Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells
Abstract
:1. Introduction
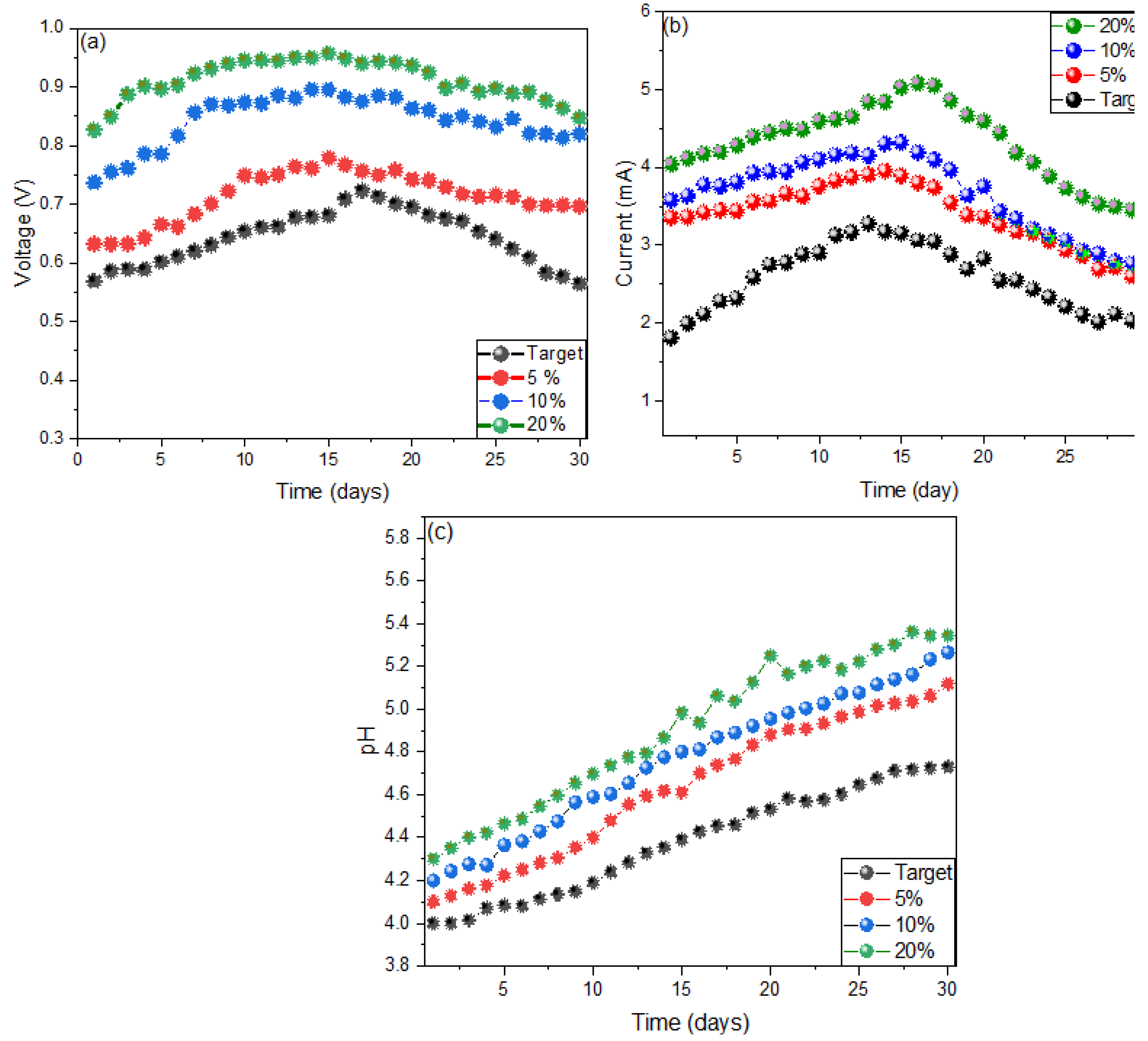
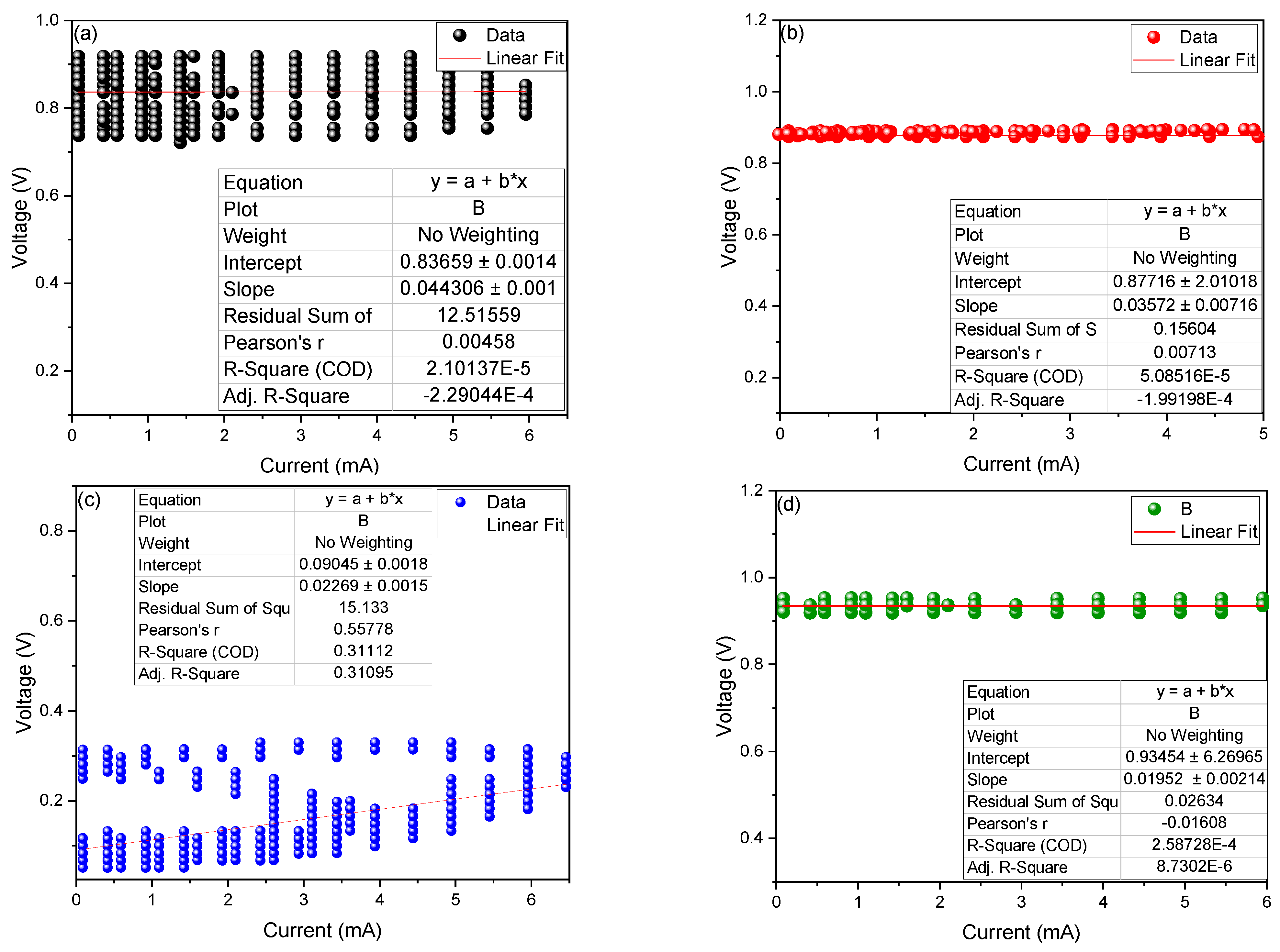
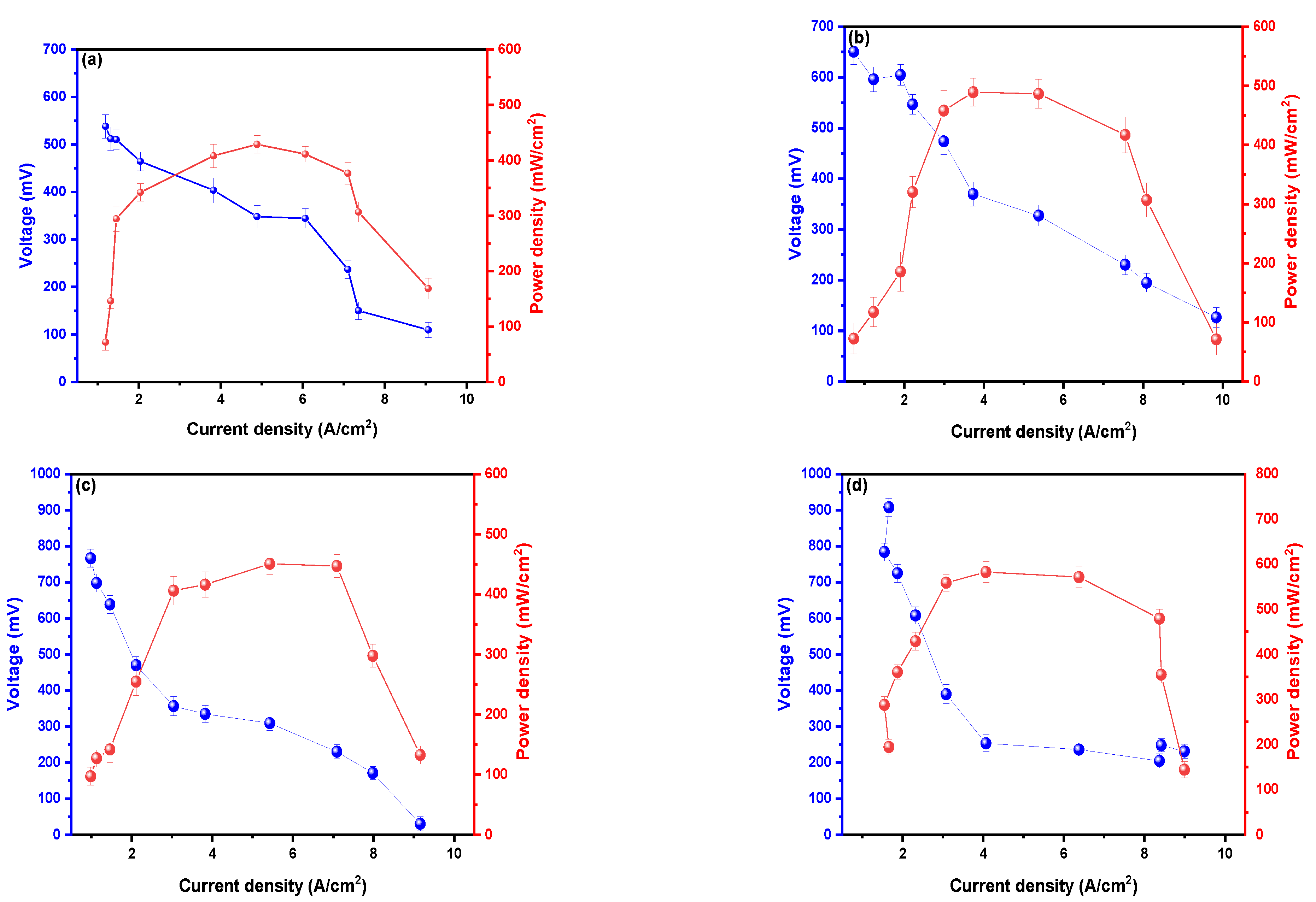
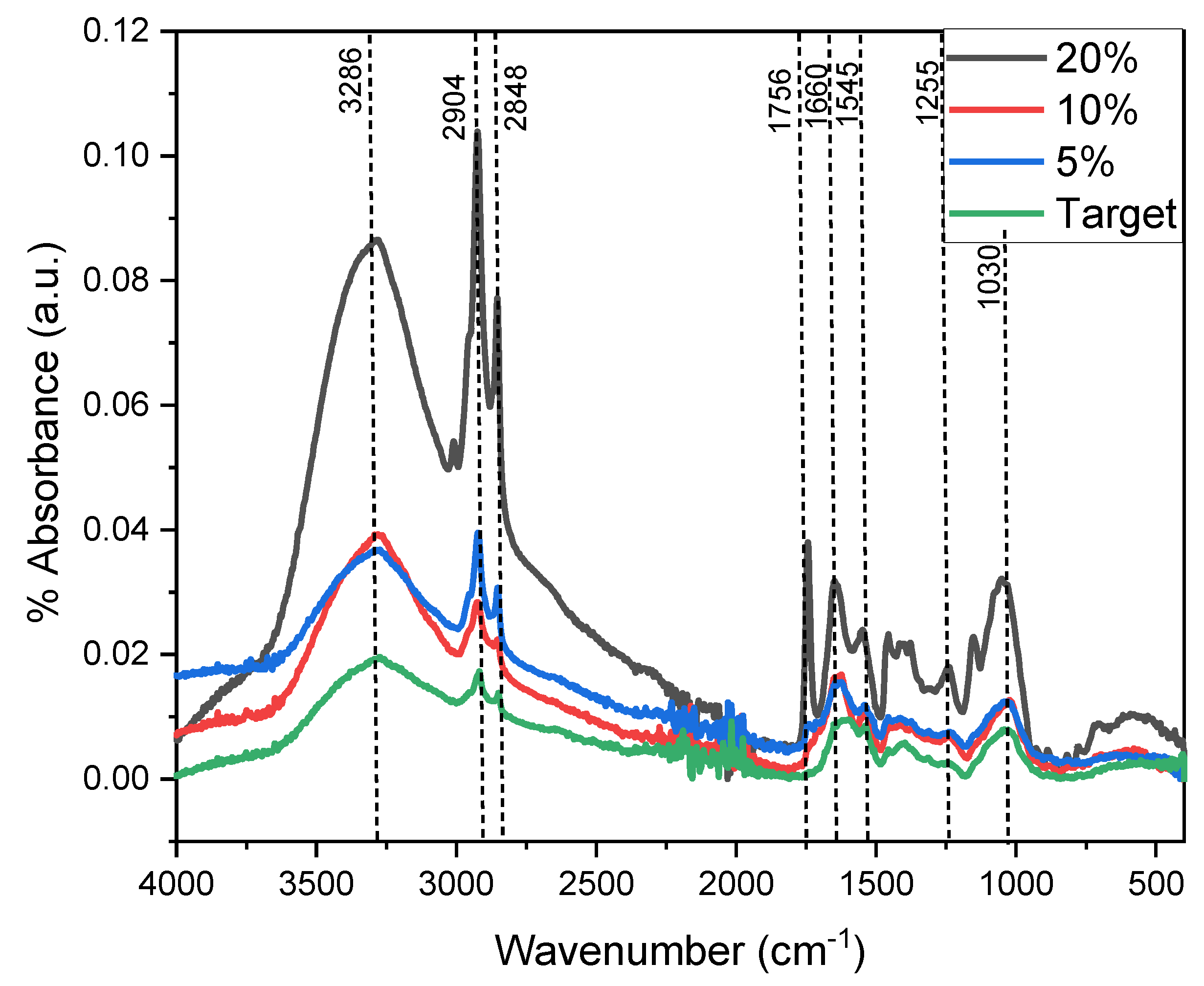
2. Results and Discussion
3. Materials and Methods
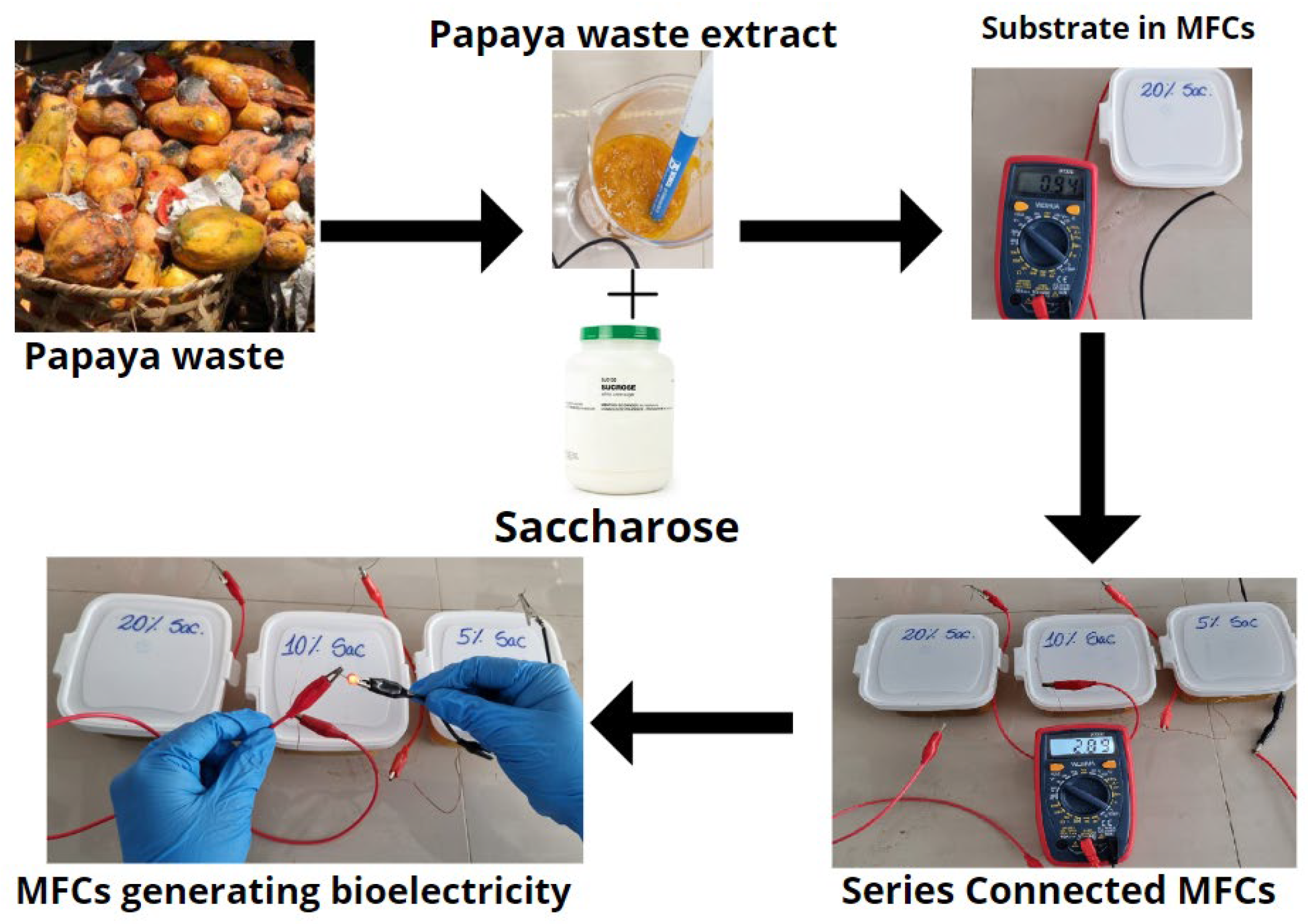
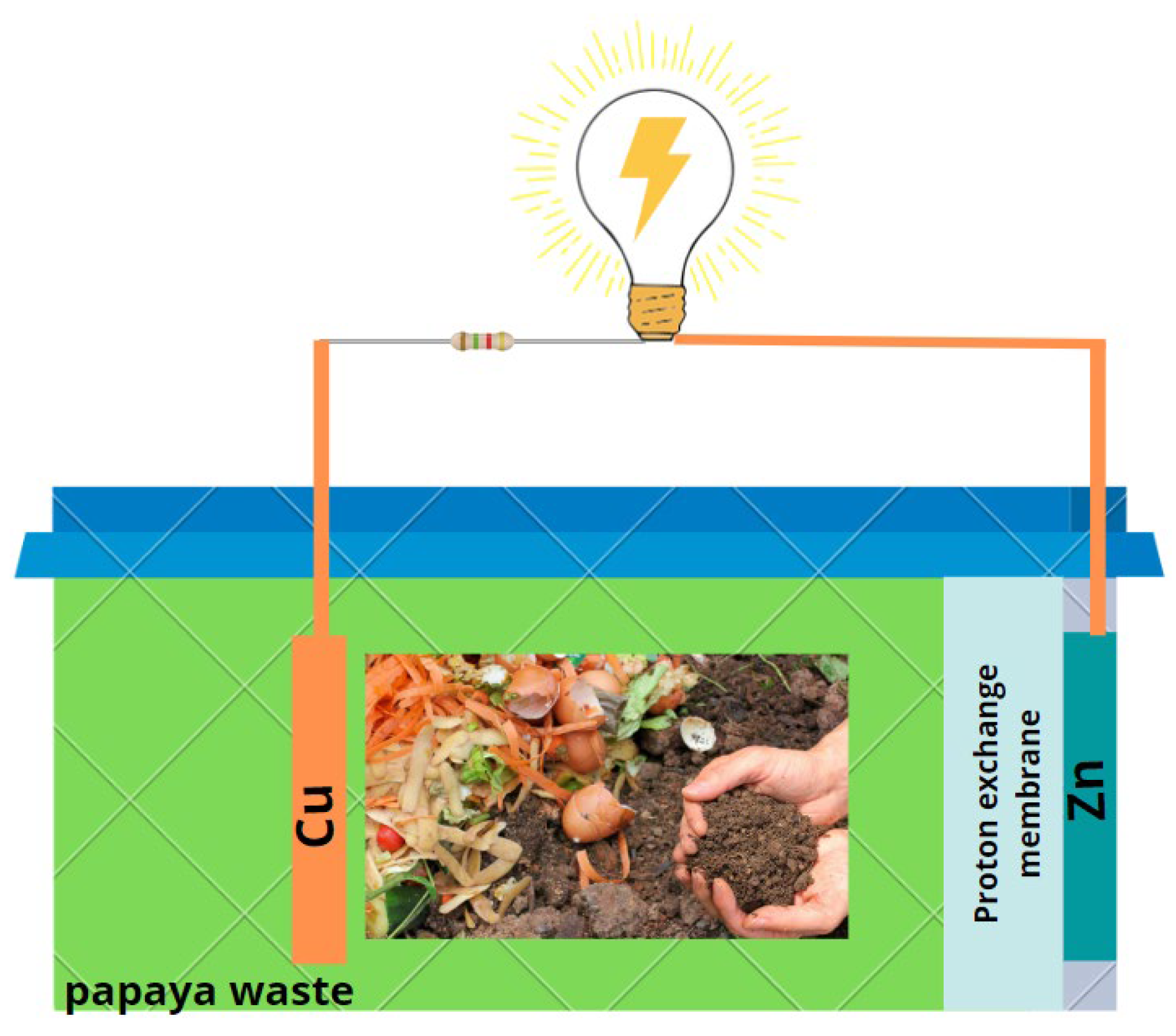
3.1. Fabrication of Single-Chamber Microbial Fuel Cells
3.2. Collection of Papaya Waste
3.3. Characterization of Microbial Fuel Cells
3.4. Molecular Identification of Microorganisms by Sequencing the 16S rRNA Genes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, B.; Jang, N.; Lee, M.; Jang, J.K.; Chang, I.S. Microbial fuel cell driven mineral rich wastewater treatment process for circular economy by creating virtuous cycles. Bioresour. Technol. 2021, 320, 124254. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Rojas-Villacorta, W.; Romero, C.V. Bioelectricity through microbial fuel cells using avocado waste. Energy Rep. 2022, 8, 376–382. [Google Scholar] [CrossRef]
- Pandit, S.; Savla, N.; Sonawane, J.M.; Sani, A.M.; Gupta, P.K.; Mathuriya, A.S.; Rai, A.K.; Jadhav, D.A.; Jung, S.P.; Prasad, R. Agricultural Waste and Wastewater as Feedstock for Bioelectricity Generation Using Microbial Fuel Cells: Recent Advances. Fermentation 2021, 7, 169. [Google Scholar] [CrossRef]
- Segundo, R.-F.; Magaly, D.L.C.-N.; Benites, S.M.; Daniel, D.-N.; Angelats-Silva, L.; Díaz, F.; Luis, C.-C.; Fernanda, S.-P. Increase in Electrical Parameters Using Sucrose in Tomato Waste. Fermentation 2022, 8, 335. [Google Scholar] [CrossRef]
- Montalván, G.; Lizeth, D. Efecto de los ácidos acético y cítrico para control de antracnosis (Colletotrichum sp.) en poscosecha de papaya (Carica papaya L.). Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecudaor, 2018. [Google Scholar]
- Cornejo-Condori, G.B.; Lima-Medina, I.; Bravo-Portocarrero, R.Y.; Barzola-Tito, K.; Casa-Coila, V.H. Nematodos asociados a la papaya andina (Carica pubescens L.), en el distrito Sandia, Puno, Perú. BIOAGRO 2021, 33, 191–203. [Google Scholar] [CrossRef]
- Kung, C.A.L.L.; Panduro, S.K.D.; Sangama, E.D. Mermelada a base de papaya enriquecida con pulpa de camu camu. J. Agro-Ind. Sci. 2021, 3, 55–61. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Benites, S.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Valdiviezo-Dominguez, F.; Álvarez, M.Q.; Vega-Ybañez, V.; Angelats-Silva, L. Bioelectricity Production from Blueberry Waste. Processes 2021, 9, 1301. [Google Scholar] [CrossRef]
- Ghazali, N.F.; Mahmood, N.A.N.; Abu Bakar, N.F.; Ibrahim, K.A. Temperature dependence of power generation of empty fruit bunch (EFB) based microbial fuel cell. Malays. J. Fundam. Appl. Sci. 2019, 15, 489–491. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Ma, F.; Yin, Y.; Pang, S.; Liu, J.; Chen, W. A Data-Driven Based Framework of Model Optimization and Neural Network Modeling for Microbial Fuel Cells. IEEE Access 2019, 7, 162036–162049. [Google Scholar] [CrossRef]
- Flores, S.R.; Pérez-Delgado, O.; Naveda-Renny, N.; Benites, S.M.; De La Cruz–Noriega, M.; Narciso, D.A.D. Generation of Bioelectricity Using Molasses as Fuel in Microbial Fuel Cells. Environ. Res. Eng. Manag. 2022, 78, 19–27. [Google Scholar] [CrossRef]
- Abbassi, R.; Yadav, A.K. Introduction to microbial fuel cells: Challenges and opportunities. Integr. Microb. Fuel Cells Wastewater Treat. 2020, 2020, 3–27. [Google Scholar] [CrossRef]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A mini review on biofouling on air cathode of single chamber microbial fuel cell; prevention and mitigation strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.-K.; Lee, W. Microbial fuel cells for bioelectricity generation through reduction of hexavalent chromium in wastewater: A review. Int. J. Hydrogen Energy 2021, 46, 11458–11481. [Google Scholar] [CrossRef]
- Prathiba, S.; Kumar, P.S.; Vo, D.-V.N. Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere 2022, 286, 131856. [Google Scholar] [CrossRef]
- Khandaker, S.; Das, S.; Hossain, T.; Islam, A.; Miah, M.R.; Awual, R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation—A comprehensive review. J. Mol. Liq. 2021, 344, 117795. [Google Scholar] [CrossRef]
- Li, F.; An, X.; Wu, D.; Xu, J.; Chen, Y.; Li, W.; Cao, Y.; Guo, X.; Lin, X.; Li, C.; et al. Engineering Microbial Consortia for High-Performance Cellulosic Hydrolyzates-Fed Microbial Fuel Cells. Front. Microbiol. 2019, 10, 409. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D.; Accardo, G.; Yoon, S.P.; Kwon, Y. Optimization of glucose concentration and glucose/yeast ratio in yeast microbial fuel cell using response surface methodology approach. J. Power Sources 2018, 402, 402–412. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Ahmad, A. Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl. Nanosci. 2021, 11, 1949–1961. [Google Scholar] [CrossRef]
- Mbugua, J.K.; Mbui, D.N.; Mwaniki, J.; Mwaura, F.; Sheriff, S. Influence of Substrate Proximate Properties on Voltage Production in Microbial Fuel Cells. J. Sustain. Bioenergy Syst. 2020, 10, 43–51. [Google Scholar] [CrossRef]
- Kalagbor Ihesinachi, A.; Akpotayire Stephen, I. Electricity Generation from Waste Tropical Fruits—Watermelon (Citrullus lanatus) and Paw-paw (Carica papaya) using Single Chamber Microbial Fuel Cells. Int. J. Energy Inf. Commun. 2020, 11, 11–20. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Waste to Wealth: A Case Study of Papaya Peel. Waste Biomass Valorization 2018, 10, 1755–1766. [Google Scholar] [CrossRef]
- Utami, L.; Yenti, E. View of Produksi Energi Listrik dari Limbah Kulit Pepaya (Carica papaya) Menggunakan Teknologi Microbial Fuel Cells. Al-Kimia 2018, 6, 56–62. Available online: https://journal3.uin-alauddin.ac.id/index.php/al-kimia/article/view/4681/pdf (accessed on 21 July 2022).
- Rojas-Flores, S.; Noriega, M.D.L.C.; Benites, S.M.; Gonzales, G.A.; Salinas, A.S.; Palacios, F.S. Generation of bioelectricity from fruit waste. Energy Rep. 2020, 6, 37–42. [Google Scholar] [CrossRef]
- Rojas Flores, S.; Naveda, R.N.; Paredes, E.A.; Orbegoso, J.A.; Céspedes, T.C.; Salvatierra, A.R.; Rodríguez, M.S. Agricultural wastes for electricity generation using microbial fuel cells. Open Biotechnol. J. 2020, 14, 52–58. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 18. [Google Scholar] [CrossRef]
- Yoon, K.-J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; Jimenez-Cyrus, D.; Su, Y.; Kim, N.-S.; Zhu, Y.; Zheng, L.; et al. Temporal Control of Mammalian Cortical Neurogenesis by m6A Methylation. Cell 2017, 171, 877–889.e17. [Google Scholar] [CrossRef]
- Fujimura, S.; Kamitori, K.; Kamei, I.; Nagamine, M.; Miyoshi, K.; Inoue, K. Performance of stacked microbial fuel cells with barley–shochu waste. J. Biosci. Bioeng. 2022, 133, 467–473. [Google Scholar] [CrossRef]
- Buñay, A.; Miguel, L. Modelado y Simulación del Proceso de Generación de Bio-Electricidad en una Celda Microbiana (MFC) con los Sustratos: Glucosa y Lixiviados. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecudaor, 2019. [Google Scholar]
- Santiago, B.; Rojas-Flores, S.; De La Cruz Noriega, M.; Cabanillas-Chirinos, L.; Otiniano, N.M.; Silva-Palacios, F.; Luis, A.S. Bioelectricity from Saccharomyces cerevisiae yeast through low-cost microbial fuel cells. In Proceedings of the 18th LACCEI International Multi-Conference for Engineering, Education, and Technology: Engineering, Integration, and Alliances for a Sustainable Development, Virtual, 27–31 July 2020; pp. 27–31. [Google Scholar]
- Leiva, E.; Leiva-Aravena, E.; Rodríguez, C.; Serrano, J.; Vargas, I. Arsenic removal mediated by acidic pH neutralization and iron precipitation in microbial fuel cells. Sci. Total Environ. 2018, 645, 471–481. [Google Scholar] [CrossRef]
- Pietrelli, A.; Bavasso, I.; Lovecchio, N.; Ferrara, V.; Allard, B. MFCs as biosensor, bioreactor and bioremediator. In Proceedings of the IEEE 8th International Workshop on Advances in Sensors and Interfaces (IWASI), Otranto, Italy, 13–14 June 2019; pp. 302–306. [Google Scholar]
- Ueda, M.; Tojo, S.; Chosa, T.; Uchigasaki, M. Decomposition characteristics of propionate when changing the electrode material, external resistance and reactor temperature of microbial fuel cells. Int. J. Hydrogen Energy 2022, 47, 2783–2793. [Google Scholar] [CrossRef]
- Yang, X.-L.; Wang, Q.; Li, T.; Xu, H.; Song, H.-L. Antibiotic removal and antibiotic resistance genes fate by regulating bioelectrochemical characteristics in microbial fuel cells. Bioresour. Technol. 2022, 348, 126752. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.N.; Hiraman, P.A.; Muthukumar, K.; Jayabalan, T. Bioelectricity production from kitchen wastewater using microbial fuel cell with photosynthetic algal cathode. Bioresour. Technol. 2020, 295, 122226. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, S.; Mohanakrishna, G.; Kumar, A.; Lai, C.; Lee, J.-K.; Kalia, V.C. Exploitation of Citrus Peel Extract as a Feedstock for Power Generation in Microbial Fuel Cell (MFC). Indian J. Microbiol. 2019, 59, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Sharma, N.; Khajuria, Y.; Singh, V.K.; Kumar, S.; Lee, Y.; Rai, P.K.; Singh, V.K. Study of Molecular and Elemental Changes in Nematode-infested Roots in Papaya Plant Using FTIR, LIBS and WDXRF Spectroscopy. At. Spectrosc. 2020, 41, 110–118. [Google Scholar] [CrossRef]
- Ahlawat, J.; Kumar, V.; Gopinath, P. Carica papaya loaded poly (vinyl alcohol)-gelatin nanofibrous scaffold for potential application in wound dressing. Mater. Sci. Eng. C 2019, 103, 109834. [Google Scholar] [CrossRef]
- Kale, R.; Barwar, S.; Kane, P.; More, S. Green synthesis of silver nanoparticles using papaya seed and its characterization. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 168–174. [Google Scholar] [CrossRef]
- Hedbavna, P.; Rolfe, S.A.; Huang, W.E.; Thornton, S.F. Biodegradation of phenolic compounds and their metabolites in contaminated groundwater using microbial fuel cells. Bioresour. Technol. 2016, 200, 426–434. [Google Scholar] [CrossRef]
- Shen, J.; Du, Z.; Li, J.; Cheng, F. Co-metabolism for enhanced phenol degradation and bioelectricity generation in microbial fuel cell. Bioelectrochemistry 2020, 134, 107527. [Google Scholar] [CrossRef]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Tangarife García, N.S. Control Biológico, la Nueva era de la Agricultura; Universidad de Ciencias Aplicadas y Ambientales: Bogota, Colombia, 2021. [Google Scholar]
- Romo, D.M.R.; Gutiérrez, N.H.H.; Pazos, J.O.R.; Figueroa, L.V.P.; Ordóñez, L.A.O. Bacterial diversity in the Cr (VI) reducing biocathode of a Microbial Fuel Cell with salt bridge. Rev. Argent. Microbiol. 2019, 51, 110–118. [Google Scholar]
- Umar, M.F.; Abbas, S.Z.; Ibrahim, M.N.M.; Ismail, N.; Rafatullah, M. Insights into Advancements and Electrons Transfer Mechanisms of Electrogens in Benthic Microbial Fuel Cells. Membranes 2020, 10, 205. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Lam, C.H.; Subramanian, K.; Qin, Z.-H.; Mou, J.-H.; Jin, M.; Chopra, S.S.; Singh, V.; Ok, Y.S.; et al. Emerging waste valorisation techniques to moderate the hazardous impacts, and their path towards sustainability. J. Hazard. Mater. 2022, 423, 127023. [Google Scholar] [CrossRef]
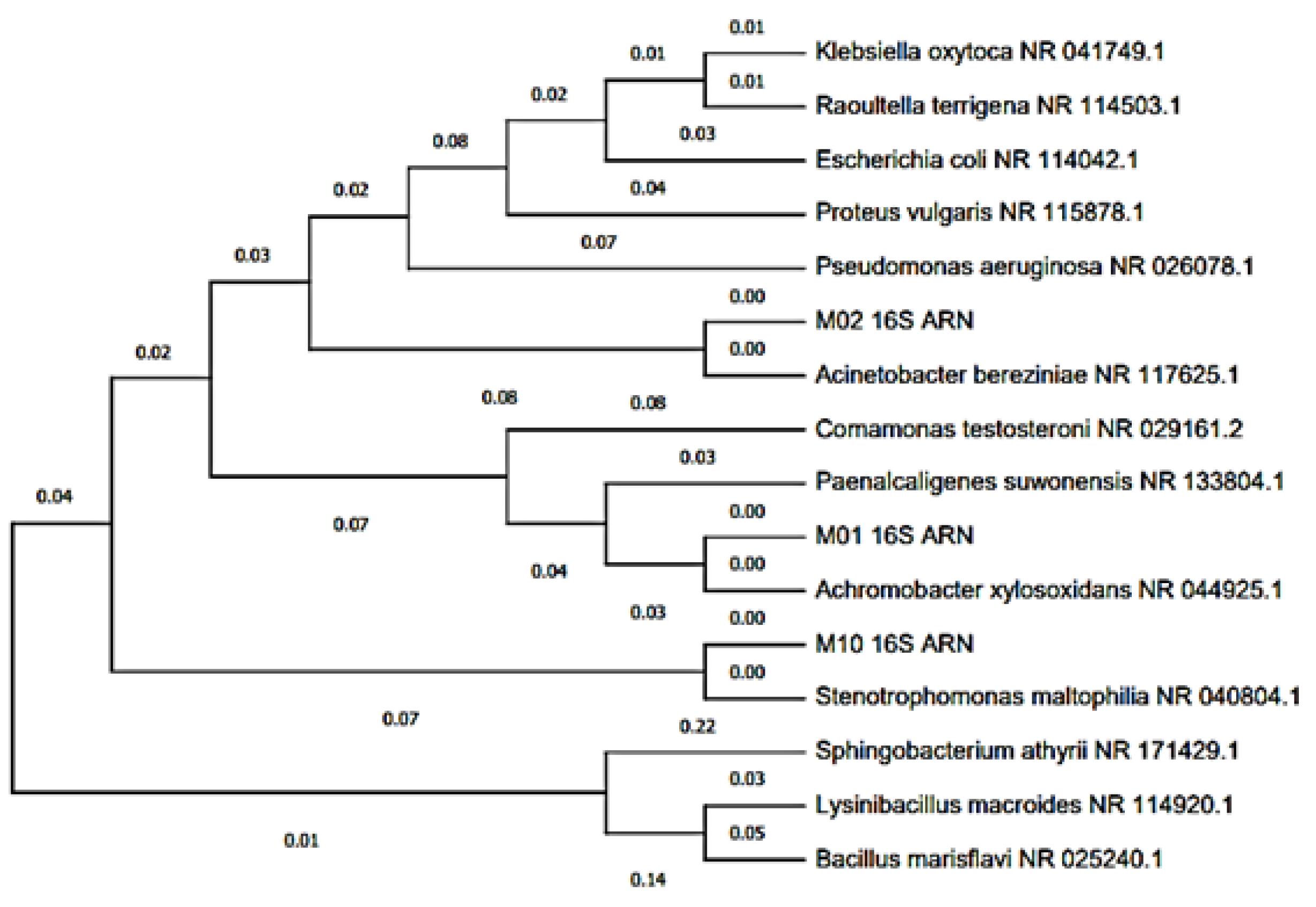
| BLAST Characterization | Consensus Sequence Length (nt) | % Maximum Identity | Accession Number | Phylogeny |
|---|---|---|---|---|
| Achromobacter xylosoxidans | 1451 | 99.32% | CP053617.1 | Cellular organisms; Bacterium; Proteobacteria; Betaproteobacteria; burkholderials; Alcaligenaceae; Achromo-bacter |
| Acinetobacter bereziniae | 1468 | 99.93% | CP018259.1 | Cellular organisms; Bacteria; Proteobacteria; Gammaproteobacteria; Pseudomonadales; Moraxellaceae; Acinetobacter |
| Stenotrophomonas maltophilia | 1477 | 100.00% | NR_041577.1 | Cellular organisms; Bacteria; Proteobacteria; Gammaproteobacteria; Xanthomonadales; Xanthomonadaceae; Stenotrophomonas; Stenotrophomonas maltophilia group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Flores, S.; De La Cruz-Noriega, M.; Benites, S.M.; Delfín-Narciso, D.; Luis, A.-S.; Díaz, F.; Luis, C.-C.; Moises, G.C. Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells. Molecules 2022, 27, 5198. https://doi.org/10.3390/molecules27165198
Rojas-Flores S, De La Cruz-Noriega M, Benites SM, Delfín-Narciso D, Luis A-S, Díaz F, Luis C-C, Moises GC. Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells. Molecules. 2022; 27(16):5198. https://doi.org/10.3390/molecules27165198
Chicago/Turabian StyleRojas-Flores, Segundo, Magaly De La Cruz-Noriega, Santiago M. Benites, Daniel Delfín-Narciso, Angelats-Silva Luis, Felix Díaz, Cabanillas-Chirinos Luis, and Gallozzo Cardenas Moises. 2022. "Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells" Molecules 27, no. 16: 5198. https://doi.org/10.3390/molecules27165198
APA StyleRojas-Flores, S., De La Cruz-Noriega, M., Benites, S. M., Delfín-Narciso, D., Luis, A.-S., Díaz, F., Luis, C.-C., & Moises, G. C. (2022). Electric Current Generation by Increasing Sucrose in Papaya Waste in Microbial Fuel Cells. Molecules, 27(16), 5198. https://doi.org/10.3390/molecules27165198







