Highly Sensitive Electrochemical Sensor for Sunset Yellow Based on Electrochemically Activated Glassy Carbon Electrode
Abstract
:1. Introduction
2. Results and Discussions
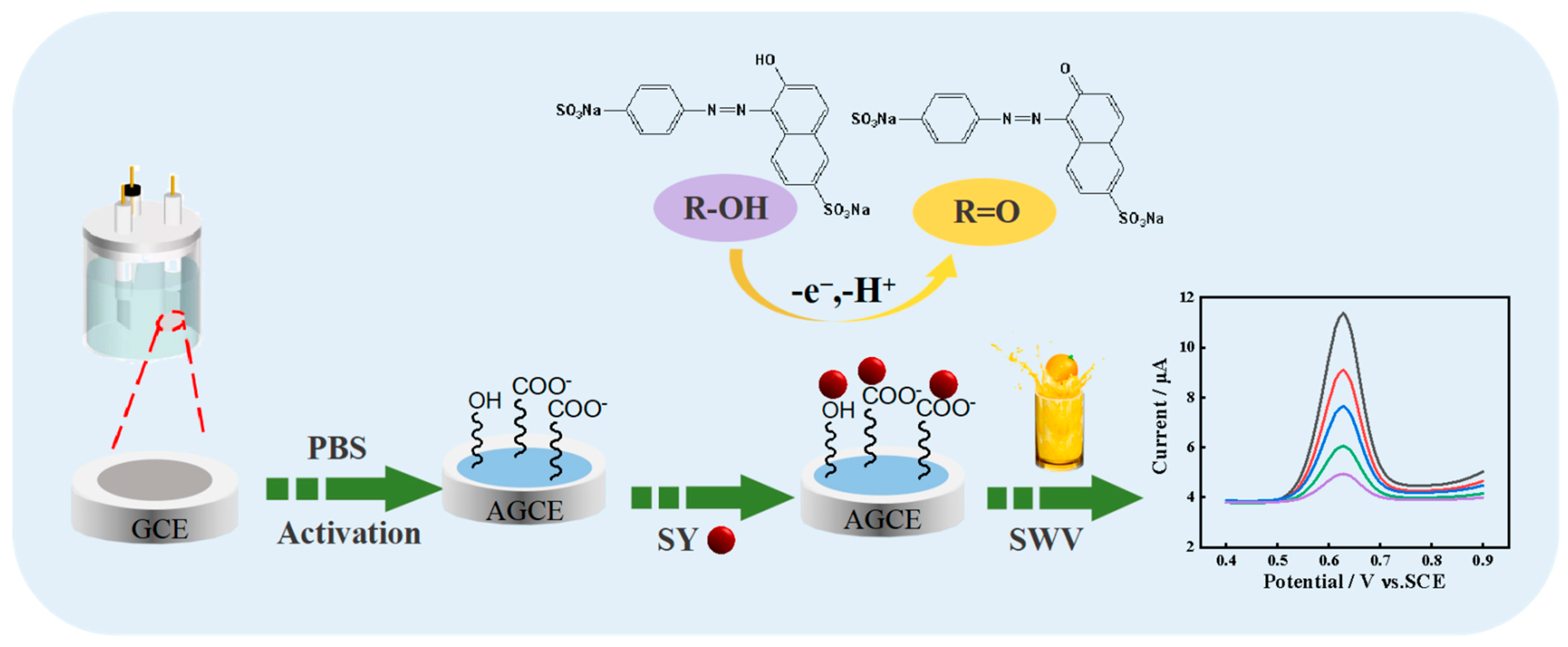
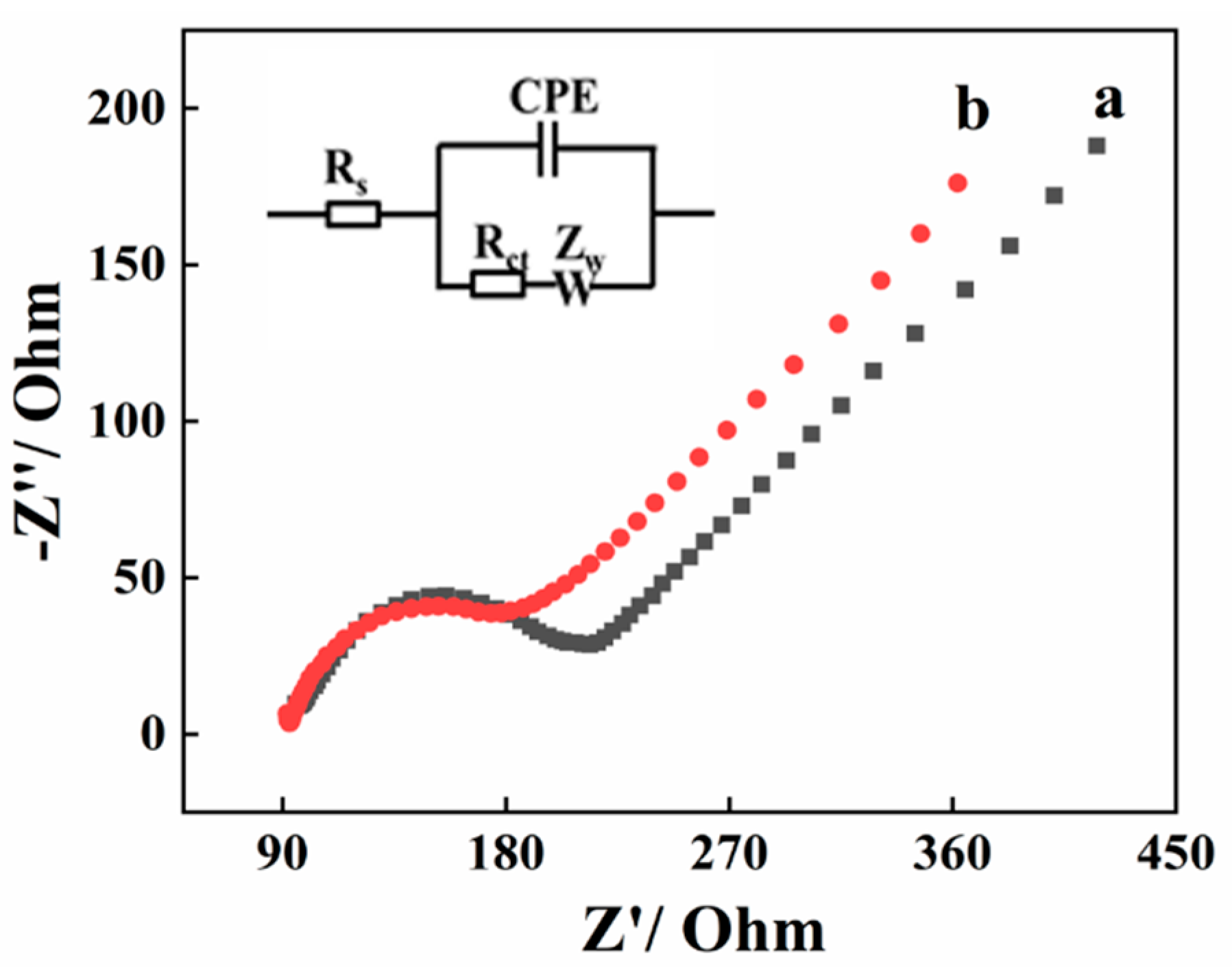
2.1. Characterization of the AGCE
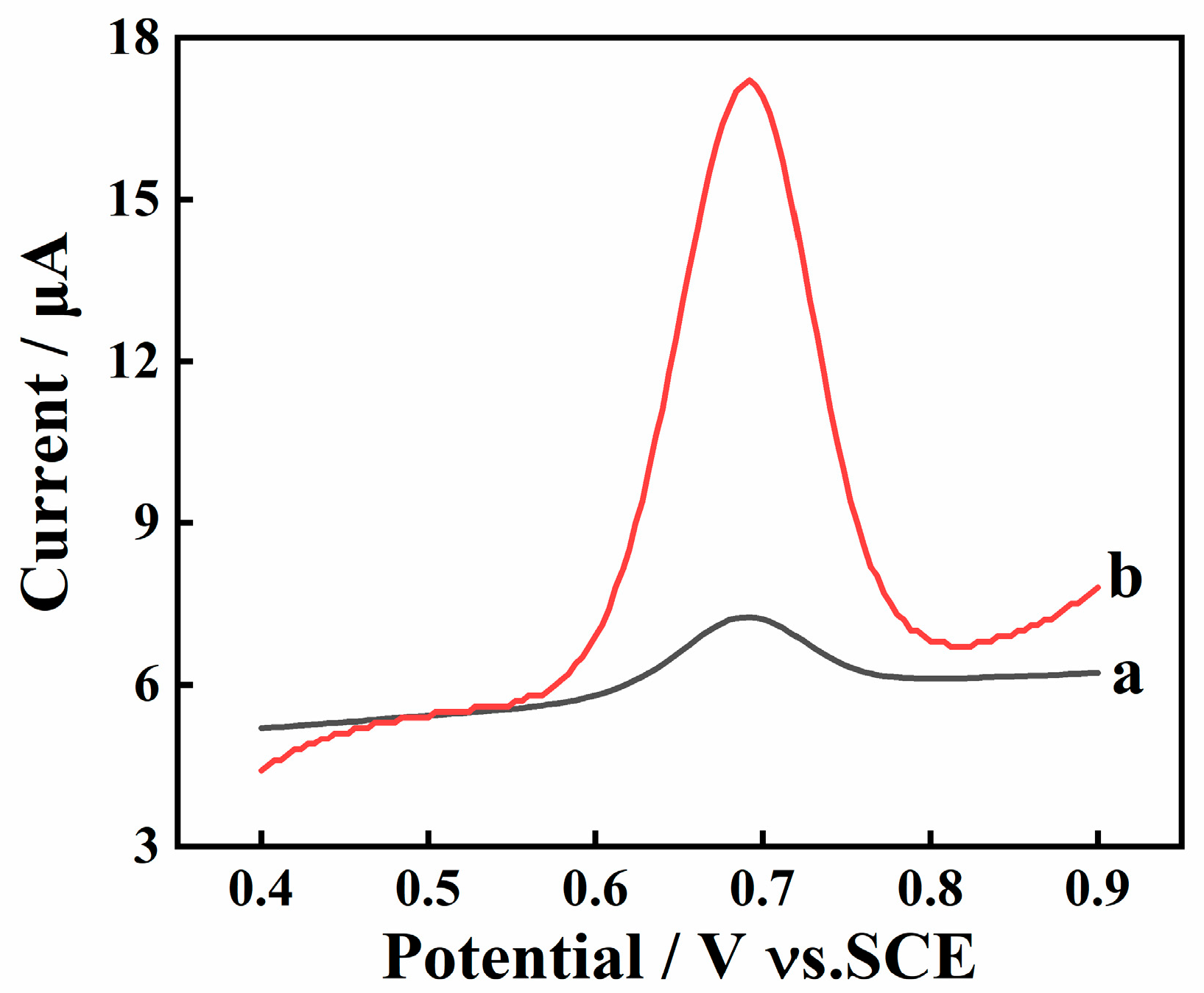
2.2. Electrochemical Behavior of SY on Different Electrodes
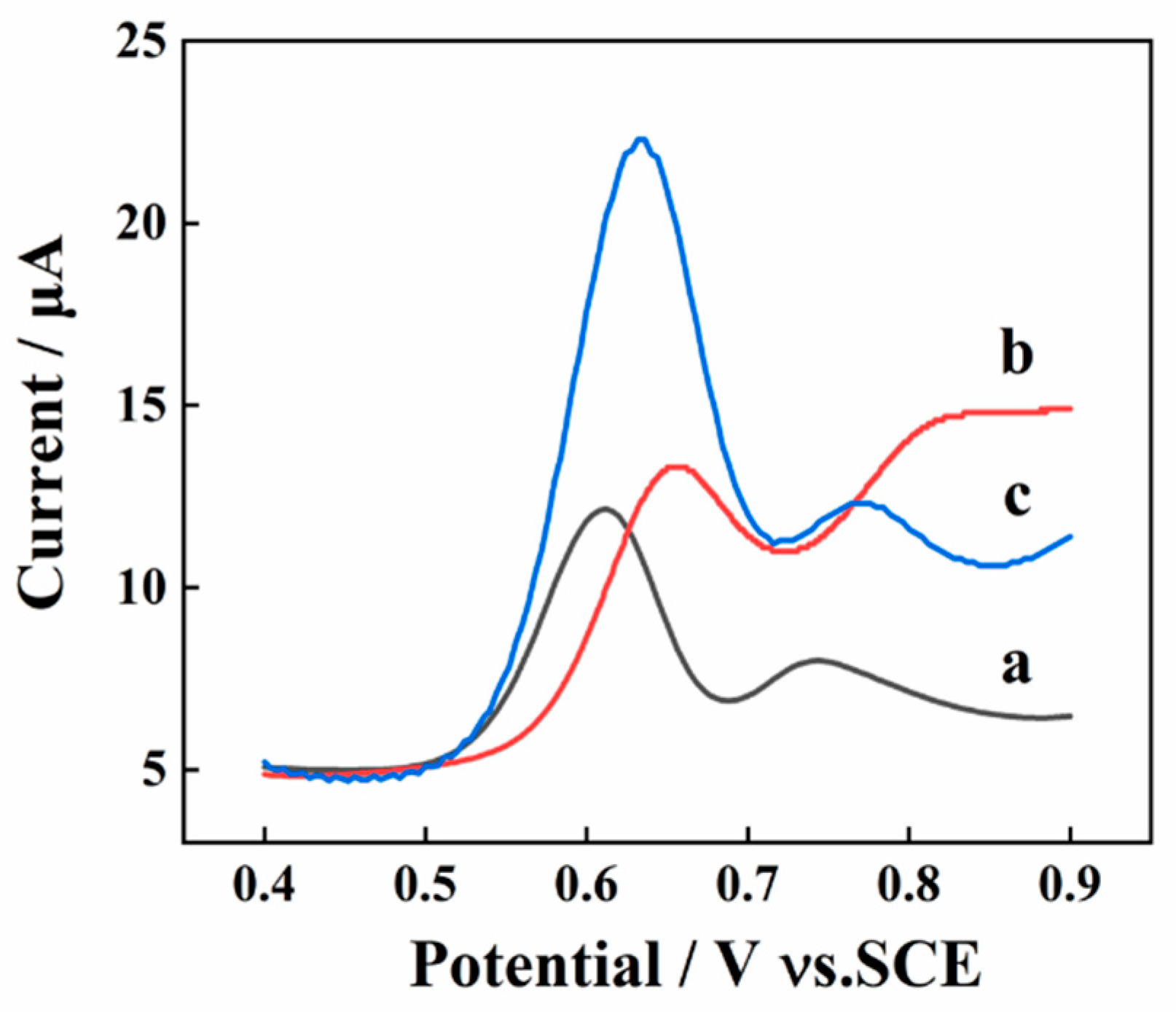
2.3. Effect of Electrochemical Detection Technique
2.4. Optimization of the Experimental Conditions
2.4.1. Effects of Accumulation Time
2.4.2. Effects of Electrochemical Activation pH
2.4.3. Effects of pH
2.4.4. Effects of Scan Rate
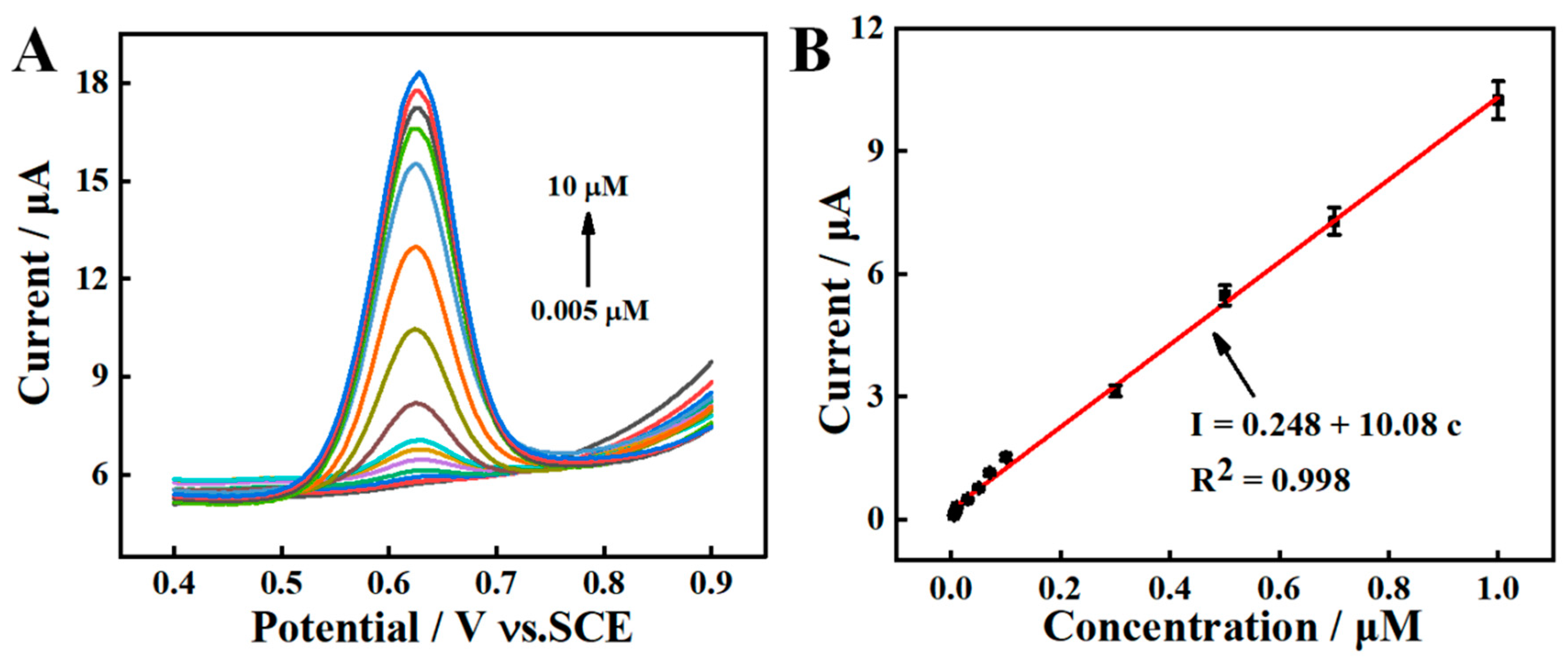
2.5. Electrochemical Detection of SY
2.6. Repeatability, Reproducibility, and Anti-Interference
2.7. Practical Application
3. Experimental
3.1. Reagents and Apparatus
3.2. Preparation of AGCE
3.3. Electrochemical Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaur, A.; Gupta, U.; Hasan, I.; Muhammad, R.; Khan, R.A. Synthesis of highly fluorescent carbon dots from spices for determination of sunset yellow in beverages. Microchem. J. 2021, 170, 106720. [Google Scholar] [CrossRef]
- Rovina, K.; Acung, L.A.; Siddiquee, S.; Akanda, J.H.; Shaarani, S. Extraction and Analytical Methods for Determination of Sunset Yellow (E110)—A Review. Food Anal. Methods 2016, 10, 773–787. [Google Scholar] [CrossRef]
- Tran, Q.T.; Phung, T.T.; Nguyen, Q.T.; Le, T.G.; Lagrost, C. Highly sensitive and rapid determination of sunset yellow in drinks using a low-cost carbon material-based electrochemical sensor. Anal. Bioanal. Chem. 2019, 28, 7539–7549. [Google Scholar] [CrossRef] [PubMed]
- Dorraji, P.S.; Jalali, F. Electrochemical fabrication of a novel ZnO/cysteic acid nanocomposite modified electrode and its application to simultaneous determination of sunset yellow and tartrazine. Food. Chem. 2017, 273, 73–97. [Google Scholar] [CrossRef]
- Ya, Y.; Jiang, C.; Li, T.; Liao, J.; Fan, Y.; Wei, Y.; Yan, F.; Xie, L. A Zinc Oxide Nanoflower-Based Electrochemical Sensor for Trace Detection of Sunset Yellow. Sensors 2017, 3, 545. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, A.; Dwivedi, P.D.; Tripathi, A.; Das, M. In vitro studies on immunotoxic potential of Orange II in splenocytes. Toxicol. Lett. 2012, 3, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.; Le, T.; Bergonzo, P.; Tran, Q. One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks. Appl. Sci. 2022, 5, 2614. [Google Scholar] [CrossRef]
- Zou, T.; He, P.; Yasen, A.; Li, Z. Determination of seven synthetic dyes in animal feeds and meat by high performance liquid chromatography with diode array and tandem mass detectors. Food. Chem. 2013, 138, 1742–1748. [Google Scholar] [CrossRef]
- Ji, L.; Peng, L.; Chen, T.; Li, X.; Zhu, X.; Hu, P. Facile synthesis of Fe-BTC and electrochemical enhancement effect for sunset yellow determination. Talanta Open 2022, 5, 100084–100091. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, X.; Qiao, M.; Zhu, J.; Liu, S.; Yang, J.; Hu, X. Determination of sunset yellow in soft drinks based on fluorescence quenching of carbon dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 167, 106–124. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Yang, L. Designing of the functional paper-based surface-enhanced Raman spectroscopy substrates for colorants detection. Mater. Res. Bull. 2015, 63, 199–204. [Google Scholar] [CrossRef]
- Llamas, N.E.; Garrido, M.; Nezio, M.S.D.; Band, B.S.F. Second order advantage in the determination of amaranth, sunset yellow FCF and tartrazine by UV–vis and multivariate curve resolution-alternating least squares. Anal. Chim. Acta. 2009, 655, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Deb, M.K. Modified silver nanoparticles-enhanced single drop microextraction of tartrazine in food samples coupled with diffuse reflectance Fourier transform infrared spectroscopic analysis. Anal. Methods 2019, 11, 3552–3562. [Google Scholar] [CrossRef]
- Yu, T.; Fenelon, O.; Herdman, K.M.; Breslin, C.B. The Electrochemical Detection of 4-chloro-2-methylphenoxyacetic Acid (MCPA) Using a Simple Activated Glassy Carbon Electrode. J. Electrochem. Soc. 2022, 169, 037514. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P.; Chen, D. Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow. Molecules 2018, 23, 2130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Luo, P.; Wu, J.; Wang, W.; Ye, B. Highly sensitive determination of Sunset Yellow in drink using a poly (L-cysteine) modified glassy carbon electrode. Anal. Methods 2013, 19, 5044–5050. [Google Scholar] [CrossRef]
- Flores-López, S.L.; Villanueva, S.F.; Montes-Morán, M.A.; Cruz, G.; Garrido, J.J.; Arenillas, A. Advantages of microwave-assisted synthesis of silica gels. Colloids Surf. A 2020, 604, 125248–125258. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Z.; Xu, Q.; Hu, X. Simple and sensitive determination of hydroxyl radical in atmosphere based on an electrochemically activated glassy carbon electrode. Int. J. Eeviron. Anal. Chem. 2018, 5, 477–492. [Google Scholar] [CrossRef]
- Márquez-Mijares, M.; Lepetitcc, B.; Lemoine, D. Carbon adsorption on tungsten and electronic field emission. Surf. Sci. 2016, 645, 56–62. [Google Scholar] [CrossRef]
- Phelan, B.T.; Zhang, J.; Huang, G.; Wu, Y.; Zarea, M.; Young, R.M.; Wasielewski, M.R. Quantum Coherence Enhances Electron Transfer Rates to Two Equivalent Electron Acceptors. J. Am. Chem. Soc. 2019, 141, 12236–12239. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Sun, J.; Qiao, S.; Zhang, M.; Li, J. A facile, inexpensive and green electrochemical sensor for sensitive detection of imidacloprid residue in rice using activated electrodes. Anal. Methods 2021, 13, 3649–3658. [Google Scholar] [CrossRef]
- Shi, K.; Shiu, K.-K. Determination of Uric Acid at Electrochemically Activated Glassy Carbon Electrode. Electroanalysis 2001, 16, 1319–1325. [Google Scholar] [CrossRef]
- Chiavazza, E.; Berto, S.; Giacomino, A.; Malandrino, M.; Barolo, C.; Prenesti, E.; Vione, D.; Abollino, O. Electrocatalysis in the oxidation of acetaminophen with an electrochemically activated glassy carbon electrode. Electrochim. Acta. 2016, 192, 139–147. [Google Scholar] [CrossRef]
- Watanabe, H.; Omoto, S.; Hoshi, Y.; Shitanda, I.; Itagaki, M. Electrochemical impedance analysis on positive electrode in lithium-ion battery with galvanostatic control. J. Power Source 2021, 507, 230258–230267. [Google Scholar] [CrossRef]
- Bystron, T.; Sramkova, E.; Dvorak, F.; Bouzek, K. Glassy carbon electrode activation—A way towards highly active, reproducible and stable electrode surface. Electrochim. Acta. 2019, 299, 963–970. [Google Scholar] [CrossRef]
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today. 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Curtolo, F.; Arantes, G.M. Mechanisms for Flavin-Mediated Oxidation: Hydride or Hydrogen Atom Transfer. J. Chem. Inf. Model. 2020, 12, 6282–6287. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lv, M.; Lu, K.; Gao, L.; Liu, J. Electrochemical Behaviors of Baicalin at an Electrochemically Activated Glassy Carbon Electrode and Its Determination in Human Blood Serum. J. Chin. Chem. Soc. 2012, 7, 829–835. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Leng, J.; Yu, Y.; Wang, W.; Jiang, M.; Bai, L. An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine. Food Chem. 2016, 190, 889–895. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Wu, Q.; Jing, Q.; Yu, S. Graphene Decorated with Nickel Nanoparticles as a Sensitive Substrate for Simultaneous Determination of Sunset Yellow and Tartrazine in Food Samples. Electroanalysis 2013, 25, 1505–1512. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Zhang, K.; Bin, D.; Shiraishi, Y.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on the ultrafine Au-Pd and reduced graphene oxide nanocomposites. J. Colloid. Interf. Sci. 2016, 481, 229–235. [Google Scholar] [CrossRef] [PubMed]



| Electrode Material | Linear Range (μM) | LOD (μM) | Reference |
|---|---|---|---|
| ZnO-cysteic acid/GCE | 0.1–3.0 | 0.030 | [4] |
| GO a-MWCNT b/GCE | 0.09–8.0 | 0.025 | [29] |
| poly(L-cysteine)/GCE | 0.008–0.7 | 0.004 | [16] |
| GN c-Ni/GCE | 0.0074–0.442 | 0.0022 | [30] |
| rGO d/Au-Pd/GCE | 0.331–0.686 | 0.0015 | [31] |
| AGCE | 0.005–1.0 | 0.00167 | This work |
| Samples | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 1 | 0.976 | 97.6 | 4.46 |
| 2 | 3 | 3.036 | 101.2 | 2.83 |
| 3 | 5 | 4.865 | 97.3 | 3.69 |
| 4 | 7 | 7.243 | 103.47 | 4.2 |
| 5 | 9 | 8.657 | 96.19 | 3.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Bao, C.; Zou, J.; Xiao, J.; Zhong, W.; Gao, Y. Highly Sensitive Electrochemical Sensor for Sunset Yellow Based on Electrochemically Activated Glassy Carbon Electrode. Molecules 2022, 27, 5221. https://doi.org/10.3390/molecules27165221
Lu Y, Bao C, Zou J, Xiao J, Zhong W, Gao Y. Highly Sensitive Electrochemical Sensor for Sunset Yellow Based on Electrochemically Activated Glassy Carbon Electrode. Molecules. 2022; 27(16):5221. https://doi.org/10.3390/molecules27165221
Chicago/Turabian StyleLu, Yan, Chengqi Bao, Jin Zou, Jinli Xiao, Wei Zhong, and Yansha Gao. 2022. "Highly Sensitive Electrochemical Sensor for Sunset Yellow Based on Electrochemically Activated Glassy Carbon Electrode" Molecules 27, no. 16: 5221. https://doi.org/10.3390/molecules27165221
APA StyleLu, Y., Bao, C., Zou, J., Xiao, J., Zhong, W., & Gao, Y. (2022). Highly Sensitive Electrochemical Sensor for Sunset Yellow Based on Electrochemically Activated Glassy Carbon Electrode. Molecules, 27(16), 5221. https://doi.org/10.3390/molecules27165221





