Overview of Research into mTOR Inhibitors
Abstract
:1. Introduction
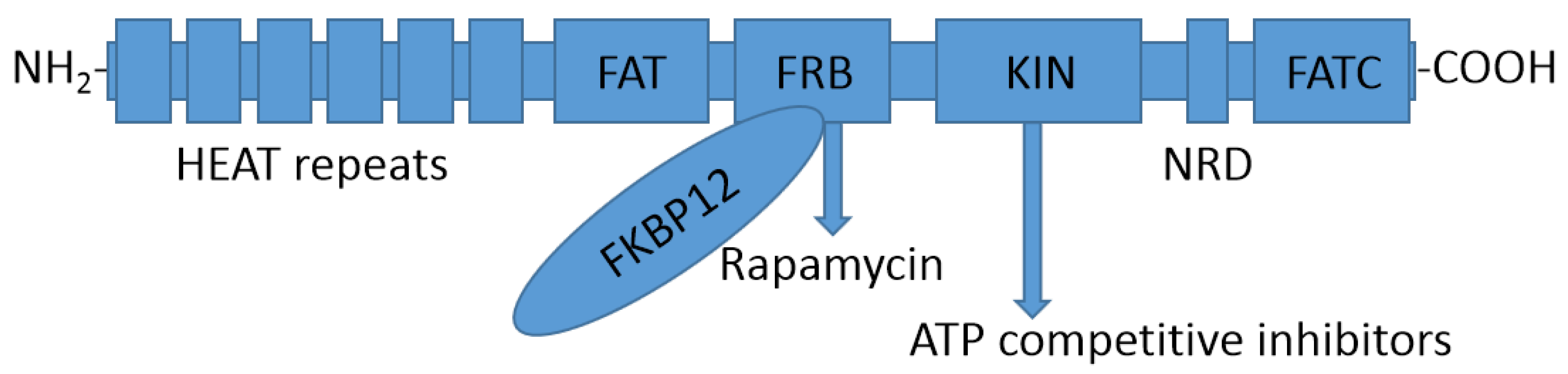
2. The Structure and Function of mTOR
2.1. Structure of the mTOR Protein
2.2. Signaling Pathway and Function of mTOR
2.2.1. The Composition of mTORC1 and Its Signaling Pathway
2.2.2. The Composition of mTORC2 and Its Signaling Pathway
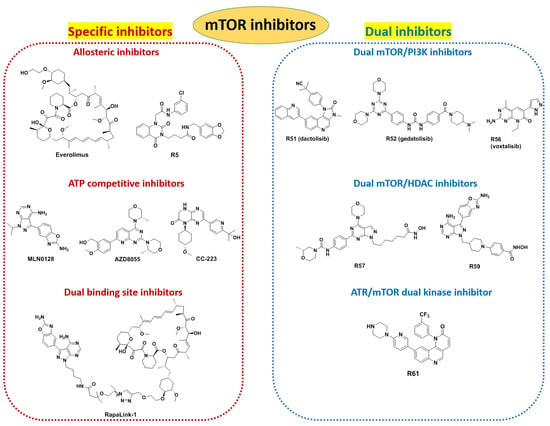
3. Inhibitors of mTOR
3.1. Allosteric Inhibitors of mTOR
3.1.1. Rapamycin and Its Derivatives
3.1.2. Non-Rapalog Allosteric Inhibitor
3.2. ATP Competitive Inhibitors
3.2.1. Morpholine-Substituted Heterocyclic Skeleton Inhibitors
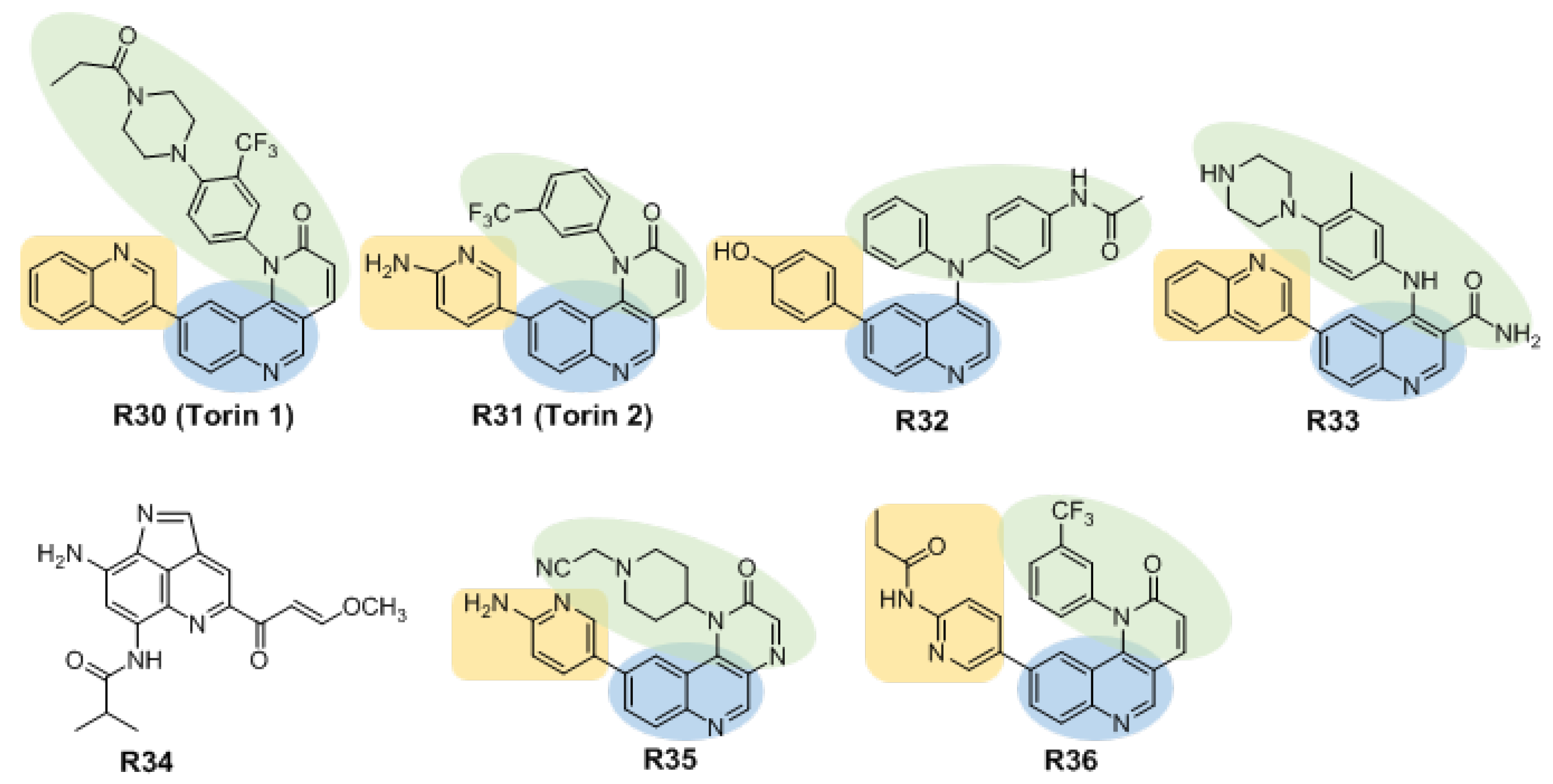
3.2.2. Inhibitors Based on Quinoline Structures
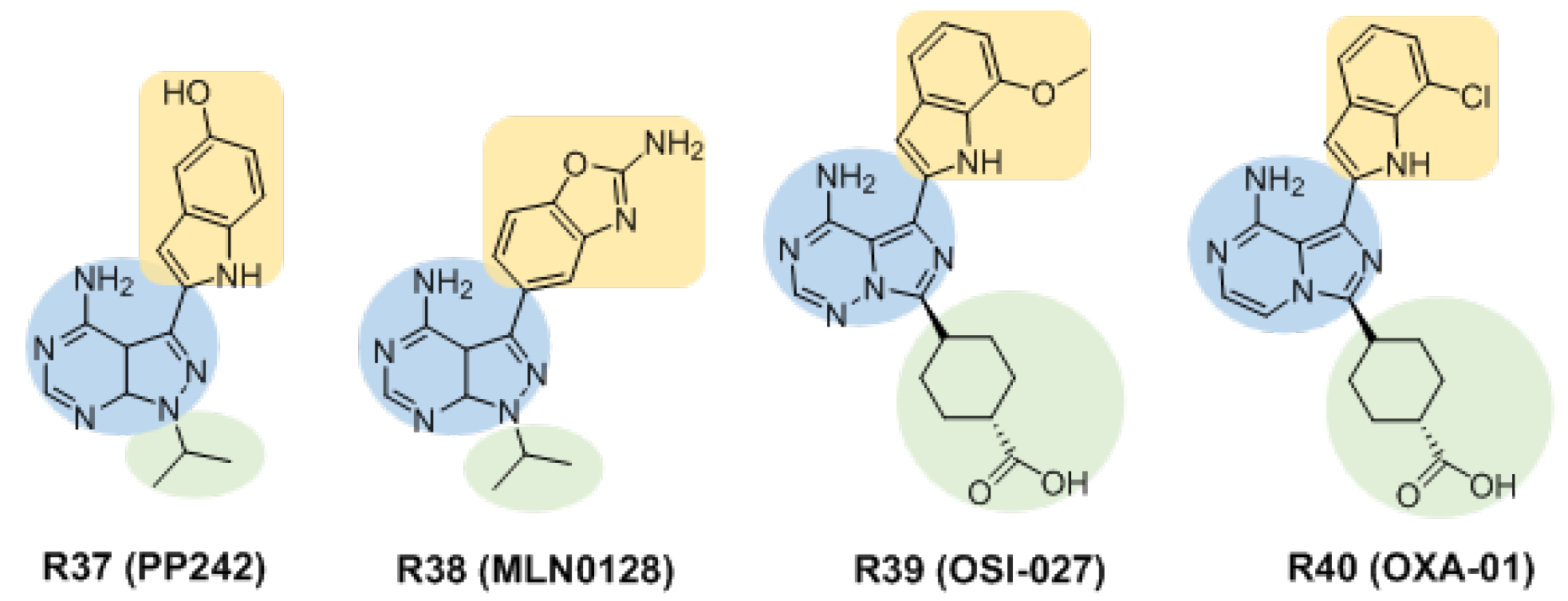
3.2.3. Inhibitors Based on Pyrazolo [3,4-d]pyrimidin-4-amine and Its Derivatives
3.2.4. Other Structural Skeletal Inhibitors
3.3. Dual Binding Site Inhibitors
3.4. Dual-Target mTOR Inhibitors
3.4.1. Dual mTOR/PI3K Inhibitors
3.4.2. Dual mTOR/HDAC Inhibitors
3.4.3. ATR/mTOR Dual Kinase Inhibitor
4. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | protein kinase-B |
| AMBRA1 | autophagy/beclin 1 regulator 1 |
| AMP | adenosine monophosphate |
| AMPK | adenosine monophosphate-activated protein kinase |
| ATG | autophagy-related gene |
| ATM | ataxia telangiectasia mutated |
| ATP | adenosine triphosphate |
| ATR | ataxia telangiectasia mutated and Rad-3 related |
| eIF4E | eukaryotic translation initiation factor 4E |
| EV71 | enterovirus 71 |
| FAT | focal adhesion targeting domain |
| FATC | focal adhesion targeting domain at the C-terminus |
| FDA | Food and Drug Administration |
| FKBP12 | FK506-binding protein of 12 kD |
| FRB | FKBP12-rapamycin binding domain |
| GDP | guanosine diphosphate |
| GEF | guanylate exchange factor |
| GTP | guanosine triphosphate |
| HDACs | histone deacetylases |
| hERG | human ether-a-go-go related gene |
| HFMD | hand-foot-and-mouth disease |
| HGMCS | 3-hydroxy 3-methyl glutaryl CoA (HMGCoA) synthase |
| HMGCoA | 3-hydroxy 3-methyl glutaryl CoA |
| KIN | kinase domain |
| MEK | mitogen-activated protein kinase kinase |
| mLST8 | mammalian lethal with sec-13 protein 8 |
| mSIN1 | mammalian stress-activated map kinase-interacting protein 1 |
| mTOR | mammalian target of rapamycin |
| mTORC | mTOR complex |
| mTORΔN | mTOR N-terminal truncation mutant |
| NRD | negative regulatory domain |
| PI3K | phosphoinositide 3-kinase |
| PIKK | phosphatidylinositol-3-kinase-related kinase |
| PIP2 | phosphatidylinositol (4,5)-biphosphate |
| PIP3 | phosphatidylinositol (3,4,5)-triphosphate |
| PKCα | protein kinase Cα |
| PRAS40 | prolin-rich Akt substrate of 40 kD |
| REDD1 | regulated in development and DNA damage responses-1 |
| Rheb | ras homolog enriched in brain 1 |
| S6K1 | ribosomal protein S6 kinase 1 |
| SGK1 | serum- and glucocorticoid-induced protein kinase 1 |
| SUPT6H | suppressor of Ty homologue-6 |
| TFEB | transcription factor EB |
| TNBC | triple-negative breast cancer |
| TSC | tuberous sclerosis complex |
| ULK | unc-51-like kinase |
| VPS34 | vacuolar protein sorting 34 |
| 4E-BP1 | eukaryotic translation initiation factor 4E-binding protein 1 |
| 5′ TOP | 5′ terminal oligopyrimidine tract mRNA translation protein |
References
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.T.; Schreiber, S.L. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science 1995, 270, 50–51. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Ruegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Evangelisti, C.; McCubrey, J.A.; Martelli, A.M. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol. Sci. 2015, 36, 124–135. [Google Scholar] [CrossRef]
- Lv, X.; Ma, X.; Hu, Y. Furthering the design and the discovery of small molecule ATP-competitive mTOR inhibitors as an effective cancer treatment. Expert Opin. Drug Discov. 2013, 8, 991–1012. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.C.; Raught, B.; Sonenberg, N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001, 15, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Hudson, C.C.; Homme, J.L.; Yin, P.; Otterness, D.M.; Karnitz, L.M.; Abraham, R.T. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000, 60, 3504–3513. [Google Scholar] [PubMed]
- Kallen, J.A.; Sedrani, R.; Cottens, S. X-ray crystal structure of 28-O-methylrapamycin complexed with FKBP12: Is the cyclohexyl moiety part of the effector domain of rapamycin? J. Am. Chem. Soc. 1996, 118, 5857–5861. [Google Scholar] [CrossRef]
- Choi, J.; Chen, J.; Schreiber, S.L.; Clardy, J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science 1996, 273, 239–242. [Google Scholar] [CrossRef]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Thoreen, C.C.; Peterson, T.R.; Lindquist, R.A.; Kang, S.A.; Spooner, E.; Carr, S.A.; Sabatini, D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 2007, 25, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, P.; Wei, W. mTOR signaling in tumorigenesis. Biochim. Biophys. Acta 2014, 1846, 638–654. [Google Scholar] [CrossRef]
- Vignot, S.; Faivre, S.; Aguirre, D.; Raymond, E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann. Oncol. 2005, 16, 525–537. [Google Scholar] [CrossRef]
- Qian, D.C.; Xiao, X.; Byun, J.; Suriawinata, A.A.; Her, S.C.; Amos, C.I.; Barth, R.J., Jr. PI3K/Akt/mTOR signaling and plasma membrane proteins are implicated in responsiveness to adjuvant dendritic cell vaccination for metastatic colorectal cancer. Clin. Cancer Res. 2017, 23, 399–406. [Google Scholar] [CrossRef]
- Lee, M.; Minaskan, N.; Wiedemann, T.; Irmler, M.; Beckers, J.; Yousefi, B.H.; Kaissis, G.; Braren, R.; Laitinen, I.; Pellegata, N.S. Targeting PI3K/mTOR signaling exerts potent antitumor activity in pheochromocytoma in vivo. Endocr. Relat. Cancer. 2017, 24, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.; Wang, L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 2016, 7, 33440–33450. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, L.; Song, H.; Xu, P.; Sun, Y.; Wu, K. Akt/AMPK/mTOR pathway was involved in the autophagy induced by vitamin E succinate in human gastric cancer SGC-7901 cells. Mol. Cell. Biochem. 2017, 424, 173–183. [Google Scholar] [CrossRef]
- Zheng, X.T.; Wu, Z.H.; Wei, Y.; Dai, J.J.; Yu, G.F.; Yuan, F.; Ye, L.C. Induction of autophagy by salidroside through the AMPK-mTOR pathway protects vascular endothelial cells from oxidative stress-induced apoptosis. Mol. Cell. Biochem. 2017, 425, 125–138. [Google Scholar] [CrossRef]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Huang, W.; Chen, W.; Tang, S.; Shi, Y.; Martin, F.L.; Yang, K. 4-Nonylphenol induces autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating AMPK activation in Sertoli cells. Toxicol. Lett. 2017, 267, 21–31. [Google Scholar] [CrossRef]
- Brugarolas, J.; Lei, K.; Hurley, R.L.; Manning, B.D.; Reiling, J.H.; Hafen, E.; Witters, L.A.; Ellisen, L.W.; Kaelin, W.G. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004, 18, 2893–2904. [Google Scholar] [CrossRef]
- DeYoung, M.P.; Horak, P.; Sofer, A.; Sgroi, D.; Ellisen, L.W. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008, 22, 239–251. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, H.; Levine, A.J.; Jin, S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. USA 2005, 102, 8204–8209. [Google Scholar] [CrossRef]
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T.W. Regulation of PTEN transcription by p53. Mol. Cell 2001, 8, 317–325. [Google Scholar] [CrossRef]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.L. Differential regulation of mTORC1 by leucine and glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef]
- Varea, O.; Escoll, M.; Diez, H.; Garrido, J.J.; Wandosell, F. Oestradiol signalling through the Akt-mTORC1-S6K1. Biochim. Biophys. Acta 2013, 1833, 1052–1064. [Google Scholar] [CrossRef]
- Ben-Hur, V.; Denichenko, P.; Siegfried, Z.; Maimon, A.; Krainer, A.; Davidson, B.; Karni, R. S6K1 alternative splicing modulates its oncogenic activity and regulates mTORC1. Cell Rep. 2013, 3, 103–115. [Google Scholar] [CrossRef]
- Siroky, B.J.; Bitzer, M. The growing importance of mTORC1-S6K1 signaling in kidney. Am. J. Physiol. Renal. Physiol. 2009, 297, F583–F584. [Google Scholar] [CrossRef] [PubMed]
- Josse, L.; Xie, J.; Proud, C.G.; Smales, C.M. mTORC1 signalling and eIF4E/4E-BP1 translation initiation factor stoichiometry influence recombinant protein productivity from GS-CHOK1 cells. Biochem. J. 2016, 473, 4651–4664. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.; Bidinosti, M. Rapamycin-insensitive mTORC1 activity controls eIF4E:4E-BP1 binding. F1000Res. 2012, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Contu, R.; Latronico, M.V.; Zhang, J.; Rizzi, R.; Catalucci, D.; Miyamoto, S.; Huang, K.; Ceci, M.; Gu, Y.; et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Investig. 2010, 120, 2805–2816. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- White, E. The role for autophagy in cancer. J. Clin. Investig. 2015, 125, 42–46. [Google Scholar] [CrossRef]
- Zhao, J.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc. Natl. Acad. Sci. USA 2015, 112, 15790–15797. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, H.P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Pearce, L.R.; Sommer, E.M.; Sakamoto, K.; Wullschleger, S.; Alessi, D.R. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem. J. 2011, 436, 169–179. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.M.; Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 2008, 416, 375–385. [Google Scholar] [CrossRef]
- Liu, Q.; Thoreen, C.; Wang, J.; Sabatini, D.; Gray, N.S. mTOR mediated anti-cancer drug discovery. Drug Discov. Today Ther. Strateg. 2009, 6, 47–55. [Google Scholar] [CrossRef]
- Wander, S.A.; Hennessy, B.T.; Slingerland, J.M. Next-generation mTOR inhibitors in clinical oncology: How pathway complexity informs therapeutic strategy. J. Clin. Investig. 2011, 121, 1231–1241. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., 3rd; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Mita, M.M.; Gong, J.; Chawla, S.P. Ridaforolimus in advanced or metastatic soft tissue and bone sarcomas. Expert Rev. Clin. Pharmacol. 2013, 6, 465–482. [Google Scholar] [CrossRef]
- Shams, R.; Matsukawa, A.; Ochi, Y.; Ito, Y.; Miyatake, H. In Silico and In Cell Hybrid Selection of Nonrapalog Ligands to Allosterically Inhibit the Kinase Activity of mTORC1. J. Med. Chem. 2022, 65, 1329–1341. [Google Scholar] [CrossRef]
- Yu, K.; Toral-Barza, L.; Shi, C.; Zhang, W.G.; Lucas, J.; Shor, B.; Kim, J.; Verheijen, J.; Curran, K.; Malwitz, D.J.; et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer. Res. 2009, 69, 6232–6240. [Google Scholar] [CrossRef]
- Cohen, F.; Bergeron, P.; Blackwood, E.; Bowman, K.K.; Chen, H.; Dipasquale, A.G.; Epler, J.A.; Koehler, M.F.; Lau, K.; Lewis, C.; et al. Potent, selective, and orally bioavailable inhibitors of mammalian target of rapamycin (mTOR) kinase based on a quaternary substituted dihydrofuropyrimidine. J. Med. Chem. 2011, 54, 3426–3435. [Google Scholar] [CrossRef]
- Koehler, M.F.; Bergeron, P.; Blackwood, E.; Bowman, K.K.; Chen, Y.H.; Deshmukh, G.; Ding, X.; Epler, J.; Lau, K.; Lee, L.; et al. Potent, selective, and orally bioavailable inhibitors of the mammalian target of rapamycin kinase domain exhibiting single agent antiproliferative activity. J. Med. Chem. 2012, 55, 10958–10971. [Google Scholar] [CrossRef]
- Pei, Z.; Blackwood, E.; Liu, L.; Malek, S.; Belvin, M.; Koehler, M.F.; Ortwine, D.F.; Chen, H.; Cohen, F.; Kenny, J.R.; et al. Discovery and biological profiling of potent and selective mTOR inhibitor GDC-0349. ACS Med. Chem. Lett. 2013, 4, 103–107. [Google Scholar] [CrossRef]
- Estrada, A.A.; Shore, D.G.; Blackwood, E.; Chen, Y.H.; Deshmukh, G.; Ding, X.; Dipasquale, A.G.; Epler, J.A.; Friedman, L.S.; Koehler, M.F.; et al. Pyrimidoaminotropanes as potent, selective, and efficacious small molecule kinase inhibitors of the mammalian target of rapamycin (mTOR). J. Med. Chem. 2013, 56, 3090–3101. [Google Scholar] [CrossRef]
- Liu, K.K.; Bailey, S.; Dinh, D.M.; Lam, H.; Li, C.; Wells, P.A.; Yin, M.J.; Zou, A. Conformationally-restricted cyclic sulfones as potent and selective mTOR kinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5114–5117. [Google Scholar] [CrossRef]
- Malagu, K.; Duggan, H.; Menear, K.; Hummersone, M.; Gomez, S.; Bailey, C.; Edwards, P.; Drzewiecki, J.; Leroux, F.; Quesada, M.J.; et al. The discovery and optimisation of pyrido[2,3-d]pyrimidine-2,4-diamines as potent and selective inhibitors of mTOR kinase. Bioorg. Med. Chem. Lett. 2009, 19, 5950–5953. [Google Scholar] [CrossRef]
- Pike, K.G.; Malagu, K.; Hummersone, M.G.; Menear, K.A.; Duggan, H.M.; Gomez, S.; Martin, N.M.; Ruston, L.; Pass, S.L.; Pass, M. Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: The discovery of AZD8055 and AZD2014. Bioorg. Med. Chem. Lett. 2013, 23, 1212–1216. [Google Scholar] [CrossRef]
- Huang, S.; Yang, Z.J.; Yu, C.; Sinicrope, F.A. Inhibition of mTOR kinase by AZD8055 can antagonize chemotherapy-induced cell death through autophagy induction and down-regulation of p62/sequestosome 1. J. Biol. Chem. 2011, 286, 40002–40012. [Google Scholar] [CrossRef]
- Holt, S.V.; Logie, A.; Davies, B.R.; Alferez, D.; Runswick, S.; Fenton, S.; Chresta, C.M.; Gu, Y.; Zhang, J.; Wu, Y.L.; et al. Enhanced apoptosis and tumor growth suppression elicited by combination of MEK (selumetinib) and mTOR kinase inhibitors (AZD8055). Cancer Res. 2012, 72, 1804–1813. [Google Scholar] [CrossRef]
- Marshall, G.; Howard, Z.; Dry, J.; Fenton, S.; Heathcote, D.; Gray, N.; Keen, H.; Logie, A.; Holt, S.; Smith, P.; et al. Benefits of mTOR kinase targeting in oncology: Pre-clinical evidence with AZD8055. Biochem. Soc. Trans. 2011, 39, 456–459. [Google Scholar] [CrossRef]
- Lee, W.; Ortwine, D.F.; Bergeron, P.; Lau, K.; Lin, L.; Malek, S.; Nonomiya., J.; Pei, Z.; Robarge, K.D.; Schmidt, S.; et al. A hit to lead discovery of novel N-methylated imidazolo-, pyrrolo-, and pyrazolo-pyrimidines as potent and selective mTOR inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5097–5104. [Google Scholar] [CrossRef]
- Finlay, M.R.; Buttar, D.; Critchlow, S.E.; Dishington, A.P.; Fillery, S.M.; Fisher, E.; Glossop, S.C.; Graham, M.A.; Johnson, T.; Lamont, G.M.; et al. Sulfonyl-morpholino-pyrimidines: SAR and development of a novel class of selective mTOR kinase inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 4163–4168. [Google Scholar] [CrossRef]
- Pike, K.G.; Morris, J.; Ruston, L.; Pass, S.L.; Greenwood, R.; Williams, E.J.; Demeritt, J.; Culshaw, J.D.; Gill, K.; Pass, M.; et al. Discovery of AZD3147: A potent, selective dual inhibitor of mTORC1 and mTORC2. J. Med. Chem. 2015, 58, 2326–2349. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, C.; Xu, S.; Wu, C.; Wu, J.; Xu, M.; Zhao, H.; Chen, L.; Zeng, W.; Zheng, P. Design, synthesis, anticancer activity and docking studies of novel 4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine derivatives as mTOR inhibitors. Bioorg. Med. Chem. 2014, 22, 6746–6754. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Gao, S.; Weng, Y.; Zhang, L.; Zhang, L. Design, synthesis, and biological evaluation of imidazo[1,2-b]pyridazine derivatives as mTOR inhibitors. Eur. J. Med. Chem. 2017, 129, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Cansfield, A.D.; Ladduwahetty, T.; Sunose, M.; Ellard, K.; Lynch, R.; Newton, A.L.; Lewis, A.; Bennett, G.; Zinn, N.; Thomson, D.W.; et al. CZ415, a highly selective mTOR inhibitor showing in vivo efficacy in a collagen induced arthritis model. ACS Med. Chem. Lett. 2016, 7, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Borsari, C.; Rageot, D.; Dall’Asen, A.; Bohnacker, T.; Melone, A.; Sele, A.M.; Jackson, E.; Langlois, J.B.; Beaufils, F.; Hebeisen, P.; et al. A Conformational Restriction Strategy for the Identification of a Highly Selective Pyrimido-pyrrolo-oxazine mTOR Inhibitor. J. Med. Chem. 2019, 62, 8609–8630. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, H.; Bravi, G.; Campbell, I.; Convery, M.; Davies, H.; Inglis, G.; Pal, S.; Peace, S.; Redmond, J.; Summers, D. Discovery of 3-oxabicyclo[4.1.0]heptane, a non-nitrogen containing morpholine isostere, and its application in novel inhibitors of the PI3K-AKT-mTOR pathway. J. Med. Chem. 2019, 62, 6972–6984. [Google Scholar] [CrossRef] [PubMed]
- Rageot, D.; Bohnacker, T.; Melone, A.; Langlois, J.B.; Borsari, C.; Hillmann, P.; Sele, A.M.; Beaufils, F.; Zvelebil, M.; Hebeisen, P.; et al. Discovery and preclinical characterization of 5-[4,6-bis({3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-1,3,5-triazin-2-yl]-4-(difluoromethyl)pyridin-2-amine (PQR620), a highly potent and selective mtorc1/2 inhibitor for cancer and neurological disorders. J. Med. Chem. 2018, 61, 10084–10105. [Google Scholar] [CrossRef]
- Borsari, C.; Keles, E.; Rageot, D.; Treyer, A.; Bohnacker, T.; Bissegger, L.; De Pascale, M.; Melone, A.; Sriramaratnam, R.; Beaufils, F.; et al. 4-(Difluoromethyl)-5-(4-((3R,5S)-3,5-dimethylmorpholino)-6-((R)-3-methylmorpholino)-1,3,5-triazin-2-yl)pyridin-2-amine (PQR626), a Potent, Orally Available, and Brain-Penetrant mTOR Inhibitor for the Treatment of Neurological Disorders. J. Med. Chem. 2020, 63, 13595–13617. [Google Scholar] [CrossRef]
- Bonazzi, S.; Goold, C.P.; Gray, A.; Thomsen, N.M.; Nunez, J.; Karki, R.G.; Gorde, A.; Biag, J.D.; Malik, H.A.; Sun, Y.; et al. Discovery of a Brain-Penetrant ATP-Competitive Inhibitor of the Mechanistic Target of Rapamycin (mTOR) for CNS Disorders. J. Med. Chem. 2020, 63, 1068–1083. [Google Scholar] [CrossRef]
- Liu, Q.; Chang, J.W.; Wang, J.; Kang, S.A.; Thoreen, C.C.; Markhard, A.; Hur, W.; Zhang, J.; Sim, T.; Sabatini, D.M.; et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J. Med. Chem. 2010, 53, 7146–7155. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Kang, S.A.; Chang, J.W.; Liu, Q.; Zhang, J.; Gao, Y.; Reichling, L.J.; Sim, T.; Sabatini, D.M.; Gray, N.S. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009, 284, 8023–8032. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Kang, S.A.; Thoreen, C.C.; Hur, W.; Ahmed, T.; Sabatini, D.M.; Gray, N.S. Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[H][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J. Med. Chem. 2011, 54, 1473–1480. [Google Scholar] [CrossRef]
- Venkateswarlu, V.; Pathania, A.S.; Kumar, K.A.A.; Mahajan, P.; Nargotra, A.; Vishwakarma, R.A.; Malik, F.A.; Sawant, S.D. 4-(N-Phenyl-N′-substituted benzenesulfonyl)-6-(4-hydroxyphenyl)quinolines as inhibitors of mammalian target of rapamycin. Bioorg. Med. Chem. 2015, 23, 4237–4247. [Google Scholar] [CrossRef]
- Ma, X.; Lv, X.; Qiu, N.; Yang, B.; He, Q.; Hu, Y. Discovery of novel quinoline-based mTOR inhibitors via introducing intra-molecular hydrogen bonding scaffold (iMHBS): The design, synthesis and biological evaluation. Bioorg. Med. Chem. 2015, 23, 7585–7596. [Google Scholar] [CrossRef]
- Miyanaga, A.; Janso, J.E.; McDonald, L.; He, M.; Liu, H.; Barbieri, L.; Eustáquio, A.S.; Fielding, E.N.; Carter, G.T.; Jensen, P.R.; et al. Discovery and assembly-line biosynthesis of the lymphostin pyrroloquinoline alkaloid family of mTOR inhibitors in Salinispora bacteria. J. Am. Chem. Soc. 2011, 133, 13311–13313. [Google Scholar] [CrossRef]
- Guo, Q.; Yu, C.; Zhang, C.; Li, Y.; Wang, T.; Huang, Z.; Wang, X.; Zhou, W.; Li, Y.; Qin, Z.; et al. Highly Selective, Potent, and Oral mTOR Inhibitor for Treatment of Cancer as Autophagy Inducer. J. Med. Chem. 2018, 61, 881–904. [Google Scholar] [CrossRef]
- Hao, T.; Li, Y.; Fan, S.; Li, W.; Wang, S.; Li, S.; Cao, R.; Zhong, W. Design, synthesis and pharmacological evaluation of a novel mTOR-targeted anti-EV71 agent. Eur. J. Med. Chem. 2019, 175, 172–186. [Google Scholar] [CrossRef]
- Apsel, B.; Blair, J.A.; Gonzalez, B.; Nazif, T.M.; Feldman, M.E.; Aizenstein, B.; Hoffman, R.; Williams, R.L.; Shokat, K.M.; Knight, Z.A. Targeted polypharmacology: Discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat. Chem. Biol. 2008, 4, 691–699. [Google Scholar] [CrossRef]
- Hayman, T.J.; Wahba, A.; Rath, B.H.; Bae, H.; Kramp, T.; Shankavaram, U.T.; Camphausen, K.; Tofilon, P.J. The ATP-competitive mTOR inhibitor INK128 enhances in vitro and in vivo radiosensitivity of pancreatic carcinoma cells. Clin. Cancer Res. 2014, 20, 110–119. [Google Scholar] [CrossRef]
- Guo, Y.; Kwiatkowski, D.J. Equivalent benefit of rapamycin and a potent mTOR ATP-competitive inhibitor, MLN0128 (INK128), in a mouse model of tuberous sclerosis. Mol. Cancer Res. 2013, 11, 467–473. [Google Scholar] [CrossRef]
- Janes, M.R.; Vu, C.; Mallya, S.; Shieh, M.P.; Limon, J.J.; Li, L.S.; Jessen, K.A.; Martin, M.B.; Ren, P.; Lilly, M.B.; et al. Efficacy of the investigational mTOR kinase inhibitor MLN0128/INK128 in models of B-cell acute lymphoblastic leukemia. Leukemia 2013, 27, 586–594. [Google Scholar] [CrossRef]
- Crew, A.P.; Bhagwat, S.V.; Dong, H.; Bittner, M.A.; Chan, A.; Chen, X.; Coate, H.; Cooke, A.; Gokhale, P.C.; Honda, A.; et al. Imidazo[1,5-a]pyrazines: Orally efficacious inhibitors of mTORC1 and mTORC2. Bioorg. Med. Chem. Lett. 2011, 21, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.V.; Gokhale, P.C.; Crew, A.P.; Cooke, A.; Yao, Y.; Mantis, C.; Kahler, J.; Workman, J.; Bittner, M.; Dudkin, L.; et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: Distinct from rapamycin. Mol. Cancer Ther. 2011, 10, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.V.; Crew, A.P. Novel inhibitors of mTORC1 and mTORC2. Curr. Opin. Investig. Drugs. 2010, 11, 638–645. [Google Scholar] [PubMed]
- Takeuchi, C.S.; Kim, B.G.; Blazey, C.M.; Ma, S.; Johnson, H.W.; Anand, N.K.; Arcalas, A.; Baik, T.G.; Buhr, C.A.; Cannoy, J.; et al. Discovery of a novel class of highly potent, selective, ATP-competitive, and orally bioavailable inhibitors of the mammalian target of rapamycin (mTOR). J. Med. Chem. 2013, 56, 2218–2234. [Google Scholar] [CrossRef]
- Peterson, E.A.; Boezio, A.A.; Andrews, P.S.; Boezio, C.M.; Bush, T.L.; Cheng, A.C.; Choquette, D.; Coats, J.R.; Colletti, A.E.; Copeland, K.W.; et al. Discovery and optimization of potent and selective imidazopyridine and imidazopyridazine mTOR inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4967–4974. [Google Scholar] [CrossRef]
- Mortensen, D.S.; Sapienza, J.; Lee, B.G.; Perrin-Ninkovic, S.M.; Harri, R.; Shevlin, G.; Parnes, J.S.; Whitefield, B.; Hickman, M.; Khambatta, G.; et al. Use of core modification in the discovery of CC214-2, an orally available, selective inhibitor of mTOR kinase. Bioorg. Med. Chem. Lett. 2013, 23, 1588–1591. [Google Scholar] [CrossRef]
- Mortensen, D.S.; Perrin-Ninkovic, S.M.; Shevlin, G.; Zhao, J.; Packard, G.; Bahmanyar, S.; Correa, M.; Elsner, J.; Harris, R.; Lee, B.G.; et al. Discovery of mammalian target of rapamycin (mTOR) kinase inhibitor CC-223. J. Med. Chem. 2015, 58, 5323–5333. [Google Scholar] [CrossRef]
- Mortensen, D.S.; Perrin-Ninkovic, S.M.; Shevlin, G.; Elsner, J.; Zhao, J.; Whitefield, B.; Tehrani, L.; Sapienza, J.; Riggs, J.R.; Parnes, J.S.; et al. Optimization of a series of triazole containing mammalian target of rapamycin (mTOR) kinase inhibitors and the discovery of CC-115. J. Med. Chem. 2015, 58, 5599–5608. [Google Scholar] [CrossRef]
- Reddy, G.L.; Guru, S.K.; Srinivas, M.; Pathania, A.S.; Mahajan, P.; Nargotra, A.; Bhushan, S.; Vishwakarma, R.A.; Sawant, S.D. Synthesis of 5-substituted-1H-pyrazolo[4,3-d]pyrimidin-7(6H)-one analogs and their biological evaluation as anticancer agents: mTOR inhibitors. Eur. J. Med. Chem. 2014, 80, 201–208. [Google Scholar] [CrossRef]
- Fouque, A.; Delalande, O.; Jean, M.; Castellano, R.; Josselin, E.; Malleter, M.; Shoji, K.F.; Hung, M.D.; Rampanarivo, H.; Collette, Y.; et al. A novel covalent mTOR inhibitor, DHM25, shows in vivo antitumor activity against triple-negative breast cancer cells. J. Med. Chem. 2015, 58, 6559–6573. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Yang, C.; Pluta, R.; Wang, G.; Ye, T.; Ouyang, L. Identification and optimization of 3-bromo-N’-(4-hydroxybenzylidene)-4-methylbenzohydrazide derivatives as mTOR inhibitors that induce autophagic cell death and apoptosis in triple-negative breast cancer. Eur. J. Med. Chem. 2021, 219, 113424. [Google Scholar] [CrossRef]
- Jin, S.; Mikami, S.; Scorah, N.; Chen, Y.; Halkowycz, P.; Shi, L.; Kahana, J.; Vincent, P.; de Jong, R.; Atienza, J.; et al. Rational discovery of a highly novel and selective mTOR inhibitor. Bioorg. Med. Chem. Lett. 2019, 29, 126659. [Google Scholar] [CrossRef]
- Rodrik-Outmezguine, V.S.; Okaniwa, M.; Yao, Z.; Novotny, C.J.; McWhirter, C.; Banaji, A.; Won, H.; Wong, W.; Berger, M.; de Stanchina, E.; et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 2016, 534, 272–276. [Google Scholar] [CrossRef]
- Serra, V.; Markman, B.; Scaltriti, M.; Eichhorn, P.J.; Valero, V.; Guzman, M.; Botero, M.L.; Llonch, E.; Atzori, F.; Di Cosimo, S.; et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008, 68, 8022–8030. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Dehnhardt, C.M.; Santos, E.D.; Chen, Z.; Santos, O.D.; Ayral-Kaloustian, S.; Khafizova, G.; Brooijmans, N.; Mallon, R.; Hollander, I.; et al. Bis(morpholino-1,3,5-triazine) derivatives: Potent adenosine 5′-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: Discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J. Med. Chem. 2010, 58, 2636–2645. [Google Scholar] [CrossRef]
- Freitag, H.; Christen, F.; Lewens, F.; Grass, I.; Briest, F.; Iwaszkiewicz, S.; Siegmund, B.; Grabowski, P. Inhibition of mTOR’s catalytic site by PKI-587 is a promising therapeutic option for gastroenteropancreatic neuroendocrine tumor disease. Neuroendocrinology 2017, 105, 90–104. [Google Scholar] [CrossRef]
- Knight, S.D.; Adams, N.D.; Burgess, J.L.; Chaudhari, A.M.; Darcy, M.G.; Donatelli, C.A.; Luengo, J.I.; Newlander, K.A.; Parrish, C.A.; Ridgers, L.H.; et al. Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med. Chem. Lett. 2010, 1, 39–43. [Google Scholar] [CrossRef]
- Sutherlin, D.P.; Bao, L.; Berry, M.; Castanedo, G.; Chuckowree, I.; Dotson, J.; Folks, A.; Friedman, L.; Goldsmith, R.; Gunzner, J. Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J. Med. Chem. 2011, 54, 7579–7587. [Google Scholar] [CrossRef]
- Beaufils, F.; Cmiljanovic, N.; Cmiljanovic, V.; Bohnacker, T.; Melone, A.; Marone, R.; Jackson, E.; Zhang, X.; Sele, A.; Borsari, C. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a potent, brain-penetrant, orally bioavailable, pan-class I PI3K/mTOR inhibitor as clinical candidate in oncology. J. Med. Chem. 2017, 60, 7524–7538. [Google Scholar] [CrossRef]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenet. 2012, 4, 5. [Google Scholar] [CrossRef]
- Rehan, M. Anticancer compound XL765 as PI3K/mTOR dual inhibitor: A structural insight into the inhibitory mechanism using computational approaches. PLoS ONE 2019, 14, e0219180. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Hamadani, M.; Hayslip, J.; Janssens, A.; Wagner-Johnston, N.; Ottmann, O.; Arnason, J.; Tilly, H.; Millenson, M.; Offner, F.; et al. Voxtalisib (XL765) in patients with relapsed or refractory non-Hodgkin lymphoma or chronic lymphocytic leukaemia: An open-label, phase 2 trial. Lancet Haematol. 2018, 5, 170–180. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, X.; Zhang, W.; Tang, M.; Zheng, L.; Wang, F.; Yan, W.; Yang, S.; Wei, Y.; He, J.; et al. Discovery of Novel Dual Histone Deacetylase and Mammalian Target of Rapamycin Target Inhibitors as a Promising Strategy for Cancer Therapy. J. Med. Chem. 2019, 62, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Jiang, J.; Zhang, H.; Huang, Y.; Huang, J.; Wang, J. Design, synthesis and biological evaluation of dual mTOR/HDAC6 inhibitors in MDA-MB-231 cells. Bioorg. Med. Chem. Lett. 2021, 47, 128204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, W.; Peng, C.; Ma, X.; He, X.; Zhang, H.; Zhou, M. Discovery of novel pyrazolopyrimidine derivatives as potent mTOR/HDAC bi-functional inhibitors via pharmacophore-merging strategy. Bioorg. Med. Chem. Lett. 2021, 49, 128286. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Zhang, H.; Chen, R.; Wu, J.; Ai, D.; Tao, S.; Cai, Y.; Zhang, J.Q.; Wang, L. Design, synthesis and biological evaluation of novel hybrids targeting mTOR and HDACs for potential treatment of hepatocellular carcinoma. Eur. J. Med. Chem. 2021, 225, 113824. [Google Scholar] [CrossRef]
- Bhakuni, R.; Shaik, A.; Priya, B.; Kirubakaran, S. Characterization of SPK 98, a Torin2 analog, as ATR and mTOR dual kinase inhibitor. Bioorg. Med. Chem. Lett. 2020, 30, 127517. [Google Scholar] [CrossRef]



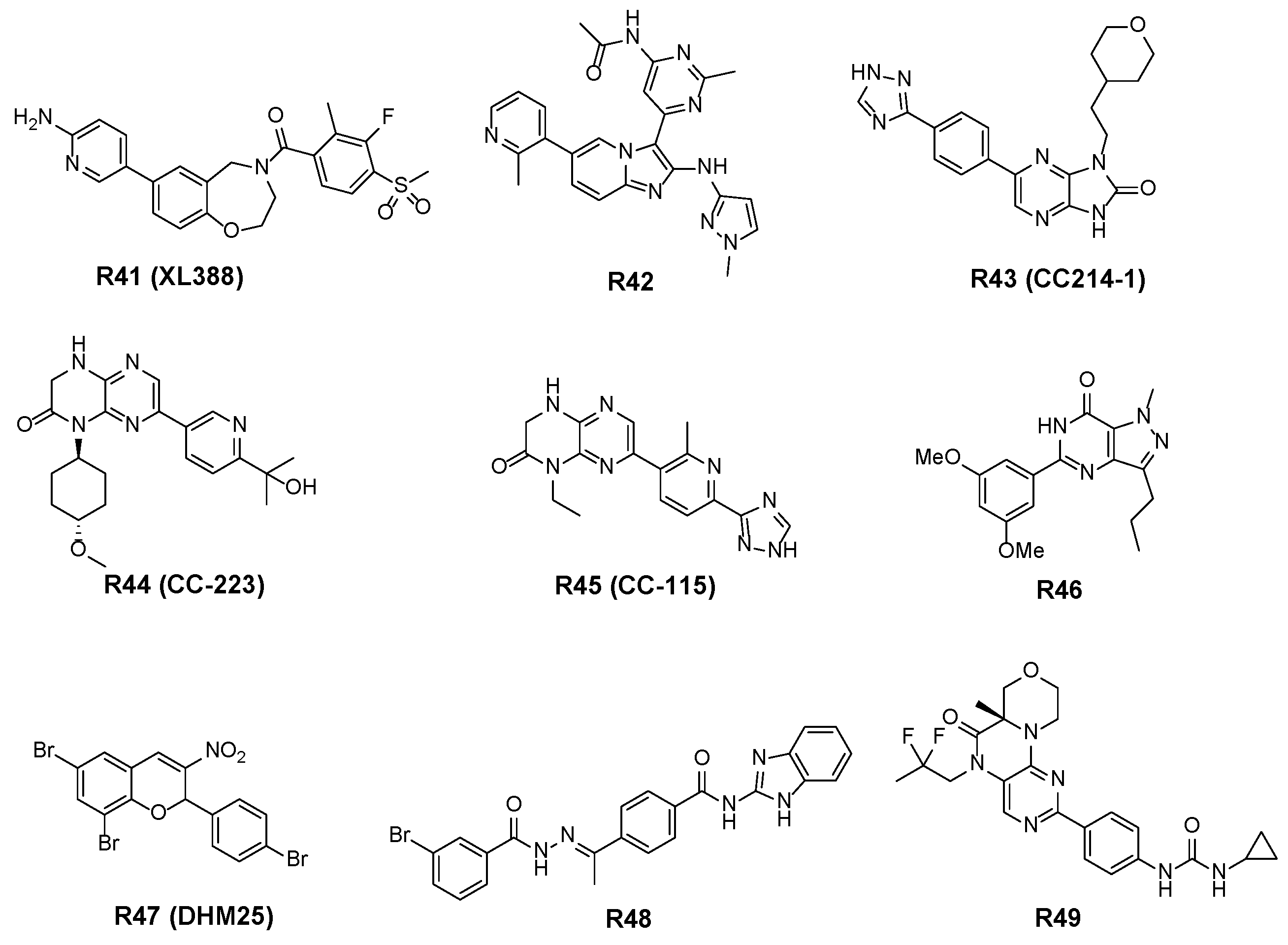
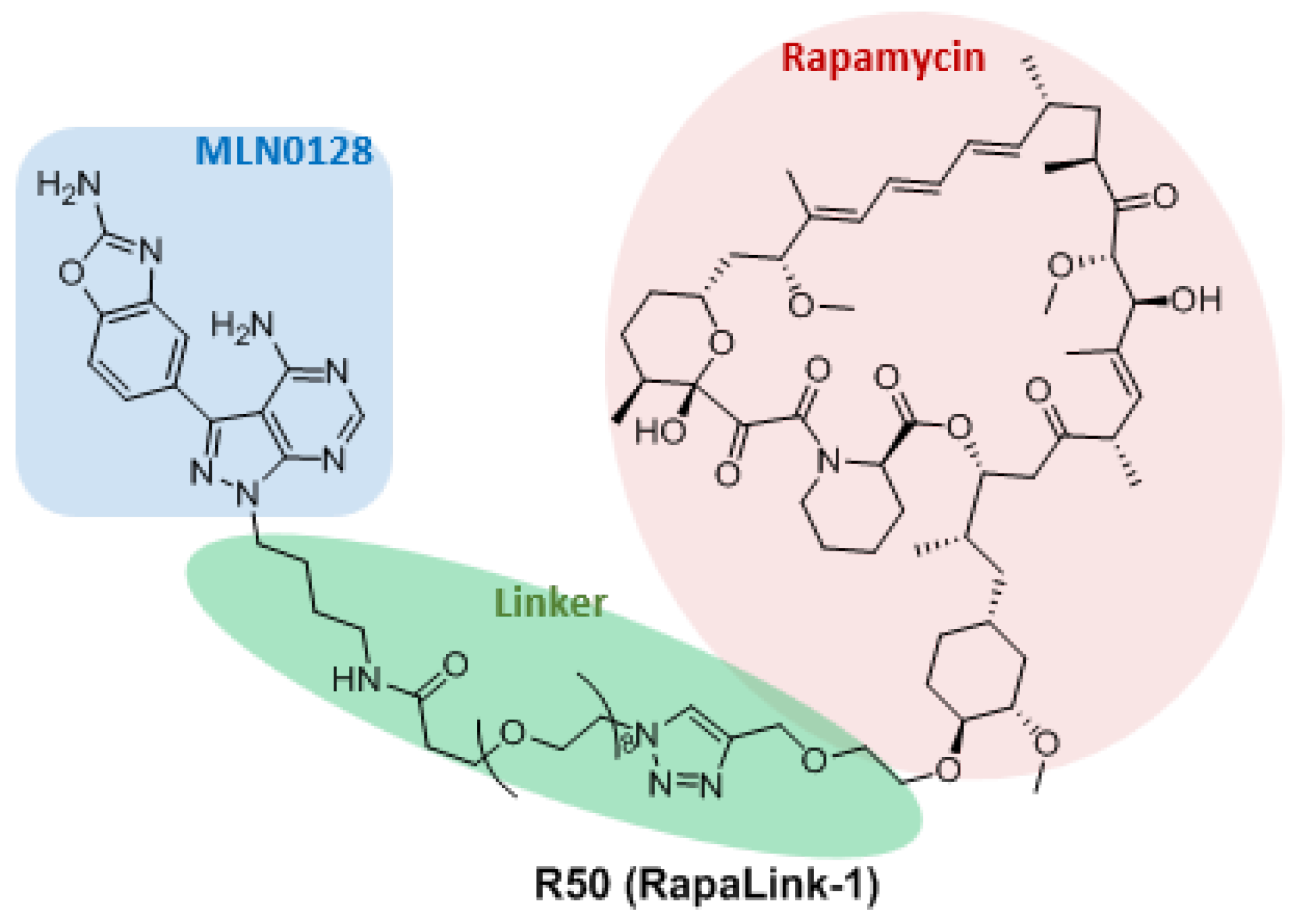

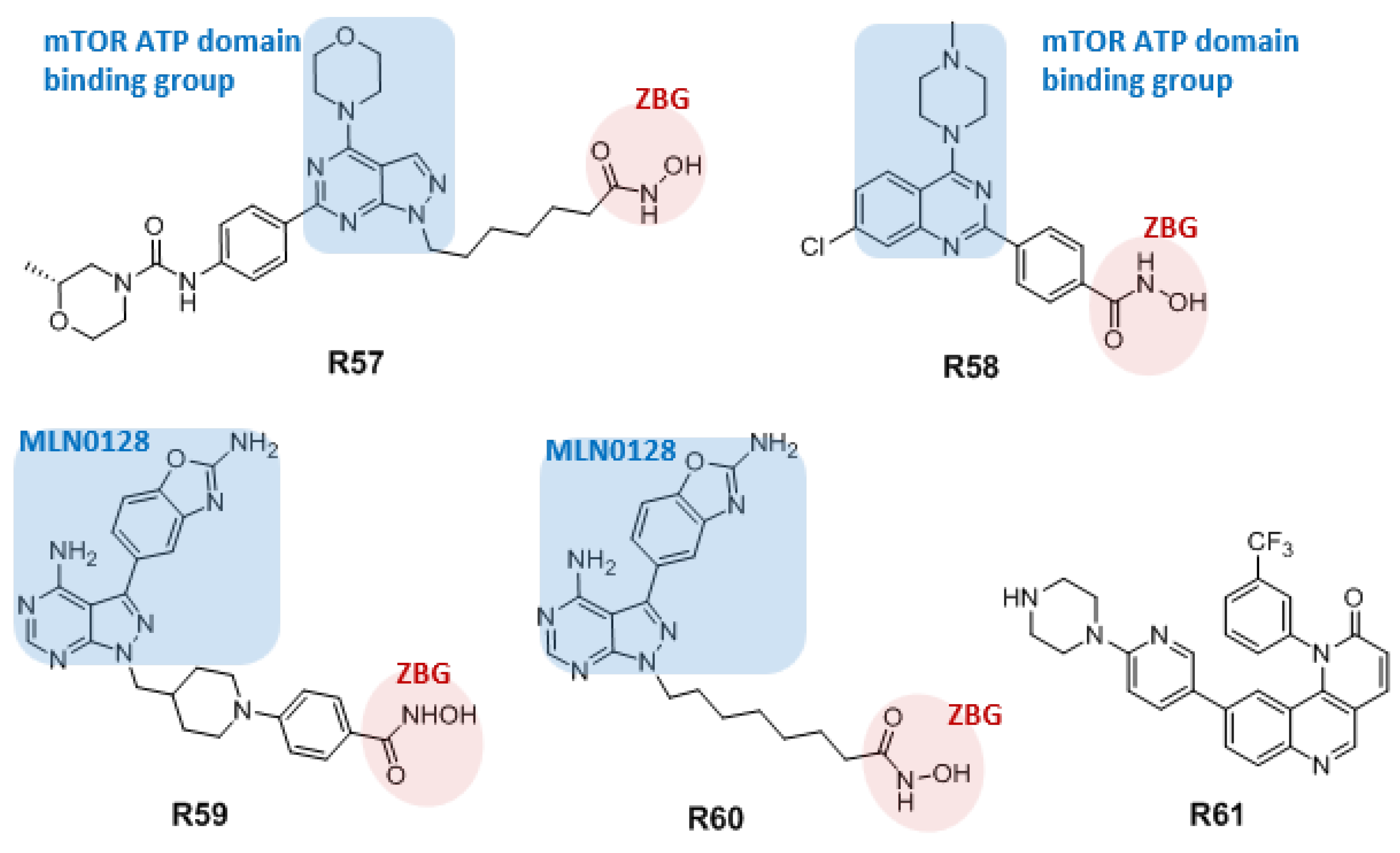
| Inhibitors | Type of Study | Inhibitors’ Applications |
|---|---|---|
| R1 | clinical use | lymphangioleiomyomatosis |
| R2 | clinical use | advanced renal cell carcinoma (RCC) |
| R3 | clinical use | advanced RCC, subependymal giant cell astrocytoma, and tuberous sclerosis complex (TSC) |
| R4 | clinical studies | osteosarcoma |
| R5 | animal studies | breast cancer |
| R6 | cells | breast cancer, glioma |
| R7 | cells | breast cancer, glioma |
| R8 | cells | breast cancer, glioma |
| R9 | animal studies | prostate cancer, glioma |
| R10 | animal studies | ovarian cancer, colon cancer |
| R11 | animal studies | ovarian cancer, colon cancer |
| R12 | animal studies | ovarian cancer, prostate cancer |
| R13 | animal studies | Non-Hodgkin’s lymphoma, solid tumor |
| R14 | animal studies | prostate tumor |
| R15 | animal studies | prostate cancer, breast cancer |
| R16 | animal studies | breast cancer |
| R17 | clinical studies | hepatocellular carcinoma (HCC) |
| R18 | clinical studies | colorectal cancer, advanced RCC |
| R19 | cells | breast cancer, bladder cancer |
| R20 | cells | prostate cancer, breast cancer |
| R21 | animal studies | glioma |
| R22 | cells | breast cancer |
| R23 | animal studies | breast cancer, endometrial cancer |
| R24 | animal studies | inflammation |
| R25 | animal studies | systemic tumor |
| R26 | kinase assays | cancer |
| R27 | animal studies | ovarian cancer, chronic epilepsy |
| R28 | animal studies | TSC-induced epilepsy, CNS disorders |
| R29 | animal studies | TSC-induced epilepsy, CNS disorders |
| R30 | animal studies | glioblastoma tumor |
| R31 | animal studies | papillary thyroid carcinoma |
| R32 | cells | ovarian cancer, colon cancer |
| R33 | cells | prostate cancer, colon cancer, breast cancer |
| R34 | cells | acute promyelocytic leukemia, pancreatic cancer, liver cancer, prostate cancer, colon cancer |
| R35 | animal studies | breast cancer, cervical cancer |
| R36 | cells | hand-foot-and-mouth disease |
| R37 | animal studies | bladder cancer |
| R38 | clinical studies | TS, acute lymphoblastic leukemia |
| R39 | clinical studies | colon cancer, breast cancer |
| R40 | cells | rhabdomyosarcoma |
| R41 | animal studies | breast cancer |
| R42 | animal studies | endometrial carcinoma, esophageal carcinoma |
| R43 | animal studies | prostatic cancer |
| R44 | clinical studies | diffuse large B cell lymphoma, breast cancer, lung cancer |
| R45 | clinical studies | breast cancer, lung cancer |
| R46 | cells | ovarian cancer, prostate cancer |
| R47 | animal studies | triple-negative breast cancer (TNBC) |
| R48 | cells | TNBC, breast cancer |
| R49 | animal studies | prostate cancer, endometrial cancer |
| R50 | animal studies | breast cancer |
| R51 | clinical studies | bladder cancer, pancreatic cancer, breast cancer, renal cell carcinoma, solid tumor, prostate cancer |
| R52 | clinical studies | metastatic breast cancer, pancreatic cancer, colon cancer |
| R53 | clinical studies | solid tumor, lymphoma |
| R54 | clinical studies | breast cancer, pancreatic cancer, non-small cell lung cancer, colon cancer |
| R55 | clinical studies | advanced solid tumor, refractory lymphoma |
| R56 | clinical studies | relapsed or refractory non-Hodgkin’s lymphoma, chronic lymphocytic leukemia |
| R57 | cells | leukemia, myeloma |
| R58 | cells | TNBC |
| R59 | cells | monocytic leukemia, prostate cancer, colon cancer |
| R60 | cells | liver cancer, breast cancer, solid tumor, prostate cancer |
| R61 | animal studies | carcinoma of colon |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, B.; Zhang, Q.; Ma, L.; Zhao, D.-S.; Zhao, P.; Yan, P. Overview of Research into mTOR Inhibitors. Molecules 2022, 27, 5295. https://doi.org/10.3390/molecules27165295
Mao B, Zhang Q, Ma L, Zhao D-S, Zhao P, Yan P. Overview of Research into mTOR Inhibitors. Molecules. 2022; 27(16):5295. https://doi.org/10.3390/molecules27165295
Chicago/Turabian StyleMao, Beibei, Qi Zhang, Li Ma, Dong-Sheng Zhao, Pan Zhao, and Peizheng Yan. 2022. "Overview of Research into mTOR Inhibitors" Molecules 27, no. 16: 5295. https://doi.org/10.3390/molecules27165295
APA StyleMao, B., Zhang, Q., Ma, L., Zhao, D.-S., Zhao, P., & Yan, P. (2022). Overview of Research into mTOR Inhibitors. Molecules, 27(16), 5295. https://doi.org/10.3390/molecules27165295