Biocatalysts Synthesized with Lipase from Pseudomonas cepacia on Glycol-Modified ZIF-8: Characterization and Utilization in the Synthesis of Green Biodiesel
Abstract
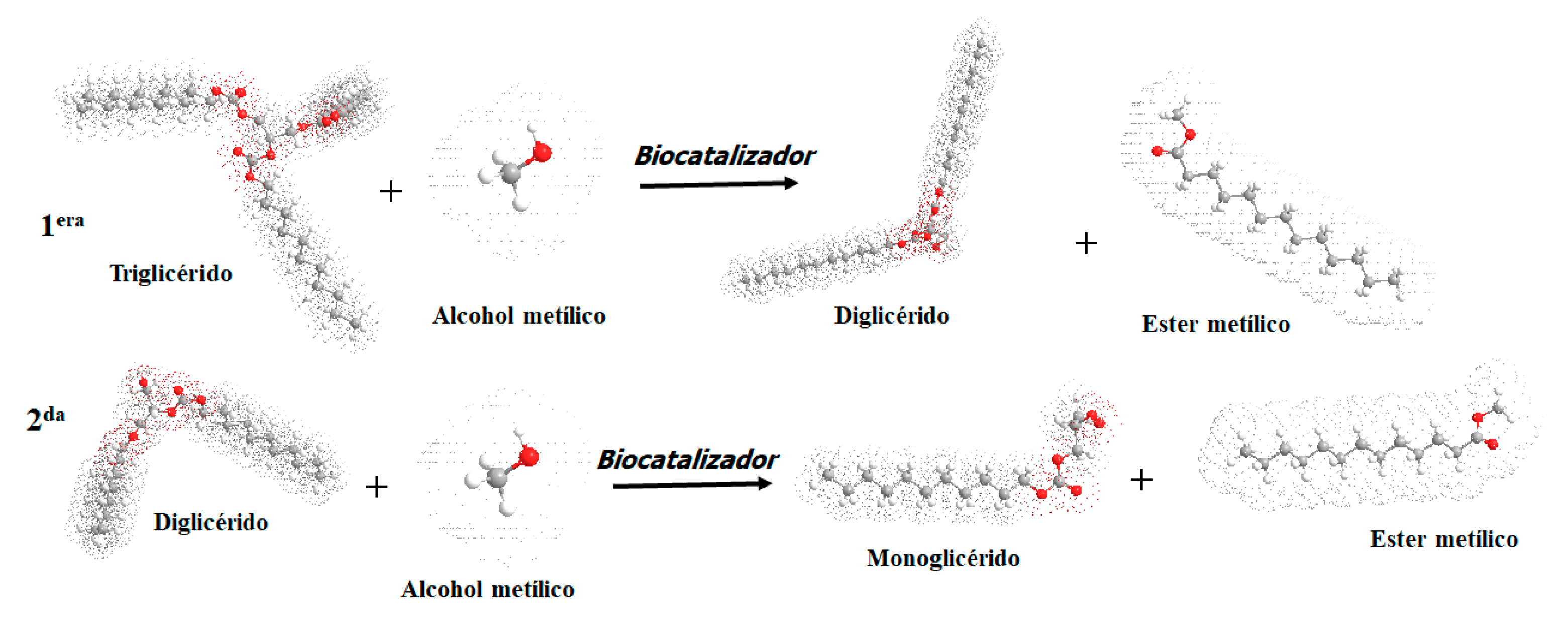
:1. Introduction
2. Results
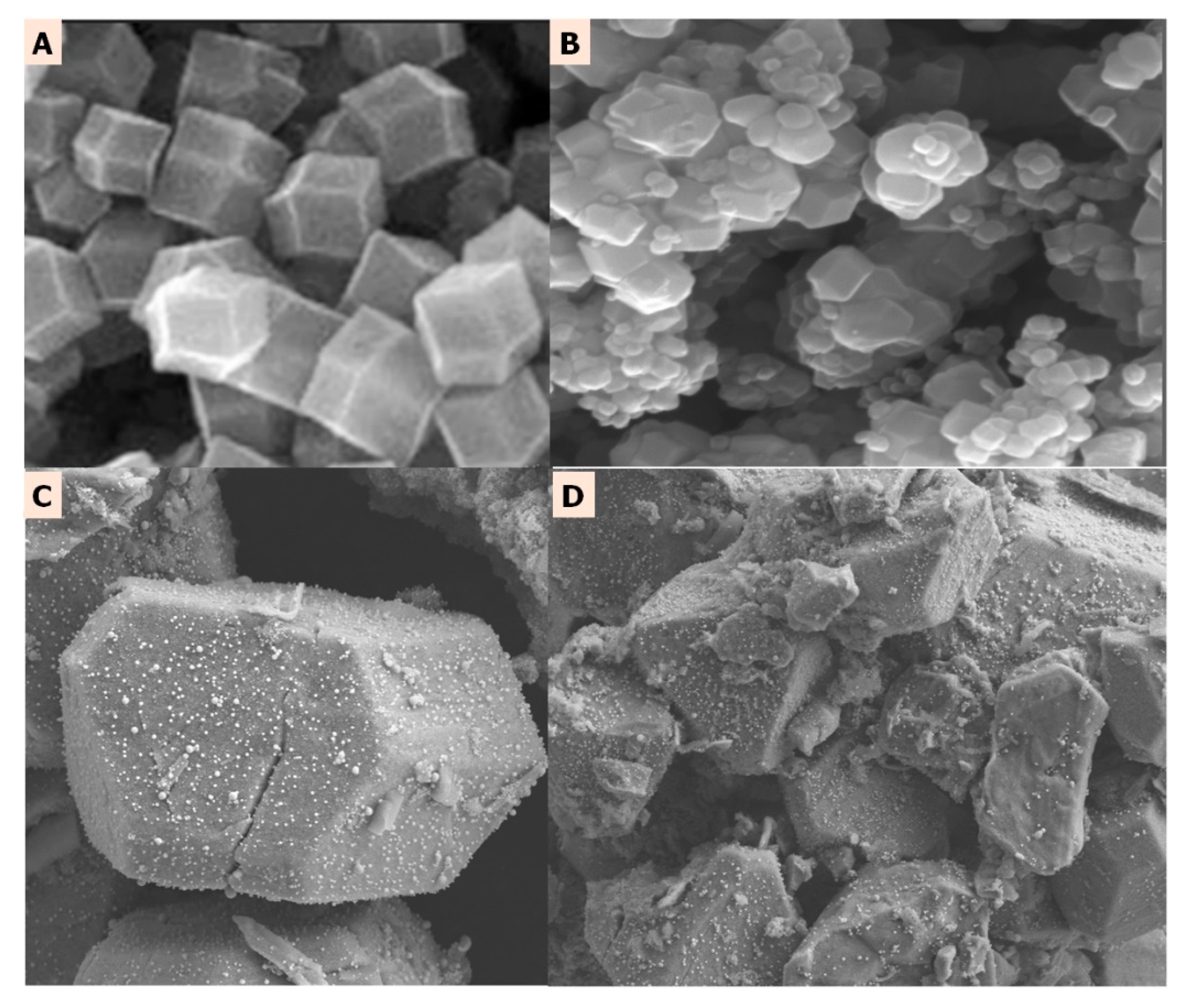
2.1. Textural Analysis of Gly@ZIF-8
2.2. Fourier Transform Infrared Spectrophotometry (FTIR)
Starting Material (ZIF-8) and Biocatalysts ZIF-8-CPL and Gly@ZIF-8-CPL
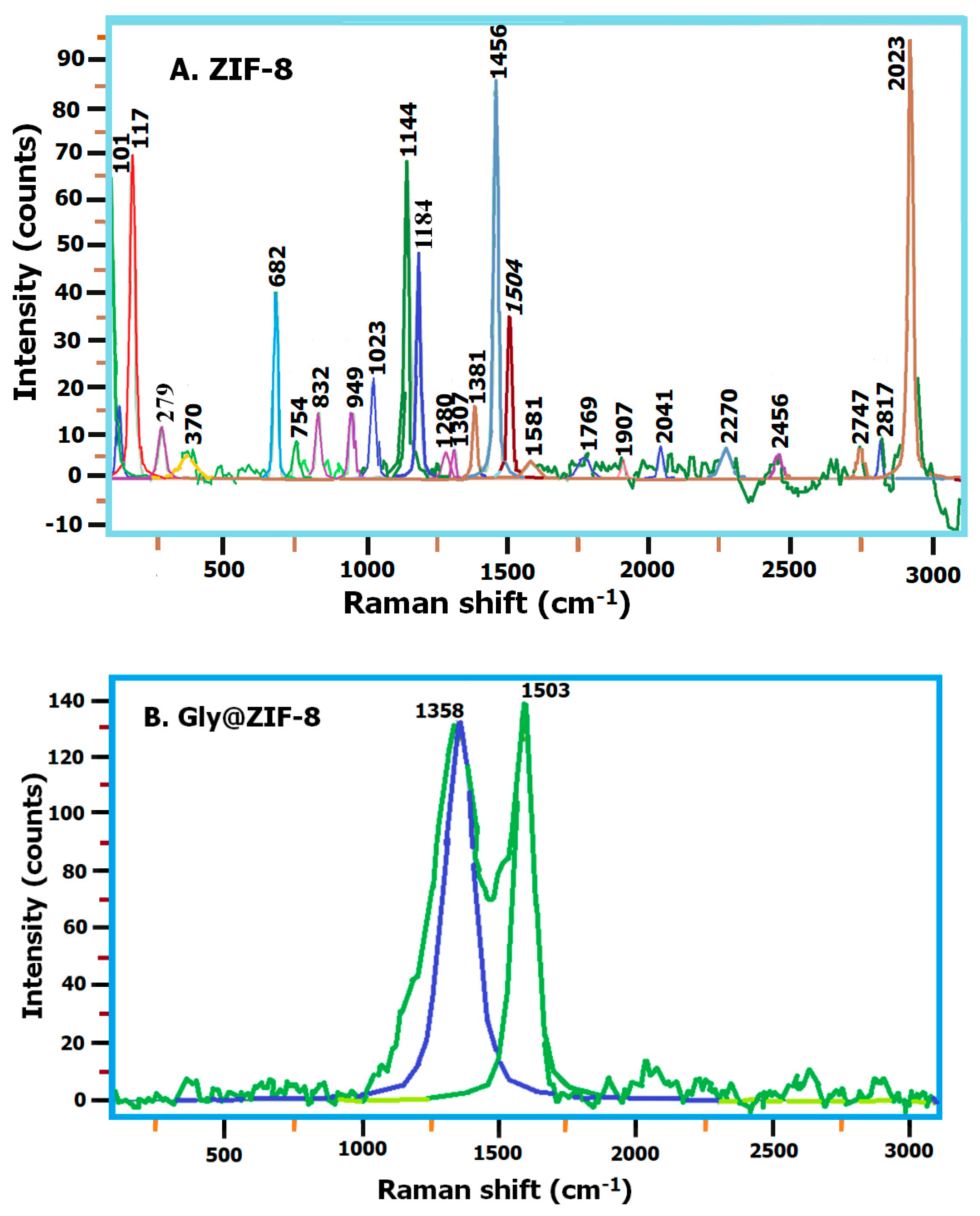
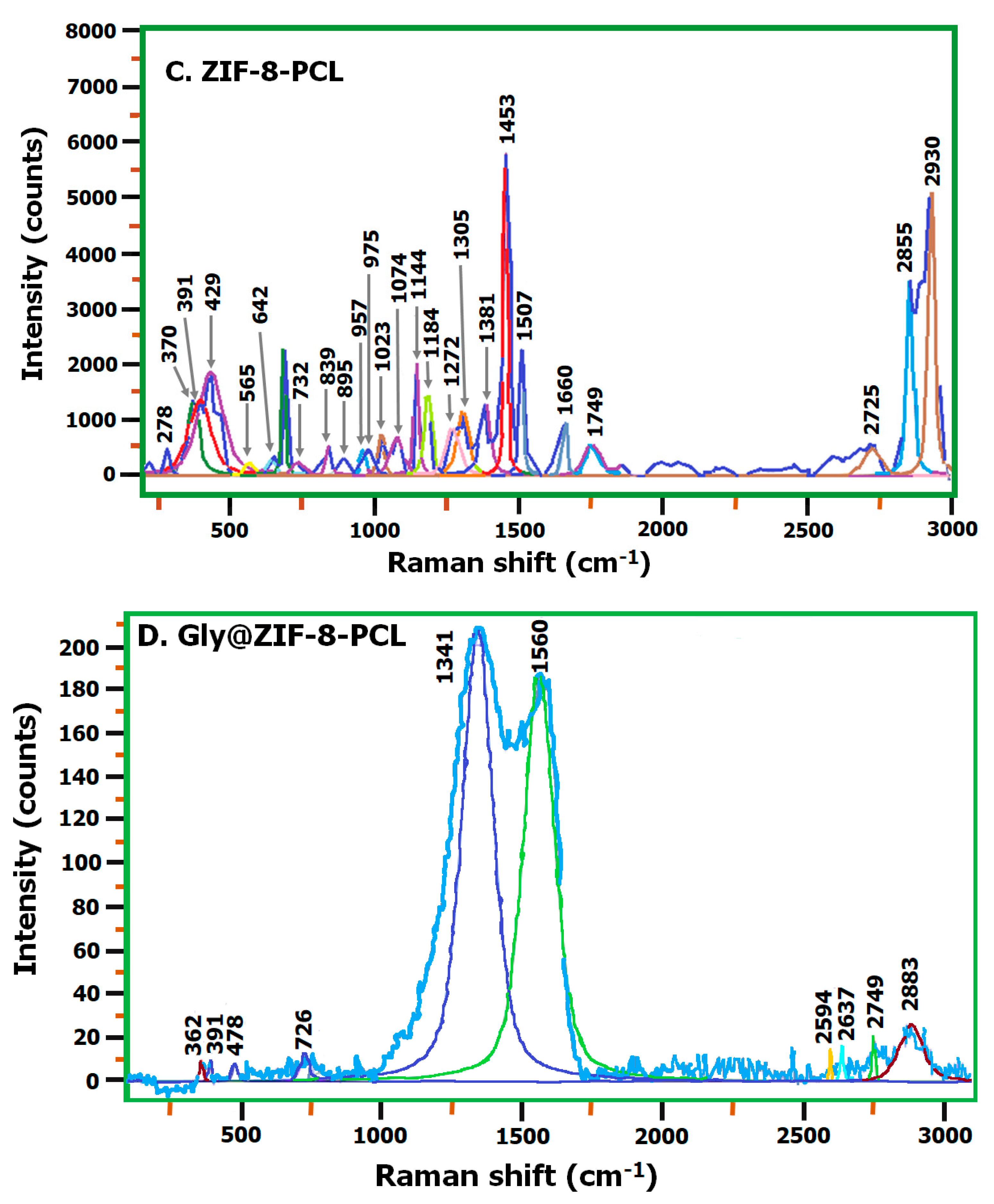
2.3. Raman Spectroscopy: ZIF-8, Gly@ZIF-8, ZIF-8-CPL, and Gly@ZIF-8-CPL
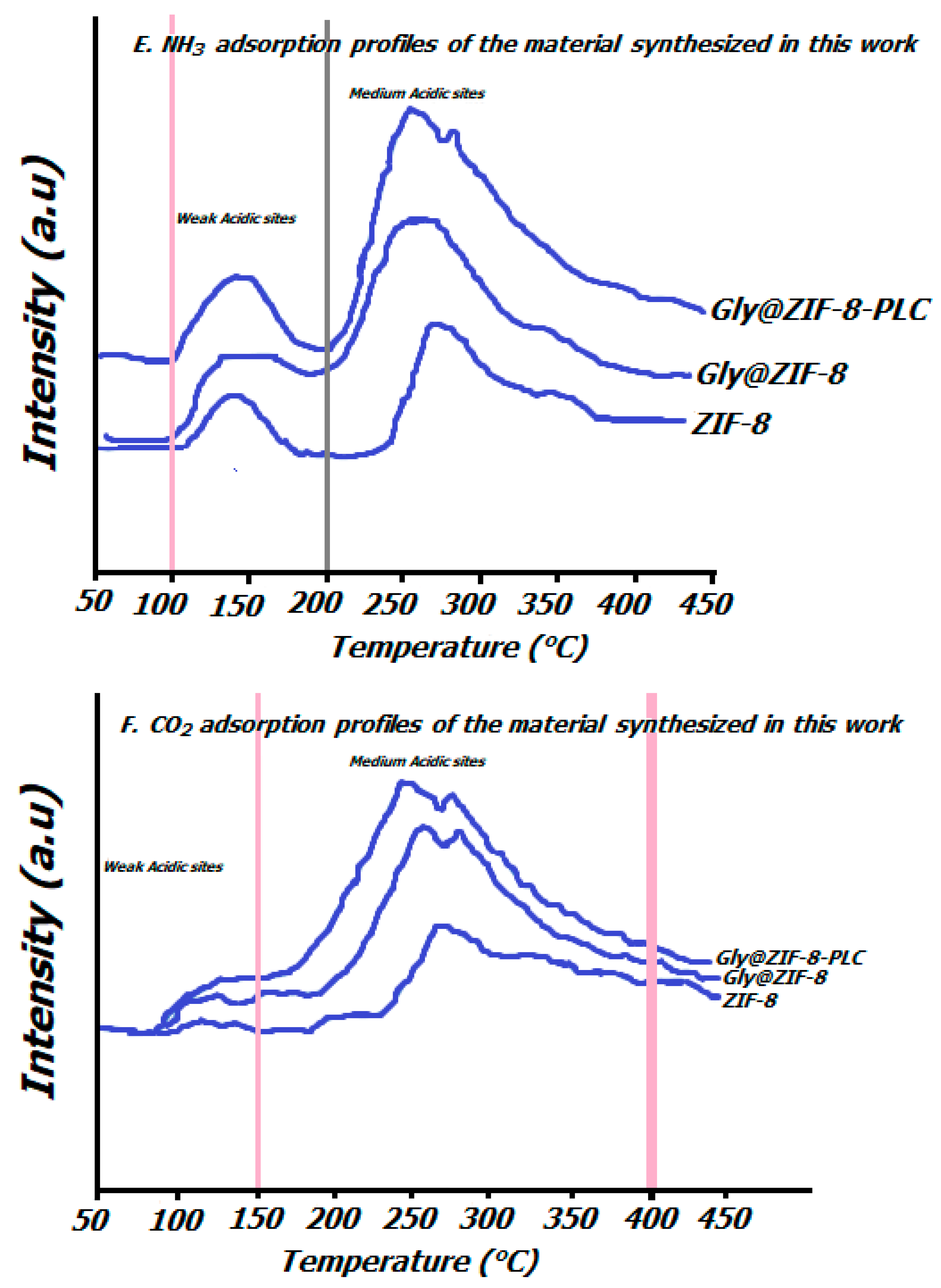
2.4. Characteristics of Acidity and Basicity of the Catalyst
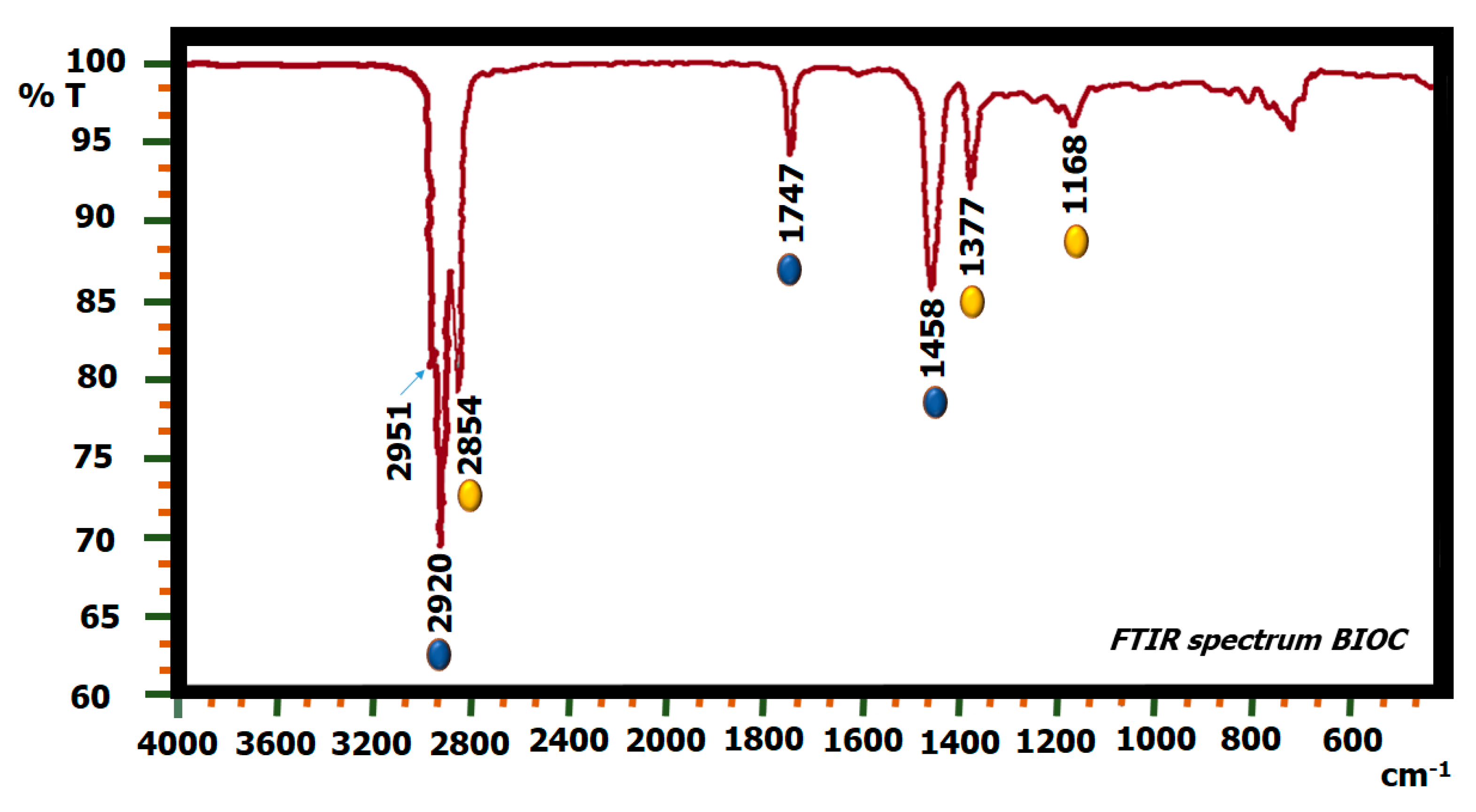
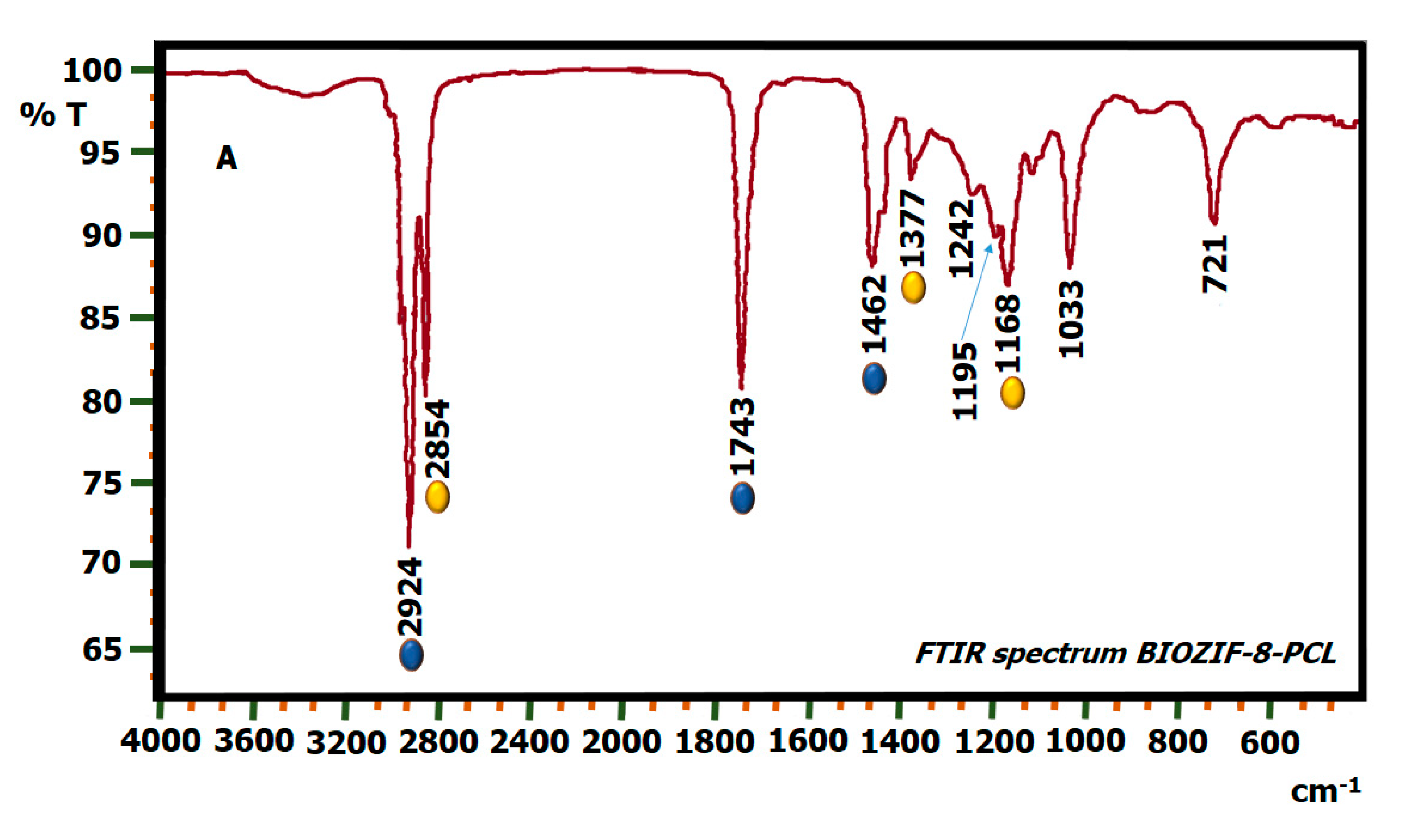
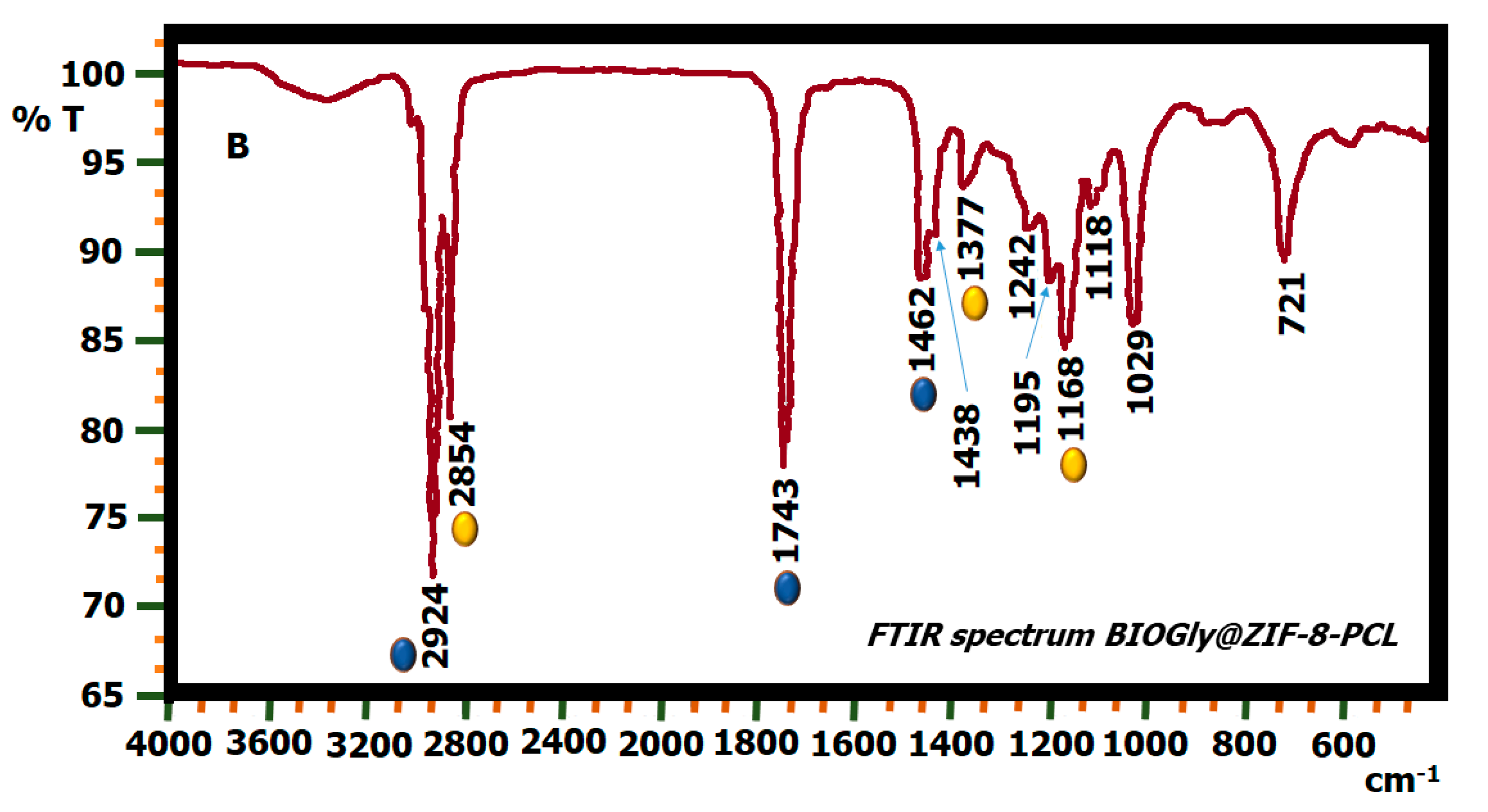
2.5. FTIR Biodiesel: Commercial Biodiesel (BIOC) and Biodiesel by Biocatalysis (BIOZIF-8-PCL and BIOGly@ZIF-8-CPL
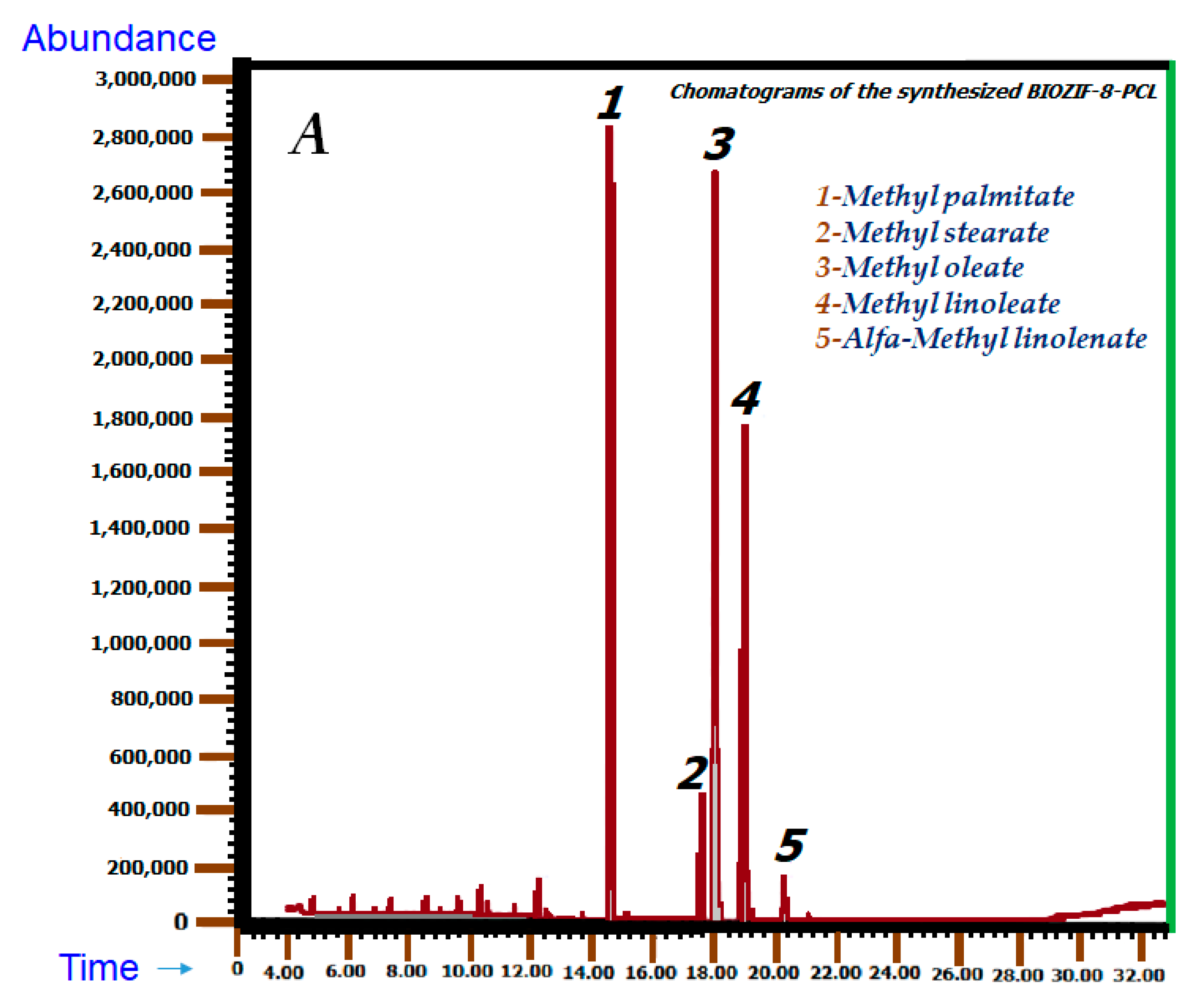
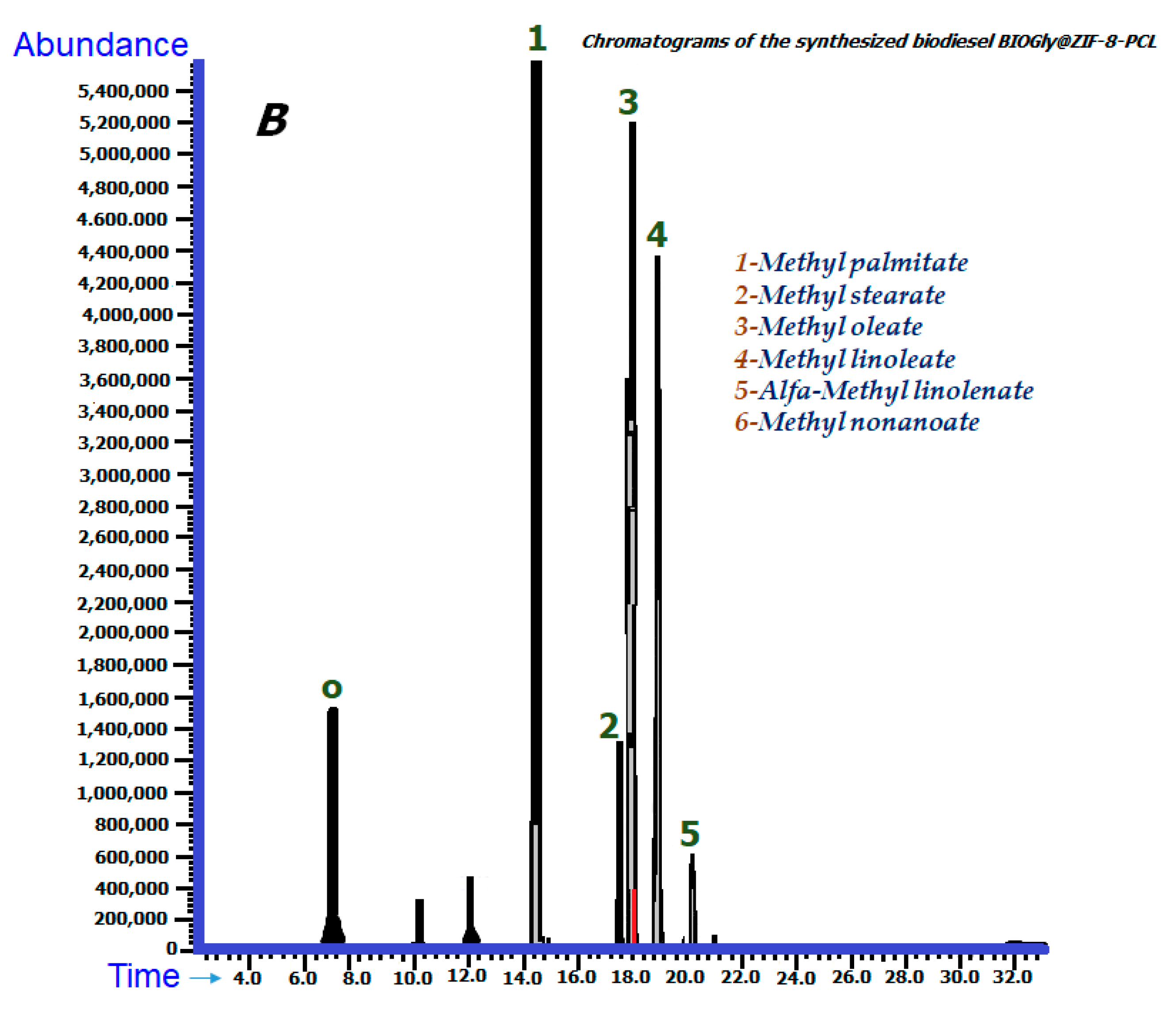
2.6. Gas Chromatography Coupled to Mass Spectrometry
2.6.1. Chromatographic Identification
2.6.2. Mass Analysis
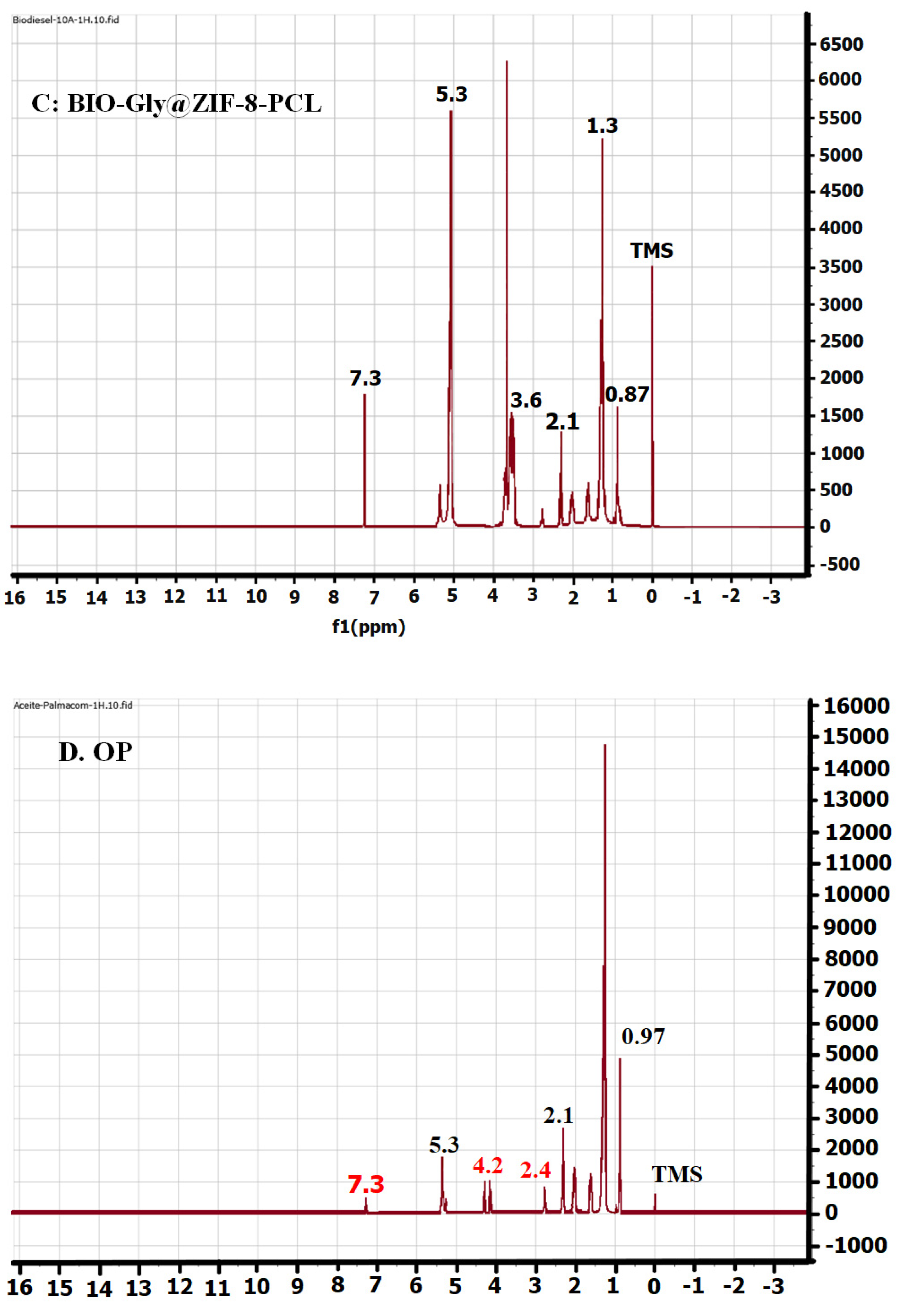
2.7. Nuclear Magnetic Resonance (NMR)
3. Discussion
4. Methods and Materials
4.1. Reagents Used
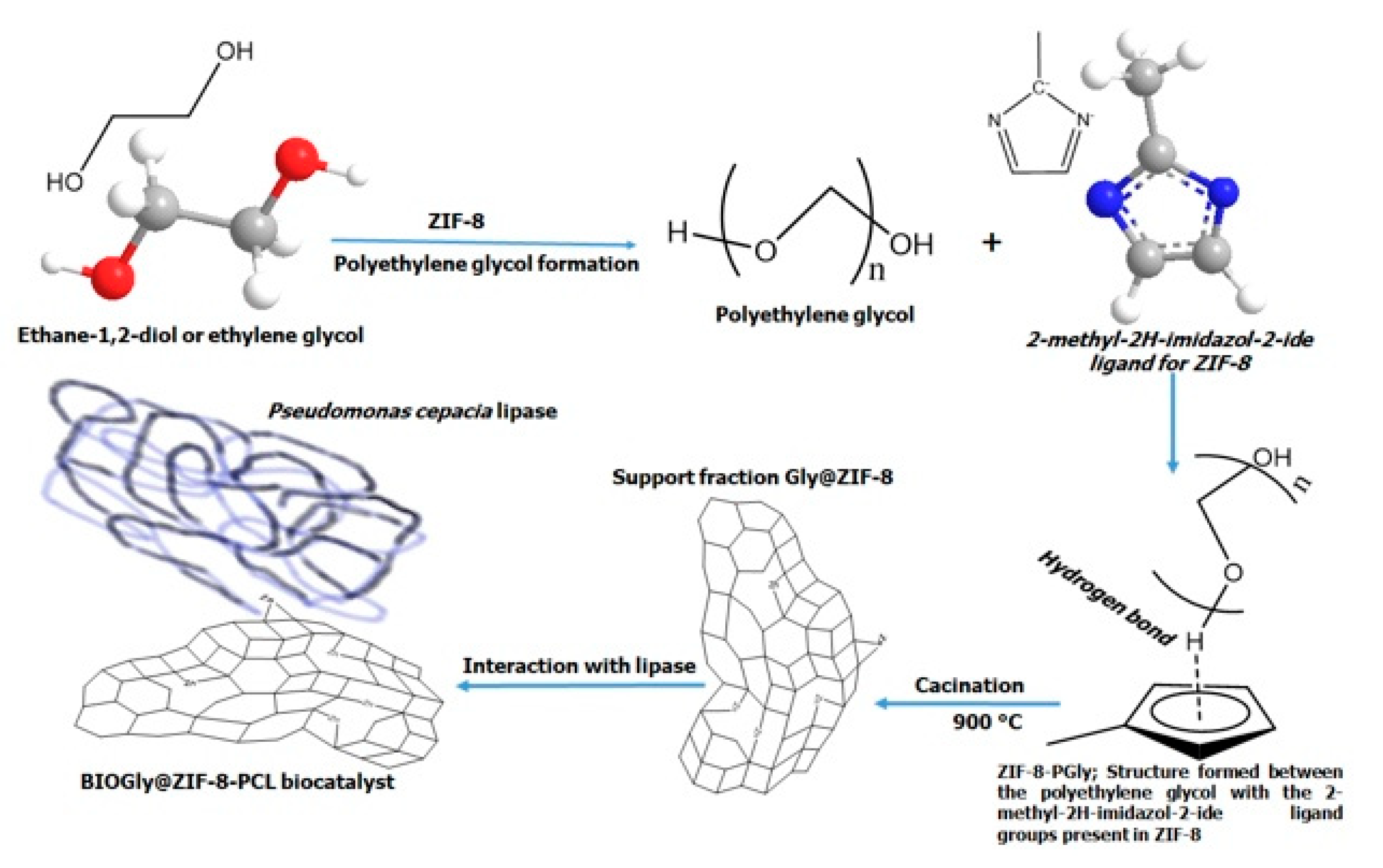
4.2. Thermal Modification of the ZIF-8
4.3. Isotherms of N2 at 77 K
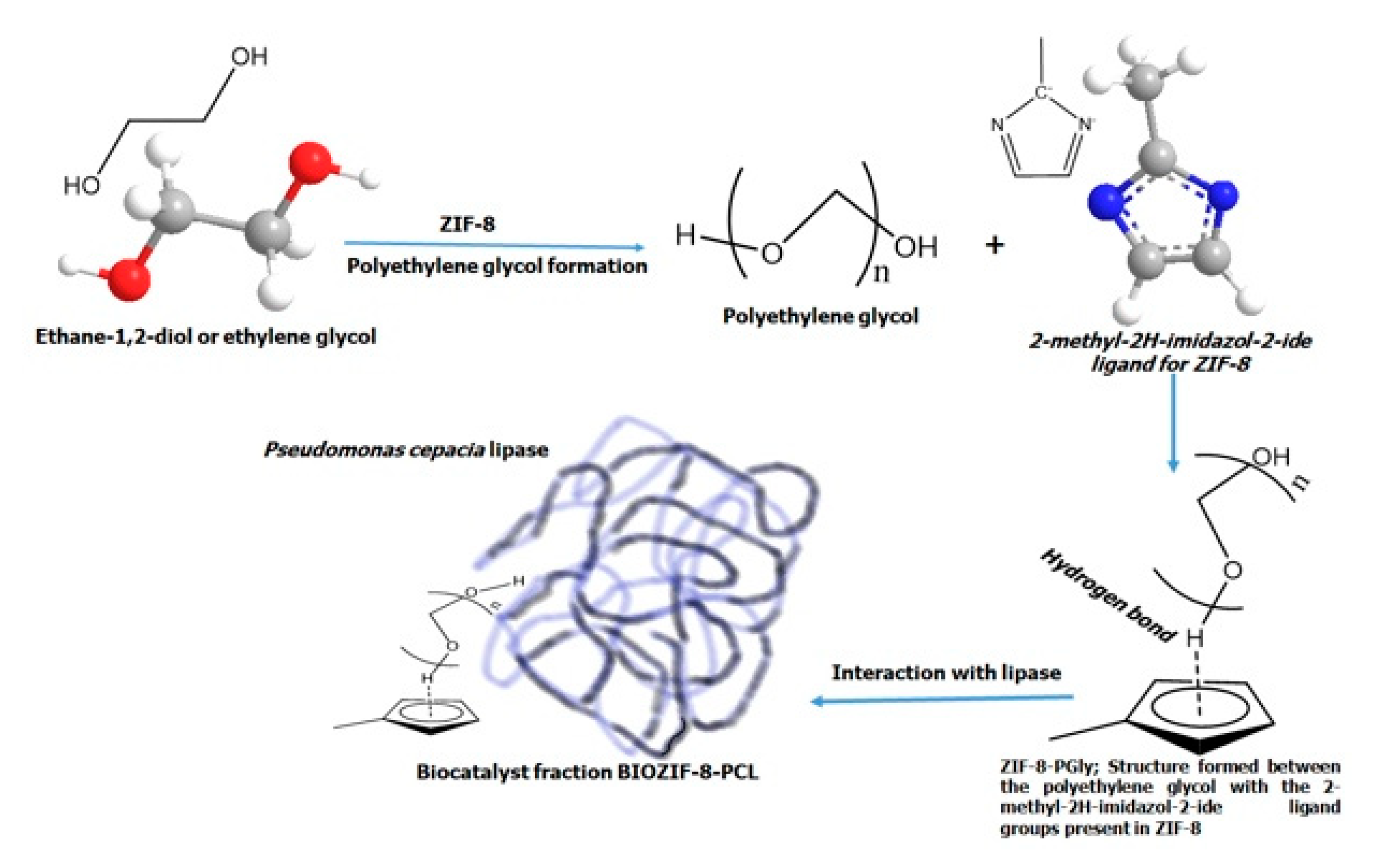
4.4. Pseudomonas Cepacia Lipase Immobilization
4.5. Derivatization of Palm Oil for Mass Analysis
4.6. Transesterification Reaction: Lipase from Pseudomonas Cepacia Supported
4.7. Fourier Transform Infrared Spectrophotometry (FTIR)
4.8. Gas Chromatography Coupled to Mass Spectrometry
4.9. Nuclear Magnetic Resonance (NMR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masaquiza Moposita, D.A.; Pereda Mouso, J.; Curbelo Rodríguez, L.M.; Figueredo Calvo, R.; Cervantes Mena, M. Intensifi-cation of agricultural systems and its relationship with productivity and efficiency. Results with your application: Review Article. Anim. Prod. Mag. 2017, 29, 57–64. [Google Scholar]
- Padilla-Rivera, A.; Paredes, M.G.; Güereca, L.P. A systematic review of the sustainability assessment of bioenergy: The case of gaseous biofuels. Biomass-Bioenergy 2019, 125, 79–94. [Google Scholar] [CrossRef]
- Vargas, E.M.; Villamizar, D.O.; Neves, M.C.; Nunes, M.I. Pelletized biomass fly ash for FAME production: Optimization of a continuous process. Fuel 2021, 293, 120425. [Google Scholar] [CrossRef]
- Stagnaro, P.; Brunengo, E.; Conzatti, L. ED-ROP of macrocyclic oligomers: A synthetic tool for multicomponent polymer materials. ARKIVOC Online J. Org. Chem. 2021, 2021, 174–221. [Google Scholar] [CrossRef]
- Antonetti, C.; Gori, S.; Licursi, D.; Pasini, G.; Frigo, S.; López, M.; Raspolli Galletti, A.M. One-Pot Alcoholysis of the Lignocellulosic Eucalyptus nitens Biomass to n-Butyl Levulinate, a Valuable Additive for Diesel Motor Fuel. Catalysts 2020, 10, 509. [Google Scholar] [CrossRef]
- Rozzi, E.; Minute, F.D.; Lanzini, A.; Leone, P. Green Synthetic Fuels: Renewable pathways for the Conversion of Non-Fossil Feedstocks into Gaseous Fuels and Their End Uses. Energies 2020, 13, 420. [Google Scholar] [CrossRef]
- Alsultan, A.G.; Asikin-Mijan, N.; Ibrahim, Z.; Yunus, R.; Razali, S.Z.; Mansir, N.; Taufiq-Yap, Y.H. A Short Review on Catalyst, Feedstock, Modernised Process, Current State and Challenges on Biodiesel Production. Catalysts 2021, 11, 1261. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Z.; Wang, R.; Zhou, C.; Sun, C. Study on ultrasound-assisted precipitation for preparing Ni/Al2O3 catalyst. Ultrason. Sonochemistry 2020, 67, 105107. [Google Scholar] [CrossRef]
- Shibasaki-Kitakawa, N.; Hiromori, K. Sustainable Production of Biodiesel Using Ion-Exchange Resin Catalysts. Biodiesel Prod. Feedstocks Catal. Technol. 2022, 193–207. [Google Scholar] [CrossRef]
- Araujo, M.N.; dos Santos, K.C.; do Carmo Diniz, N.; de Carvalho, J.C.; Corazza, M.L. A biorefinery approach for spent coffee grounds valorization using pressurized fluid extraction to produce oil and bioproducts: A systematic review. Bioresour. Technol. Rep. 2022, 18, 101013. [Google Scholar] [CrossRef]
- Lowe, B.; Gardy, J.; Wu, K.; Hassanpour, A. Mixed Metal Oxide Catalysts in Biodiesel Production. Biodiesel Prod. Feedstocks Catal. Technol. 2022, 143–166. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Mustapha, E.; Theng, Y.Y.; Razak, N.A.A.; Bar, N.A.; Baharudin, K.B.; Derawi, D. Advanced biofuels from waste cooking oil via solventless and hydrogen-free catalytic deoxygenation over mesostructured Ni-Co/SBA-15, Ni-Fe/SBA-15, and Co-Fe/SBA-15 catalysts. Fuel 2022, 313, 122695. [Google Scholar] [CrossRef]
- De Sousa, M.H.; da Silva, A.S.F.; Correia, R.C.; Leite, N.P.; Bueno, C.E.G.; dos Santos Pinheiro, R.L.; Menezes, R.S.C. Valorizing municipal organic waste to produce biodiesel, biogas, organic fertilizer, and value-added chemicals: An integrated biorefinery approach. Biomass-Convers. Biorefinery 2022, 12, 827–841. [Google Scholar] [CrossRef]
- Hayyan, A.; Yeow, A.T.; Alkahli, N.A.M.; Saleh, J.; Aldeehani, A.K.; Alkandari, K.H.; Abdelrahman, M.A. Application of acidic ionic liquids for the treatment of acidic low grade palm oil for biodiesel production. J. Ion. Liq. 2022, 2, 100023. [Google Scholar] [CrossRef]
- Badgujar, V.C.; Bhanage, B.M. Recent update on use of ionic liquids for enzyme immobilization, activation and catalysis: A partnership for sustainability. Curr. Opin. Green Sustain. Chem. 2022, 36, 100621. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Domínguez, Y.D.; Machín, L.R.; Pohl, S.; Lapuerta, M.; Piloto-Rodríguez, R. Biomass-based heterogeneous catalysts for biodiesel production: A comprehensive review. Int. J. Energy Res. 2022, 46, 3782–3809. [Google Scholar] [CrossRef]
- El-Galad, M.I.; El-Khatib, K.M.; El-Sheltawy, S.T. Techno-economic and Sensitivity Analyses of Different Biodiesel Production Pathways by Adding Tetrahydrofuran as a Cosolvent. BioEnergy Res. 2022, 1–15. [Google Scholar] [CrossRef]
- Hussain, F.; Alshahrani, S.; Abbas, M.M.; Khan, H.M.; Jamil, A.; Yaqoob, H.; Munir, M. Animal bone waste as catalysts for biodiesel production; a short review. Catalysts 2021, 11, 630. [Google Scholar] [CrossRef]
- Hijazi, A.; Khalaf, N.; Kwapinski, W.; Leahy, J.J. Catalytic valorisation of biomass levulinic acid into gamma valerolactone using formic acid as a H2 donor: A critical review. RSC Adv. 2022, 12, 13673–13694. [Google Scholar] [CrossRef]
- Sitepu, E.K.; Sembiring, Y.; Supeno, M.; Tarigan, K.; Ginting, J.; Karo-karo, J.A.; Tarigan, J.B. Homogenizer-intensified room temperature biodiesel production using heterogeneous palm bunch ash catalyst. S. Afr. J. Chem. Eng. 2022, 40, 240–245. [Google Scholar] [CrossRef]
- Helmi, M.; Tahvildari, K.; Hemmati, A.; Azar, P.A.; Safekordi, A. Converting waste cooking oil into biodiesel using phosphomolybdic acid/clinoptilolite as an innovative green catalyst via electrolysis procedure; optimization by response surface methodology (RSM). Fuel Process. Technol. 2022, 225, 107062. [Google Scholar] [CrossRef]
- Helmi, F.; Helmi, M.; Hemmati, A. Phosphomolybdic acid/chitosan as acid solid catalyst using for biodiesel production from pomegranate seed oil via microwave heating system: RSM optimization and kinetic study. Renew. Energy 2022, 189, 881–898. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gebeyehu, K.B.; Selvakumar, K.V.; Parvathy, S.; Kim, W.; Karmegam, N. Waste Ox bone based heterogeneous catalyst synthesis, characterization, utilization and reaction kinetics of biodiesel generation from Jatropha curcas oil. Chemosphere 2021, 288, 132534. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Mahdinia, S.; Oryani, B.; Cho, J.; Kwon, E.E.; Bozorgian, A.; Mehranzamir, K. Biodiesel production from wild mustard (Sinapis Arvensis) seed oil using a novel heterogeneous catalyst of LaTiO3 nanoparticles. Fuel 2022, 307, 121759. [Google Scholar] [CrossRef]
- Pasupulety, N.; Al-zahrani, A.A.; Daous, M.A.; Driss, H.; Alhumade, H. Highly efficient tantalum-zirconium oxide catalysts for biodiesel production from higher water and FFA containing soybean oil or yellowgrease feedstocks. Fuel 2022, 324, 124513. [Google Scholar] [CrossRef]
- Gufrana, T.; Islam, H.; Khare, S.; Pandey, A. In-situ transesterification of single-cell oil for biodiesel production: A review. Prep. Biochem. Biotechnol. 2022, 1–16. [Google Scholar] [CrossRef]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Li, J.; Tong, W.; Gao, C. One-pot synthesis of poly(ethylene glycol) modified zeolitic imidazolate framework-8 nanoparticles: Size control, surface modification and drug encapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 224–230. [Google Scholar] [CrossRef]
- Wickenheisser, M.; Jeremias, F.; Henninger, S.K.; Janiak, C. Grafting of hydrophilic ethylene glycols or ethylenediamine on coordinatively unsaturated metal sites in MIL-100 (Cr) for improved water adsorption characteristics. Inorganica Chim. Acta 2013, 407, 145–152. [Google Scholar] [CrossRef]
- Kim, K.K.; Song, H.K.; Shin, D.H.; Hwang, K.Y.; Suh, S.W. The crystal structure of a triacylglycerol lipase from Pseudomonas cepacia reveals a highly open conformation in the absence of a bound inhibitor. Structure 1997, 5, 173–185. [Google Scholar] [CrossRef]
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Burkholderia cepacia lipase: A versatile catalyst in synthesis reactions. Biotechnol. Bioeng. 2018, 115, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Dulęba, J.; Siódmiak, T.; Marszałł, M.P. The influence of substrate systems on the enantioselective and lipolytic activity of immobilized Amano PS from Burkholderia cepacia lipase (APS-BCL). Process Biochem. 2022, 120, 126–137. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Chan, E.-S.; Ravindra, P. Physical and stability characteristics of Burkholderia cepacia lipase encapsulated in κ-carrageenan. J. Mol. Catal. B: Enzym. 2009, 58, 78–83. [Google Scholar] [CrossRef]
- Matinja, A.I.; Zain, N.A.M.; Suhaimi, M.S.; Alhassan, A.J. Optimization of biodiesel production from palm oil mill effluent using lipase immobilized in PVA-alginate-sulfate beads. Renew. Energy 2019, 135, 1178–1185. [Google Scholar] [CrossRef]
- Chen, G.; Liu, J.; Qi, Y.; Yao, J.; Yan, B. Biodiesel production using whole-cell magnetic biocatalysts by immobilization of Pseudomonas mendocina in Fe3O4-chitosan microspheres. Biochem. Eng. J. 2016, 113, 86–92. [Google Scholar] [CrossRef]
- Liu, Y.; Dave, D. Recent advances in immobilization technology in the enzymatic conversion of marine by-products into concentrated omega-3 fatty acids. Green Chem. 2022, 24, 1049. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Yousefi, P.; Mohammadi, J.; Brask, J. Enzymatic production of biodiesel using lipases immobilized on silica nanoparticles as highly reusable biocatalysts: Effect of water, t-butanol and blue silica gel contents. Renew. Energy 2016, 91, 196–206. [Google Scholar] [CrossRef]
- Pitzalis, F.; Carucci, C.; Naseri, M.; Fotouhi, L.; Magner, E.; Salis, A. Lipase Encapsulation onto ZIF-8: A Comparison between Biocatalysts Obtained at Low and High Zinc/2-Methylimidazole Molar Ratio in Aqueous Medium. ChemCatChem 2018, 10, 1578–1585. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Dai, L.; Liu, D.; Al-Zuhair, S.; Du, W. Immobilization of Lipase from Thermomyces lanuginosus in Magnetic Macroporous ZIF-8 Improves Lipase Reusability in Biodiesel Preparation. ACS Omega 2021, 7, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Entrapment of surfactant modified lipase within zeolitic imidazolate framework (ZIF)-8. Int. J. Biol. Macromol. 2020, 146, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Pitzalis, F.; Carucci, C.; Medda, L.; Fotouhi, L.; Magner, E.; Salis, A. Lipase and Laccase Encapsulated on Zeolite Imidazolate Framework: Enzyme Activity and Stability from Voltammetric Measurements. ChemCatChem 2018, 10, 5425–5433. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Yan, Y. Recent developments of lipase immobilization technology and application of immobilized lipase mixtures for biodiesel production. Biofuels Bioprod. Biorefining 2022, 16, 1062–1094. [Google Scholar] [CrossRef]
- Shomal, R.; Du, W.; Al-Zuhair, S. Immobilization of Lipase on Metal-Organic frameworks for biodiesel production. J. Environ. Chem. Eng. 2022, 10, 107265. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Encapsulation of lipase within metal-organic framework (MOF) with enhanced activity intensified under ultrasound. Enzym. Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef]
- Metz, P.C.; Ryder, M.R.; Ganesan, A.; Bhattacharyya, S.; Purdy, S.C.; Nair, S.; Page, K. Structure Evolution of Chemically Degraded ZIF-8. J. Phys. Chem. C 2022, 126, 9736–9741. [Google Scholar] [CrossRef]
- Dong, X.; Su, Y.; Lu, T.; Zhang, L.; Wu, L.; Lv, Y. MOFs-derived dodecahedra porous Co3O4: An efficient cataluminescence sensing material for H2S. Sensors Actuators B: Chem. 2018, 258, 349–357. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, K.J.; Cheon, J.Y.; Lee, J.H.; Joo, S.H.; Moon, H.R. Nanoporous metal oxides with tunable and nanocrystalline structures through the conversion of metal-organic structures. J. Am. Chem. Soc. 2013, 135, 8940–8946. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S.; Tuller, H.L.; Kim, I.-D. Heterogeneous Sensitization of Metal–Organic Framework Driven Metal@Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef]
- Díaz-Duran, A.K.; Roncaroli, F. The Influence of Particle Size and Shape in Cobalt 2-Methylimidazolate Polymers on Catalytic Properties. Eur. J. Inorg. Chem. 2021, 2021, 2830–2839. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Al-Mansouri, R.; Du, W.; Al-Zuhair, S. Reaction-diffusion model to describe biodiesel production using lipase encapsulated in ZIF-8. Fuel 2022, 311, 122630. [Google Scholar] [CrossRef]
- Jian, O.; Xin, Y.; Yu, L.; Panliang, Z.; Weifeng, X.; Kewen, T. Lipase from Pseudomonas cepacia immobilized into ZIF-8 as bio-catalyst for enantioselective hydrolysis and transesterification. Process Biochem. 2021, 102, 132–140. [Google Scholar] [CrossRef]
- Suman, C.; Li-Fang, W.; Himanshu, A.; Ming-Fong, T.; Siva, S.S.; Suresh, S.; Srinath, G.; Sripal, R.G.; Venkatathri, N. Rapid synthesis of a novel nano-crystalline mesoporous faujasite type metal-organic framework, ZIF-8 catalyst, its detailed characterization, and NaBH4 assisted, enhanced catalytic Rhodamine B degradation. Mater. Today Commun. 2021, 26, 101993. [Google Scholar] [CrossRef]
- Duan, Y.; Qiu, M.; Xu, S.; Li, D.; Wu, H.; Chang, L.; Zeng, H. Zwitterionic ionic liquids modulating two-dimensional hierarchically porous zeolitic imidazolate framework composites. J. Colloid Interface Sci. 2022, 620, 365–375. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. El Manual de Frecuencias Características Infrarrojas y Raman de Mo-Léculas Orgánicas; Elsevier: Amsterdam, The Netherlands, 1991; ISBN 0-12451160-0. [Google Scholar]
- Ivanoiu, A.; Schmidt, A.; Peter, F.; Rusnac, L.M.; Ungurean, M. Comparative study on biodiesel synthesis from different veg-etables oils. Chem. Bull. 2011, 56, 94–98. [Google Scholar]
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2020, 11, 42. [Google Scholar] [CrossRef]
- Ayala, S.; Bentz, K.C.; Cohen, S.M. Block co-polyMOFs: Morphology control of polymer–MOF hybrid materials. Chem. Sci. 2019, 10, 1746–1753. [Google Scholar] [CrossRef]
- An, T.B.; Linh, D.H.T.; Anh, N.P.; An, T.T.T.; Tri, N. Inmovilización y Desempeño de Celulasa en Hidrotalcitas Magnéticas Reciclables. Rev. Tecnol. Bioquímica 2022, 13, 109365. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Timucin, E.; Sezerman, O.U. Zinc Modulates Self-Assembly of Bacillus thermocatenulatus Lipase. Biochemistry 2015, 54, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huan, X.; Jia, X.; Sui, G.; Wu, L.; Cai, Q.; Yang, X. 3D printing nanocomposites with enhanced mechanical property and excellent electromagnetic wave absorption capability via the introduction of ZIF-derivative modified carbon fibers. Compos. Part B Eng. 2022, 233, 109658. [Google Scholar] [CrossRef]
- Guo, X.; Geng, S.; Zhuo, M.; Chen, Y.; Zaworotko, M.J.; Cheng, P.; Zhang, Z. The utility of the template effect in metal-organic frameworks. Co-ord. Chem. Rev. 2019, 391, 44–68. [Google Scholar] [CrossRef]
- Mateos, P.S.; Navas, M.B.; Morcelle, S.R.; Ruscitti, C.; Matkovic, S.R.; Briand, L.E. Perspectives in the biocatalyzed hydrolysis, esterification and transesterification of used cooking oil with a vegetable lipase. Catal. Today 2021, 372, 211–219. [Google Scholar] [CrossRef]
- Wancura, J.H.; Fantinel, A.L.; Ugalde, G.A.; Donato, F.F.; de Oliveira, J.V.; Tres, M.V.; Jahn, S.L. Semi-continuous production of biodiesel on pilot scale via enzymatic hydroesterification of waste material: Process and economics considerations. J. Clean. Prod. 2021, 285, 124838. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, Z.; Sentosun, K.; Pérez-Juste, I.; Bals, S.; Liz-Marzán, L.M.; Hong, M. Shape control in ZIF-8 nanocrystals and metal nanoparticles@ZIF-8 heterostructures. Nanoscale 2017, 9, 16645–16651. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y. Synthesis of zeolitic imidazolate-8 framework in polyester fiber for removal of PM 2.5. RSC Adv. 2018, 8, 31471–31477. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is BET equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A.; Neimark, A.V. Advanced Physical Adsorption Characterization of Nanoporous Carbons. In Novels Carbons Adsorbent; Tascón, J.M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
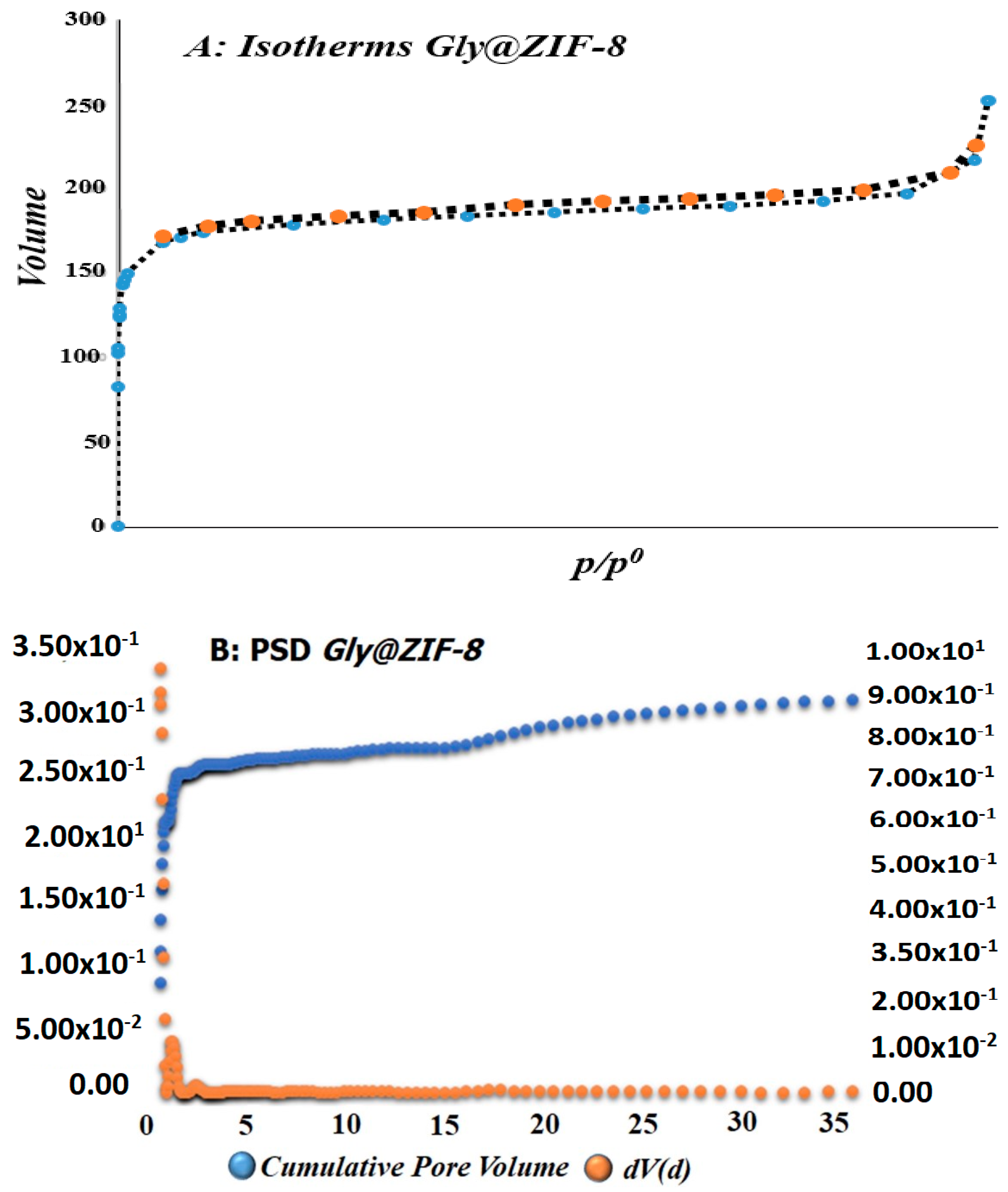
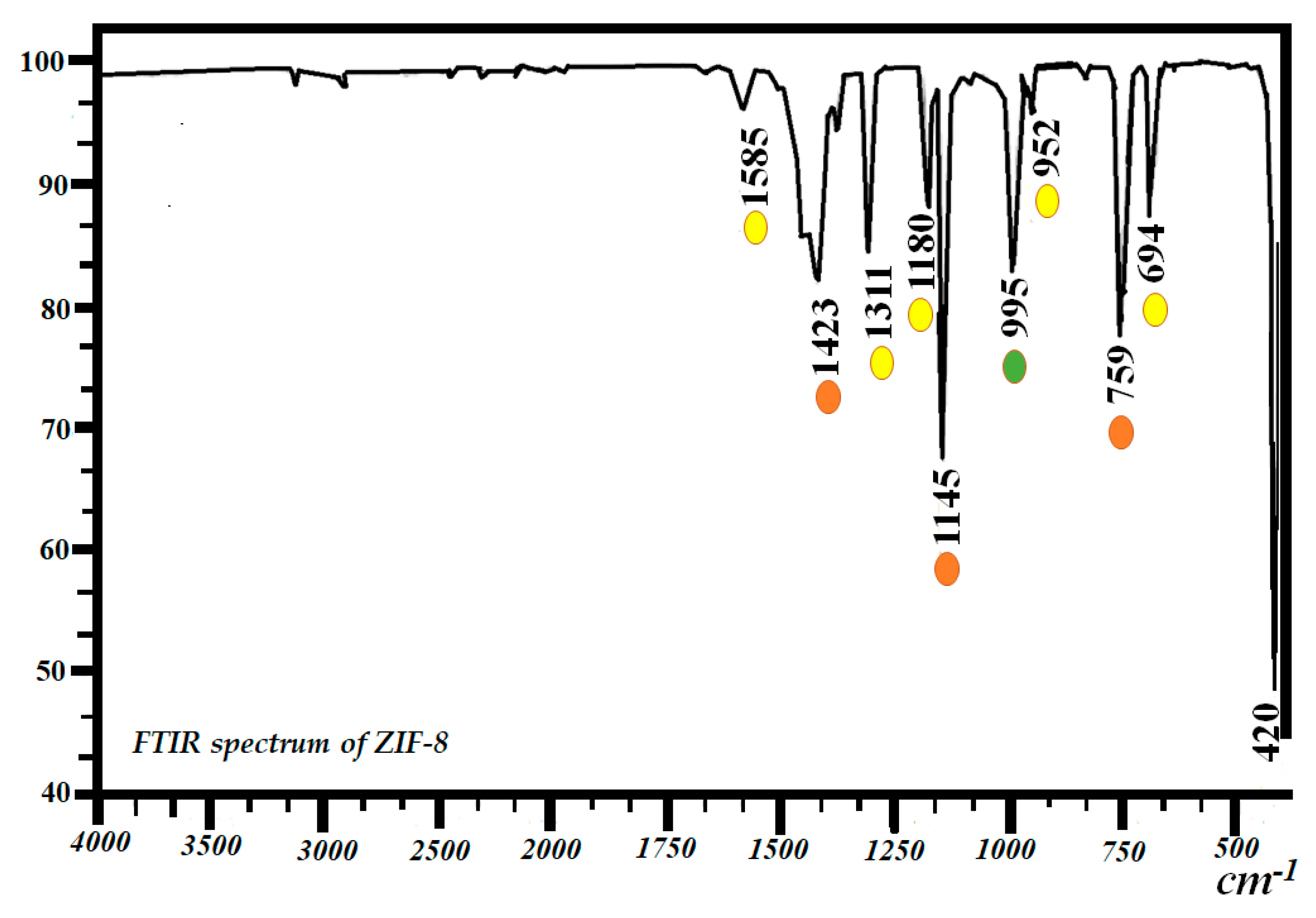
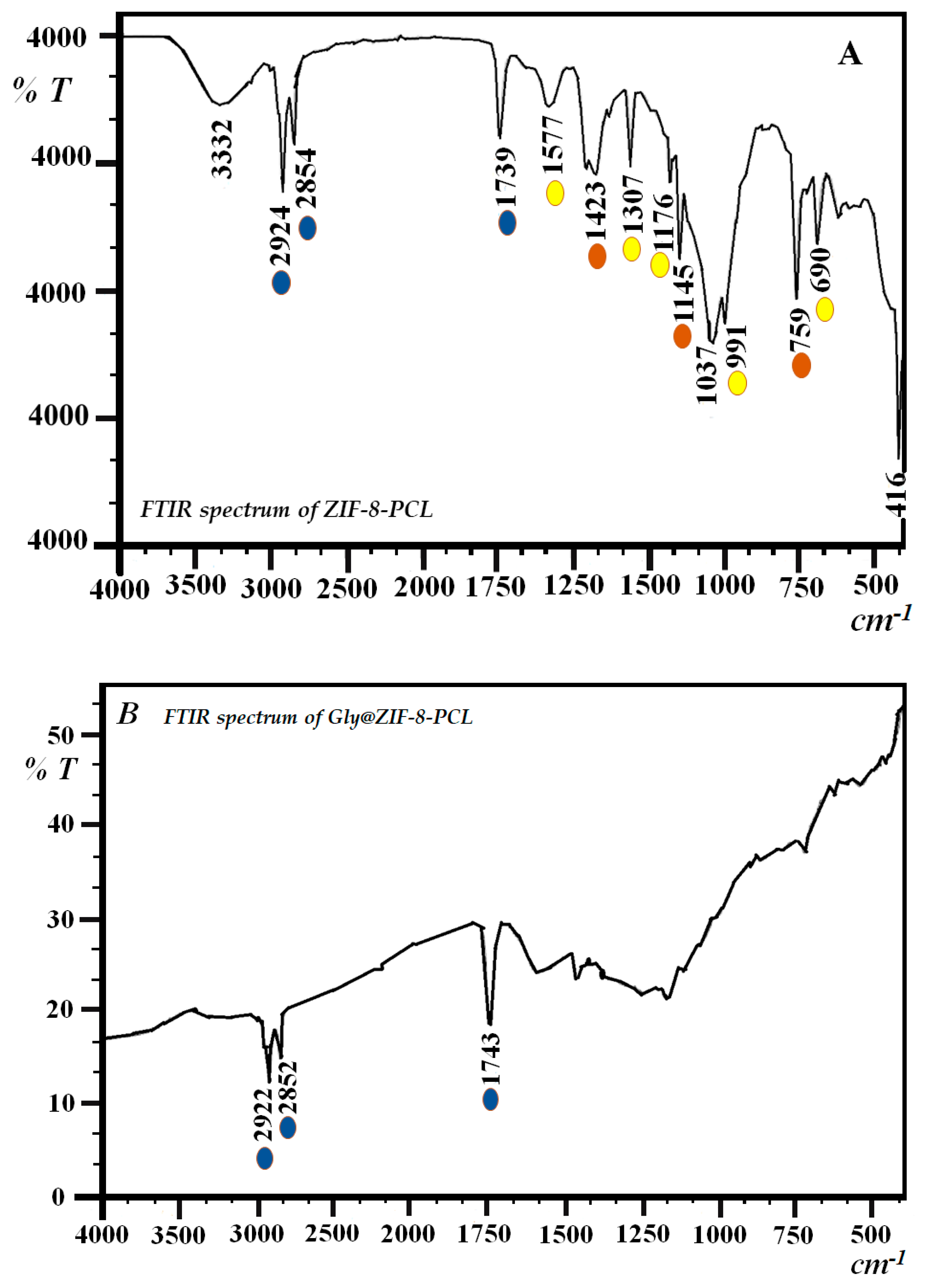

| Sample | SBET a (m2g−1) | Vpore b (cm3∙g−1) | Pore Size c (nm) | Acid d Amount (mmol∙g−1) | Base e (mmol∙g−1) |
|---|---|---|---|---|---|
| ZIF-8 | 1733 | 0.609 | 0.64 | 0.24 | 0.08 |
| Gly@ZIF-8 | 702 | 0.306 | 0.69 | 0.35 | 0.13 |
| Gly@ZIF-8-PCL | 654 | 0.424 | 0.53 | 0.42 | 0.17 |
| Asigne Compound | BIOZIF-8-PCL | BIOGly@ZIF-8-PCL | Molecular Formula | ||||
|---|---|---|---|---|---|---|---|
| Retention Time (min) | Area | % Mass | Retention Time (min) | Area | % Mass | ||
| Methyl palmitate (1) | 14.448 | 106,823,904 | 29.12 | 14.504 | 263,962,120 | 24.24 | C₁₇H₃₄O₂ |
| Methyl stearate (2) | 17.471 | 18,268,908 | 4.98 | 17.556 | 57,724,642 | 5.30 | C19H38O2 |
| Methyl oleate (3) | 17.903 | 136,362,997 | 37.17 | 18.007 | 372,716,433 | 34.23 | C₁₉H₃₆O₂ |
| Methyl linoleate (4) | 18.862 | 85,866,896 | 23.41 | 18.954 | 283,399,032 | 26.03 | C19H34O2 |
| Alfa-methyl linolenate (5) | 20.156 | 7,094,967 | 1.93 | 20.200 | 31,862,269 | 2.93 | C19H32O2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Gil, J.M.; Vivas Reyes, R.; Bastidas Barranco, M.J.; Giraldo, L.; Moreno-Piraján, J.C. Biocatalysts Synthesized with Lipase from Pseudomonas cepacia on Glycol-Modified ZIF-8: Characterization and Utilization in the Synthesis of Green Biodiesel. Molecules 2022, 27, 5396. https://doi.org/10.3390/molecules27175396
Martínez Gil JM, Vivas Reyes R, Bastidas Barranco MJ, Giraldo L, Moreno-Piraján JC. Biocatalysts Synthesized with Lipase from Pseudomonas cepacia on Glycol-Modified ZIF-8: Characterization and Utilization in the Synthesis of Green Biodiesel. Molecules. 2022; 27(17):5396. https://doi.org/10.3390/molecules27175396
Chicago/Turabian StyleMartínez Gil, José Manuel, Ricardo Vivas Reyes, Marlon José Bastidas Barranco, Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2022. "Biocatalysts Synthesized with Lipase from Pseudomonas cepacia on Glycol-Modified ZIF-8: Characterization and Utilization in the Synthesis of Green Biodiesel" Molecules 27, no. 17: 5396. https://doi.org/10.3390/molecules27175396
APA StyleMartínez Gil, J. M., Vivas Reyes, R., Bastidas Barranco, M. J., Giraldo, L., & Moreno-Piraján, J. C. (2022). Biocatalysts Synthesized with Lipase from Pseudomonas cepacia on Glycol-Modified ZIF-8: Characterization and Utilization in the Synthesis of Green Biodiesel. Molecules, 27(17), 5396. https://doi.org/10.3390/molecules27175396







