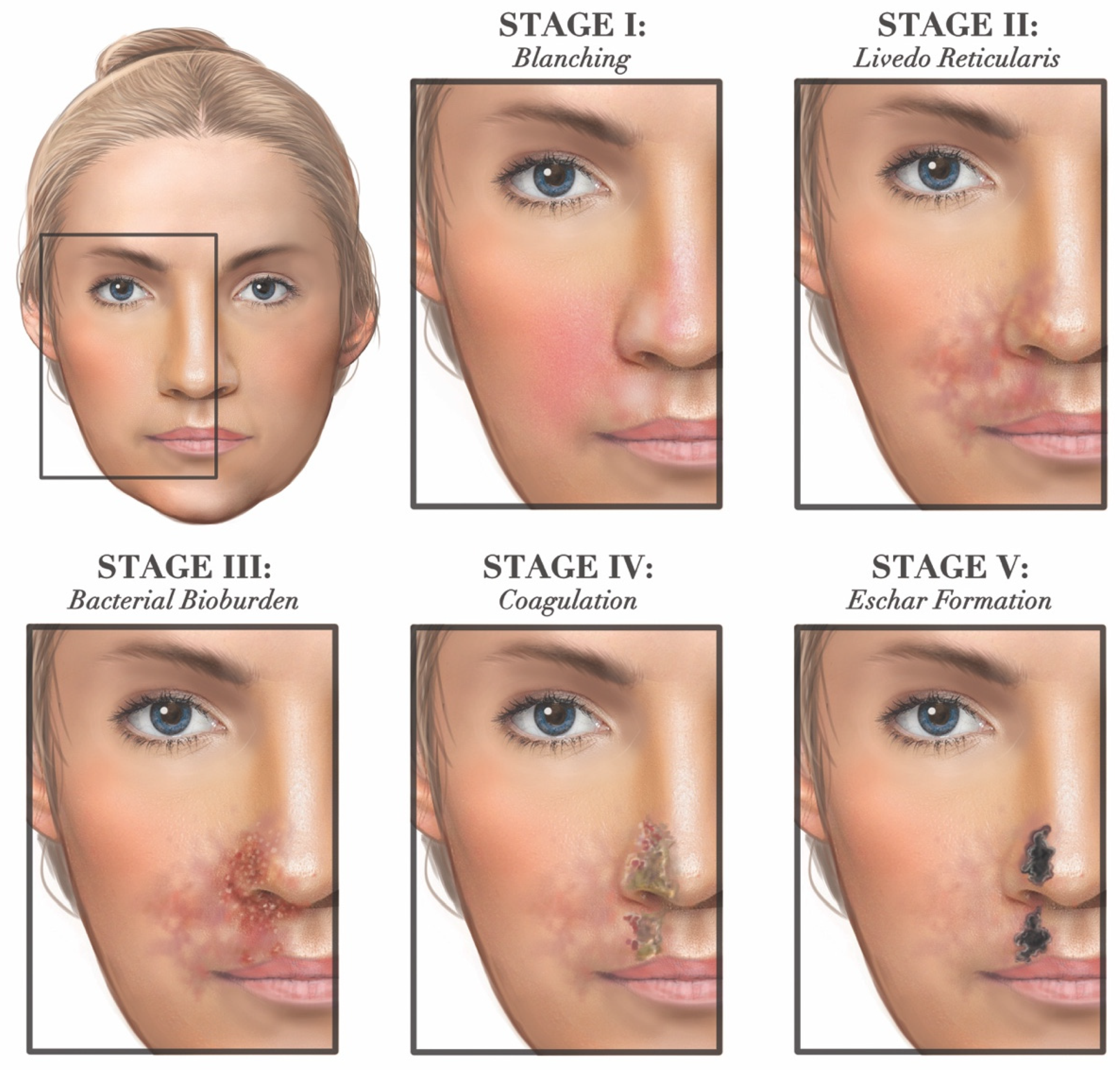
3.5.1. Ischemic Skin Injury
3.5.2. Ischemic Cerebroretinal Injury (CRI)
| Anastomosis | Reference |
|---|---|
| Dorsal nasal a. (ICA-OA)—Angular a. (ECA-FA) | [264] |
| Dorsal nasal a. (ICA-OA)—Infraorbital a. (ECA-IMA) | [264] |
| Supratrochlear a. (ICA-OA)—Anterior branch (ECA-STA) | [264] |
| Supraorbital a. (ICA-OA)—Anterior branch (ECA-STA) | [264] |
| Zygomaticofacial a. (ICA-OA)—Anterior branch (ECA-STA) | [264] |
| Zygomaticofacial a. (ICA-OA)—Transverse facial a. (ECA) | [264] |
| Zygomaticotemporal a. (ICA-OA)—Anterior branch (ECA-STA) | [264] |
| Lacrimal a. (ICA-OA)—Deep temporal a. (ECA-IMA) | [264] |
| Inferior palpebral a. (ICA-OA)—Infraorbital a. (ECA-IMA) | [264] |
| Anterior ethmoid a. (ICA-OA)—Sphenopalatine a (ECA-IMA) | [264] |
| Posterior ethmoid a. (ICA-OA)—Sphenopalatine a (ECA-IMA) | [264] |
| Meningo-ophthalmic a. (ICA-OA)—Middle meningeal a. (ECA-IMA) | [264] |
| Ophthalmic artery (ICA)—Artery of superior orbital fissure (ECA-IMA) | [265] |
| Petrous branch (ICA)—Middle meningeal a. (ECA-IMA) | [266] |
| Superior/tentorial branch (ICA-ILT)—Cavernous branches of MMA (ECA-IMA) | [266] |
| Anterolateral branch (ICA-ILT)—Orbital branches of MMA (ECA-IMA) | [266] |
| Anterolateral branch (ICA-ILT)—Artery of foramen rotundum (ECA-IMA) | [266] |
| Posteromedial branch (ICA-ILT)—Accessory meningeal a. (ECA-IMA) | [266] |
| Petrous ICA—Vidian a. (ECA-IMA) | [266] |
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical modification of hyaluronan and their biomedical applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- American Society of Plastic Surgeons. 2020 Plastic Surgery Statistics Report. 2021. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 15 June 2022).
- Facial Injectables Market. Available online: https://www.fortunebusinessinsights.com/industry-reports/facial-injectables-market-100603 (accessed on 15 June 2022).
- Global Facial Injectable Market Size Report, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/facial-injectables-industry (accessed on 15 June 2022).
- Ballenger, W.L. Chapter 15: The surgical correction of external nasal deformities. In Diseases of the Nose, Throat and Ear, Medical and Surgical; Ballenger, W.L., Ed.; Lea & Febiger: Palo Alto, CA, USA, 1911; pp. 289–301. Available online: https://www.google.com/books/edition/Diseases_of_the_Nose_Throat_and_Ear_Medi/mTxKAAAAMAAJ?hl=en&gbpv=0 (accessed on 15 June 2022).
- Kolle, F. Chapter XVIII: Case reporting methods. In Plastic and Cosmetic Surgery; Kolle, F., Ed.; D. Appleton and Company: New York, NY, USA, 1911; pp. 491–497. Available online: https://www.google.com/books/edition/Plastic_and_Cosmetic_Surgery/_VpDAQAAMAAJ?hl=en&gbpv=0 (accessed on 15 June 2022).
- Miller, C.C. The limitations and the use of paraffin in cosmetic surgery. Wisc. Med. Rec. 1908, 11, 333–338. Available online: https://www.google.com/books/edition/The_Wisconsin_Medical_Recorder/edJXAAAAMAAJ?hl=en&gbpv=0 (accessed on 15 June 2022).
- Robinson, E.P. The use of paraffin in surgery. York State J. Med. 1902, 2, 150–154. [Google Scholar]
- Ritchie, M.D. Paraffin as a cosmetic remedy. Laryngoscope 1904, 14, 622–630. [Google Scholar] [CrossRef]
- Smith, H. Subcutaneous injection of paraffin in the correction of nasal deformities. N. Y. Med. J. 1902, 75, 52–66. [Google Scholar]
- Berry, G.A.; Sym, W.G. Sudden blindness following paraffin injection. Edinb. Med. J. 1903, 56, 283–284. [Google Scholar]
- Ritchie, M.D. Paraffin as a surgical medium. Pa. Med. J. 1905, 8, 276–287. [Google Scholar]
- Bazin, A.T. Two cases of paraffinoma. Br. Med. J. 1929, 2, 1101–1102. [Google Scholar] [CrossRef]
- Chasan, P.E. The history of injectable silicone fluids for soft-tissue augmentation. Plast. Reconstr. Surg. 2007, 120, 2034–2040. [Google Scholar] [CrossRef]
- Cockerham, K.; Hsu, V.J. Collagen-based dermal fillers: Past, present, future. Facial Plast. Surg. 2009, 25, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kontis, T.C.; Rivkin, A. The history of injectable facial fillers. Facial Plast. Surg. 2009, 25, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Sykes, J.M. Hyaluronic acid fillers: History and overview. Facial Plast. Surg. 2011, 27, 523–528. [Google Scholar] [CrossRef]
- ASPS. American Society of Plastic Surgeons: Plastic Surgery National Cosmetic Procedures: Overall Trends 2000/2019/2020. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/cosmetic-procedure-trends-2020.pdf (accessed on 15 June 2022).
- ASPS. American Society of Plastic Surgeons: Plastic Surgery Statistics Report 2005: Minimally-Invasive Procedures. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics?sub=2005+Plastic+Surgery+Statistics (accessed on 15 June 2022).
- Dermal Fillers Market. Available online: https://www.fortunebusinessinsights.com/industry-reports/dermal-fillers-market-100939 (accessed on 15 June 2022).
- Hyaluronic Acid Based Dermal Fillers Market. Available online: https://www.fortunebusinessinsights.com/industry-reports/hyaluronic-acid-based-dermal-fillers-market-100951 (accessed on 15 June 2022).
- Beleznay, K.; Carruthers, J.D.; Humphrey, S.; Jones, D. Avoiding and treating blindness from fillers: A review of the world literature. Dermatol. Surg. 2015, 41, 1097–1117. [Google Scholar] [CrossRef]
- Beleznay, K.; Carruthers, J.D.A.; Humphrey, S.; Carruthers, A.; Jones, D. Update on avoiding and treating blindness from fillers: A recent review of the world literature. Aesthet. Surg. J. 2019, 39, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.J.; Bowhay, A.; Blevins, L.W.; Patel, S.M.; Zuliani, G.F. Patterns of filler-induced facial skin necrosis: A systematic review of 243 cases and introduction of the F.O.E.M. scoring system and grading scale. Plast. Reconstr. Surg. 2022, in press.
- Wang, H.C.; Yu, N.; Wang, X.; Dong, R.; Long, X.; Feng, X.; Li, J.; Wu, W.T.L. Cerebral embolism as a result of facial filler injections: A literature review. Aesthet. Surg. J. 2022, 42, 162–175. [Google Scholar] [CrossRef] [PubMed]
- FDA. Labeling Change Request for Soft Tissue Fillers 2015. Available online: https://www.fda.gov/media/92608/download (accessed on 15 June 2022).
- Ortiz, A.E.; Ahluwalia, J.; Song, S.S.; Avram, M.M. Analysis of U.S. food and drug administration data on soft-tissue filler complications. Dermatol. Surg. 2020, 46, 958–961. [Google Scholar] [CrossRef]
- Soares, D.J.; Blevins, L.W. Distal internal maxillary artery occlusion with palatal necrosis following cheek injection with calcium hydroxylapatite. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4164. [Google Scholar] [CrossRef]
- Tran, A.Q.; Lee, W.W. Vision loss and blindness following fillers. J. Dermatol. Skin. Sci. 2021, 3, 1–4. [Google Scholar]
- Yang, Y.; Sheng, H.; Gu, Q.; Su, L.; Tong, H.; Chen, J.; Qi, X. Death caused by vaginal injection of hyaluronic acid and collagen: A case report. Aesthet. Surg. J. 2020, 40, 263–268. [Google Scholar] [CrossRef]
- Gabrielpillai, J.; Salamat, A.; Schaefer, C.; Kania, A.; Lunatschek, C.; Eichhorn, K.W.; Bootz, F.; Send, T. Hyaluronic acid-based filler injection: Late-onset thrombosis of the frontal vein. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3216. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.G.; Hong, K.S.; Choi, E.Y. A case of nonthrombotic pulmonary embolism after facial injection of hyaluronic Acid in an illegal cosmetic procedure. Tuberc. Respir. Dis. 2014, 77, 90–93. [Google Scholar] [CrossRef]
- Tao, L.; Wu, Q.; Wang, J.; Xu, K.; Yu, G.; Wan, F.; Qian, H.; Tang, J. A patient with cerebral venous sinus thrombosis induced by facial hyaluronic acid injection. Ann. Hematol. 2021, 100, 2403–2405. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Ahmad, S.A. Use and Application of MAUDE in Patient Safety. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570582/ (accessed on 15 June 2022).
- FDA. Food and Drug Administration: Executive Summary General Issues Panel Meeting on Dermal Filler. Available online: https://www.fda.gov/media/146870/download (accessed on 15 June 2022).
- Svider, P.F.; Keeley, B.R.; Zumba, O.; Mauro, A.C.; Setzen, M.; Eloy, J.A. From the operating room to the courtroom: A comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope 2013, 123, 1849–1853. [Google Scholar] [CrossRef]
- Dey, J.K.; Ishii, L.E.; Byrne, P.J.; Boahene, K.D.; Ishii, M. The social penalty of facial lesions: New evidence supporting high-quality reconstruction. JAMA Facial Plast. Surg. 2015, 17, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Dey, J.K.; Ishii, L.E.; Joseph, A.W.; Goines, J.; Byrne, P.J.; Boahene, K.D.; Ishii, M. The cost of facial deformity: A health utility and valuation study. JAMA Facial Plast. Surg. 2016, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Palmer, J. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Weissman, B.; Meyer, K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J. Am. Chem. Soc. 1954, 76, 1753–1757. [Google Scholar] [CrossRef]
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef]
- de Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Selyanin, M.A.; Boykov, P.Y.; Khabarov, V.N.; Polyak, F. The history of hyaluronic acid discovery, foundational research and initial use. In Hyaluronic Acid; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Greco, T.M.; Antunes, M.B.; Yellin, S.A. Injectable fillers for volume replacement in the aging face. Facial Plast. Surg. 2012, 28, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, A.; Blevins, L.W.; Wills-Kofford, J.C.; Soares, D.J. Dermal filler re-contouring of the aged jawline: A useful non-surgical modality in facial rejuvenation. J. Aesthetic Nurs. 2022, 11, 70–78. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N.; Schiller, J.; Stern, R.; Soltés, L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
- O’Regan, M.; Martini, I.; Crescenzi, F.; De Luca, C.; Lansing, M. Molecular mechanisms and genetics of hyaluronan biosynthesis. Int. J. Biol. Macromol. 1994, 16, 283–286. [Google Scholar] [CrossRef]
- Laurent, U.B.G.; Reed, R.K. Turnover of hyaluronan in the tissues. Adv. Drug Del. Rev. 1991, 7, 237–256. [Google Scholar] [CrossRef]
- Cowman, M.K.; Lee, H.G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The content and size of hyaluronan in biological fluids and tissues. Front. Immunol. 2015, 2, 261. [Google Scholar] [CrossRef]
- Scott, J.E.; Heatley, F. Hyaluronan forms specific stable tertiary structures in aqueous solution: A 13C NMR study. Proc. Natl. Acad. Sci. USA 1999, 27, 4850–4855. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Dea, I.C.; Moorhouse, R.; Rees, D.A.; Arnott, S.; Guss, J.M.; Balazs, E.A. Hyaluronic acid: A novel, double helical molecule. Science 1973, 179, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Maytin, E.V. Hyaluronan: More than just a wrinkle filler. Glycobiology 2016, 26, 553–559. [Google Scholar] [CrossRef]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Wang, S.J.; Bourguignon, L.Y. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 2011, 178, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.; Jenkins, R.H.; Meran, S.; Phillips, A.; Steadman, R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am. J. Pathol. 2009, 175, 148–160. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Caon, I.; Parnigoni, A.; Viola, M.; Karousou, E.; Passi, A.; Vigetti, D. Cell Energy metabolism and hyaluronan synthesis. J. Histochem. Cytochem. 2021, 69, 35–47. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef]
- Viola, M.; Vigetti, D.; Karousou, E.; D’Angelo, M.L.; Caon, I.; Moretto, P.; De Luca, G.; Passi, A. Biology and biotechnology of hyaluronan. Glycoconj. J. 2015, 32, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Tammi, R.H.; Passi, A.G.; Rilla, K.; Karousou, E.; Vigetti, D.; Makkonen, K.; Tammi, M.I. Transcriptional and posttranslational regulation of hyaluronan synthesis. FEBS J. 2011, 278, 1419–1428. [Google Scholar] [CrossRef]
- Viola, M.; Bartolini, B.; Vigetti, D.; Karousou, E.; Moretto, P.; Deleonibus, S.; Sawamura, T.; Wight, T.N.; Hascall, V.C.; De Luca, G.; et al. Oxidized low density lipoprotein (LDL) affects hyaluronan synthesis in human aortic smooth muscle cells. J. Biol. Chem. 2013, 288, 29595–29603. [Google Scholar] [CrossRef] [PubMed]
- Bastow, E.R.; Byers, S.; Golub, S.B.; Clarkin, C.E.; Pitsillides, A.; Fosang, A.J. Hyaluronan synthesis and degradation in cartilage and bone. Cell. Mol. Life Sci. 2008, 65, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwon, S.B.; Whang, K.U.; Lee, J.S.; Park, Y.L.; Lee, S.Y. The duration of hyaluronidase and optimal timing of hyaluronic acid (HA) filler reinjection after hyaluronidase injection. J. Cosmet. Laser Ther. 2018, 20, 52–57. [Google Scholar] [CrossRef]
- Kwak, S.S.; Yoon, K.H.; Kwon, J.H.; Kang, W.H.; Rhee, C.H.; Yang, G.H.; Cruz, D.J.M.; Son, W.C. Comparative analysis of hyaluronidase-mediated degradation among seven hyaluronic acid fillers in hairless mice. Clin. Cosmet. Investig. Dermatol. 2021, 8, 241–248. [Google Scholar] [CrossRef]
- Kim, D.-W.; Yoon, E.-S.; Ji, Y.-H.; Park, S.-H.; Lee, B.-I.; Dhong, E.-S. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1590–1595. [Google Scholar] [CrossRef]
- Weigel, J.A.; Weigel, P.H. Characterization of the recombinant rat 175-kDa hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2003, 278, 42802–42811. [Google Scholar] [CrossRef]
- Yoshida, H.; Okada, Y. Role of HYBID (hyaluronan binding protein involved in hyaluronan depolymerization), Alias KIAA1199/CEMIP, in hyaluronan degradation in normal and photoaged skin. Int. J. Mol. Sci. 2019, 20, 5804. [Google Scholar] [CrossRef] [PubMed]
- Mikulic, M.U.S. Hyaluronic acid raw material market size 2014–2024, by application Hyaluronic acid raw material market size in the U.S from 2014 to 2024, by application. Statista 2018, 212, 1–2. [Google Scholar]
- Huerta-Ángeles, G.; Nešporová, K.; Ambrožová, G.; Kubala, L.; Velebný, V. An effective translation: The development of hyaluronan-based medical products from the physicochemical, and preclinical aspects. Front. Bioeng. Biotechnol. 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Ucm, R.; Aem, M.; Lhb, Z.; Kumar, V.; Taherzadeh, M.J.; Garlapati, V.K.; Chandel, A.K. Comprehensive review on biotechnological production of hyaluronic acid: Status, innovation, market and applications. Bioengineered 2022, 13, 9645–9661. [Google Scholar] [CrossRef]
- Widner, B.; Behr, R.; von Dollen, S.; Tang, M.; Heu, T.; Sloma, A.; Sternberg, D.; DeAngelis, P.L.; Weigel, P.H.; Brown, S. Hyaluronic acid production in Bacillus subtilis. Appl. Environ. Microbiol. 2005, 71, 3747–3752. [Google Scholar] [CrossRef]
- Jing, W.; Deangelis, P.L. Dissection of the two transferase activities of the Pasteurella multocida hyaluronan synthase: Two active sites exist in one polypeptide. Glycobiology 2000, 10, 883–889. [Google Scholar] [CrossRef]
- Jing, W.; Deangelis, P.L. Synchronized chemoenzymatic synthesis of monodisperse hyaluronan polymers. J. Biol. Chem. 2004, 279, 42345–42349. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Santana, M.H.A.; Petrides, D. Technoeconomic analysis of a hyaluronic acid production process utilizing streptococcal fermentation. Processes 2021, 9, 241. [Google Scholar] [CrossRef]
- Silverstein, S.M.; Greenbaum, S.; Stern, R. Hyaluronidase in ophthalmology. J. Appl. Res. 2012, 1, 12–13. [Google Scholar]
- Murray, G.; Convery, C.; Walker, L.; Davies, E. Guideline for the safe use of hyaluronidase in aesthetic medicine, including modified high-dose protocol. J. Clin. Aesthet. Dermatol. 2021, 14, 69–75. [Google Scholar]
- Murray, G.; Convery, C.; Walker, L.; Davies, E. Guideline for the management of hyaluronic acid filler-induced vascular occlusion. J. Clin. Aesthet. Dermatol. 2021, 14, 61–69. [Google Scholar]
- Walker, L.; Convery, C.; Davies, E.; Murray, G.; Croasdell, B. Consensus opinion for the management of soft tissue filler induced vision loss. J. Clin. Aesthet. Dermatol. 2021, 14, 84–94. [Google Scholar]
- Urdiales-Gálvez, F.; Delgado, N.E.; Figueiredo, V.; Lajo-Plaza, J.V.; Mira, M.; Moreno, A.; Ortíz-Martí, F.; Del Rio-Reyes, R.; Romero-Álvarez, N.; Del Cueto, S.R.; et al. Treatment of soft tissue filler complications: Expert consensus recommendations. Aesthet. Plast. Surg. 2018, 42, 498–510. [Google Scholar] [CrossRef]
- Guarise, C.; Barbera, C.; Pavan, M.; Panfilo, S.; Beninatto, R.; Galesso, D. HA-based dermal filler: Downstream process comparison, impurity quantitation by validated HPLC-MS analysis, and in vivo residence time study. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019867075. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Bartlett, E.L.; Dayan, E. Practical approach and safety of hyaluronic acid fillers. Plast. Reconstr. Surg. Glob. Open 2019, 7, 2172. [Google Scholar] [CrossRef]
- Edsman, K.; Nord, L.I.; Ohrlund, A.; Lärkner, H.; Kenne, A.H. Gel properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2012, 38, 1170–1179. [Google Scholar] [CrossRef]
- Fagien, S.; Bertucci, V.; von Grote, E.; Mashburn, J.H. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast. Reconstr. Surg. 2019, 143, 707–720. [Google Scholar] [CrossRef]
- Faivre, J.; Gallet, M.; Tremblais, E.; Trévidic, P.; Bourdon, F. Advanced concepts in rheology for the evaluation of hyaluronic acid-based soft tissue fillers. Dermatol. Surg. 2021, 47, 159–167. [Google Scholar] [CrossRef]
- Chun, C.; Lee, D.Y.; Kim, J.-T.; Kwon, M.-K.; Kim, Y.-Z.; Kim, S.-S. Effect of molecular weight of hyaluronic acid (HA) on viscoelasticity and particle texturing feel of HA dermal biphasic fillers. Biomater. Res. 2016, 20, 24. [Google Scholar] [CrossRef]
- Bertucci, V.; Lynde, C.B. Current concepts in the use of small-particle hyaluronic acid. Plast. Reconstr. Surg. 2015, 136, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Y.; Fei, X.; Fan, Q.; Mao, J. Monophasic and biphasic hyaluronic acid fillers for esthetic correction of nasolabial folds: A meta-analysis of randomized controlled trials. Aesthet. Plast. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.C.; Sarazin, D.; Bezzola, A.; Terrani, C.; Micheels, P. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol. Surg. 2011, 37, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Kablik, J.; Monheit, G.D.; Yu, L.; Chang, G.; Gershkovich, J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009, 35, 302–312. [Google Scholar] [CrossRef]
- Ugradar, S. Quantifying the digestion of cross-linked hyaluronic acid fillers with hyaluronidase. Dermatol. Surg. 2021, 47, 1233–1236. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, T.-R.; Lee, S.E.; Jang, Y.N.; Han, H.S.; Mun, S.K.; Kim, B.J. Comparative evaluation of the effectiveness of novel hyaluronic acid-polynucleotide complex dermal filler. Sci. Rep. 2020, 10, 5127. [Google Scholar] [CrossRef]
- Hee, C.K.; Shumate, G.T.; Narurkar, V.; Bernardin, A.; Messina, D.J. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol. Surg. 2015, 41, 373–381. [Google Scholar] [CrossRef]
- De La Guardia, C.; Virno, A.; Musumeci, M.; Bernardin, A.; Silberberg, M.B. Rheologic and physicochemical characteristics of hyaluronic acid fillers: Overview and relationship to product performance. Facial Plast. Surg. 2022, 38, 116–123. [Google Scholar] [CrossRef]
- Edsman, K.L.M.; Öhrlund, Å. Cohesion of hyaluronic acid fillers: Correlation between cohesion and other physicochemical properties. Dermatol. Surg. 2018, 44, 557–562. [Google Scholar] [CrossRef]
- Sundaram, H.; Rohrich, R.J.; Liew, S.; Sattler, G.; Talarico, S.; Trévidic, P.; Molliard, S.G. Cohesivity of hyaluronic acid fillers: Development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six u.s. food and drug administration-approved fillers. Plast. Reconstr. Surg. 2015, 136, 678–686. [Google Scholar] [CrossRef]
- Ryu, C.; Lu, J.E.; Zhang-Nunes, S. Response of twelve different hyaluronic acid gels to varying doses of recombinant human hyaluronidase. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Kin, M.; Walker, L.; Convery, C.; Davies, E. Management of a vascular occlusion associated with cosmetic injections. J. Clin. Aesthet. Dermatol. 2020, 13, 53–58. [Google Scholar]
- Nikolis, A.; Enright, K.M.; Öhrlund, Å.; Winlöf, P.; Cotofana, S. A randomized, split-face, double-blind, comparative study of the safety and efficacy of small- and large-particle hyaluronic acid fillers for the treatment of nasolabial folds. J. Cosmet. Dermatol. 2021, 20, 1450–1458. [Google Scholar] [CrossRef]
- Lee, W.; Oh, W.; Moon, H.-J.; Koh, I.-S.; Yang, E.-J. Soft tissue filler properties can be altered by a small-diameter needle. Dermatol. Surg. 2020, 46, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.G.F.; Regattieri, N.A.T.; Pompeu, M.F.; Costa, I.M.C. External vascular compression by hyaluronic acid filler documented with high-frequency ultrasound. J. Cosmet. Dermatol. 2019, 18, 1629–1631. [Google Scholar] [CrossRef]
- Chang, S.-H.; Yousefi, S.; Qin, J.; Tarbet, K.; Dziennis, S.; Wang, R.; Chappell, M.C. External compression versus intravascular injection: A mechanistic animal model of filler-induced tissue ischemia. Ophthalmic Plast. Reconstr. Surg. 2016, 32, 261–266. [Google Scholar] [CrossRef]
- Hwang, C.J.; Morgan, P.V.; Pimentel, A.; Sayre, J.W.; Goldberg, R.A.; Duckwiler, G. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: An animal model with ICG imaging. Ophthalmic Plast. Reconstr. Surg. 2016, 32, 118–122. [Google Scholar] [CrossRef]
- Rocha, P.S.; Guerra, T.A.; Teixeira, D.A. Description of a safe doppler ultrasound-guided technique for hyaluronic acid filler in the face-A method to avoid adverse vascular events. J. Cosmet. Dermatol. 2021, 21, 2783–2787. [Google Scholar] [CrossRef]
- Wolferman, A.; Dwyer, F.P., Jr. Unilateral and bilateral ligation of the external carotid for epistaxis. AMA Arch. Otolaryngol. 1955, 62, 310–315. [Google Scholar] [CrossRef]
- Hassard, A.D.; Kirkpatrick, D.A.; Wong, F.S. Ligation of the external carotid and anterior ethmoidal arteries for severe or unusual epistaxis resulting from facial fractures. Can. J. Surg. 1986, 29, 447–449. [Google Scholar]
- Cooke, E.T. An evaluation and clinical study of severe epistaxis treated by arterial ligation. J. Laryngol. Otol. 1985, 99, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Schelke, L.; Decates, T.; Kadouch, J.; Velthuis, P. Incidence of vascular obstruction after filler injections. Aesthet. Surg. J. 2020, 40, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Goldman, A. Facial vascular danger zones for filler injections. Dermatol. Ther. 2020, 3, 4285. [Google Scholar] [CrossRef] [PubMed]
- Humzah, M.D.; Ataullah, S.; Chiang, C.; Malhotra, R.; Goldberg, R. The treatment of hyaluronic acid aesthetic interventional induced visual loss (AIIVL): A consensus on practical guidance. J. Cosmet. Dermatol. 2019, 18, 71–76. [Google Scholar] [CrossRef]
- Cohen, J.L.; Biesman, B.S.; Dayan, S.H.; de Lorenzi, C.; Lambros, V.L.; Nestor, M.S.; Sadick, N.; Sykes, J. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: Consensus recommendations. Aesthet. Surg. J. 2015, 35, 844–849. [Google Scholar] [CrossRef]
- Hexsel, D.; Soirefmann, M.; Porto, M.D.; Siega, C.; Schilling-Souza, J.; Brum, C. Double-blind, randomized, controlled clinical trial to compare safety and efficacy of a metallic cannula with that of a standard needle for soft tissue augmentation of the nasolabial folds. Dermatol. Surg. 2012, 38, 207–214. [Google Scholar] [CrossRef]
- van Loghem, J.A.J.; Humzah, D.; Kerscher, M. Cannula versus sharp needle for placement of soft tissue fillers: An observational cadaver study. Aesthet. Surg. J. 2017, 38, 73–88. [Google Scholar] [CrossRef]
- Pavicic, T.; Frank, K.; Erlbacher, K.; Neuner, R.; Targosinski, S.; Schenck, T.; Gotkin, H.; Cotofana, S. Precision in dermal filling: A comparison between needle and cannula when using soft tissue fillers. J. Drugs. Dermatol. 2017, 16, 866–872. [Google Scholar]
- Jones, D.; Palm, M.; Cox, S.E.; McDermott, M.; Sartor, M.; Chawla, S. Safety and effectiveness of hyaluronic acid filler, VYC-20L, via cannula for cheek augmentation: A randomized, single-blind, controlled study. Dermatol. Surg. 2021, 47, 1590–1594. [Google Scholar] [CrossRef]
- Alam, M.; Kakar, R.; Dover, J.S.; Harikumar, V.; Kang, B.Y.; Wan, H.T.; Poon, E.; Jones, D.H. Rates of vascular occlusion associated with using needles vs cannulas for filler injection. JAMA Dermatol. 2021, 157, 174–180. [Google Scholar] [CrossRef]
- Ugradar, S.; Hoenig, J. Measurement of the force required by blunt-tipped microcannulas to perforate the facial artery. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, T.; Webb, K.L.; Frank, K.; Gotkin, R.H.; Tamura, B.; Cotofana, S. Arterial wall penetration forces in needles versus cannulas. Plast. Reconstr. Surg. 2019, 143, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, A.; Prescher, A.; Riediger, D.; Axer, H. Anatomy of the SMAS revisited. Aesthet. Plast. Surg. 2003, 27, 258–264. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, B.; Guo, N.; Li, L.; Wang, Y.; Ma, X.; Su, Y. Fatal cerebral infarction and ophthalmic artery occlusion after nasal augmentation with hyaluronic acid—A case report and review of literature. Aesthet. Plast. Surg. 2020, 44, 543–548. [Google Scholar] [CrossRef]
- Thanasarnaksorn, W.; Cotofana, S.; Rudolph, C.; Kraisak, P.; Chanasumon, N.; Suwanchinda, A. Severe vision loss caused by cosmetic filler augmentation: Case series with review of cause and therapy. J. Cosmet. Dermatol. 2018, 17, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Z.; Hu, J.Y.; Wu, P.S.; Yu, S.B.; Kikkawa, D.O.; Lu, W. Posterior ciliary artery occlusion caused by hyaluronic acid injections into the forehead: A case report. Medicine 2016, 95, e3124. [Google Scholar] [CrossRef]
- Darling, M.D.; Peterson, J.D.; Fabi, S.G. Impending necrosis after injection of hyaluronic acid and calcium hydroxylapatite fillers: Report of 2 cases treated with hyperbaric oxygen therapy. Dermatol. Surg. 2014, 40, 1049–1052. [Google Scholar] [CrossRef]
- Wibowo, A.; Kapoor, K.M.; Philipp-Dormston, W.G. Reversal of post-filler vision loss and skin ischaemia with high-dose pulsed hyaluronidase injections. Aesthet. Plast. Surg. 2019, 43, 1337–1344. [Google Scholar] [CrossRef]
- Goodman, G.J.; Magnusson, M.R.; Callan, P.; Roberts, S.; Hart, S.; McDonald, C.B.; Liew, S.; Porter, C.; Corduff, N.; Clague, M. Neither positive nor negative aspiration before filler injection should be relied upon as a safety maneuver. Aesthet. Surg. J. 2021, 41, 134–136. [Google Scholar] [CrossRef]
- Casabona, G. Blood aspiration test for cosmetic fillers to prevent accidental intravascular injection in the face. Dermatol. Surg. 2015, 41, 841–847. [Google Scholar] [CrossRef]
- Goodman, G.J.; Magnusson, M.R.; Callan, P.; Roberts, S.; Hart, S.; Lin, F.; Rahman, E.; McDonald, C.B.; Liew, S.; Porter, C.; et al. Aspiration before tissue filler-an exercise in futility and unsafe practice. Aesthet. Surg. J. 2022, 42, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, K.M.; Murthy, R.; Hart, S.L.A.; Cattin, T.A.; Nola, P.F.; Rossiter, A.P.; Singh, R.; Singh, S. Factors influencing pre-injection aspiration for hyaluronic acid fillers: A systematic literature review and meta-analysis. Dermatol. Ther. 2021, 34, e14360. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-J.; Lee, W.; Kim, J.-S.; Yang, E.-J.; Sundaram, H. Aspiration revisited: Prospective evaluation of a physiologically pressurized model with animal correlation and broader applicability to filler complications. Aesthet. Surg. J. 2021, 41, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Schelke, L.W.; Decates, T.S.; Velthuis, P.J. Ultrasound to improve the safety of hyaluronic acid filler treatments. J. Cosmet. Dermatol. 2018, 17, 1019–1024. [Google Scholar] [CrossRef]
- Lee, G.S.K. Use of handheld ultrasound Doppler to prevent complications from intra-arterial injection of dermal fillers: Clinical experience. J. Cosmet. Dermatol. 2019, 18, 1267–1270. [Google Scholar] [CrossRef]
- Jagus, D.; Skrzypek, E.; Migda, B.; Wozniak, W.; Mlosek, R.K. Usefulness of Doppler sonography in aesthetic medicine. J. Ultrason. 2021, 20, 268–272. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J.-S.; Moon, H.-J.; Yang, E.-J. A safe doppler ultrasound-guided method for nasolabial fold correction with hyaluronic acid filler. Aesthet. Surg. J. 2021, 41, 486–492. [Google Scholar] [CrossRef]
- Iwayama, T.; Hashikawa, K.; Osaki, T.; Yamashiro, K.; Horita, N.; Fukumoto, T. Ultrasonography-guided cannula method for hyaluronic acid filler injection with evaluation using laser speckle flowgraphy. Plast. Reconstr. Surg. Glob. Open 2018, 6, 1776. [Google Scholar] [CrossRef]
- Quezada-Gaón, N.; Wortsman, X. Ultrasound-guided hyaluronidase injection in cosmetic complications. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 39–40. [Google Scholar] [CrossRef]
- Munia, M.A.; Munia, C.G.; Parada, M.B.; Ben-Hurferraz Parente, J.; Wolosker, N. Doppler ultrasound in the management of vascular complications associated with hyaluronic acid dermal fillers. J. Clin. Aesthet. Dermatol. 2022, 15, 40–43. [Google Scholar]
- Levy, J.; Barrett, D.L.; Harris, N.; Jeong, J.J.; Yang, X.; Chen, S.C. High-frequency ultrasound in clinical dermatology: A review. Ultrasound J. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzi, C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthet. Surg. J. 2017, 37, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Hoey, M.; Egbert, J. Pressure measurements during injection of corticosteroids: In vivo studies. Med. Biol. Eng. Comput. 1999, 37, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Pozza, E.D.; Toth, G.; Gharb, B.B.; Zins, J.E. Pathophysiology study of filler-induced blindness. Aesthet. Surg. J. 2019, 39, 96–106. [Google Scholar] [CrossRef]
- Townsend, J.M.; Beck, E.C.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019, 91, 126–140. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, S.M.; Lee, W.; Yang, E.J. Comparison of hyaluronic acid filler ejection pressure with injection force for safe filler injection. J. Cosmet. Dermatol. 2021, 20, 1551–1556. [Google Scholar] [CrossRef]
- Nie, F.; Xie, H.; Wang, G.; An, Y. Risk Comparison of filler embolism between polymethyl methacrylate (PMMA) and hyaluronic acid (HA). Aesthet. Plast. Surg. 2019, 43, 853–860. [Google Scholar] [CrossRef]
- Koziej, M.; Polak, J.; Wnuk, J.; Trybus, M.; Walocha, J.; Chrapusta, A.; Brzegowy, P.; Mizia, E.; Popiela, T.; Hołda, M. The transverse facial artery anatomy: Implications for plastic surgery procedures. PLoS ONE 2019, 14, 0211974. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ha, T.-J.; Koh, K.-S.; Song, W.-C. External and internal diameters of the facial artery relevant to intravascular filler injection. Plast. Reconstr. Surg. 2019, 143, 1031–1037. [Google Scholar] [CrossRef]
- Kim, B.S.; Jung, Y.J.; Chang, C.H.; Choi, B.Y. The anatomy of the superficial temporal artery in adult koreans using 3-dimensional computed tomographic angiogram: Clinical research. J. Cerebrovasc. Endovasc. Neurosurg. 2013, 15, 145–151. [Google Scholar] [CrossRef]
- Phumyoo, T.; Jiirasutat, N.; Jitaree, B.; Rungsawang, C.; Uruwan, S.; Tansatit, T. Anatomical and ultrasonography-based investigation to localize the arteries on the central forehead region during the glabellar augmentation procedure. Clin. Anat. 2020, 33, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Money, S.M.; Wall, W.B.; Davis, L.S.; Edmondson, A.C. Lumen diameter and associated anatomy of the superior labial artery with a clinical application to dermal filler injection. Dermatol. Surg. 2020, 46, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.T.; Colon-Acevedo, B.; Mettu, P.; DeLorenzi, C.; Woodward, J.A. An anatomical analysis of the supratrochlear artery: Considerations in facial filler injections and preventing vision loss. Aesthet. Surg. J. 2017, 37, 203–208. [Google Scholar] [CrossRef] [PubMed]
- van Loghem, J.; Sattler, S.; Casabona, G.; Cotofana, S.; Fabi, S.G.; Goldie, K.; Gout, U.; Kerscher, M.; Lim, T.S.; de Sanctis Pecora, C.; et al. Consensus on the use of hyaluronic acid fillers from the cohesive polydensified matrix range: Best practice in specific facial indications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1175–1199. [Google Scholar] [CrossRef]
- Goodman, G.J.; Liew, S.; Callan, P.; Hart, S. Facial aesthetic injections in clinical practice: Pretreatment and posttreatment consensus recommendations to minimise adverse outcomes. Australas. J. Dermatol. 2020, 61, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.-L.; Luo, S.-K. Experimentally induced arterial embolism by hyaluronic acid injection: Clinicopathologic observations and treatment. Plast. Reconstr. Surg. 2019, 143, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, X.; Shi, H.; Wu, W.T.L.; Wu, S. Blindness after facial filler injections: The role of extravascular hyaluronidase on intravascular hyaluronic acid embolism in the rabbit experimental model. Aesthet. Surg. J. 2020, 40, 319–326. [Google Scholar] [CrossRef]
- Nie, F.; Xie, H. Effect of local massage on prevention and treatment of intra-arterial polymethyl methacrylate embolism complications: An experimental animal study. Chin. J. Plast. Reconstr. Surg. 2022, 4, 6–12. [Google Scholar] [CrossRef]
- Taylor, G.I.; Shoukath, S.; Gascoigne, A.; Corlett, R.J.; Ashton, M.W. The functional anatomy of the ophthalmic angiosome and its implications in blindness as a complication of cosmetic facial filler procedures. Plast. Reconstr. Surg. 2020, 146, 745. [Google Scholar] [CrossRef]
- Abduljabbar, M.H.; Basendwh, M.A. Complications of hyaluronic acid fillers and their managements. J. Derm. Derm. Surg. 2016, 20, 100–106. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, K.H.; Kim, S.Y.; Lee, J.H.; Jeong, J.Y.; Kim, J.H. Hyaluronic acid pulmonary embolism: A critical consequence of an illegal cosmetic vaginal procedure. Thorax 2010, 65, 360–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhuang, Y.; Yang, M.; Liu, C. An islanded rabbit auricular skin flap model of hyaluronic acid injection-induced embolism. Aesthet. Plast. Surg. 2016, 40, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I.; Palmer, J.H. The vascular territories (angiosomes) of the body: Experimental study and clinical applications. Br. J. Plast. Surg. 1987, 40, 113–141. [Google Scholar] [CrossRef]
- Taylor, G.I.; Corlett, R.J.; Ashton, M.W. The functional angiosome: Clinical implications of the anatomical concept. Plast. Reconstr. Surg. 2017, 140, 721–733. [Google Scholar] [CrossRef]
- Jajoria, H.; Venkataram, A.; Mysore, V. Importance of choke vessels in injectable fillers. J. Cutan. Aesthet. Surg. 2020, 13, 185–190. [Google Scholar] [CrossRef]
- Gascoigne, A.C.; Taylor, G.I.; Corlett, R.J.; Briggs, C.; Ashton, M.W. Increasing perfusion pressure does not distend perforators or anastomoses but reveals arteriovenous shuntings. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2857. [Google Scholar] [CrossRef]
- Ashton, M.W.; Taylor, G.I.; Corlett, R.J. The role of anastomotic vessels in controlling tissue viability and defining tissue necrosis with special reference to complications following injection of hyaluronic acid fillers. Plast. Reconstr. Surg. 2018, 141, 818–830. [Google Scholar] [CrossRef]
- Ogawa, R.; Oki, K.; Hyakusoku, H. Skin perforator freeways and pathways: Understanding the role of true and choke anastomoses between perforator angiosomes and their impact on skin flap planning and outcomes. Plast. Reconstr. Surg. 2014, 133, 719–720. [Google Scholar] [CrossRef]
- Mao, Y.; Li, H.; Ding, M.; Hao, X.; Pan, J.; Tang, M.; Chen, S. Comparative study of choke vessel reconstruction with single and multiple perforator-based flaps on the murine back using delayed surgery. Ann. Plast. Surg. 2019, 82, 93–98. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Wang, Y.; Hsu, Y.; Wang, P.; Li, J. The role of hyaluronidase for the skin necrosis caused by hyaluronic acid injection-induced embolism: A rabbit auricular model study. Aesthet. Plast. Surg. 2019, 43, 1362–1370. [Google Scholar] [CrossRef]
- Baley-Spindel, I.; Villaseñor-Villalpando, E.; Márquez-Espriella, C.; Rivera-Salgado, M.I.; Dávila-Díaz, R. Perivascular hyaluronidase with alteplase as treatment for hyaluronic acid thrombosis. Aesthet. Surg. J. 2020, 40, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-F.; Huang, Y.-H. Partial vision recovery after iatrogenic retinal artery occlusion. BMC Ophthalmol. 2014, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.; Braverman, I.M. Ultrastructure of the human dermal microcirculation: The horizontal plexus of the papillary dermis. J. Investig. Dermatol. 1976, 66, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M. The cutaneous microcirculation: Ultrastructure and microanatomical organization. Microcirculation. 1997, 4, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M. The cutaneous microcirculation. J. Investig. Dermatol. Symp. Proc. 2000, 5, 3–9. [Google Scholar] [CrossRef]
- Intengan, H.D.; Schiffrin, E.L. Structure and mechanical properties of resistance arteries in hypertension: Role of adhesion molecules and extracellular matrix determinants. Hypertension 2000, 36, 312–318. [Google Scholar] [CrossRef]
- Oh, B.-L.; Jung, C.; Park, K.H.; Hong, Y.J.; Woo, S.J. Therapeutic intra-arterial hyaluronidase infusion for ophthalmic artery occlusion following cosmetic facial filler (hyaluronic acid) injection. Neuroophthalmology 2014, 38, 39–43. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H. Facial injections and blindness: A review on anatomy. Ann. Plast. Surg. 2022, 88, 233–236. [Google Scholar] [CrossRef]
- Toshiharu, M.; Katsumata, S.; Ogo, K.; Taylor, G.I. The function of ‘choke vessels’ to the blood flow: Angiographic and laser flow-graphic study on the rat flap model. Wound Repair Regen. 2004, 12, 15. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hu, S.; Wu, D.; Tang, M.; Xu, D.C. A novel in vivo technique for observations of choke vessels in a rat skin flap model. Plast. Reconstr. Surg. 2012, 130, 308–317. [Google Scholar] [CrossRef]
- Han, J.; He, Y.; Liu, K.; Yang, Q. Necrosis of the glabella after injection with hyaluronic acid into the forehead. J. Craniofac. Surg. 2018, 29, 726–727. [Google Scholar] [CrossRef] [PubMed]
- Davidova, P.; Müller, M.; Wenner, Y.; König, C.; Kenikstul, N.; Kohnen, T. Ophthalmic artery occlusion after glabellar hyaluronic acid filler injection. Am. J. Ophthalmol. Case Rep. 2022, 26, 101407. [Google Scholar] [CrossRef]
- Lucaciu, A.; Samp, P.F.; Hattingen, E.; Kestner, R.-I.; Davidova, P.; Kohnen, T.; Rudolph, J.; Dietz, A.; Steinmetz, H.; Strzelczyk, A. Sudden vision loss and neurological deficits after facial hyaluronic acid filler injection. Neurol. Res. Pract. 2022, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, G.; Fu, Q.; Zhang, L.; Yu, Y.; Dong, Y.; Liang, L.; Chen, M. Efficacy of intra-arterial thrombolytic therapy for vision loss resulting from hyaluronic acid filler embolization. J. Cosmet. Dermatol. 2021, 20, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Yatziv, Y.; Leibovitch, I.; Kesler, A.; Cnaan, R.B.; Klein, A.; Goldenberg, D.; Habot-Wilner, Z. A case report of ophthalmic artery emboli secondary to calcium hydroxylapatite filler injection for nose augmentation—Long-term outcome. BMC Ophthalmol. 2016, 16, 98. [Google Scholar] [CrossRef]
- Grzybinski, S.; Temin, E. Vascular occlusion after hyaluronic acid filler injection. Clin. Pract. Cases Emerg. Med. 2018, 2, 167–168. [Google Scholar] [CrossRef]
- Lee, J.I.; Kang, S.J.; Sun, H. Skin necrosis with oculomotor nerve palsy due to a hyaluronic acid filler injection. Arch. Plast. Surg. 2017, 44, 340–343. [Google Scholar] [CrossRef][Green Version]
- Chen, Q.; Liu, Y.; Fan, D. Serious vascular complications after nonsurgical rhinoplasty: A case report. Plast. Reconstr. Surg. Glob. Open 2016, 4, e683. [Google Scholar] [CrossRef]
- Eldweik, L. Orbital infarction syndrome following hyaluronic acid filler rhinoplasty. Am. J. Ophthalmol. Case Rep. 2021, 22, 101063. [Google Scholar] [CrossRef]
- Koziej, M.; Polak, J.; Hołda, J.; Trybus, M.; Hołda, M.; Kluza, P.; Moskała, A.; Chrapusta, A.; Walocha, J.; Woźniak, K. The arteries of the central forehead: Implications for facial plastic surgery. Aesthet. Surg. J. 2020, 40, 1043–1050. [Google Scholar] [CrossRef]
- Kapoor, K.M.; Kapoor, P.; Heydenrych, I.; Bertossi, D. Vision loss associated with hyaluronic acid fillers: A systematic review of literature. Aesthet. Plast. Surg. 2020, 44, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.H. Blood supply of the retina. Ophthalmic Res. 1997, 29, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Acute retinal arterial occlusive disorders. Prog. Retin. Eye Res. 2011, 30, 359–394. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Marco, R.D.; Goldhardt, R.; Modi, Y. Central retinal artery occlusion: Acute management and treatment. Curr. Ophthalmol. Rep. 2017, 5, 149–159. [Google Scholar] [CrossRef]
- Hayreh, S.S. Orbital vascular anatomy. Eye 2006, 20, 1130–1144. [Google Scholar] [CrossRef]
- Schneider, M.; Molnar, A.; Angeli, O.; Szabo, D.; Bernath, F.; Hajdu, D.; Gombocz, E.; Mate, B.; Jiling, B.; Nagy, B.V.; et al. Prevalence of cilioretinal arteries: A systematic review and a prospective cross-sectional observational study. Acta. Ophthalmol. 2021, 99, 310–318. [Google Scholar] [CrossRef]
- DeLorenzi, C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol. Surg. 2014, 40, 832–841. [Google Scholar] [CrossRef]
- Rauso, R.; Zerbinati, N.; Fragola, R.; Nicoletti, G.F.; Tartaro, G. Transvascular hydrolysis of hyaluronic acid filler with hyaluronidase: An ex vivo study. Dermatol. Surg. 2021, 47, 370–372. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Zhang, Y.; Tian, W.; Wang, H. Comparison of intra-arterial and subcutaneous testicular hyaluronidase injection treatments and the vascular complications of hyaluronic acid filler. Dermatol. Surg. 2017, 43, 246–254. [Google Scholar] [CrossRef]
- Lee, W.; Oh, W.; Oh, S.M.; Yang, E.J. Comparative effectiveness of different interventions of perivascular hyaluronidase. Plast. Reconstr. Surg. 2020, 145, 957–964. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016, 21, 5. [Google Scholar] [CrossRef]
- Zheng, C.; Fu, Q.; Zhou, G.-W.; Lai, L.-Y.; Zhang, L.-X.; Zhang, D.-Q.; Chen, G.-J.; Liang, L.-M.; Chen, M.-L. Efficacy of percutaneous intraarterial facial/supratrochlear arterial hyaluronidase injection for treatment of vascular embolism resulting from hyaluronic acid filler cosmetic injection. Aesthet. Surg. J. 2022, 42, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Oh, W.; Ko, H.-S.; Lee, S.-Y.; Kim, K.W.; Yang, E.-J. Effectiveness of retrobulbar hyaluronidase injection in an iatrogenic blindness rabbit model using hyaluronic acid filler injection. Plast. Reconstr. Surg. 2019, 144, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, C. Restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol. Surg. 2018, 44, 435–437. [Google Scholar] [CrossRef]
- Hwang, C.J.; Mustak, H.; Gupta, A.A.; Ramos, R.M.; Goldberg, R.A.; Duckwiler, G.R. Role of retrobulbar hyaluronidase in filler-associated blindness: Evaluation of fundus perfusion and electroretinogram readings in an animal model. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-Z.; Sun, Z.-S.; Liao, W.-X.; Cai, B.; Chen, C.-L.; Zheng, H.-H.; Zeng, L.; Luo, S.-K. Efficacy of retrobulbar hyaluronidase injection for vision loss resulting from hyaluronic acid filler embolization. Aesthet. Surg. J. 2017, 38, 12–22. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Lai, L.-Y.; Zhou, G.-W.; Liang, L.-M.; Zhou, Y.-C.; Bai, X.-Y.; Dai, Q.; Yu, Y.-T.; Tang, W.-Q.; Chen, M.-L. Evaluation of intraarterial thrombolysis in treatment of cosmetic facial filler-related ophthalmic artery occlusion. Plast. Reconstr. Surg. 2020, 145, 42–50. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Liu, T.; Li, Q.; Lyu, Z.; Yu, Y. An efficacy and safety study of intra-arterial recanalization of occluded ophthalmic arteries in patients with monocular blindness caused by injection of hyaluronic acid in facial tissues. Aesthet. Plast. Surg. 2021, 45, 1573–1578. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, H.-M.; Chen, S.-J.; Lee, H.-J.; Lirng, J.-F.; Lin, C.-J.; Chang, F.-C.; Luo, C.-B.; Guo, W.-Y. Intra-arterial thrombolytic therapy is not a therapeutic option for filler-related central retinal artery occlusion. Facial Plast. Surg. 2018, 34, 325–329. [Google Scholar] [CrossRef]
- Chen, J.; Ruan, J.; Wang, W.; Chen, Z.; Huang, Q.; Yang, Y.; Li, J. Superselective arterial hyaluronidase thrombolysis is not an effective treatment for hyaluronic acid-induced retinal artery occlusion: Study in a rabbit model. Plast. Reconstr. Surg. 2021, 147, 69–75. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Z.; Li, J.; Liu, Z.; Xu, H.; Wu, M.; Wu, S. Endovascular hyaluronidase application through superselective angiography to rescue blindness caused by hyaluronic acid injection. Aesthet. Surg. J. 2021, 41, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Zhou, S.; Liu, K. Intravenous hyaluronidase with urokinase as treatment for arterial hyaluronic acid embolism. Plast. Reconstr. Surg. 2016, 137, 114–121. [Google Scholar] [CrossRef]
- Mccann, M. Intravenous hyaluronidase for visual loss secondary to filler injection: A novel therapeutic approach. J. Clin. Aesthet. Dermatol. 2019, 12, 25–27. [Google Scholar] [PubMed]
- Zamora-Alejo, K.; Moore, S.; Leatherbarrow, B.; Norris, J.H.; Lake, D.B.; Malhotra, R.; Selva, D.; Goggin, M. Hyaluronidase toxicity: A possible cause of postoperative periorbital inflammation. Clin. Exp. Ophthalmol. 2013, 41, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Wattanakrai, P.; Jurairattanaporn, N.; Rojhirunsakool, S.; Visessiri, Y.; Suwanchinda, A.; Thanasarnaksorn, W. The study of histological changes of the arterial vascular structure after hyaluronidase exposure. J. Cosmet. Dermatol. 2018, 17, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Lip, G.Y. Red vs white thrombi: Treating the right clot is crucial. Arch. Intern. Med. 2003, 163, 2534–2535. [Google Scholar] [CrossRef]
- de la Motte, C.; Nigro, J.; Vasanji, A.; Rho, H.; Kessler, S.; Bandyopadhyay, S.; Danese, S.; Fiocchi, C.; Stern, R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am. J. Pathol. 2009, 174, 2254–2264. [Google Scholar] [CrossRef]
- Kessler, S.; Rho, H.; West, G.; Fiocchi, C.; Drazba, J.; de la Motte, C. Hyaluronan (HA) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin. Transl. Sci. 2008, 1, 57–61. [Google Scholar] [CrossRef]
- Docampo, M.J.; Cabrera, J.; Bassols, A. Hyaluronan mediates the adhesion of porcine peripheral blood mononuclear cells to poly (I:C)-treated intestinal cells and modulates their cytokine production. Vet. Immunol. Immunopathol. 2017, 184, 8–17. [Google Scholar] [CrossRef]
- Kim, J.S.; Gonzales, L.; Lester, J.; Householder, N.; Boxrud, C.; Goldberg, R.; Ugradar, S. Thrombogenicity of hyaluronic acid fillers: A quantitative thrombodynamics study. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 68–72. [Google Scholar] [CrossRef]
- Hurkal, O.; Sibar, S.; Cenetoglu, S.; Tuncer, S.; Elmas, C.; Seymen, C.M. Arterial occlusion after hyaluronic acid injection: Treatment with hyaluronidase and streptokinase. Ann. Plast. Surg. 2021, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Tran, H.T.T.; Duong, Q.H.; Nguyen, M.D.; Dao, H.X.; Le, D.T. Significant vision recovery from filler-induced complete blindness with combined intra-arterial injection of hyaluronidase and thrombolytic agents. Aesthet. Plast. Surg. 2022, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, O.M.; Newman, N.J.; Biousse, V. Thrombolysis for central retinal artery occlusion in 2020: Time is vision! J. Neuroophthalmol. 2020, 40, 333–345. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Suo, Y.; Qin, D.; Ren, M.; Lei, R.; Zhang, Y.; Wang, Y. Intravenous recombinant tissue-type plasminogen activator thrombolysis for acute central retinal artery occlusion. J. Craniofac. Surg. 2021, 32, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Hakim, N.; Hakim, J. Intra-arterial thrombolysis for central retinal artery occlusion. Clin. Ophthalmol. 2019, 13, 2489–2509. [Google Scholar] [CrossRef] [PubMed]
- Bober, R.M.; Jahangir, E. What is ischemia and how should this be defined based on modern imaging? Prog. Cardiovasc. Dis. 2015, 57, 537–554. [Google Scholar] [CrossRef]
- Jennings, R.B.; Ganote, C.E.; Reimer, K.A. Ischemic tissue injury. Am. J. Pathol. 1975, 81, 179–198. [Google Scholar]
- Jennings, R.B. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ. Res. 2013, 113, 428–438. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Paciaroni, M.; Caso, V.; Agnelli, G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur. Neurol. 2009, 61, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Eckert, P.; Schnackerz, K. Ischemic tolerance of human skeletal muscle. Ann. Plast. Surg. 1991, 26, 77–84. [Google Scholar] [CrossRef]
- Wang, W.Z.; Baynosa, R.C.; Zamboni, W.A. Update on ischemia-reperfusion injury for the plastic surgeon: 2011. Plast. Reconstr. Surg. 2011, 128, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B.; Kimura, A.; Sanon, A. Central retinal artery occlusion. Retinal survival time. Exp. Eye Res. 2004, 78, 723–736. [Google Scholar] [CrossRef]
- Tobalem, S.; Schutz, J.S.; Chronopoulos, A. Central retinal artery occlusion—Rethinking retinal survival time. BMC Ophthalmol. 2018, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Varma, D.D.; Cugati, S.; Lee, A.W.; Chen, C.S. A review of central retinal artery occlusion: Clinical presentation and management. Eye 2013, 27, 688–697. [Google Scholar] [CrossRef]
- Bluhmki, E.; Chamorro, A.; Dávalos, A.; Machnig, T.; Sauce, C.; Wahlgren, N.; Wardlaw, J.; Hacke, W. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): Additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009, 8, 1095–1102. [Google Scholar] [CrossRef]
- Maricevich, M.; Carlsen, B.; Mardini, S.; Moran, S. Upper extremity and digital replantation. Hand 2011, 6, 356–363. [Google Scholar] [CrossRef]
- Sajjan, V.V.; Lunge, S.; Swamy, M.B.; Pandit, A.M. Livedo reticularis: A review of the literature. Indian Dermatol. Online J. 2015, 6, 315–321. [Google Scholar] [CrossRef]
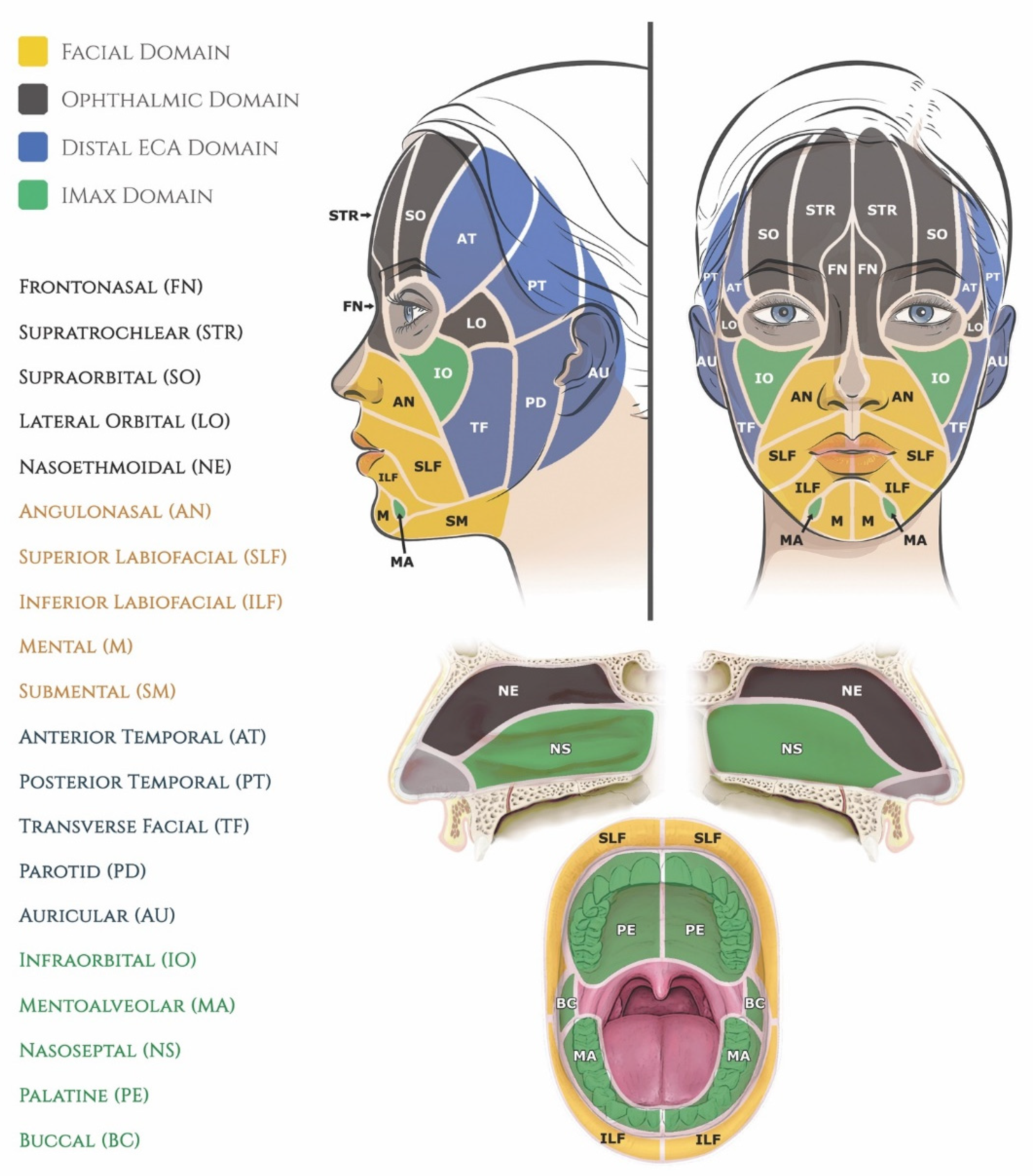
- Houseman, N.D.; Taylor, G.I.; Pan, W.R. The angiosomes of the head and neck: Anatomic study and clinical applications. Plast. Reconstr. Surg. 2000, 105, 2287–2313. [Google Scholar] [CrossRef]
- Whetzel, T.; Mathes, S.J. Arterial anatomy of the face: An analysis of vascular territories and perforating cutaneous vessels. Plast. Reconstr. Surg. 1992, 89, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The perforasome theory: Vascular anatomy and clinical implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Pierrefeu, A.; Brosset, S.; Lahon, M.; Guerid, S.; Shipkov, H.; Boucher, F.; Breton, P.; Sigaux, N.; Mojallal, A. Transverse facial artery perforators: Anatomical, two- and three-dimensional radiographic study. Plast. Reconstr. Surg. 2019, 143, 820–828. [Google Scholar] [CrossRef]
- Qassemyar, Q.; Havet, E.; Sinna, R. Vascular basis of the facial artery perforator flap: Analysis of 101 perforator territories. Plast. Reconstr. Surg. 2012, 129, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Pessa, J.E. The fat compartments of the face: Anatomy and clinical implications for cosmetic surgery. Plast. Reconstr. Surg. 2007, 119, 2219–2227. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Pessa, J.E. The retaining system of the face: Histologic evaluation of the septal boundaries of the subcutaneous fat compartments. Plast. Reconstr. Surg. 2008, 121, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Schenck, T.L.; Koban, K.C.; Schlattau, A.; Frank, K.; Sykes, J.M.; Targosinski, S.; Erlbacher, K.; Cotofana, S. The functional anatomy of the superficial fat compartments of the face: A detailed imaging study. Plast. Reconstr. Surg. 2018, 141, 1351–1359. [Google Scholar] [CrossRef]
- Hong, J.Y.; Seok, J.; Ahn, G.R.; Jang, Y.J.; Li, K.; Kim, B.J. Impending skin necrosis after dermal filler injection: A “golden time” for first-aid intervention. Dermatol. Ther. 2017, 30, e12440. [Google Scholar] [CrossRef]
- Oley, M.H.; Oley, M.C.; Mawu, F.O.; Aling, D.M.R.; Faruk, M. Hyperbaric oxygen therapy in managing minimally invasive aesthetic procedure complications: A report of three cases. Clin. Cosmet. Investig. Dermatol. 2022, 15, 63–68. [Google Scholar] [CrossRef]
- Uittenbogaard, D.; Lansdorp, C.A.; Bauland, C.G.; Boonstra, O. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: A case report. Undersea Hyperb. Med. 2019, 46, 207–210. [Google Scholar] [CrossRef]
- Henderson, R.; Reilly, D.A.; Cooper, J.S. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: Case report and issues. Plast. Reconstr. Surg. Glob. Open 2018, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Kruize, R.G.F.; Teguh, D.N.; van Hulst, R.A. Hyperbaric oxygen therapy in hyaluronic acid filler-induced dermal ischemia. Dermatol. Surg. 2020, 46, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Baynosa, R.C. Hyperbaric oxygen therapy for the compromised graft or flap. Adv. Wound Care. 2017, 6, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.S.; Basto, R.; Henriques, S.; Colaço, L.; Costa e Silva, F.; Prieto, I.; Guerreiro, I. Hyperbaric oxygen therapy in retinal arterial occlusion: Epidemiology, clinical approach, and visual outcomes. Case Rep. Ophthalmol. Med. 2019, 2019, 9765938. [Google Scholar] [CrossRef]
- Chuchvara, N.; Alamgir, M.; John, A.M.; Rao, B. Dermal filler-induced vascular occlusion successfully treated with tadalafil, hyaluronidase, and aspirin. Dermatol. Surg. 2021, 47, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Grove, E.L.; Würtz, M.; Thomas, M.R.; Kristensen, S.D. Antiplatelet therapy in acute coronary syndromes. Expert Opin. Pharmacother. 2015, 16, 2133–2147. [Google Scholar] [CrossRef]
- Li, Z.-H.; Alfertshofer, M.; Hong, W.-J.; Li, X.-R.; Zhang, Y.-L.; Moellhoff, N.; Frank, K.; Luo, S.-K.; Cotofana, S. Upper facial anastomoses between the external and internal carotid vascular territories–A 3d computed tomographic investigation. Aesthet. Surg. J. 2022. [Google Scholar] [CrossRef]
- Myung, Y.; Yim, S.; Jeong, J.H.; Kim, B.-K.; Heo, C.-Y.; Baek, R.-M.; Pak, C.-S. The classification and prognosis of periocular complications related to blindness following cosmetic filler injection. Plast. Reconstr. Surg. 2017, 140, 61–64. [Google Scholar] [CrossRef]
- Olson, E.A.; Lentz, K. Central retinal artery occlusion: A literature review and the rationale for hyperbaric oxygen therapy. Mo. Med. 2016, 113, 53–57. [Google Scholar]
- Bertelli, E.; Regoli, M.; Bracco, S. An update on the variations of the orbital blood supply and hemodynamic. Surg. Radiol. Anat. 2017, 39, 485–496. [Google Scholar] [CrossRef]
- Kiyosue, H.; Tanoue, S.; Hongo, N.; Sagara, Y.; Mori, H. Artery of the superior orbital fissure: An undescribed branch from the pterygopalatine segment of the maxillary artery to the orbital apex connecting with the anteromedial branch of the inferolateral trunk. AJNR Am. J. Neuroradiol. 2015, 36, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Geibprasert, S.; Pongpech, S.; Armstrong, D.; Krings, T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: Vessels the neurointerventionalist needs to know. AJNR Am. J. Neuroradiol. 2009, 30, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.; Rodríguez-Feliz, J. Ocular pain and impending blindness during facial cosmetic injections: Is your office prepared? Aesthet. Plast. Surg. 2017, 41, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.G.; Eom, T.K.; Kang, S.J. Severe visual loss and cerebral infarction after injection of hyaluronic acid gel. J. Craniofac. Surg. 2014, 25, 684–686. [Google Scholar] [CrossRef] [PubMed]
- He, M.-S.; Sheu, M.-M.; Huang, Z.-L.; Tsai, C.-H.; Tsai, R.-K. Sudden bilateral vision loss and brain infarction following cosmetic hyaluronic acid injection. JAMA Ophthalmol. 2013, 131, 1234–1235. [Google Scholar] [CrossRef]
- Moore, R.M.; Mueller, M.A.; Hu, A.C.; Evans, G.R.D. Asymptomatic stroke after hyaluronic acid filler injection: Case report and literature review. Aesthet. Surg. J. 2021, 41, 602–608. [Google Scholar] [CrossRef]




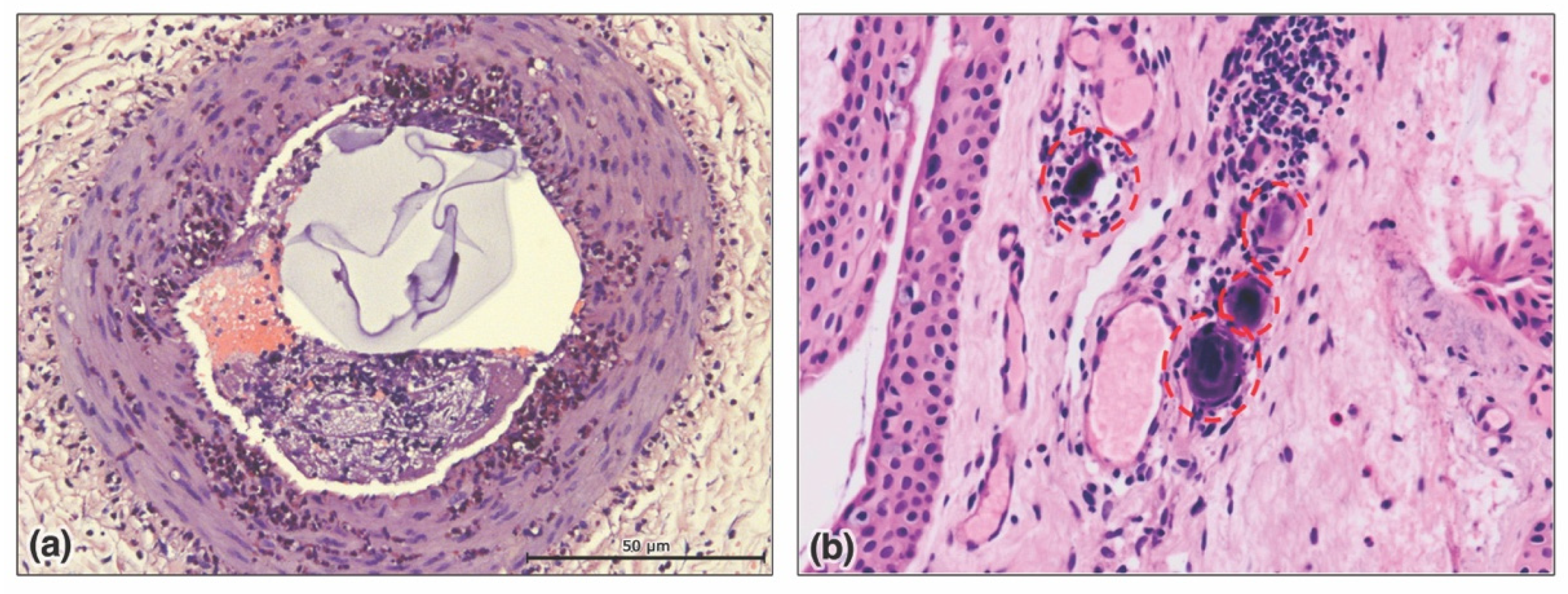
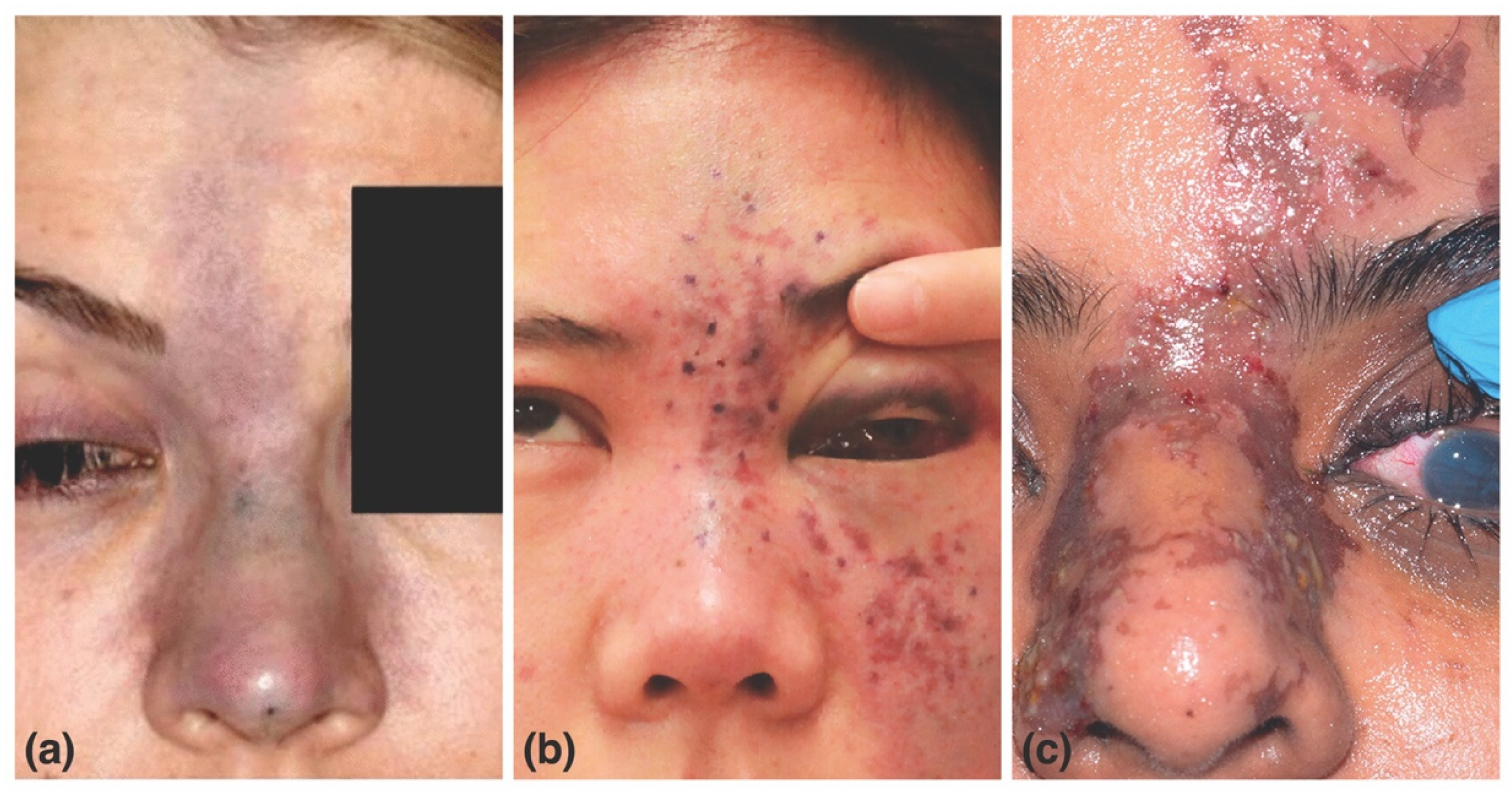
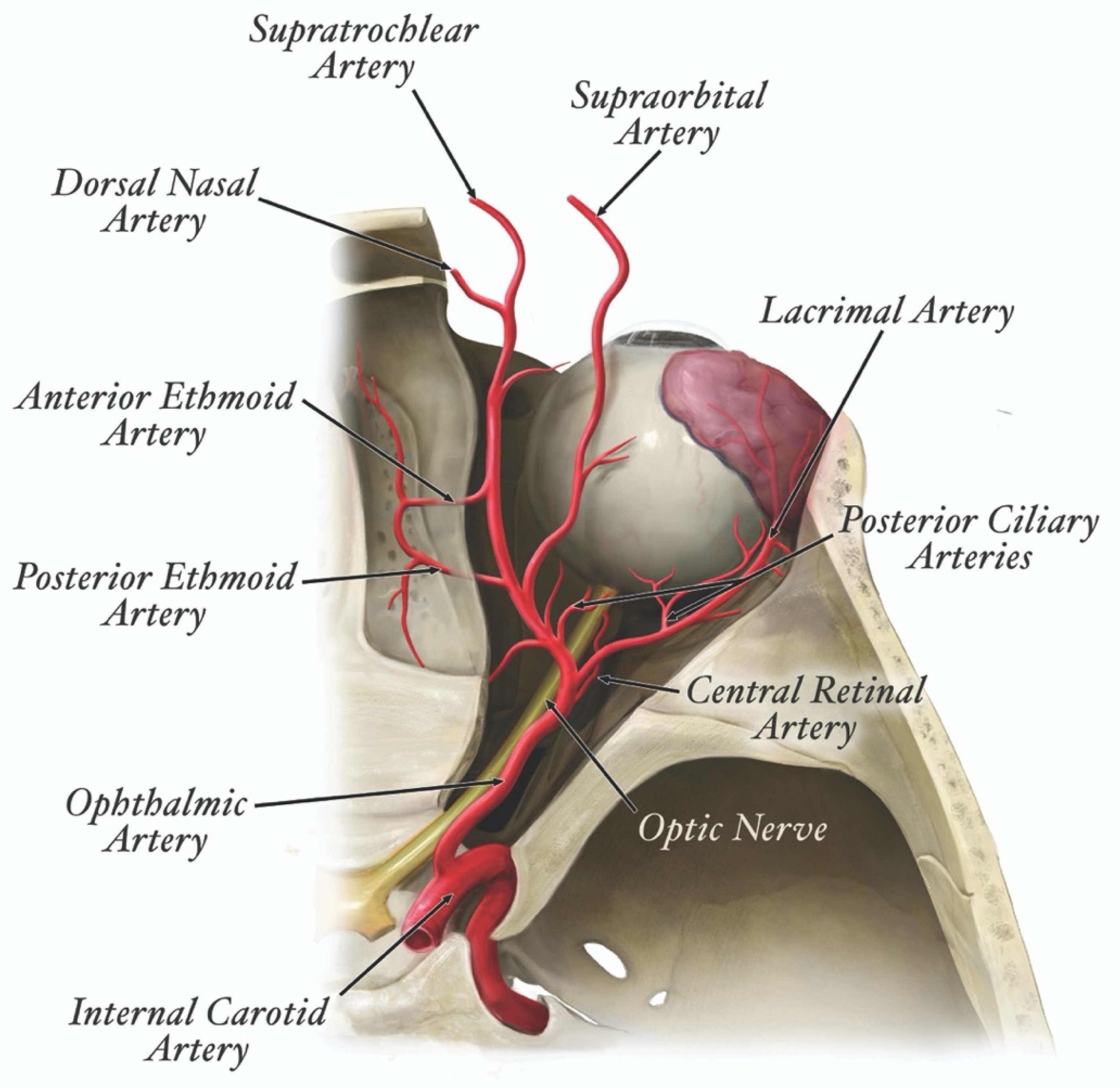
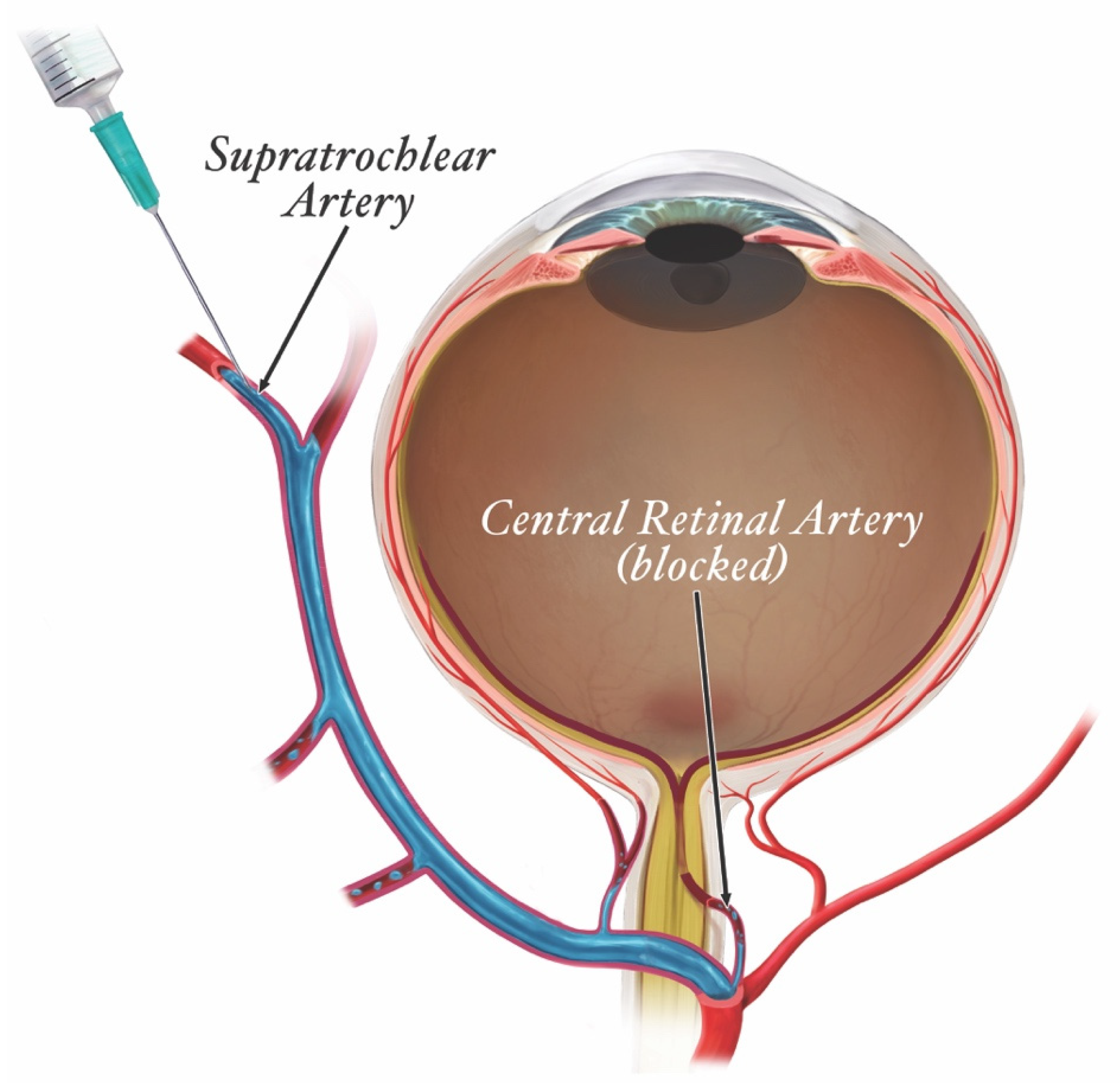

| Filler Product Name * | HA (mg/mL) | G’5Hz (Pa) | G”5Hz (Pa) | Tan δ | Cohesivity/Fn (gmf) | Swelling Factor (%) |
|---|---|---|---|---|---|---|
| Belotero Balance | 22.5 | 128 | 82 | 0.641 | 69 | 664 |
| Juvéderm Ultra | 24 | 156 | 68 | 0.436 | 96 | 580 |
| Juvéderm Ultra XC | 24 | 207 | 80 | 0.386 | 96 | 622 |
| Juvéderm Ultra Plus | 24 | 214 | 74 | 0.346 | 116 | 515 |
| Juvéderm Ultra Plus XC | 24 | 263 | 79 | 0.300 | 112 | 454 |
| Juvéderm Volbella | 15 | 271 | 39 | 0.144 | 19 | 133 |
| Juvéderm Voluma | 20 | 398 | 41 | 0.103 | 40 | 227 |
| Restylane Refyne | 20 | 116 | 50 | 0.431 | 49 | 516 |
| Restylane Defyne | 20 | 342 | 47 | 0.137 | 60 | 318 |
| Restylane Kysse | 20 | 236 | 50 | 0.212 | 85 | 373 |
| Restylane-L | 20 | 864 | 185 | 0.214 | 29 | <100 |
| Restylane Lyft | 20 | 977 | 198 | 0.203 | 32 | <100 |
| Teosyal RHA1 | 15 | 133 | 54 | 0.406 | 22 | 260 |
| Teosyal RHA2 | 23 | 319 | 99 | 0.310 | 77 | 420 |
| Teosyal RHA3 | 23 | 264 | 67 | 0.254 | 109 | 427 |
| Teosyal RHA4 | 23 | 346 | 62 | 0.179 | 115 | 366 |
| Arterial * | Venous |
|---|---|
| Muco-Cutaneous/Soft Tissue Necrosis | Local Venous Thrombophlebitis |
| Vision Loss +/− Ophthalmoplegia | Cerebral Sinus Thrombosis |
| Ischemic Cerebral Stroke | Pulmonary Embolism |
| Facial Paralysis/Peripheral Nerve Injury | Myocardial Infarction (PFO **) |
| Grade I: Limited Mucocutaneous Injury—No more than one FOEM Segment Affected |
| Ia: Ophthalmic domain not involved |
| Ib: Ophthalmic domain involved |
| Grade II: Moderate Mucocutaneous Injury—Two FOEM segments affected |
| IIa: Ophthalmic domain not involved |
| IIb: Ophthalmic domain involved |
| Grade III: Extensive Mucocutaneous Injury—Three or more FOEM segments affected |
| IIIa: Ophthalmic domain not involved |
| IIIb: Ophthalmic domain involved |
| Grade IV: Visual Deficits—Any segmental skin injury with visual deficits |
| IVa: Unilateral visual deficits |
| IVb: Bilateral visual deficits |
| Grade V: Stroke—Any segmental skin injury with ischemic stroke |
| Va: Unilateral ischemic stroke |
| Vb: Bilateral ischemic stroke |
| Type 0 | Ptosis and/or Ophthalmoplegia without Blindness |
| Type I | Blindness without Ophthalmoplegia and without Ptosis |
| Type II | Blindness with Ptosis but without Ophthalmoplegia |
| Type III | Blindness with Ophthalmoplegia but without Ptosis |
| Type IV | Blindness with Ophthalmoplegia and Ptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, D.J. Bridging a Century-Old Problem: The Pathophysiology and Molecular Mechanisms of HA Filler-Induced Vascular Occlusion (FIVO)—Implications for Therapeutic Interventions. Molecules 2022, 27, 5398. https://doi.org/10.3390/molecules27175398
Soares DJ. Bridging a Century-Old Problem: The Pathophysiology and Molecular Mechanisms of HA Filler-Induced Vascular Occlusion (FIVO)—Implications for Therapeutic Interventions. Molecules. 2022; 27(17):5398. https://doi.org/10.3390/molecules27175398
Chicago/Turabian StyleSoares, Danny J. 2022. "Bridging a Century-Old Problem: The Pathophysiology and Molecular Mechanisms of HA Filler-Induced Vascular Occlusion (FIVO)—Implications for Therapeutic Interventions" Molecules 27, no. 17: 5398. https://doi.org/10.3390/molecules27175398
APA StyleSoares, D. J. (2022). Bridging a Century-Old Problem: The Pathophysiology and Molecular Mechanisms of HA Filler-Induced Vascular Occlusion (FIVO)—Implications for Therapeutic Interventions. Molecules, 27(17), 5398. https://doi.org/10.3390/molecules27175398





