Comprehensive Review on Medicinal Applications of Coumarin-Derived Imine–Metal Complexes
Abstract
:Simple Summary
Abstract
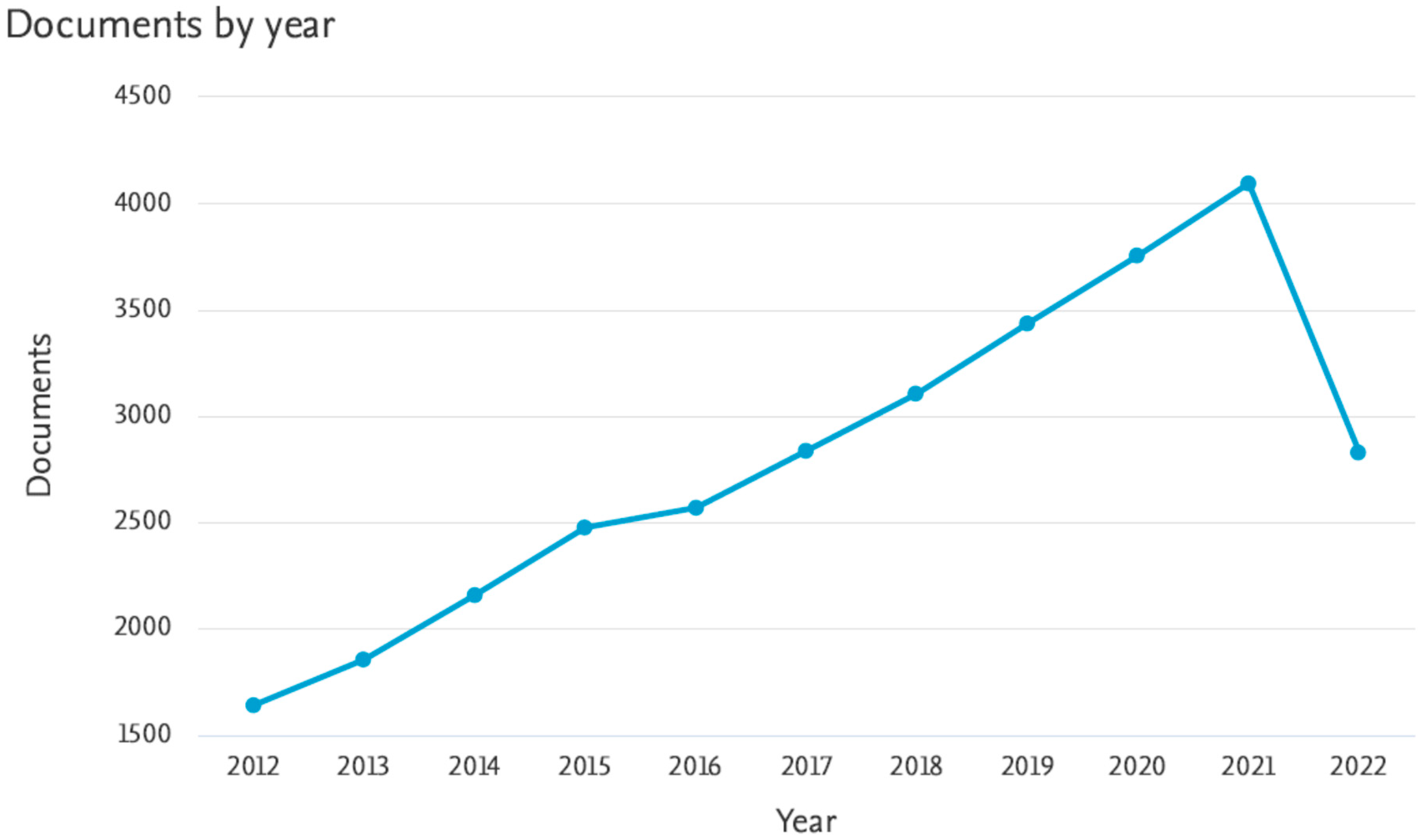
1. Introduction
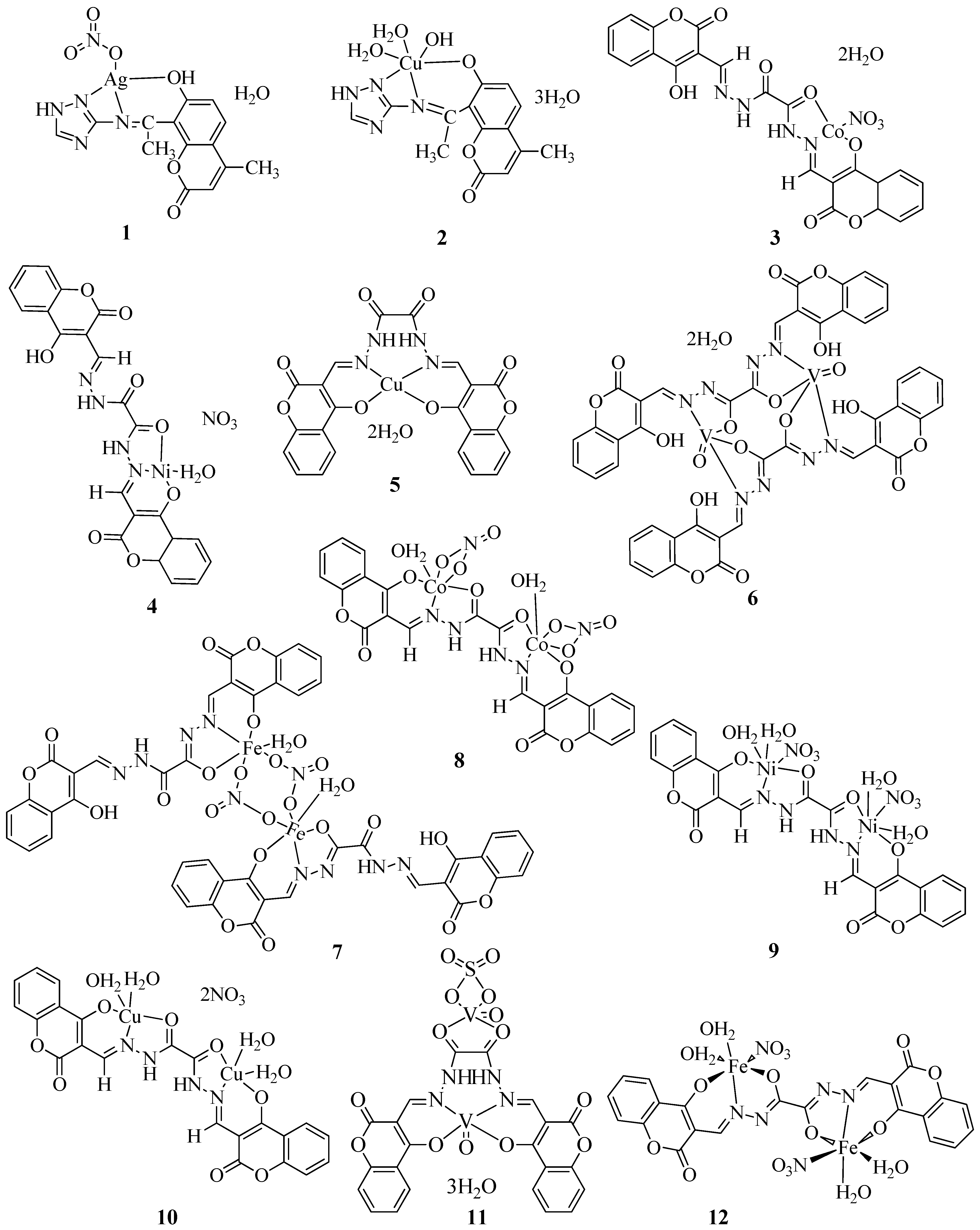
2. Chemistry
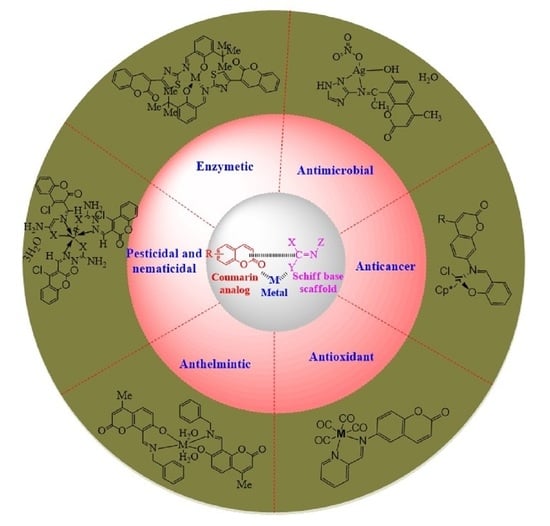
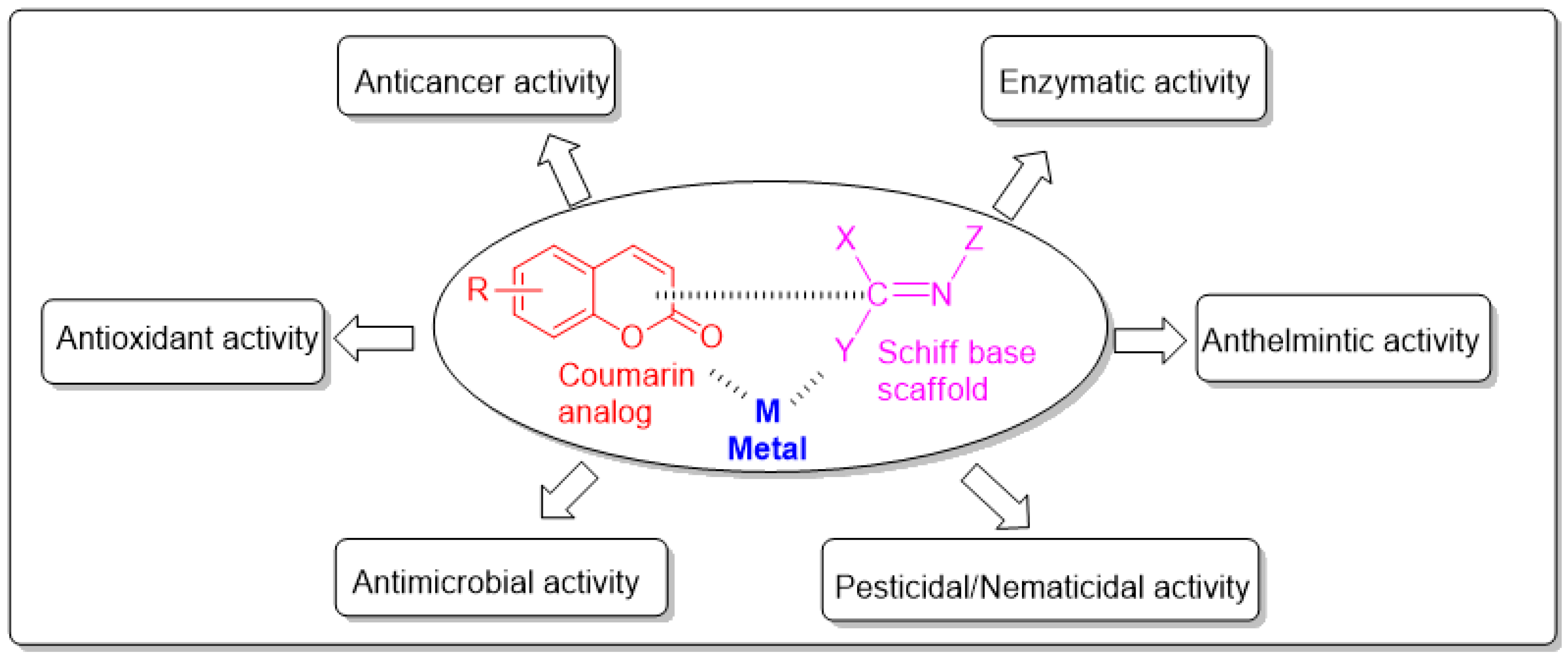
3. Medicinal Applications of Coumarin-Derived Imine–Metal Complexes
3.1. Antimicrobial Activity of Coumarin-Derived Imine–Metal Complexes
3.2. Anticancer Activity of Coumarin-Derived Imine–Metal Complexes
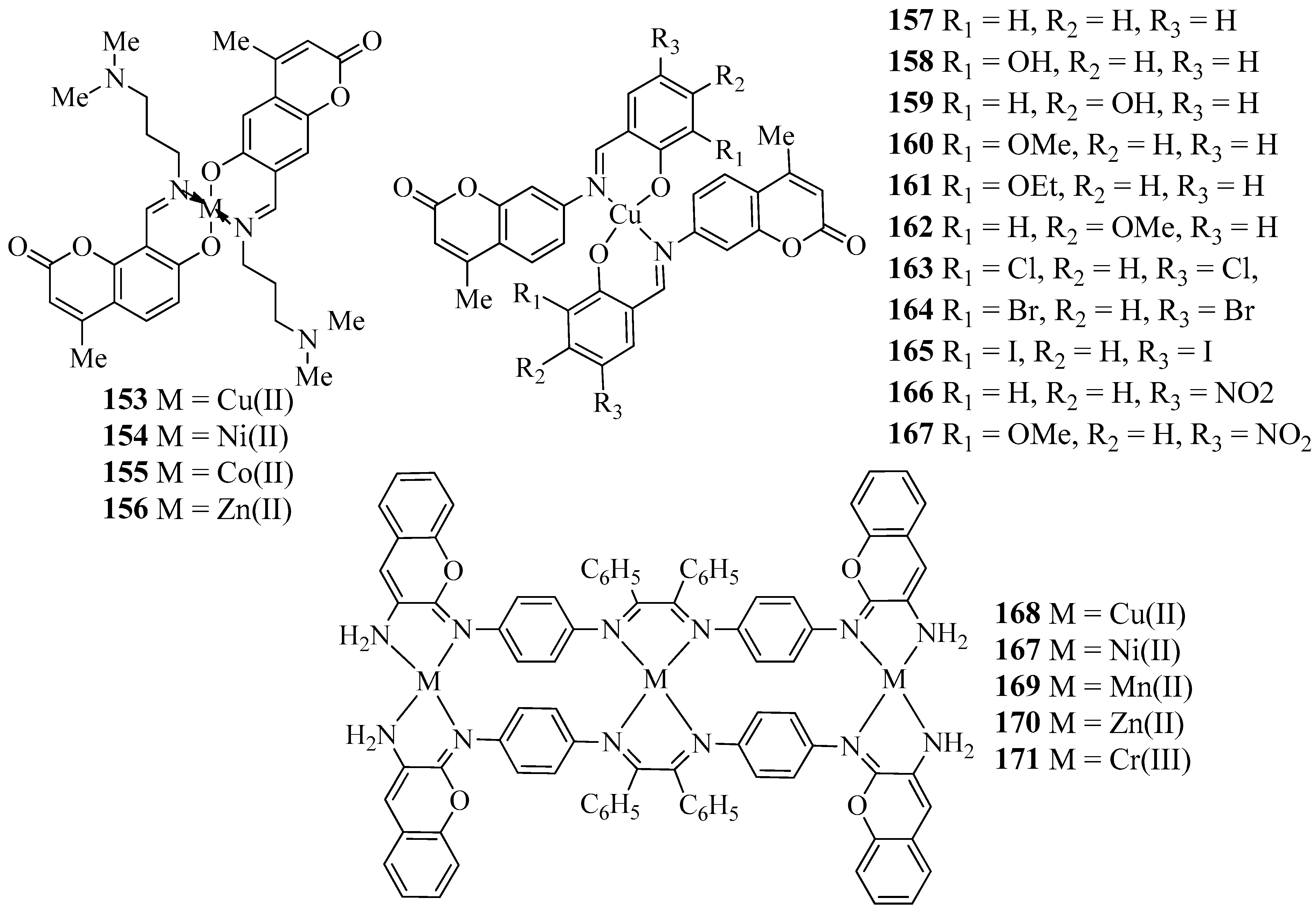
| Compound | Cell Line | IC50 (µM) | Ref. | Compound No. | Cell Line | IC50 (µM) | Ref. |
|---|---|---|---|---|---|---|---|
| 172 | MCF-7 | no reported | [58] | 187 | HeLa | 86.9 ± 9.0 | [63] |
| 173 | MCF-7 | no reported | [58] | 188 | HeLa | 3.5 ± 1.2 | [63] |
| 174 | MCF-7 | no reported | [58] | 189 | HeLa | 52.5 ± 1.0 | [63] |
| 175 | MCF-7 | no reported | [58] | 190 | HeLa | 72.7 ± 8.1 | [63] |
| 176 | MCF-7 | no reported | [58] | 191 | HeLa | 90.7 ± 2.5 | [63] |
| 177 | MCF-7 | no reported | [58] | 192 | HeLa | 4.1 ± 0.9 | [63] |
| 178 | MCF-7 | no reported | [58] | 193 | HeLa | 83.5 ± 4.7 | [63] |
| 179 | MCF-7 | no reported | [58] | 194 | HeLa | 85.1 ± 1.6 | [63] |
| 180 | MCF-7 | 79.8 ± 5 | [58] | Cisplatin | HeLa | 11.6 ± 2.7 | [63] |
| 181 | MCF-7 | no reported | [58] | 195 | A549 | 30.9 ± 1.6 | [63] |
| 182 | MCF-7 | 34.5 ± 6 | [58] | 196 | HeLa | 9.9 ± 0.1 | [64] |
| Mitoxantrone | MCF-7 | 44 ± 3 | [58] | 197 | HeLa | 10.8 ± 0.1 | [64] |
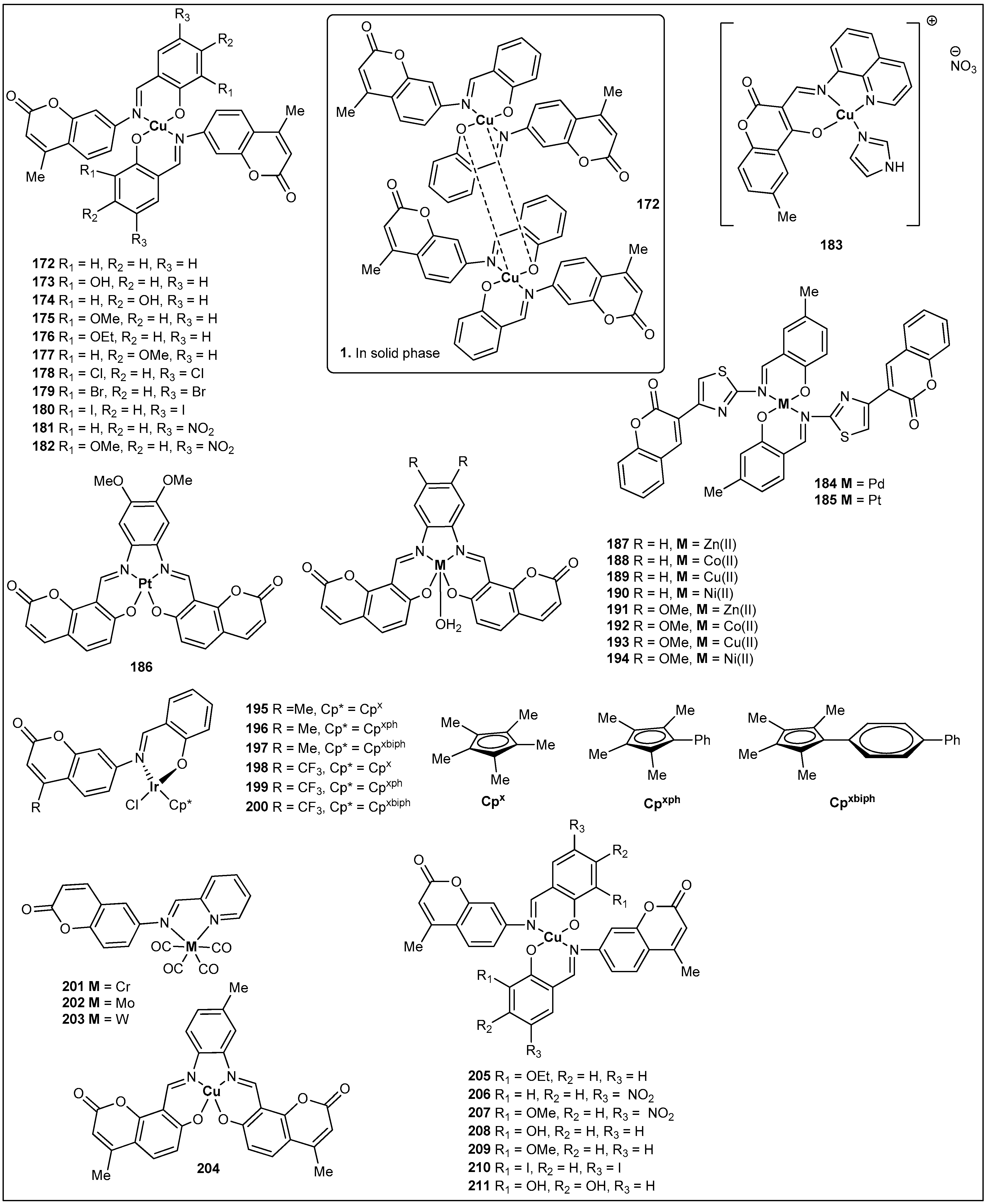
| 183 | A549 | 4.6 ± 0.3 | [59] | 198 | A549 | 39.6 ± 1.0 | [64] |
| Cisplatin | A549 | 57.7 ± 0.9 | [59] | 199 | HeLa | 22.1 ± 0.5 | [64] |
| 184 | LNCap | 10.05 | [59] | 200 | A549 | 33.4 ± 0.7 | [64] |
| 185 | LNCap | 21.53 | [59] | Cisplatin | A549 | 21.3 ± 1.7 | [64] |
| 186 | HeLa | 61.4 ± 1.1 | [59] | Cisplatin | HeLa | 7.5 ± 0.2 | [64] |
3.3. Antioxidant Activity of Coumarin-Derived Imine–Metal Complexes
3.4. Anthelmintic Activity of Coumarin-Derived Imine–Metal Complexes
3.5. Pesticidal and Nematicidal Activity of Coumarin-Derived Imine–Metal Complexes
3.6. Enzyme Activity of Coumarin-Derived Imine–Metal Complexes
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A. Darstellung von benzoesaure aus der tonka-boline und aus den meliIoten-oder steinklee-blumen. Ann. Phys. 1820, 64, 161–166. [Google Scholar] [CrossRef]
- Perkin, W.H. On the artificial production of coumarin and formation of its homologues. J. Chem. Soc. 1868, 21, 53–63. [Google Scholar] [CrossRef]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and antiinflammatory activity of coumarin derivatives. J. Med. Chem. 2005, 48, 6400–6408. [Google Scholar] [CrossRef]
- Silvan, A.M.; Abad, M.J.; Bermejo, P.; Sollhuber, M.; Villar, A. Antiinflammatory activity of coumarins from Santolina oblonglifolia. J. Nat. Prod. 1996, 59, 1183–1185. [Google Scholar] [CrossRef]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg. Med. Chem. Lett. 2004, 14, 611–614. [Google Scholar] [CrossRef]
- Melagraki, G.; Afantitis, A.; Igglessi-Markopoulou, O.; Detsi, A.; Koufaki, M.; Kontogiorgis, C.; Hadjipavlou-Litina, D.J. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur. J. Med. Chem. 2009, 44, 3020–3026. [Google Scholar] [CrossRef]
- Yoakim, C.; Bonneau, P.R.; Deziel, R.; Doyon, L.; Duan, J.; Guse, I.; Landry, S.; Malenfant, E.; Naud, J.; Ogilvie, W.W.; et al. Novel nevirapine-like inhibitors with improved activity against NNRTI-resistant HIV: 8-heteroarylthiomethyldipyridodiazepinone derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 739–742. [Google Scholar] [CrossRef]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef]
- Kamal, A.; Adil, S.F.; Tamboli, J.R.; Jaki, R.; Siddardha, B.; Murthy, U.S.N. Synthesis of coumarin linked naphthalimide conjugates as potential anticancer and antimicrobial agents. Lett. Drug. Des. Discov. 2009, 6, 201–209. [Google Scholar] [CrossRef]
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Pereira, T.M.; Franco, D.P.; Vitorio, F.; Kummerle, A.E. Coumarin Compounds in Medicinal Chemistry: Some Important Examples from the Last Years. Curr. Top. Med. Chem. 2018, 18, 124–148. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Owens, T.W.; Mandler, M.D. The Antibiotic Novobiocin binds and activates the ATPase that powers lipopolysaccharide transport. J. Am. Chem. Soc. 2017, 139, 17221–17224. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Heide, L. New aminocoumarin antibiotics from genetically engineered Streptomyces strains. Curr. Med. Chem. 2005, 12, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Eustáquio, A.S.; Gust, B.; Luft, T.; Li, S.M.; Chater, K.F.; Heide, L. Clorobiocin biosynthesis in Streptomyces: Identification of the halogenase and generation of structural analogs. Chem. Biol. 2003, 10, 279–288. [Google Scholar] [CrossRef]
- Ghanghas, P.; Choudhary, A.; Kumar, D.; Poonia, K. Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorg. Chem. Commun. 2021, 130, 108710. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, S.A.; Fariyike, T.; Marichev, K.O.; Martinez, H.M.H.; Bugarin, A. Medicinal applications of coumarins bearing azetidinone and thiazolidinone moieties. Future Med. Chem. 2021, 13, 1907–1934. [Google Scholar]
- Delcourt, M.-L.; Reynaud, C.; Turcaud, S.; Favereau, L.; Crassous, J.; Micouin, L.; Benedetti, E. 3D coumarin systems based on [2.2]paracyclophane: Synthesis, spectroscopic characterization, and chiroptical properties. J. Org. Chem. 2019, 84, 888–899. [Google Scholar] [CrossRef]
- Halter, O.; Plenio, H. Fluorescent dyes in organometallic chemistry: Coumarin-tagged NHC–metal complexes. Eur. J. Inorg. Chem. 2018, 25, 2935–2943. [Google Scholar] [CrossRef]
- Bagihalli, G.B.; Avaji, P.G.; Patil, S.A.; Badami, P.S. Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur. J. Med. Chem. 2018, 43, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Tumer, M.; Koksal, H.; Sener, M.K.; Serin, S. Antimicrobial activity studies of the binuclear metal complexes derived from tridentate Schiff base ligands. Transit. Metal. Chem. 1999, 24, 414–420. [Google Scholar] [CrossRef]
- Mladenovic, M.; Vukovic, N.; Sukdolak, S.; Solujic, S. Design of novel 4-hydroxy-chromene-2-one derivatives as antimicrobial agents. Molecules 2010, 15, 4294–4308. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N. An efficient synthesis and antioxidant properties of novel imino and amino derivatives of 4-hydroxy coumarin. Arch. Pharmacal Res. 2010, 33, 5–15. [Google Scholar] [CrossRef]
- Gilani, A.H.; Shaheen, F.; Saeed, S.A.; Bibi, S.; Sadiq, M.; Faizi, S. Hypotensive action of coumarin glycosides from Daucus carota. Phytomedicine 2000, 7, 423–426. [Google Scholar] [CrossRef]
- Sandhya, B.; Giles, D.; Mathew, V.; Basavarajaswamy, G.; Abraham, R. Synthesis, pharmacological evaluation and docking studies of coumarin derivatives. Eur. J. Med. Chem. 2011, 46, 4696–4701. [Google Scholar] [CrossRef]
- Cavettos, G.; Nano, G.M.; Palmisano, G.; Tagliapietra, S. An asymmetric approach to coumarin anticoagulants via hetero-Diels–Alder cycloaddition. Tetrahedron Asymmetry 2001, 12, 707–709. [Google Scholar]
- Stanchev, S.; Momekov, G.; Jensen, F.; Manolov, I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2008, 43, 694–706. [Google Scholar] [CrossRef]
- Zabradnik, M. The Production and Application of Fluorescent Brightening Agents; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef]
- Abdel-Kader, N.S.; Moustafa, H.; El-Ansary, A.L.; Sherifa, O.E.; Farghaly, A.M. A coumarin Schiff base and its Ag (I) and Cu (II) complexes: Synthesis, characterization, DFT calculations and biological applications. New J. Chem. 2021, 45, 7714–7730. [Google Scholar] [CrossRef]
- Knittl, E.T.; Abou-Hussein, A.A.; Linert, W. Syntheses, characterization, and biological activity of novel mono-and binuclear transition metal complexes with a hydrazone Schiff base derived from a coumarin derivative and oxalyldihydrazine. Monatsh. Chem. 2018, 149, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Prabhakara, C.T.; Patil, S.A.; Kulkarni, A.D.; Naik, V.H.; Manjunatha, M.; Kinnala, S.M.; Badami, P.S. Synthesis, spectral, thermal, fluorescence, antimicrobial, anthelmintic and DNA cleavage studies of mononuclear metal chelates of bi-dentate 2H-chromene-2-one Schiff base. J. Photochem. Photobiol. B Biol. 2015, 148, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.M.; Mruthyunjayaswamy, B. Synthesis, spectroscopic characterization, electrochemistry and biological evaluation of some metal (II) complexes with ONO donor ligand containing benzo [b] thiophene and coumarin moieties. J. Mol. Struct. 2014, 1074, 572–582. [Google Scholar]
- Elhusseiny, A.F.; Aazam, E.S.; Al-Amri, H.M. Synthesis of new microbial pesticide metal complexes derived from coumarin-imine ligand. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 852–863. [Google Scholar] [CrossRef]
- Abou-Hussein, A.; Linert, W. Synthesis, spectroscopic studies and inhibitory activity against bactria and fungi of acyclic and macrocyclic transition metal complexes containing a triamine coumarine Schiff base ligand. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 223–232. [Google Scholar] [CrossRef]
- Patil, S.A.; Prabhakara, C.T.; Halasangi, B.M.; Toragalmath, S.S.; Badami, P.S. DNA cleavage, antibacterial, antifungal and anthelmintic studies of Co (II), Ni (II) and Cu (II) complexes of coumarin Schiff bases: Synthesis and spectral approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 641–651. [Google Scholar] [CrossRef]
- Halli, M.; Sumathi, R.; Kinni, M. Synthesis, spectroscopic characterization and biological evaluation studies of Schiff’s base derived from naphthofuran-2-carbohydrazide with 8-formyl-7-hydroxy-4-methyl coumarin and its metal complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 99, 46–56. [Google Scholar] [CrossRef]
- Kalwania, G.; Bajroliya, S. Synthesis, characterization and antimicrobial activities of 1, 2, 4-triazole-coumarin schiff bases and their Mn (II), Co (II) complexes. Asian, J. Chem. 2015, 27, 3956. [Google Scholar] [CrossRef]
- Al-Bayati, R.; Mahdi, F.; Al-Amiery, A. Synthesis, spectroscopic and antimicrobial studies of transition metal complexes of N-amino quinolone derivatives. Br. J. Pharmacol. Toxicol. 2011, 2, 5–11. [Google Scholar]
- Nath, P.; Dhumwad, S.D. Synthesis, characterization and antimicrobial studies of Co (II), Ni (II), Cu (II) and Zn (II) complexes derived from a Schiff base of 2-[(4-Methyl-2-oxo- 2H-chromen-7-yl) oxy] acetohydrazide with 3-formyl-2-hydroxy quinoline and 3-formyl-2-mercapto quinolone. J. Chem. Pharm. Res. 2012, 4, 851–865. [Google Scholar]
- Patil, S.A.; Unki, S.N.; Badami, P.S. In vitro antibacterial, antifungal, and DNA cleavage studies of coumarin Schiff bases and their metal complexes: Synthesis and spectral characterization. Med. Chem. Res. 2012, 21, 4017–4027. [Google Scholar] [CrossRef]
- Patil, S.A.; Naik, V.H.; Kulkarni, A.D.; Unki, S.N.; Badami, P.S. Synthesis, characterization, DNA cleavage, and in-vitro antimicrobial studies of Co(II), Ni(II), and Cu(II) complexes with Schiff bases of coumarin derivatives. J. Coord. Chem. 2011, 64, 2688–2697. [Google Scholar] [CrossRef]
- Akkasali, R.; Patil, N.; Angadi, S.D. Synthesis, characterization and microbial activities of metal complexes with coumarine derivatives. Rasayan J. Chem. 2009, 2, 81–86. [Google Scholar]
- Phaniband, M.A.; Dhumwad, S.D.; Pattan, S.R. Synthesis, characterization, antimicrobial, and DNA cleavage studies of metal complexes of coumarin Schiff bases. Med. Chem. Res. 2011, 20, 493–502. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Bagihalli, G.B.; Patil, S.A.; Badami, P.S. Synthesis, characterization, electrochemical and in-vitro antimicrobial studies of Co(II), Ni(II), and Cu(II) complexes with Schiff bases of formyl coumarin derivatives. J. Coord. Chem. 2009, 62, 3060–3072. [Google Scholar] [CrossRef]
- Bagihalli, G.B.; Badami, P.S.; Patil, S.A. Synthesis, spectral characterization and in vitro biological studies of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. J. Enzyme Inhib. Med. Chem. 2009, 24, 381–394. [Google Scholar] [CrossRef]
- Refat, M.S.; El-Deen, I.M.; Anwer, Z.M.; El-Ghol, S. Bivalent transition metal complexes of coumarin-3-yl thiosemicarbazone derivatives: Spectroscopic, antibacterial activity and thermogravimetric studies. J. Mol. Struct. 2009, 920, 149–162. [Google Scholar] [CrossRef]
- Kapoor, P.; Singh, R.V.; Fahmi, N. Coordination chemistry of rare earth metal complexes with coumarin-based imines: Ecofriendly synthesis, characterization, antimicrobial, DNA cleavage, pesticidal, and nematicidal activity evaluations. J. Coord. Chem. 2012, 65, 262–277. [Google Scholar] [CrossRef]
- Aldabaldetrecu, M.; Parra, M.; Soto, S.; Arce, P.; Tello, M.; Guerrero, J.; Modak, B. New Copper(I) Complex with a Coumarin as Ligand with Antibacterial Activity against Flavobacterium psychrophilum. Molecules 2020, 25, 3183. [Google Scholar] [CrossRef]
- Jani, G.R.; Vyas, K.B.; Franco, Z. Preparation and antimicrobial activity of s-triazine hydrazones of 7-hydroxy coumarin and their metal complexes. E-J. Chem. 2009, 6, 1228–1232. [Google Scholar] [CrossRef]
- Prabhakara, C.T.; Patil, S.A.; Toragalmath, S.S.; Kinnal, S.M.; Badami, P.S. Synthesis, characterization and biological approach of metal chelates of some first row transition metal ions with halogenated bidentate coumarin Schiff bases containing N and O donor atoms. J. Photochem. Photobiol. B Biol. 2016, 157, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Arora, E.K.; Cardoza, S. Synthesis, antioxidant, antibacterial, and DFT study on a coumarin based salen-type Schiff base and its copper complex. Chem. Pap. 2016, 70, 1493–1502. [Google Scholar] [CrossRef]
- Elsayed, S.A.; El-Gharabawy, H.M.; Butler, I.S.; Atlam, F.M. Novel metal complexes of 3-acetylcoumarin-2-hydrazinobenzothiazole Schiff base: Design, structural characterizations, DNA binding, DFT calculations, molecular docking and biological studies. Appl. Organomet. Chem. 2020, 34, e5643. [Google Scholar] [CrossRef]
- Pawar, V.; Chavan, S.V.; Yamgar, R.S.; Atram, R.G.; Thorat, B.R.; Bisht, S.; Sawant, S.S. Synthesis and characterization of novel transition metal complexes of 4-methyl-7-hydroxy 8-formyl coumarin and their biological activities. Asian J. Res. Chem. 2011, 4, 1238–1242. [Google Scholar]
- Creaven, B.S.; Devereux, M.; Karcz, D.; Kellett, A.; McCann, M.; Noble, A.; Walsh, M. Copper(II) complexes of coumarin-derived Schiff bases and their anti-Candida activity. J. Inorg. Biochem. 2009, 103, 1196–1203. [Google Scholar] [CrossRef]
- Sivakumar, K.; Chandrasekaran, V. Synthesis, characterization, DNA cleavage and biological activities of Schiff base derived from p-phenylenediamine, benzil and 3-aminocoumarin and their transition metal compound. J. Crit. Rev. 2020, 7, 2769–2778. [Google Scholar]
- Patil, S.A.; Hoagland, P.; Patil, S.A.; Bugarin, A. N-heterocyclic carbene-metal complexes as bio-organometallic antimicrobial and anticancer drugs, an update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275. [Google Scholar]
- Aazam, E.; Husseiny, A.E.L.; Hitchcock, P.; Alshehri, J. Copper coumarin complex: Synthesis and crystal structure of [{Cu(4-methyl-7-(salicylideneamino)coumarin)2}2]. Cent. Eur. J. Chem. 2008, 6, 319–323. [Google Scholar] [CrossRef]
- Creaven, B.S.; Czeglédi, E.; Devereux, M.; Enyedy, É.A.; Foltyn-Arfa Kia, A.; Karcz, D.; Kellett, A.; McClean, S.; Nagy, N.V.; Noble, A.; et al. Biological activity and coordination modes of copper(II) complexes of Schiff base-derived coumarin ligands. Dalton Trans. 2010, 39, 10854. [Google Scholar] [CrossRef]
- Usman, M.; Zaki, M.; Khan, R.A.; Alsalme, A.; Ahmad, M.; Tabassum, S. Coumarin centered copper(II) complex with appended-imidazole as cancer chemotherapeutic agents against lung cancer: Molecular insight via DFT-based vibrational analysis. RSC Adv. 2017, 7, 36056–36071. [Google Scholar] [CrossRef]
- Şahin, Ö.; Özdemir, Ü.Ö.; Seferoğlu, N.; Genc, Z.K.; Kaya, K.; Aydıner, B.; Tekin, S.; Seferoğlu, Z. New Platinum (II) and palladium (II) complexes of coumarin-thiazole Schiff base with a fluorescent chemosensor properties: Synthesis, spectroscopic characterization, X-ray structure determination, in vitro anticancer activity on various human carcinoma cell lines and computational studies. J. Photochem. Photobiol. B Biol. 2018, 178, 428–439. [Google Scholar]
- Mestizo, P.D.; Narváez, D.M.; Pinzón-Ulloa, J.A.; Bello, D.T.D.; Franco-Ulloa, S.; Macías, M.A.; Groot, H.; Miscione, G.P.; Suescun, L.; Hurtado, J.J. Novel complexes with ONNO tetradentate coumarin Schiff-base donor ligands: X-ray structures, DFT calculations, molecular dynamics and potential anticarcinogenic activity. Biometals 2021, 34, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, X.; Ge, X. Fluorescent iridium (III) coumarin-salicylaldehyde Schiff base compounds as lysosome-targeted antitumor agents. Dalton Trans. 2020, 49, 5988–5998. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Mukhopadhyay, A.P.; Manna, P.; Tiekink, E.R.T.; Chandra Sil, P.; Sinha, C. Structure, photophysics, electrochemistry, DFT calculation, and in-vitro antioxidant activity of coumarin Schiff base complexes of group 6 metal carbonyls. J. Inorg. Biochem. 2011, 105, 577–588. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.; Karcz, D.; Jenkins, H.; McClean, S.; Devereux, M.; Howe, O.; Pereira, M.D.; May, N.V.; Enyedy, É.A.; Creaven, B.S. Copper(II) complexes of coumarin-derived Schiff base ligands: Pro- or antioxidant activity in MCF-7 cells? J. Inorg. Biochem. 2019, 197, 110702. [Google Scholar] [CrossRef]
- Şahin, Ö.; Özdemir, Ü.Ö.; Seferoğlu, N.; Adem, S.; Seferoğlu, Z. Synthesis, characterization, molecular docking and in vitro screening of new metal complexes with coumarin Schiff base as anticholine esterase and antipancreatic cholesterol esterase agents. J. Biomol. Struct. Dyn. 2022, 40, 4460–4474. [Google Scholar] [CrossRef]
- Pradhan, D.A.; Koshal, A.K.; Chauhan, N. Coumarin based Schiff base: Synthesis and their antioxidant and antimicrobial activity. IOSR J. Pharm. 2019, 9, 1–5. [Google Scholar]

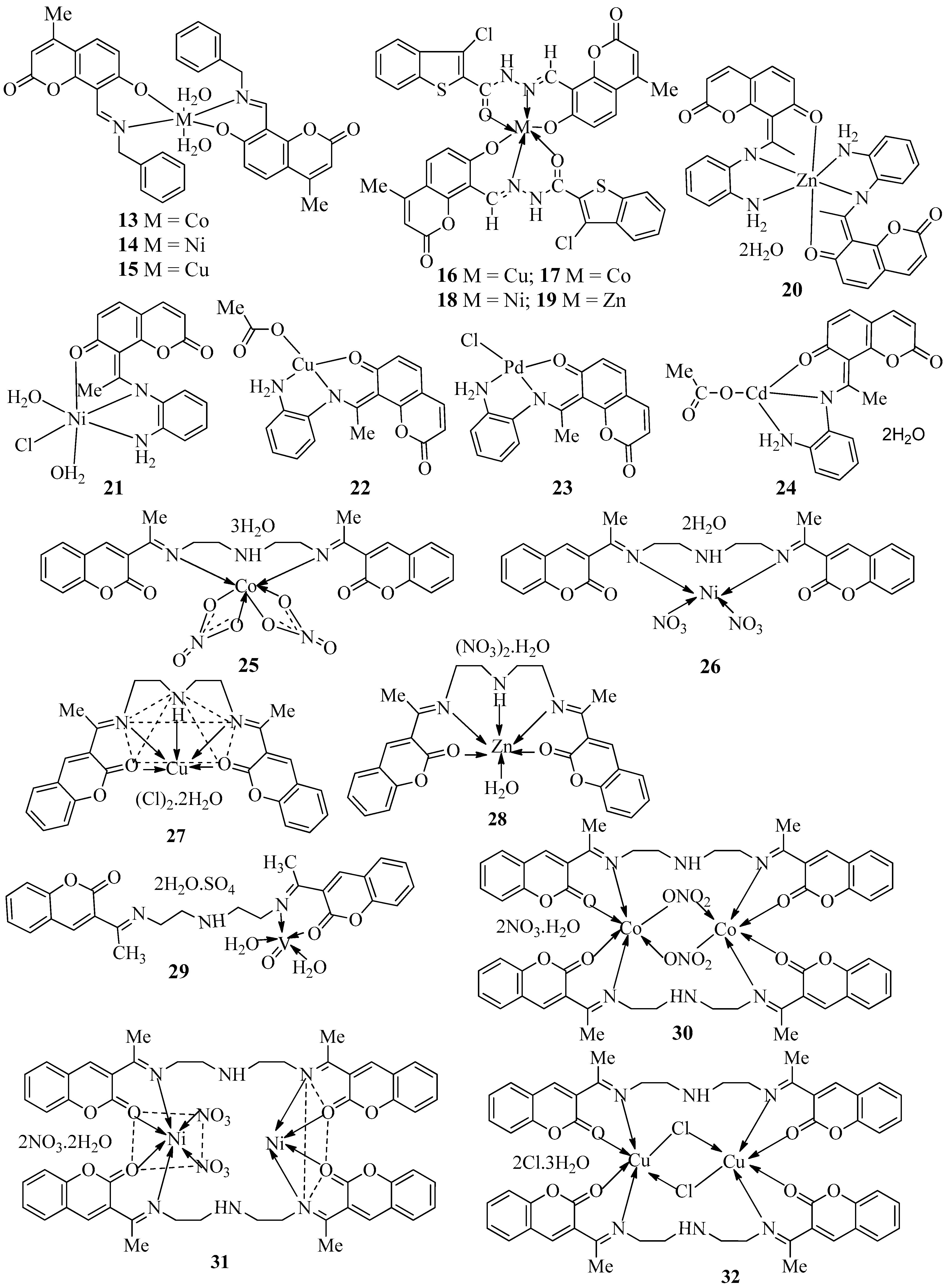

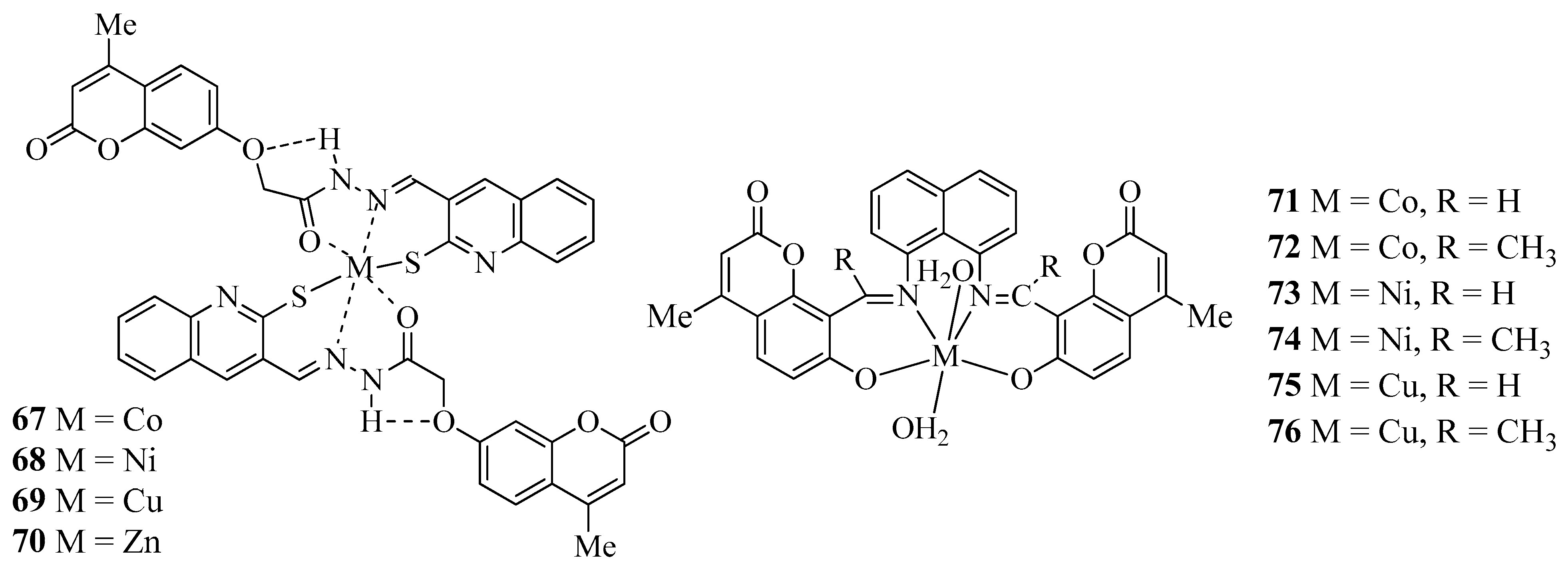
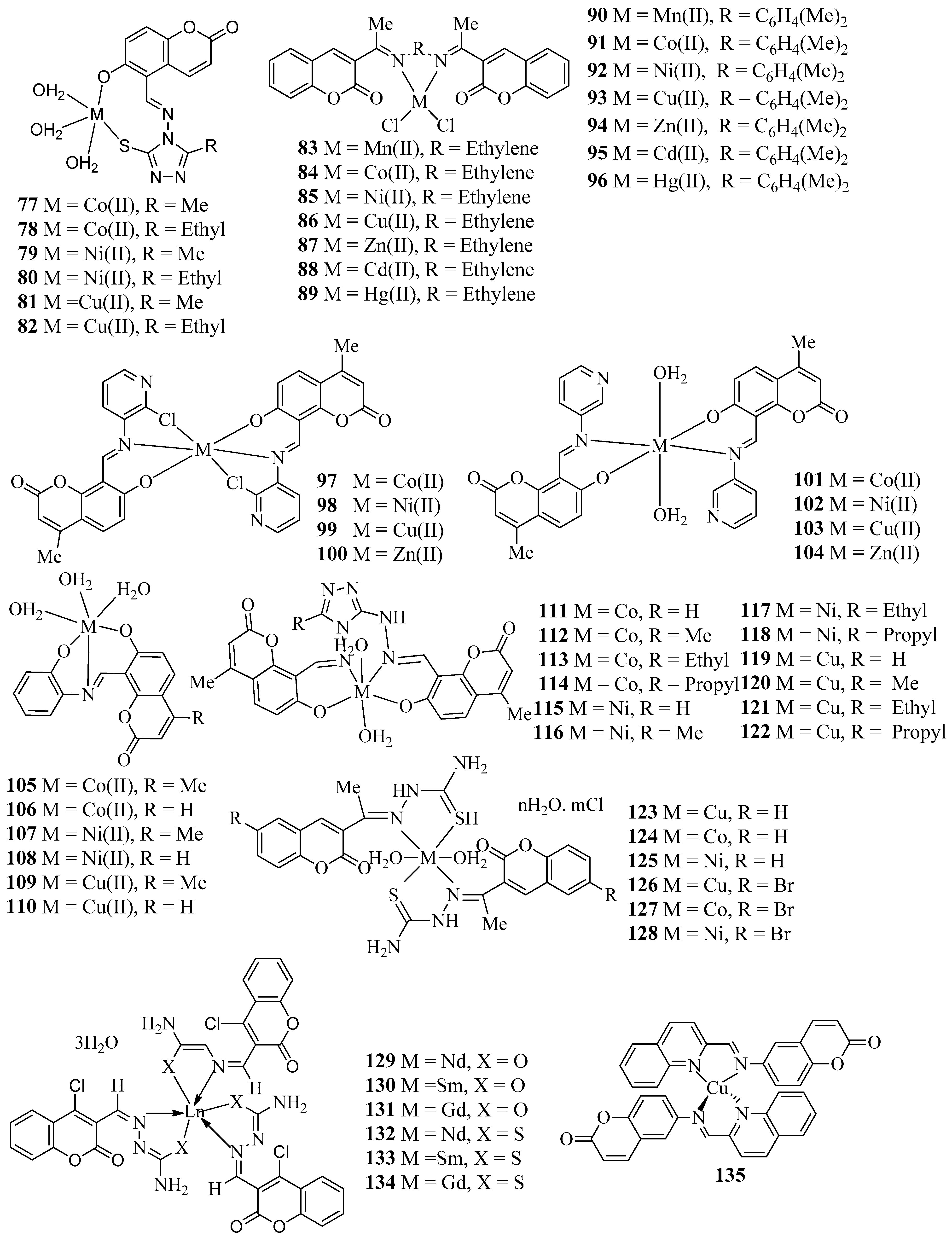
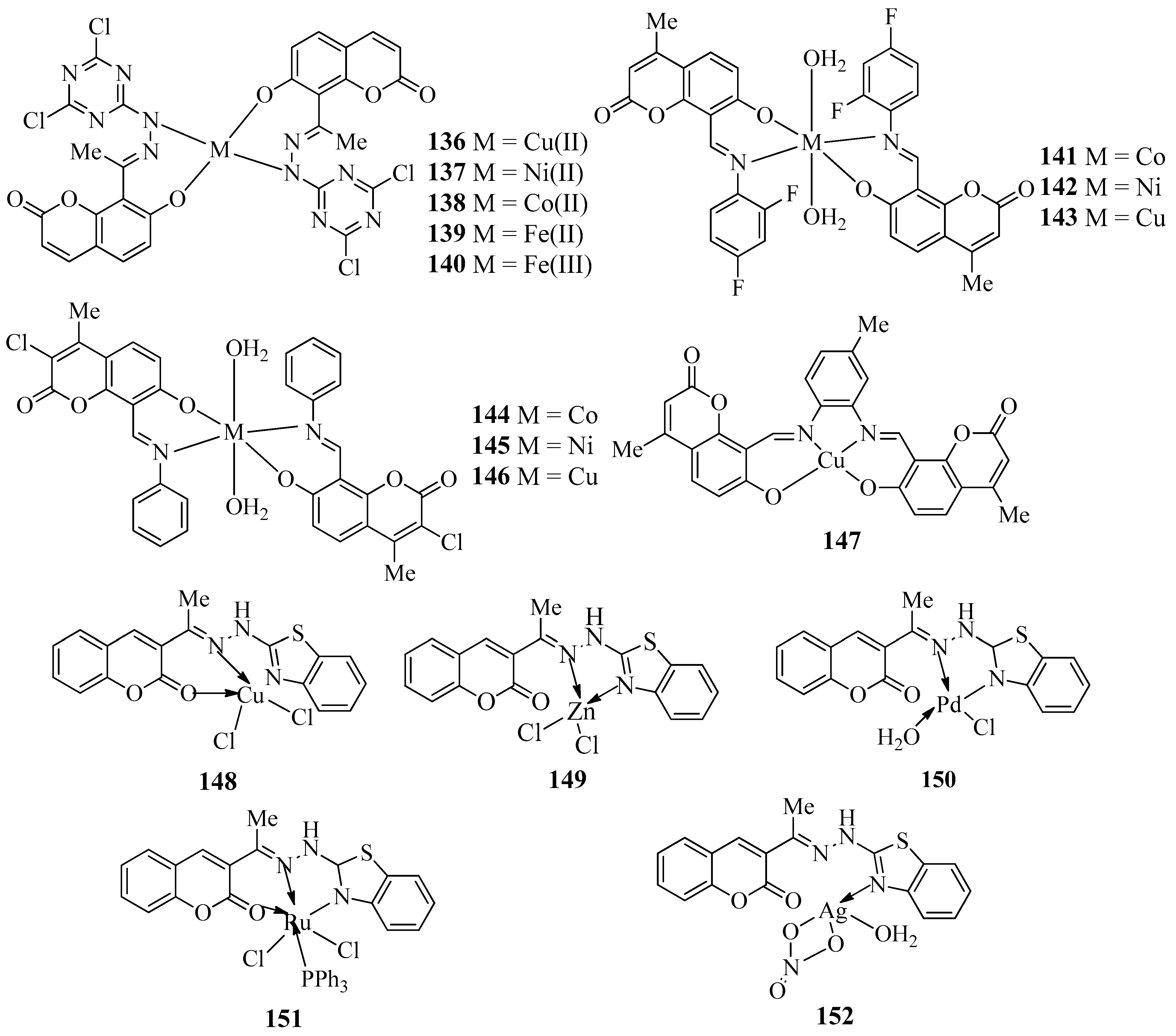
| Compound | Bacteria Screened (n) | Fungi Screened (n) | Highest Activity Against | Concentration | Activity Inhibition Zone (mm) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 5 | 3 | B. subtilis, S. aureus, S. faecalis and P. aeruginosa | NA | 13 | [31] |
| 2 | 5 | 3 | S. aureus | NA | 11 | [31] |
| 3 | 2 | 1 | F. oxysporum | NA | 26 ± 0.2 | [32] |
| 4 | 2 | 1 | P. phaseolicola | NA | 28 ± 0.3 | [32] |
| 5 | 2 | 1 | S. aureus | NA | 36 ± 0.3 | [32] |
| 6 | 2 | 1 | S. aureus | NA | 33 ± 0.1 | [32] |
| 7 | 2 | 1 | S. aureus | NA | 37 ± 0.4 | [32] |
| 8 | 2 | 1 | S. aureus | NA | 27 ± 0.4 | [32] |
| 9 | 2 | 1 | F. oxysporum | NA | 29 ± 0.3 | [32] |
| 10 | 2 | 1 | S. aureus | NA | 31 ± 0.1 | [32] |
| 11 | 2 | 1 | F. oxysporum | NA | 34 ± 0.3 | [32] |
| 12 | 2 | 1 | S. aureus | NA | 32 ± 0.2 | [32] |
| 13 | 4 | 3 | E. coli | 200 μg/mL | NA | [33] |
| 14 | 4 | 3 | S. aureus | 200 μg/mL | NA | [33] |
| 15 | 4 | 3 | S. aureus | 200 μg/mL | NA | [33] |
| 16 | 4 | 3 | B. subtilis, E. coli and A. niger | 12.5 μg/mL | MIC | [34] |
| 17 | 4 | 3 | B. subtilis and C. oxysporum | 12.5 μg/mL | MIC | [34] |
| 18 | 4 | 3 | B. subtilis | 12.5 μg/mL | MIC | [34] |
| 19 | 4 | 3 | B. subtilis, E. coli, A. niger and C. oxysporum | 12.5 μg/mL | MIC | [34] |
| 20 | 8 | 0 | B. subtilis | NA | MP | [35] |
| 21 | 8 | 0 | B. subtilis | NA | MP | [35] |
| 22 | 8 | 0 | B. subtilis | NA | MP | [35] |
| 23 | 8 | 0 | B. subtilis | NA | MP | [35] |
| 24 | 8 | 0 | B. subtilis | NA | MP | [35] |
| 25 | 0 | 8 | B. cinerea | NA | RI | [35] |
| 21 | 0 | 8 | A. alternate, A. flavus, F. moniliforme, H. tetramera and V. alboatrum | NA | RI | [35] |
| 22 | 0 | 8 | A. flavus | NA | RI | [35] |
| 23 | 0 | 8 | A. flavus, F. moniliforme, R. stolonifera and V. alboatrum | NA | RI | [35] |
| 24 | 0 | 8 | A. flavus, F. moniliforme and H. tetramera | NA | RI | [35] |
| 25 | 2 | 1 | P. phaseolicola | NA | 26 ± 0.2 | [36] |
| 26 | 2 | 1 | P. phaseolicola | NA | 22 ± 0.2 | [36] |
| 27 | 2 | 1 | P. phaseolicola | NA | 27 ± 0.2 | [36] |
| 28 | 2 | 1 | P. phaseolicola | NA | 23 ± 0.3 | [36] |
| 29 | 2 | 1 | P. phaseolicola | NA | 20 ± 0.1 | [36] |
| 30 | 2 | 1 | P. phaseolicola | NA | 32 ± 0.3 | [36] |
| 31 | 2 | 1 | P. phaseolicola | NA | 30 ± 0.1 | [36] |
| 32 | 2 | 1 | S. aureus | NA | 37 ± 0.1 | [36] |
| 33 | 2 | 1 | P. phaseolicola | NA | 32 ± 0.2 | [36] |
| 34 | 2 | 1 | P. phaseolicola | NA | 39 ± 0.1 | [36] |
| 35 | 6 | 3 | Klebsiella and Salmonella | 200 μg/mL | 11 | [37] |
| 36 | 6 | 3 | Klebsiella and A. niger | 200 μg/mL | 13 | [37] |
| 37 | 6 | 3 | S. aureus | 200 μg/mL | 14 | [37] |
| 38 | 6 | 3 | S. aureus | 200 μg/mL | 13 | [37] |
| 39 | 6 | 3 | A. niger | 200 μg/mL | 14 | [37] |
| 40 | 6 | 3 | A. niger | 200 μg/mL | 15 | [37] |
| 41 | 4 | 4 | P. aeruginosa, A. niger and Cladosporium | 12.5 μg/mL | MIC | [38] |
| 42 | 4 | 4 | E. coli, S. aureus and A. flavus | 12.5 μg/mL | MIC | [38] |
| 43 | 4 | 4 | B. subtilis, A. niger and C. albicans | 12.5 μg/mL | MIC | [38] |
| 44 | 4 | 4 | E. coli, P. aeruginosa, A. niger and Cladosporium | 12.5 μg/mL | MIC | [38] |
| 45 | 4 | 4 | S. aureus and A. flavus | 12.5 μg/mL | MIC | [38] |
| 46 | 4 | 4 | S. aureus, P. aeruginosa, A. niger, A. flavus and C. albicans | 12.5 μg/mL | MIC | [38] |
| 47 | 5 | 2 | S. typhi | 100 μg/mL | 68.13 | [39] |
| 48 | 5 | 2 | S. typhi | 100 μg/mL | 72.00 | [39] |
| 49 | 5 | 2 | S. typhi | 100 μg/mL | 79.36 | [39] |
| 50 | 5 | 2 | S. typhi | 100 μg/mL | 76.44 | [39] |
| 51 | 5 | 2 | S. typhi | 100 μg/mL | 82.05 | [39] |
| 52 | 5 | 2 | S. typhi | 100 μg/mL | 65.00 | [39] |
| 53 | 5 | 2 | C. albicans | 100 μg/mL | 71.32 | [39] |
| 54 | 5 | 2 | S. typhi | 100 μg/mL | 75.66 | [39] |
| 55 | 5 | 2 | S. typhi | 100 μg/mL | 72.22 | [39] |
| 56 | 5 | 2 | S. typhi | 100 μg/mL | 80.00 | [39] |
| 57 | 3 | 0 | P. aeruginosa | 200 μg/mL | 21 | [40] |
| 58 | 3 | 0 | P. aeruginosa | 200 μg/mL | 21 | [40] |
| 59 | 3 | 0 | P. aeruginosa | 200 μg/mL | 22 | [40] |
| 60 | 3 | 0 | P. aeruginosa | 200 μg/mL | 23 | [40] |
| 61 | 3 | 0 | E. coli | 200 μg/mL | 24 | [40] |
| 62 | 3 | 0 | P. aeruginosa | 200 μg/mL | 23 | [40] |
| 63 | 2 | 2 | E. coli | 100 μg/mL | 26 | [41] |
| 64 | 2 | 2 | E. coli | 100 μg/mL | 27 | [41] |
| 65 | 2 | 2 | S. aureus | 100 μg/mL | 21 | [41] |
| 66 | 2 | 2 | S. aureus | 100 μg/mL | 25 | [41] |
| 67 | 2 | 2 | E. coli | 100 μg/mL | 22 | [41] |
| 68 | 2 | 2 | S. aureus | 100 μg/mL | 28 | [41] |
| 69 | 2 | 2 | S. aureus | 100 μg/mL | 23 | [41] |
| 70 | 2 | 2 | E. coli, S. aureus | 100 μg/mL | 28 | [41] |
| 71 | 4 | 3 | E. coli, A. flavus | 500 μg/mL | 24 | [42] |
| 72 | 4 | 3 | Cladosporium | 500 μg/mL | 28 | [42] |
| 73 | 4 | 3 | A. niger | 500 μg/mL | 27 | [42] |
| 74 | 4 | 3 | A. flavus | 500 μg/mL | 26 | [42] |
| 75 | 4 | 3 | A. flavus | 500 μg/mL | 28 | [42] |
| 76 | 4 | 3 | A. flavus | 500 μg/mL | 29 | [42] |
| 77 | 4 | 3 | P. aeroginosa A. flavus A. niger | 10 10 10 | 80% 81% 82% | [43] |
| 78 | 4 | 3 | A. niger | 10 | 80% | [43] |
| 79 | 4 | 3 | Cladosporium | 10 | 90% | [43] |
| 80 | 4 | 3 | Cladosporium | 10 | 82% | [43] |
| 81 | 4 | 3 | Cladosporium | >100 | >93% | [43] |
| 82 | 4 | 3 | Cladosporium | >100 | >96% | [43] |
| Gentamycin | 4 | 0 | All four | 100 | 100% | [43] |
| Fluconozole | 3 | 0 | All three | 100 | 100% | [43] |
| 83 | 2 | 2 | P. aeroginosa | NA | 17 | [44] |
| 84 | 2 | 2 | P. aeroginosa | NA | 18 | [44] |
| 85 | 2 | 2 | E. coli | NA | 20 | [44] |
| 86 | 2 | 2 | P. aeroginosa | NA | 13 | [44] |
| 87 | 2 | 2 | P. aeroginosa | NA | 16 | [44] |
| 88 | 2 | 2 | P. aeroginosa | NA | 17 | [44] |
| 89 | 2 | 2 | P. aeroginosa | NA | 22 | [44] |
| 90 | 2 | 2 | P. aeroginosa | NA | 16 | [44] |
| 91 | 2 | 2 | E. coli P. aeruginosa and C. albicans | NA | 15 | [44] |
| 92 | 2 | 2 | A.niger C.albicans | NA | 18 | [44] |
| 93 | 2 | 2 | P. aeroginosa | NA | 24 | [44] |
| 94 | 2 | 2 | E. coli | NA | 17 | [44] |
| 95 | 2 | 2 | E. coli | NA | 18 | [44] |
| 96 | 2 | 2 | E. coli C. albicans | NA | 17 | [44] |
| Fluconozole | 0 | 2 | A. niger | NA | 24 | [44] |
| Ciprofloxacin | 2 | 0 | P. aeroginosa | NA | 30 | [44] |
| 97 | 2 | 2 | S. aureus | 3.12 | 21 | [45] |
| 98 | 2 | 2 | S. aureus | 19 | [45] | |
| 99 | 2 | 2 | S. aureus | 1.56 | 22 | [45] |
| 100 | 2 | 2 | S. aureus E. coli | 18 | [45] | |
| 101 | 2 | 2 | S. aureus E. coli | 3.12 | 21 | [45] |
| 102 | 2 | 2 | S. aureus | 20 | [45] | |
| 103 | 2 | 2 | S. aureus | 1.56 | 25 | [45] |
| 104 | 2 | 2 | S. aureus | 20 | [45] | |
| Norfloxacin | 2 | 0 | S. aureus E. coli | 1.56 | 26 | [45] |
| Griseofulvin | 0 | 2 | A. niger and C. albicans | 1.56 | 26 | [45] |
| 105 | 4 | 3 | Cladosporium | 10 | >59% | [46] |
| 106 | 4 | 3 | Cladosporium | 10 | >55% | [46] |
| 107 | 4 | 3 | Cladosporium | 10 | >65% | [46] |
| 108 | 4 | 3 | Cladosporium | 10 | >61% | [46] |
| 109 | 4 | 3 | E. coli and A. flavus | 25 | >20% >16% | [46] |
| 110 | 4 | 3 | E. coli | 25 | [46] | |
| Gentamycin | 4 | 0 | E. coli | 25 | 86% | [46] |
| Flucanozole | 0 | 3 | Cladosporium | 25 | 92% | [46] |
| 111 | 4 | 3 | A. flavus | 25 | 80% | [47] |
| 112 | 4 | 3 | A. flavus | 25 | 77% | [47] |
| 113 | 4 | 3 | A. niger A. flavus | 25 25 | 83% 71% | [47] |
| 114 | 4 | 3 | A. niger and S. aureus | 10 | [47] | |
| 115 | 4 | 3 | A. niger | 25 | 79% | [47] |
| 116 | 4 | 3 | A. flavus | 25 | 72% | [47] |
| 117 | 4 | 3 | A. niger and P. aeruginosa | 25 25 | 82% 66% | [47] |
| 118 | 4 | 3 | A. niger | 25 | 70% | [47] |
| 119 | 4 | 3 | A. flavus | 25 | 78% | [47] |
| 120 | 4 | 3 | A. flavus | 10 | [47] | |
| 121 | 4 | 3 | A. flavus A. niger | 25 25 | 80% 74% | [47] |
| 122 | 4 | 3 | A. flavus | 25 | 85% | [47] |
| Gentamycin | 4 | 3 | All four | 25 | 100% | [47] |
| Flucanozole | 4 | 3 | All three | 25 | 100% | [47] |
| 123 | 4 | 0 | B. subtili, E. coli and P. aeruginosa | NA | 13 mm | [48] |
| 124 | 4 | 0 | B. subtili S. aureus and P. aeruginosa | NA | 18 mm | [48] |
| 125 | 4 | 0 | B. subtili and P. aeruginosa | NA | 17 mm | [48] |
| 126 | 4 | 0 | E. coli | NA | 17 mm | [48] |
| 127 | 4 | 0 | S. aureus and E. coli | NA | 18 mm | [48] |
| 128 | 4 | 0 | B. subtili | NA | 18 mm | [48] |
| 129 | 4 | 3 | F. oxysporum and S. aureus | 100 ppm 500 ppm | 79 ± 1.4% 15 ± 0.03% | [49] |
| 130 | 4 | 3 | F. oxysporum S. aureus | 100 ppm 500 ppm | 71 ± 0.4% 13.9 ± 0.09% | [49] |
| 131 | 4 | 3 | F. oxysporum and S. aureus | 100 ppm 500 ppm | 84 ± 0.6% 14.4 ± 0.1% | [49] |
| 132 | 4 | 3 | C. albicans and S. aureus | 100 ppm 500 ppm | 59 ± 0.7% 12.6 ± 0.09% | [49] |
| 133 | 4 | 3 | C. albicans and P. aeruginosa | 100 ppm 500 ppm | 60 ± 0.4% 12.0 ± 0.03% | [49] |
| 134 | 4 | 3 | F. oxysporum and E. coli | 100 ppm 500 ppm | 69 ± 0.2% 10.5 ± 0.09% | [49] |
| Ciprofloxacin | 4 | 0 | S. aureus | 500 ppm | 18.6 ± 0.03% | [49] |
| Fluconazole | 0 | 3 | F. oxysporum, C. albicans and A. Niger | 500 ppm | 100 | [49] |
| 135 | 1 | 0 | F. psychrophilum | 32 | 16.1 ± 0.9% | [50] |
| 136 | 4 | 1 | A. Niger | NA | 4.6 mm | [51] |
| 137 | 4 | 1 | A. Niger | NA | 1.15 mm | [51] |
| 138 | 4 | 1 | S. aureus | NA | 0.81 mm | [51] |
| 139 | 4 | 1 | S. aureus | NA | 1.72 mm | [51] |
| 140 | 4 | 1 | S. aureus | NA | 1.72 mm | [51] |
| 141 | 4 | 2 | R. oryzae | 200 | 10 mm | [11] |
| 142 | 4 | 2 | A. niger | 200 | 11 mm | [11] |
| 143 | 4 | 2 | P. auregenosa A. niger R. oryzae | 200 200 200 | 12 mm 12 mm 12 mm | [52] |
| 144 | 4 | 2 | A. niger | 200 | 11mm | [52] |
| 145 | 4 | 2 | A. niger | 200 | 14 mm | [52] |
| 146 | 4 | 2 | A. niger | 200 | 15 mm | [52] |
| Gentamicin | 2 | 0 | P. auregenosa | 200 | 15 mm | [52] |
| Fluconazole | 0 | 2 | A. niger | 200 | 16 mm | [52] |
| 147 | 4 | 0 | K. pneumonia | 100 | 22 mm | [53] |
| 148 | 0 | 2 | O. latemarginatus | 100 | 82.2 ± 1.1 | [54] |
| 149 | 0 | 2 | O. latemarginatus | 100 | 74.4 ± 1.1 | [54] |
| 150 | 0 | 2 | O. latemarginatus | 100 | 83.7 ± 0.6 | [54] |
| 151 | 0 | 2 | O. latemarginatus | 100 | 76.7 ± 1.1 | [54] |
| 152 | 0 | 2 | O. latemarginatus | 100 | 67.8 ± 1.1 | [54] |
| 153 | 1 | 1 | E. coli and A. niger | 20 µg/mL | MIC | [55] |
| 154 | 1 | 1 | E. coli and A. niger | 20 µg/mL | MIC | [55] |
| 155 | 1 | 1 | E. coli and A. niger | 20 µg/mL | MIC | [55] |
| 156 | 1 | 1 | E. coli and A. niger | 20 µg/mL | MIC | [55] |
| 157 | 0 | 1 | Candida | 5.2 µM | MIC | [56] |
| 158 | 0 | 1 | Candida | 10.4 µM | MIC | [56] |
| 159 | 0 | 1 | Candida | 16.7 µM | MIC | [56] |
| 160 | 0 | 1 | Candida | 8.2 µM | MIC | [56] |
| 161 | 0 | 1 | Candida | NA | MIC | [56] |
| 162 | 0 | 1 | Candida | 14.5 µM | MIC | [56] |
| 163 | 0 | 1 | Candida | 3.6 µM | MIC | [56] |
| 164 | 0 | 1 | Candida | 4.4 µM | MIC | [56] |
| 165 | 0 | 1 | Candida | 0.7 µM | MIC | [56] |
| 166 | 0 | 1 | Candida | 9.8 µM | MIC | [56] |
| 167 | 0 | 1 | Candida | 12.6 µM | MIC | [56] |
| Amphotericin B | 1 | Candida | 0.7 µM | MIC | [56] | |
| Ketoconazole | 1 | Candida | 4.7 µM | MIC | [56] | |
| 168 | 3 | 0 | K. pneumoniae and S. aureus | 100 µg/mL | 19 ± 0 | [57] |
| 169 | 3 | 0 | K. pneumoniae and S. aureus | 100 µg/mL | 18 ± 0 | [57] |
| 170 | 3 | 0 | K. pneumoniae, and S. aureus | 100 µg/mL | 17 ± 0 | [57] |
| 171 | 3 | 0 | K. pneumoniae, E. coli, and S. aureus | 100 µg/mL | 16 ± 0 | [57] |
| Compound | Scavenging Activity (%) | Ref. |
|---|---|---|
| 41 | 44 | [38] |
| 42 | 59 | [38] |
| 43 | 48 | [38] |
| 44 | 63 | [38] |
| 45 | 58 | [38] |
| 46 | 61 | [38] |
| BHA | 70 | [38] |
| Compound | Conc.(μg/mL) | Time of Paralysis (min) | Time of Death (min) | Ref. |
|---|---|---|---|---|
| Albendazole | 10 | 3.48 ± 0.06 | 7.25 ± 0.14 | [33] |
| 13 | 10 | 7.40 ± 0.04 | 10.12 ± 0.03 | [33] |
| 14 | 10 | 9.13 ± 0.01 | 15.51 ± 0.00 | [33] |
| 15 | 10 | 5.39 ± 0.22 | 9.31 ± 0.01 | [33] |
| 13 | 2 | 12.14 ± 0.14 | 19.50 ± 0.02 | [33] |
| 14 | 2 | 16.10 ± 0.25 | 21.20 ± 0.09 | [33] |
| 15 | 2 | 10.27 ± 0.03 | 16.41 ± 0.06 | [33] |
| 35 | 10 | 4.30 ± 0.04 | 7.72 ± 0.03 | [37] |
| 36 | 10 | 3.43 ± 0.01 | 6.51 ± 0.20 | [37] |
| 37 | 10 | 3.29 ± 0.02 | 6.91 ± 0.01 | [37] |
| 38 | 10 | 54 ± 0.01 | 7.25 ± 0.01 | [37] |
| 39 | 10 | 3.25 ± 0.00 | 6.93 ± 0.10 | [37] |
| 40 | 10 | 3.20 ± 0.04 | 6.25 ± 0.12 | [37] |
| 35 | 2 | 8.24 ± 0.04 | 16.50 ± 0.09 | [37] |
| 36 | 2 | 7.20 ± 0.05 | 120.60 ± 0.09 | [37] |
| 37 | 2 | 7.17 ± 0.01 | 19.61 ± 0.06 | [37] |
| 38 | 2 | 7.10 ± 0.023 | 11.41 ± 0.05 | [37] |
| 39 | 2 | 6.10 ± 0.01 | 12.15 ± 0.09 | [37] |
| 40 | 2 | 5.14 ± 0.03 | 10.20 ± 0.09 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, S.A.; Kandathil, V.; Sobha, A.; Somappa, S.B.; Feldman, M.R.; Bugarin, A.; Patil, S.A. Comprehensive Review on Medicinal Applications of Coumarin-Derived Imine–Metal Complexes. Molecules 2022, 27, 5220. https://doi.org/10.3390/molecules27165220
Patil SA, Kandathil V, Sobha A, Somappa SB, Feldman MR, Bugarin A, Patil SA. Comprehensive Review on Medicinal Applications of Coumarin-Derived Imine–Metal Complexes. Molecules. 2022; 27(16):5220. https://doi.org/10.3390/molecules27165220
Chicago/Turabian StylePatil, Siddappa A., Vishal Kandathil, Anjali Sobha, Sasidhar B. Somappa, Max R. Feldman, Alejandro Bugarin, and Shivaputra A. Patil. 2022. "Comprehensive Review on Medicinal Applications of Coumarin-Derived Imine–Metal Complexes" Molecules 27, no. 16: 5220. https://doi.org/10.3390/molecules27165220
APA StylePatil, S. A., Kandathil, V., Sobha, A., Somappa, S. B., Feldman, M. R., Bugarin, A., & Patil, S. A. (2022). Comprehensive Review on Medicinal Applications of Coumarin-Derived Imine–Metal Complexes. Molecules, 27(16), 5220. https://doi.org/10.3390/molecules27165220