Synthesis of Functionalized Azepines via Cu(I)-Catalyzed Tandem Amination/Cyclization Reaction of Fluorinated Allenynes
Abstract
:1. Introduction
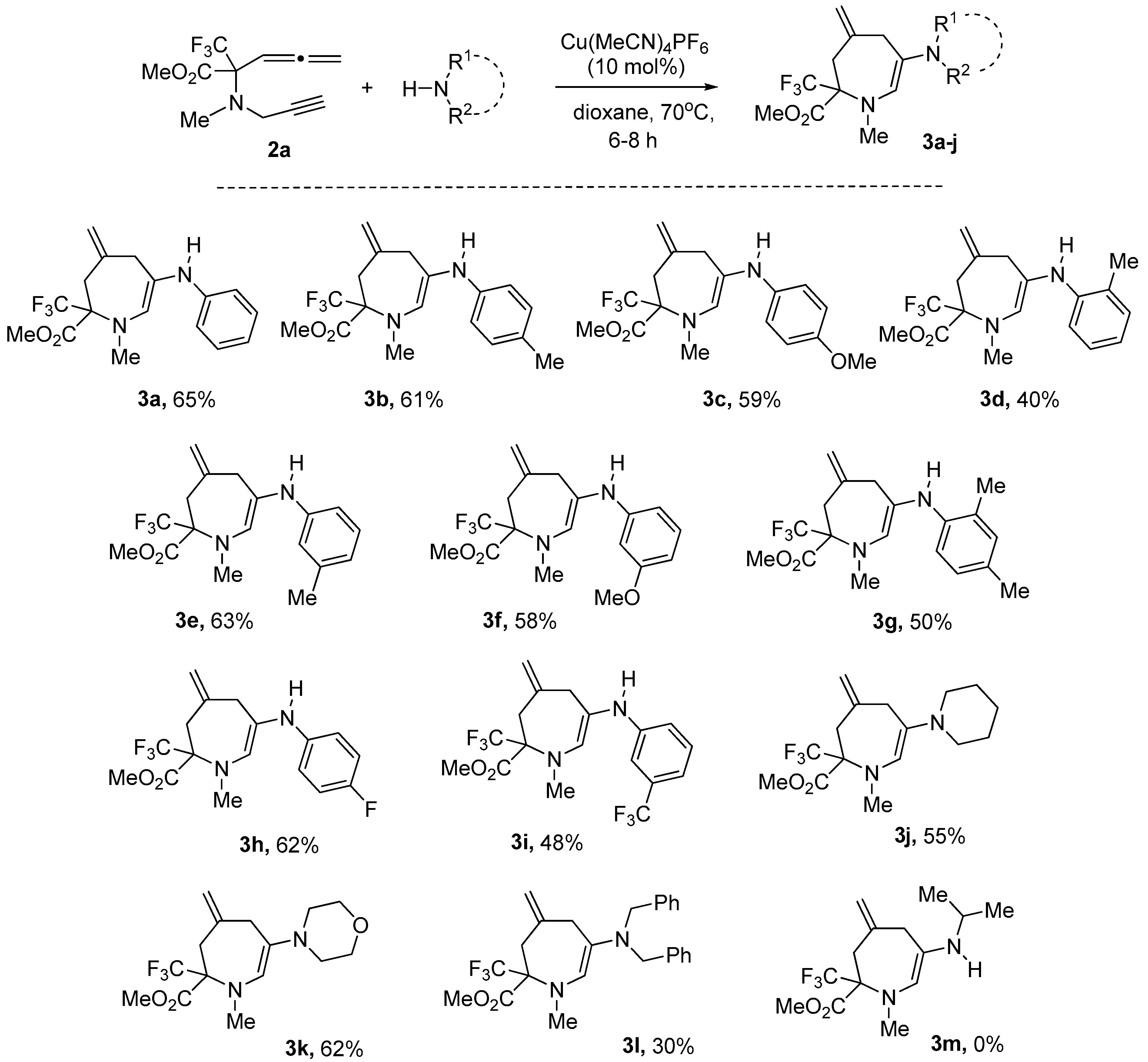
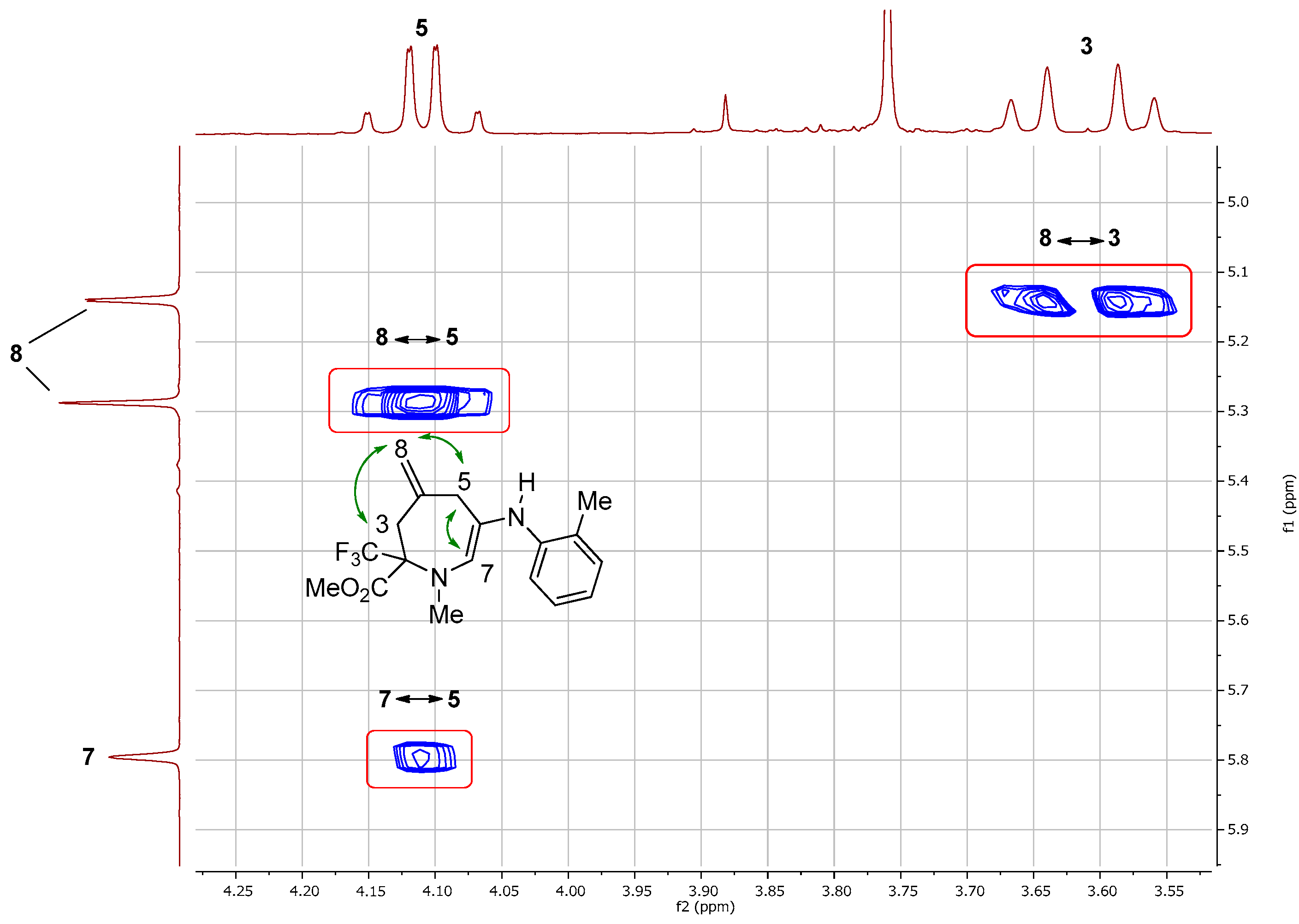
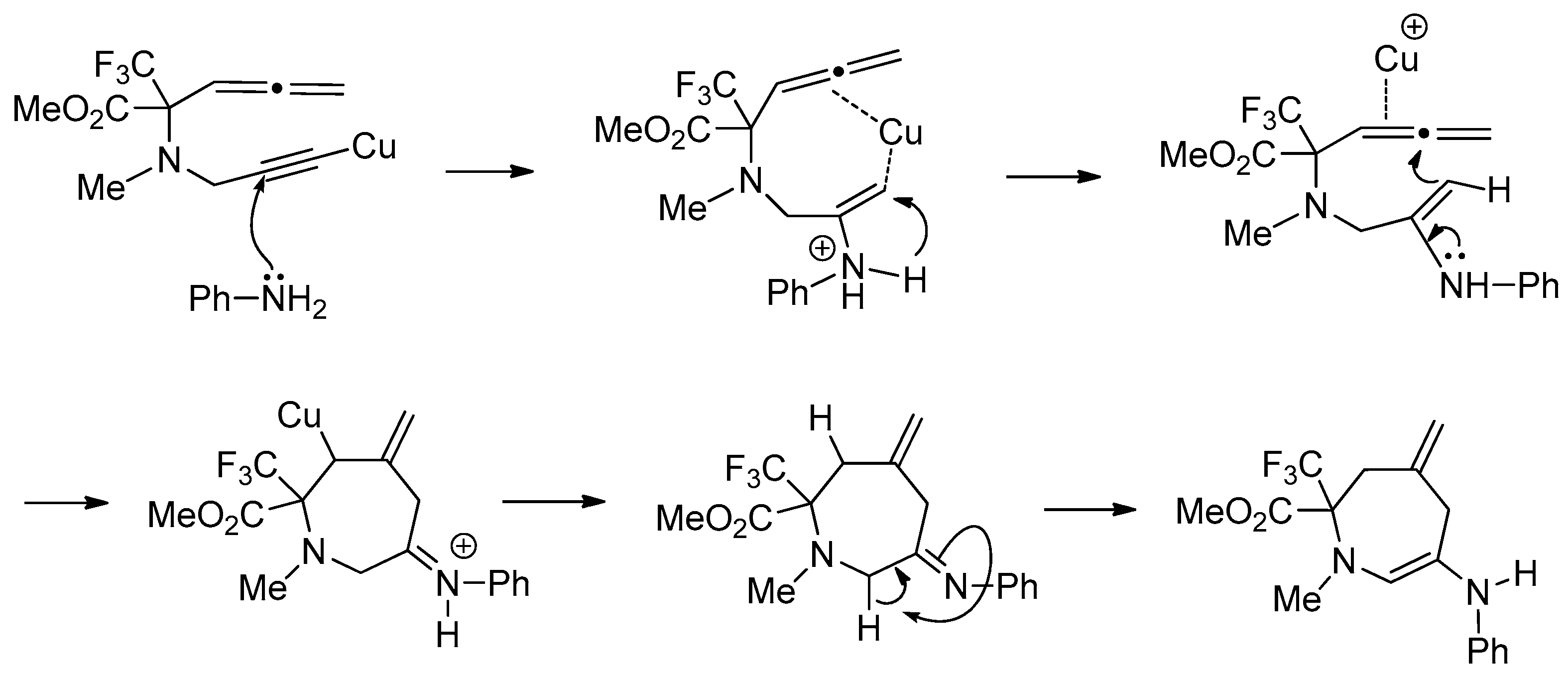
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure
- Methyl 1-methyl-4-methylene-6-(phenylamino)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3a)
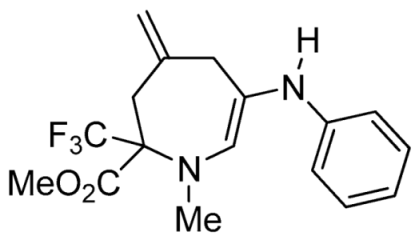
- Methyl 1-methyl-4-methylene-6-(p-tolylamino)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3b)

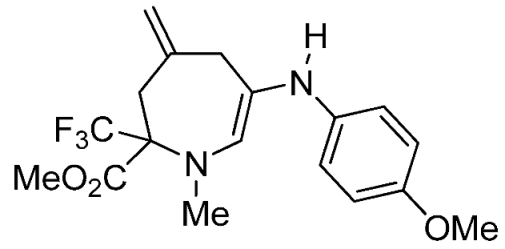
- Methyl 6-(4-methoxyphenylamino)-1-methyl-4-methylene-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3c)
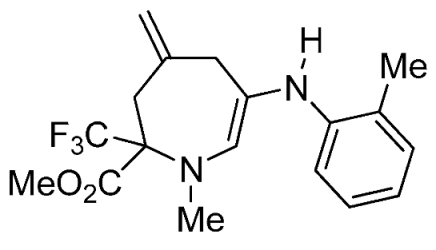
- Methyl 1-methyl-4-methylene-6-(o-tolylamino)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3d)
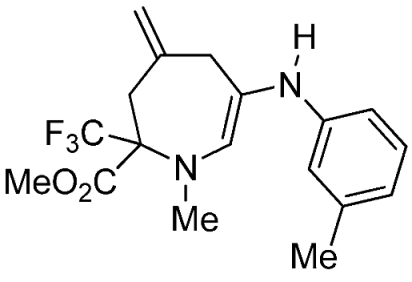
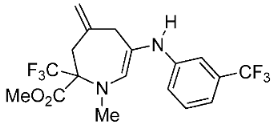
- Methyl 1-methyl-4-methylene-6-(m-tolylamino)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3e)
- Methyl 6-(3-methoxyphenylamino)-1-methyl-4-methylene-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3f)
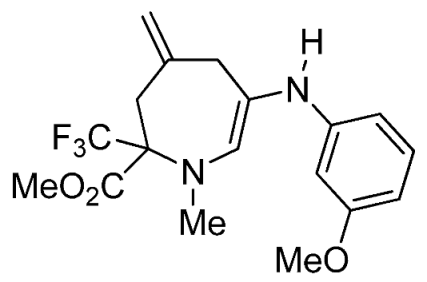
- Methyl 6-(2,4-dimethylphenylamino)-1-methyl-4-methylene-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3g)
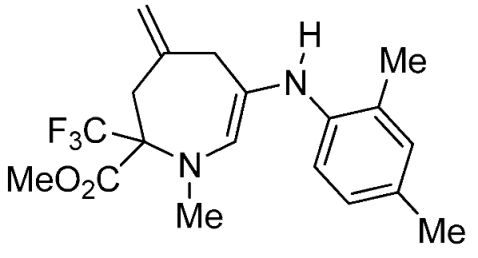
- Methyl 6-(4-fluorophenylamino)-1-methyl-4-methylene-2-(trifluoromethyl)-2,3,4,5-tetrahydro-1H-azepine-2-carboxylate (3h)
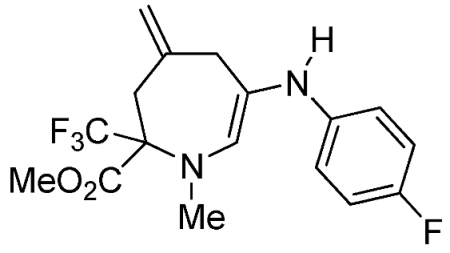
- Methyl 1-methyl-4-methylene-2-(trifluoromethyl)-6-(3-(trifluoromethyl)phenylamino)-2,3,4,5-tetrahydro-1H-azepine-2-carboxylate (3i)
- Methyl 1-methyl-4-methylene-6-(piperidin-1-yl)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3j)

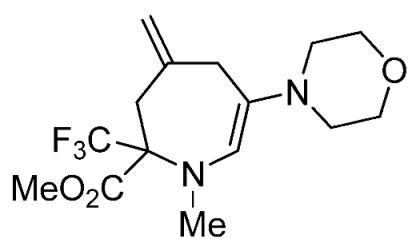
- Methyl 1-methyl-4-methylene-6-morpholino-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3k)
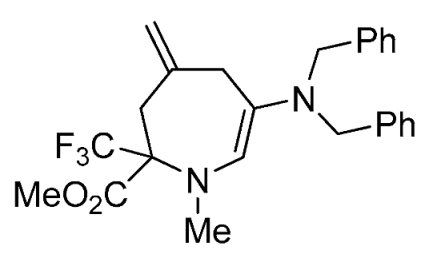
- Methyl 6-(dibenzylamino)-1-methyl-4-methylene-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepine-2-carboxylate (3l)
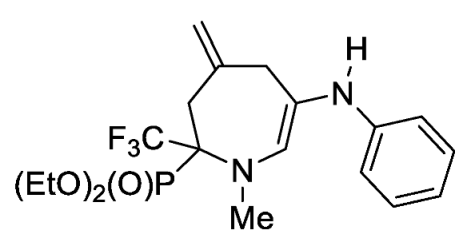
- Diethyl 1-methyl-4-methylene-6-(phenylamino)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepin-2-ylphosphonate (4a)
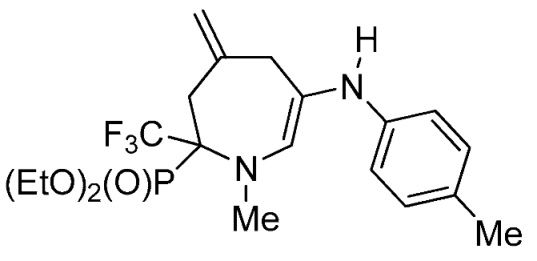
- Diethyl 1-methyl-4-methylene-6-(p-tolylamino)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepin-2-ylphosphonate (4b)
- Diethyl 6-(4-methoxyphenylamino)-1-methyl-4-methylene-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepin-2-ylphosphonate (4c)

- Diethyl 1-methyl-4-methylene-6-(piperidin-1-yl)-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepin-2-ylphosphonate (4d)

- Diethyl 1-methyl-4-methylene-6-morpholino-2-(trifluoromethyl)-2,3,4,7-tetrahydro-1H-azepin-2-ylphosphonate (4e)
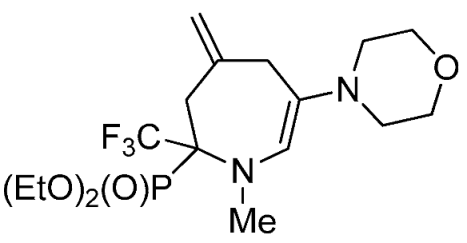
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Heathcock, C.H.; Blumenkopf, T.A.; Smith, K.M. Total Synthesis of (±)-Fawcettimine. J. Org. Chem. 1989, 54, 1548–1562. [Google Scholar] [CrossRef]
- Proctor, G.R.; Redpath, J. Monocyclic Azepines: The Synthesis and Chemical Properties of the Monocyclic Azepines; Wiley: New York, NY, USA; Chichester, UK, 1996. [Google Scholar]
- Fürstner, A.; Thiel, O.R. Formal Total Synthesis of (-)-Balanol: Concise Approach to the Hexahydroazepine Segment Based on RCM. J. Org. Chem. 2000, 65, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Heck, H.A.; Buttrill, S.E., Jr.; Flynn, N.W.; Dyer, R.L.; Anbar, M.; Cairns, T.; Dighe, S.; Cabana, B.E. Bioavailability of imipramine tablets relative to a stable isotope-labeled internal standard: Increasing the power of bioavailability tests. J. Pharmacokinet. Biopharm. 1979, 7, 233–248. [Google Scholar] [CrossRef]
- Hou, F.F.; Zhang, X.; Zhang, G.H.; Xie, D.; Chen, P.Y.; Zhang, W.R.; Jiang, J.P.; Liang, M.; Wang, G.B.; Liu, Z.R.; et al. Efficacy and Safety of Benazepril for Advanced Chronic Renal Insufficiency. N. Engl. J. Med. 2006, 354, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Bruce, T.O.; Masand, P. Review of olanzapine in the management of bipolar disorders. Neuropsychiatr. Dis. Treat. 2007, 3, 579–587. [Google Scholar]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Zha, G.-F.; Rakesh, K.; Manukumar, H.; Shantharam, C.; Long, S. Pharmaceutical significance of azepane based motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 465–494. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, M.L. Catalytic mechanism of enzymic glycosyl transfer. Chem. Rev. 1990, 90, 1171–1202. [Google Scholar] [CrossRef]
- Gura, T. Uncoupling Proteins Provide New Clue to Obesity’s Causes. Science 1998, 280, 1369–1370. [Google Scholar] [CrossRef]
- Li, H.Q.; Zhang, Y.M.; Vogel, P.; Sinay, P.; Bleriot, Y. Tandem Staudinger-azaWittig mediated ring expansion: Rapid access to new isofagomine-tetrahydroxyazepane hybrids. Chem. Commun. 2007, 183–185. [Google Scholar] [CrossRef]
- Sazak, V.W.; Ordovas, J.M.; Elbein, A.D.; Berninger, R.W. Castanospermine inhibits glucosidase I and glycoprotein secretion in human hepatoma cells. Biochem. J. 1985, 232, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Woynaroska, B.; Wilkiel, H.; Sharma, M.; Carpenter, N.; Fleet, G.W.; Bernacki, R.J. Inhibition of human ovarian carcinoma cell- and hexosaminidase-mediated degradation of extracellular matrix by sugar analogs. Anticancer Res. 1992, 12, 161–166. [Google Scholar]
- Winchester, B.; Fleet, G.W.J. Amino-sugar glycosidase inhibitors: Versatile tools for glycobiologists. Glycobiology 1992, 2, 199–210. [Google Scholar] [CrossRef]
- Cai, J.; Davison, B.E.; Ganellin, C.R.; Thaisrivongs, S.; Wibley, K.S. Potential HIV protease inhibitors: Preparation of di-N-alkylated 2-, 6-, and 2,6-aminodeoxy-derivatives of d-glucose by direct displacement and by a novel reductive-alkylation procedure. Carbohydr. Res. 1997, 300, 109–117. [Google Scholar] [CrossRef]
- Greimel, P.; Spreitz, J.; Stütz, A.E.; Wrodnigg, T.M. Iminosugars and Relatives as Antiviral and Potential Anti-infective Agents. Curr. Top. Med. Chem. 2003, 3, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Blériot, Y.; Chantereau, C.; Mallet, J.-M.; Sollogoub, M.; Zhang, Y.; Rodríguez-García, E.; Vogel, P.; Jiménez-Barbero, J.; Sinay, P. The first synthesis of substituted azepanes mimicking monosaccharides: A new class of potent glycosidase inhibitors. Org. Biomol. Chem. 2004, 2, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Kulanthaivel, P.; Hallock, Y.F.; Boros, C.; Hamilton, S.M.; Janzen, W.P.; Ballas, L.M.; Loomis, C.R.; Jiang, J.B.; Katz, B. Balanol: A novel and potent inhibitor of protein kinase C from the fungus Verticillium balanoides. J. Am. Chem. Soc. 1993, 115, 6452–6453. [Google Scholar] [CrossRef]
- Cini, E.; Bifulco, G.; Menchi, G.; Rodriquez, M.; Taddei, M. Synthesis of Enantiopure 7-Substituted Azepane-2-carboxylic Acids as Templates for Conformationally Constrained Peptidomimetics. Eur. J. Org. Chem. 2012, 2012, 2133–2141. [Google Scholar] [CrossRef]
- Zhou, J.; Yeung, Y.-Y. N-Bromosuccinimide-Induced Aminocyclization-Aziridine Ring-Expansion Cascade: An Asymmetric and Highly Stereoselective Approach toward the Synthesis of Azepane. Org. Lett. 2014, 16, 2134–2137. [Google Scholar] [CrossRef] [PubMed]
- René, O.; Stepek, I.A.; Gobbi, A.; Fauber, B.P.; Gaines, S. Palladium-Catalyzed Ring Expansion of Spirocyclopropanes to Form Caprolactams and Azepanes. J. Org. Chem. 2015, 80, 10218–10225. [Google Scholar] [CrossRef]
- Nortcliffe, A.; Moody, C.J. Seven-membered ring scaffolds for drug discovery: Access to functionalised azepanes and oxepanes through diazocarbonyl chemistry. Bioorganic Med. Chem. 2015, 23, 2730–2735. [Google Scholar] [CrossRef]
- Barbero, A.; Diez-Varga, A.; Pulido, F.J.; González-Ortega, X. Synthesis of Azepane Derivatives by Silyl-aza-Prins Cyclization of Allylsilyl Amines: Influence of the Catalyst in the Outcome of the Reaction. Org. Lett. 2016, 18, 1972–1975. [Google Scholar] [CrossRef]
- Drouillat, B.; Dorogan, I.V.; Kletskii, M.; Burov, O.N.; Couty, F. Competitive Ring Expansion of Azetidines into Pyrrolidines and/or Azepanes. J. Org. Chem. 2016, 81, 6677–6685. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kattanguru, P.; Tomashenko, O.A.; Karpowicz, R.; Siemiaszko, G.; Bhattacharya, A.; Calasans, V.; Six, Y. Synthesis of functionalised azepanes and piperidines from bicyclic halogenated aminocyclopropane derivatives. Org. Biomol. Chem. 2017, 15, 5364–5372. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Javed, S.; Noreen, R.; Huma, T.; Iqbal, S.; Umbreen, H.; Gulzar, T.; Farooq, T. Facile and Green Synthesis of Saturated Cyclic Amines. Molecules 2017, 22, 1691. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yadav, N.N.; Ha, H.-J. Preparation of a Stable Bicyclic Aziridinium Ion and Its Ring Expansion toward Piperidines and Azepanes. Asian J. Org. Chem. 2017, 6, 1292–1307. [Google Scholar] [CrossRef]
- Masson, G.; Rioton, S.; Pardo, D.G.; Cossy, J. Access to Enantio-enriched Substituted α-Trifluoromethyl Azepanes from L-Proline. Org. Lett. 2018, 20, 5019–5022. [Google Scholar] [CrossRef]
- Dupas, A.; Lhotellier, P.-A.; Guillamot, G.; Meyer, C.; Cossy, J. Synthesis of Highly Substituted Azepanones from 2H-Azirines by a Stepwise Annulation/Ring-Opening Sequence. Org. Lett. 2019, 21, 3589–3593. [Google Scholar] [CrossRef]
- Nash, A.; Soheili, A.; Tambar, U.K. Stereoselective Synthesis of Functionalized Cyclic Amino Acid Derivatives via a [2,3]-Stevens Rearrangement and Ring-Closing Metathesis. Org. Lett. 2013, 15, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Curto, J.M.; Kozlowski, M.C. α-Allyl-α-aryl α-Amino Esters in the Asymmetric Synthesis of Acyclic and Cyclic Amino Acid Derivatives by Alkene Metathesis. J. Org. Chem. 2014, 79, 5359–5364. [Google Scholar] [CrossRef] [PubMed]
- Casadei, M.A.; Galli, C.; Mandolini, L. Ring-closure reactions. 22. Kinetics of cyclization of diethyl (ω-bromoalkyl)malonates in the range of 4- to 21-membered rings. Role of ring strain. J. Am. Chem. Soc. 1984, 106, 1051–1056. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I. Exploration of fluorine chemistry at the multidisciplinary interface of chemistry and biology. J. Org. Chem. 2013, 78, 6358–6383. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N.A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Han, J.; White, S.; Graham, D.J.; Izawa, K.; Sato, T.; Fustero, S.; Meanwell, N.A.; Soloshonok, V.A. Tailor-made amino acids and fluorinated motifs as prominent traits in modern pharmaceuticals. Chem. Eur. J. 2020, 26, 11349–11390. [Google Scholar] [CrossRef]
- Smits, R.; Cadicamo, C.D.; Burger, K.; Koksch, B. Synthetic strategies to α-trifluoromethyl and α-difluoromethyl substituted α-amino acids. Chem. Soc. Rev. 2008, 37, 1727. [Google Scholar] [CrossRef] [PubMed]
- Moschner, J.; Stulberg, V.; Fernandes, R.; Huhmann, S.; Leppkes, J.; Koksch, B. Approaches to Obtaining Fluorinated α-Amino Acids. Chem. Rev. 2019, 119, 10718. [Google Scholar] [CrossRef]
- Mykhailiuk, P.K. Fluorine-Containing Prolines: Synthetic Strategies, Applications, and Opportunities. J. Org. Chem. 2022, 87, 6961–7005. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Mailyan, A.K.; Peregudov, A.S.; Karimova, N.M.; Vasilyeva, T.P.; Bushmarinov, I.S.; Bruneau, C.; Dixneuf, P.H.; Osipov, S.N. Synthesis of functionalized CF3-containing heterocycles via [2,3]-sigmatropic rearrangement and sequential catalytic carbocyclization. Tetrahedron 2011, 67, 3524–3532. [Google Scholar] [CrossRef]
- Philippova, A.N.; Vorobyeva, D.V.; Monnier, F.; Osipov, S.N. Synthesis of α-CF3-substituted E-dehydroornithine derivatives via copper(I)-catalyzed hydroamination of allenes. Org. Biomol. Chem. 2020, 18, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, D.V.; Philippova, A.N.; Gribanov, P.S.; Nefedov, S.E.; Novikov, V.V.; Osipov, S.N. Ruthenium-catalyzed dimerization of CF3-containing functional allenes. J. Organometallic Chem. 2021, 951, 121998. [Google Scholar] [CrossRef]
- Mailyan, A.K.; Peregudov, A.S.; Dixneuf, P.H.; Bruneau, C.; Osipov, S.N. Cyclobutene Ring-Opening of Bicyclo[4.2.0]octa-1,6-dienes: Access to CF3-Substituted 5,6,7,8-Tetrahydro-1,7-naphthyridines. J. Org. Chem. 2012, 77, 8518–8526. [Google Scholar] [CrossRef] [PubMed]
- Mailyan, A.K.; Krylov, I.M.; Bruneau, C.; Dixneuf, P.H.; Osipov, S.N. Thermal [2+2] Cycloaddition of CF3-Substituted Allenynes: Access to Novel Cyclobutene-Containing α-Amino Acids. Synlett 2011, 16, 2321–2324. [Google Scholar] [CrossRef]
- Eckert, M.; Monnier, F.; Shchetnikov, G.T.; Titanyuk, I.D.; Osipov, S.N.; Toupet, L.; Dérien, S.; Dixneuf, P.H. Dixneuf, Tandem Catalytic Carbene Addition/Bicyclization of Enynes. One-Step Synthesis of Fluorinated Bicyclic Amino Esters by Ruthenium Catalysis. Org. Lett. 2005, 7, 3741–3743. [Google Scholar] [CrossRef]
- Shchetnikov, G.T.; Peregudov, A.S.; Osipov, S.N. Effective Pathway to the α-CF3-Substituted Azahistidine Analogues. Synlett 2007, 2007, 136–140. [Google Scholar] [CrossRef]
- Shchetnikov, G.T.; Osipov, S.N.; Bruneau, C.; Dixneuf, P.H. Ruthenium-Catalyzed Cyclotrimerization of 1,6- and 1,7-Azadiynes: New Access to Fluorinated Bicyclic Amino Acids. Synlett 2008, 2008, 578–582. [Google Scholar] [CrossRef]
- Eckert, M.; Moulin, S.; Monnier, F.; Titanyuk, I.D.; Osipov, S.N.; Roisnel, T.; Derien, S.; Dixneuf, P.H. Ruthenium-Catalysed Synthesis of Fluorinated Bicyclic Amino Esters through Tandem Carbene Addition/Cyclopropanation of Enynes. Chem. Eur. J. 2011, 17, 9457–9462. [Google Scholar] [CrossRef]
- Mailyan, A.K.; Krylov, I.M.; Bruneau, C.; Dixneuf, P.H.; Osipov, S.N. Access to Cyclic α-CF3-Substituted α-Amino Acid Derivatives by Ring-Closing Metathesis of Functionalized 1,7-Enynes. Eur. J. Org. Chem. 2013, 2013, 5353–5363. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Peregudov, A.S.; Röschenthaler, G.-V.; Osipov, S.N. Synthesis of α-CF3-containing triazolyl amino acids as potential neurotransmitters via click-reaction. J. Fluor. Chem. 2015, 175, 60–67. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Petropavlovskikh, D.A.; Godovikov, I.A.; Nefedov, S.E.; Osipov, S.N. Rh(III)-Catalyzed C-H Activation/Annulation of Aryl Hydroxamates with CF3-Containing α-Propargyl α-Amino Acid Derivatives. Eur. J. Org. Chem. 2021, 2021, 1883–1890. [Google Scholar] [CrossRef]
- Petropavlovskikh, D.A.; Vorobyeva, D.V.; Godovikov, I.A.; Nefedov, S.E.; Filippov, O.A.; Osipov, S.N. Lossen rearrangement by Rh(III)-catalyzed C-H activation/annulation of aryl hydroxamates with alkynes: Access to quinolone-containing amino acid derivatives. Org. Biomol. Chem. 2021, 19, 9421–9426. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective Ligation of Azides and Terminal Alkynes. Angew. Chem. 2002, 41, 2708–2711. [Google Scholar] [CrossRef]
- Yoo, E.J.; Ahlquist, M.; Bae, I.; Sharpless, K.B.; Fokin, V.V.; Chang, S. Mechanistic Studies on the Cu-Catalyzed Three-Component Reactions of Sulfonyl Azides, 1-Alkynes and Amines, Alcohols, or Water: Dichotomy via a Common Pathway. J. Org. Chem. 2008, 73, 5520–5528. [Google Scholar] [CrossRef]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Amino-Phosphinic Acids—Chemistry and Biological Activity; Wiley: Chichester, UK, 2000; pp. 1–660. [Google Scholar]
- Romanenko, V.D.; Kukhar, V.P. Fluorinated Phosphonates: Synthesis and Biomedical Application. Chem. Rev. 2006, 106, 3868–3935. [Google Scholar] [CrossRef]
- Ordonez, M.; Sayago, F.J.; Cativiela, C. Synthesis of quaternary α-aminophosphonic acids. Tetrahedron 2012, 68, 6369–6412. [Google Scholar] [CrossRef]
- Naydenova, E.D.; Todorov, P.T.; Troev, K.D. Recent synthesis of aminophosphonic acids as potential biological importance. Amino Acids 2010, 38, 23–30. [Google Scholar] [CrossRef]
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic acids and derivatives. Synthesis and biological applications. Curr. Med. Chem. 2010, 17, 264–289. [Google Scholar] [CrossRef]
- Bhagat, S.; Shah, P.; Garg, S.K.; Mishra, S.P.; Kaur, K.; Singh, S.; Chakraborti, A.K. α-Aminophosphonates as novel anti-leishmanial chemotypes: Synthesis, biological evaluation, and CoMFA studies. MedChemComm 2014, 5, 665–670. [Google Scholar] [CrossRef]
- Ramírez-Marroquín, O.A.; Romero-Estudillo, I.; Viveros-Ceballos, J.L.; Cativiela, C.; Ordóñez, M. Convenient Synthesis of Cyclic α-Aminophosphonates by Alkylation-Cyclization Reaction of Iminophosphoglycinates Using Phase-Transfer Catalysis. Eur. J. Org. Chem. 2016, 2016, 308–313. [Google Scholar] [CrossRef]



| Entry | Amine (Equiv.) | Catalyst (mol%) | Solv./Temp. (°C) | Time (h) | Yield 2 (%) |
|---|---|---|---|---|---|
| 1 | 2.0 | Cu(MeCN)4PF6 (10) | dioxane/90 | 8 | 65 (43 3) |
| 2 | 2.0 | Cu(MeCN)4PF6 (10) | dioxane/90 | 16 | 60 |
| 3 | 2.0 | Cu(MeCN)4PF6 (5) | dioxane/90 | 8 | 35 |
| 4 | 1.5 | Cu(MeCN)4PF6 (10) | dioxane/80 | 8 | 77 |
| 5 | 1.5 | Cu(MeCN)4PF6 (10) | DCE/80 | 16 | 35 |
| 6 | 1.5 | Cu(MeCN)4PF6 (10) | toluene/80 | 16 | 43 |
| 7 | 1.5 | Cu(MeCN)4PF6 (10) | THF/70 | 16 | 75 |
| 8 | 2.0 | CuI (10) | dioxane/90 | 8 | NR |
| 9 | 2.0 | CuCl (10) | dioxane/90 | 8 | NR |
| 10 | 1.2 | Cu(MeCN)4PF6 (10) | dioxane/70 | 6 | 91 (65 3) |
| 11 | 2.0 | - | dioxane/90 | 16 | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippova, A.N.; Vorobyeva, D.V.; Gribanov, P.S.; Godovikov, I.A.; Osipov, S.N. Synthesis of Functionalized Azepines via Cu(I)-Catalyzed Tandem Amination/Cyclization Reaction of Fluorinated Allenynes. Molecules 2022, 27, 5195. https://doi.org/10.3390/molecules27165195
Philippova AN, Vorobyeva DV, Gribanov PS, Godovikov IA, Osipov SN. Synthesis of Functionalized Azepines via Cu(I)-Catalyzed Tandem Amination/Cyclization Reaction of Fluorinated Allenynes. Molecules. 2022; 27(16):5195. https://doi.org/10.3390/molecules27165195
Chicago/Turabian StylePhilippova, Anna N., Daria V. Vorobyeva, Pavel S. Gribanov, Ivan A. Godovikov, and Sergey N. Osipov. 2022. "Synthesis of Functionalized Azepines via Cu(I)-Catalyzed Tandem Amination/Cyclization Reaction of Fluorinated Allenynes" Molecules 27, no. 16: 5195. https://doi.org/10.3390/molecules27165195
APA StylePhilippova, A. N., Vorobyeva, D. V., Gribanov, P. S., Godovikov, I. A., & Osipov, S. N. (2022). Synthesis of Functionalized Azepines via Cu(I)-Catalyzed Tandem Amination/Cyclization Reaction of Fluorinated Allenynes. Molecules, 27(16), 5195. https://doi.org/10.3390/molecules27165195






