Antitumour Effects of Selected Pyridinium Salts on Sensitive Leukaemia HL60 Cells and Their Multidrug Resistant Topoisomerase II-Defective HL60/MX2 Counterparts
Abstract
:1. Introduction
2. Results
2.1. The Ability of MNP and MDION to Inhibit the Growth of Sensitive HL60 and Resistant HL60/MX2 Leukaemia Cells
2.2. The Effect of MNP and MDION on the ROS Level in Sensitive HL60 and Resistant HL60/MX2 Leukaemia Cells
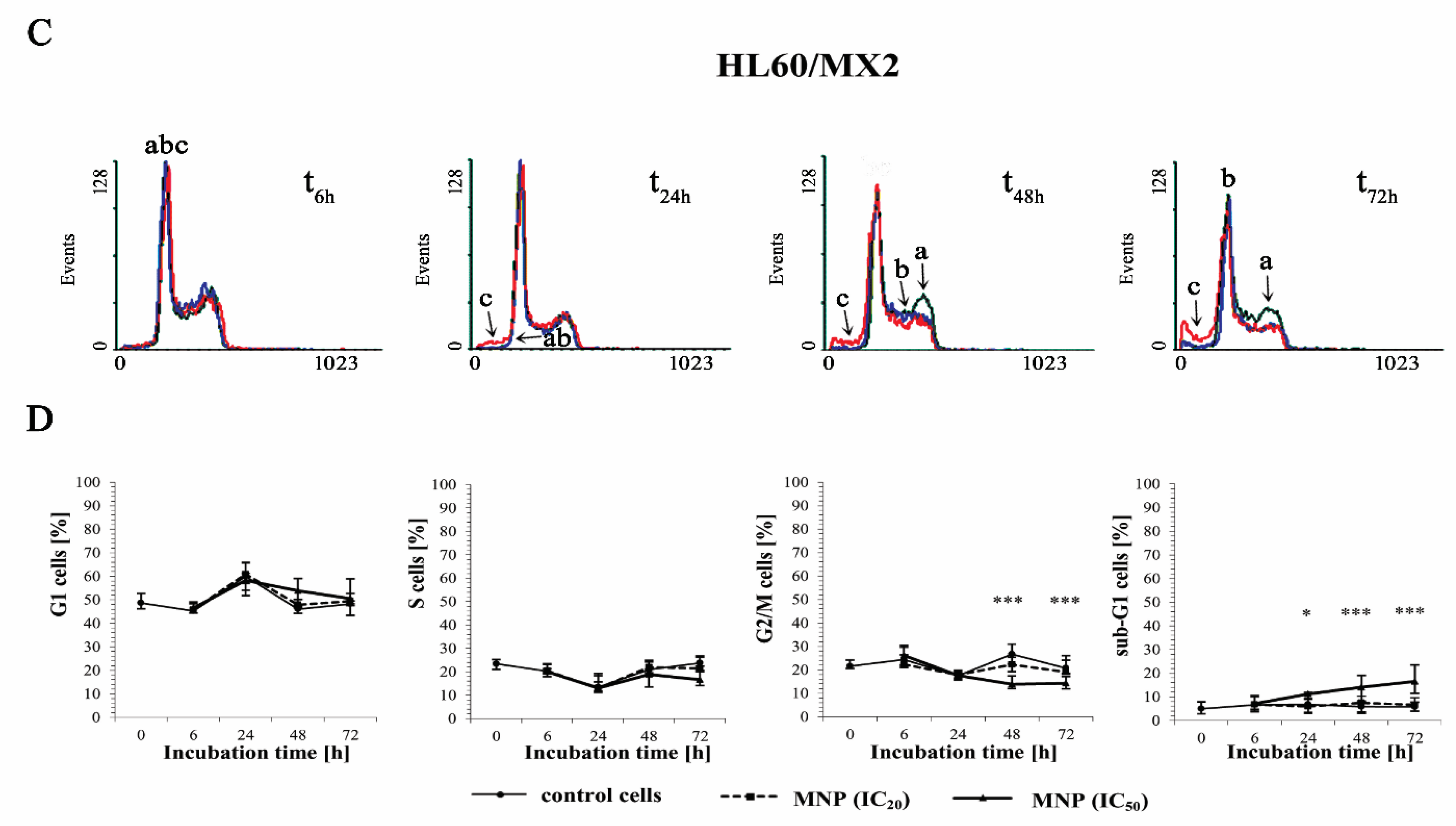
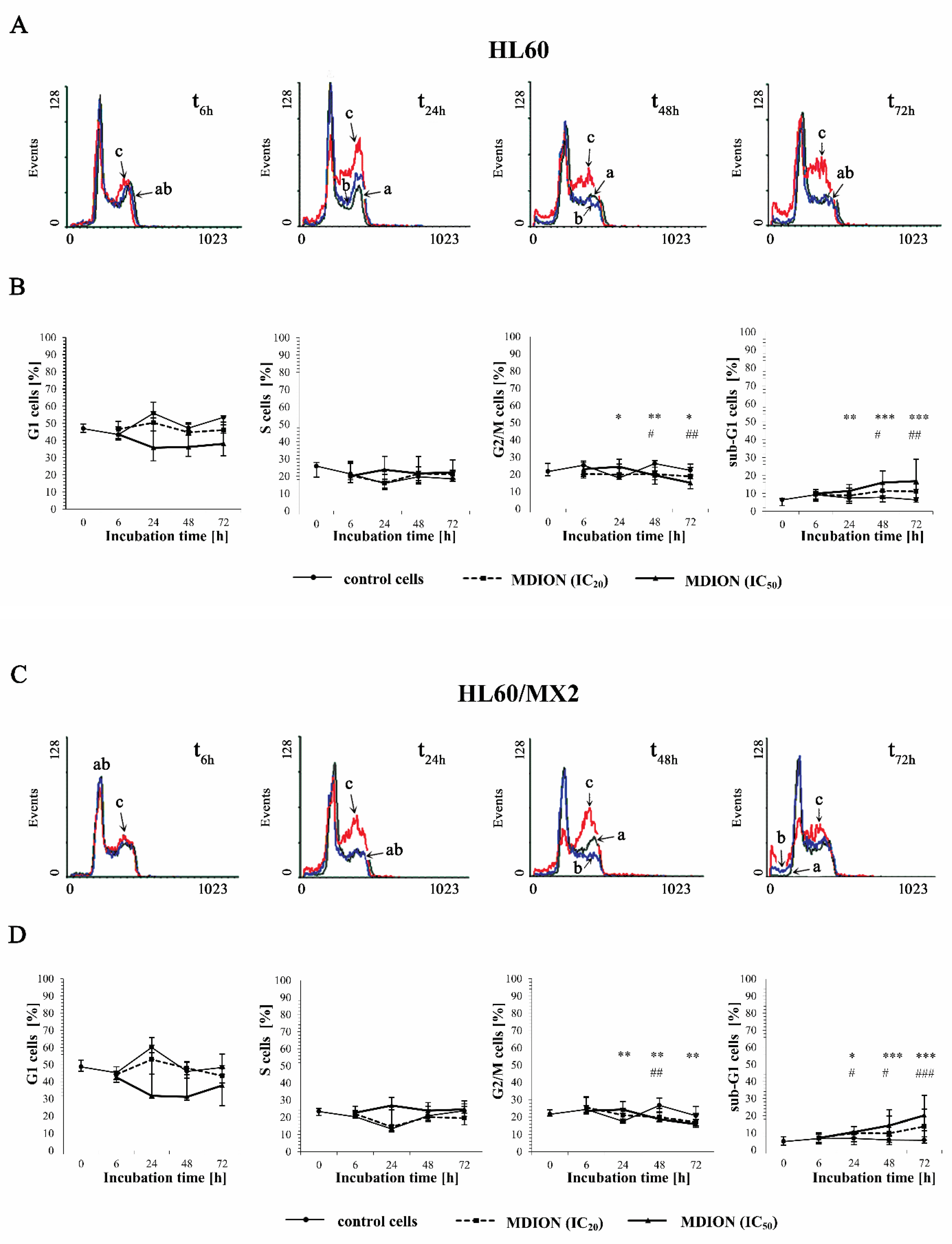
2.3. The Effect of MNP and MDION on Cell Cycle Distribution of Sensitive HL60 and Resistant HL60/MX2 Leukaemia Cells
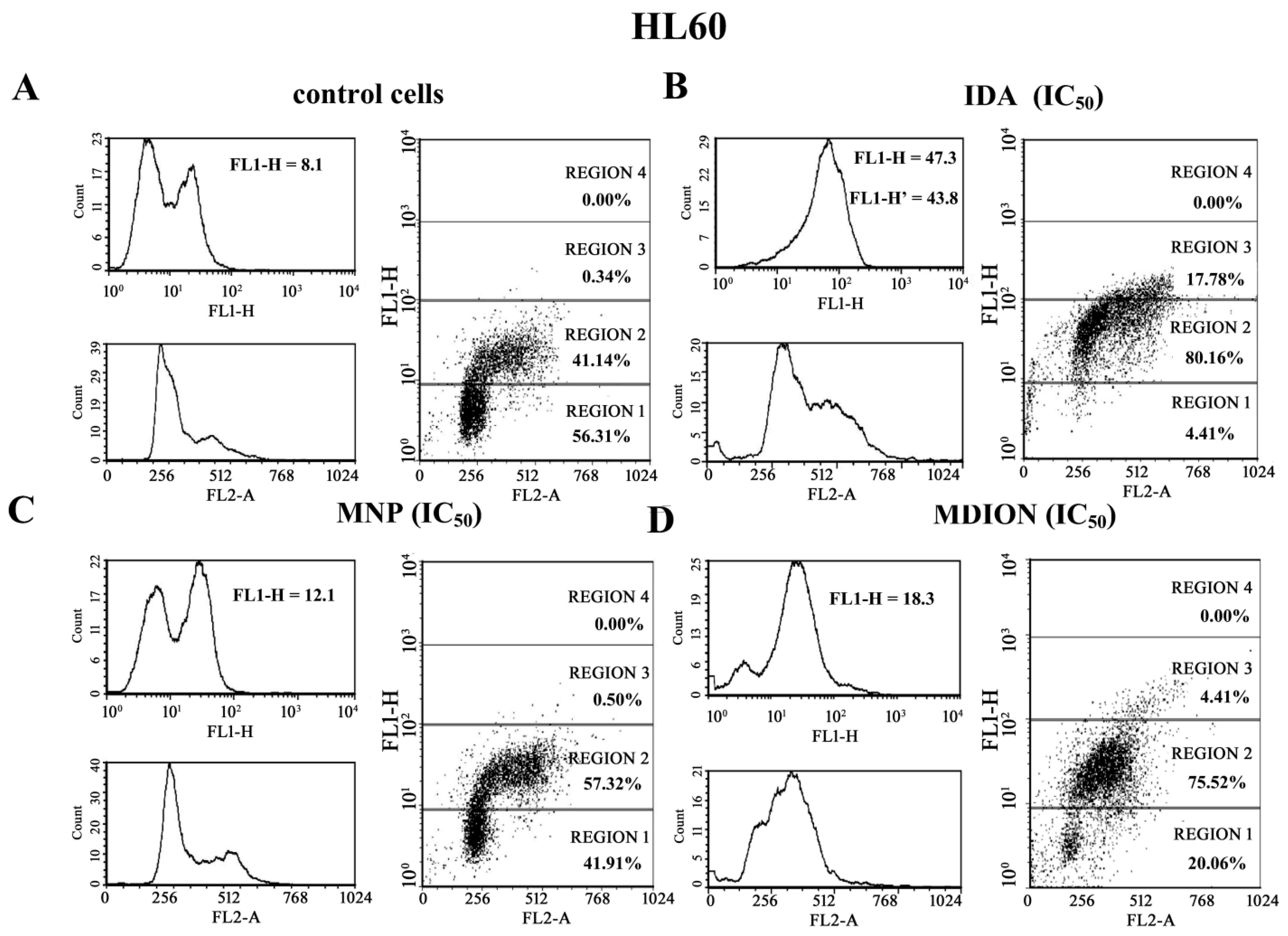
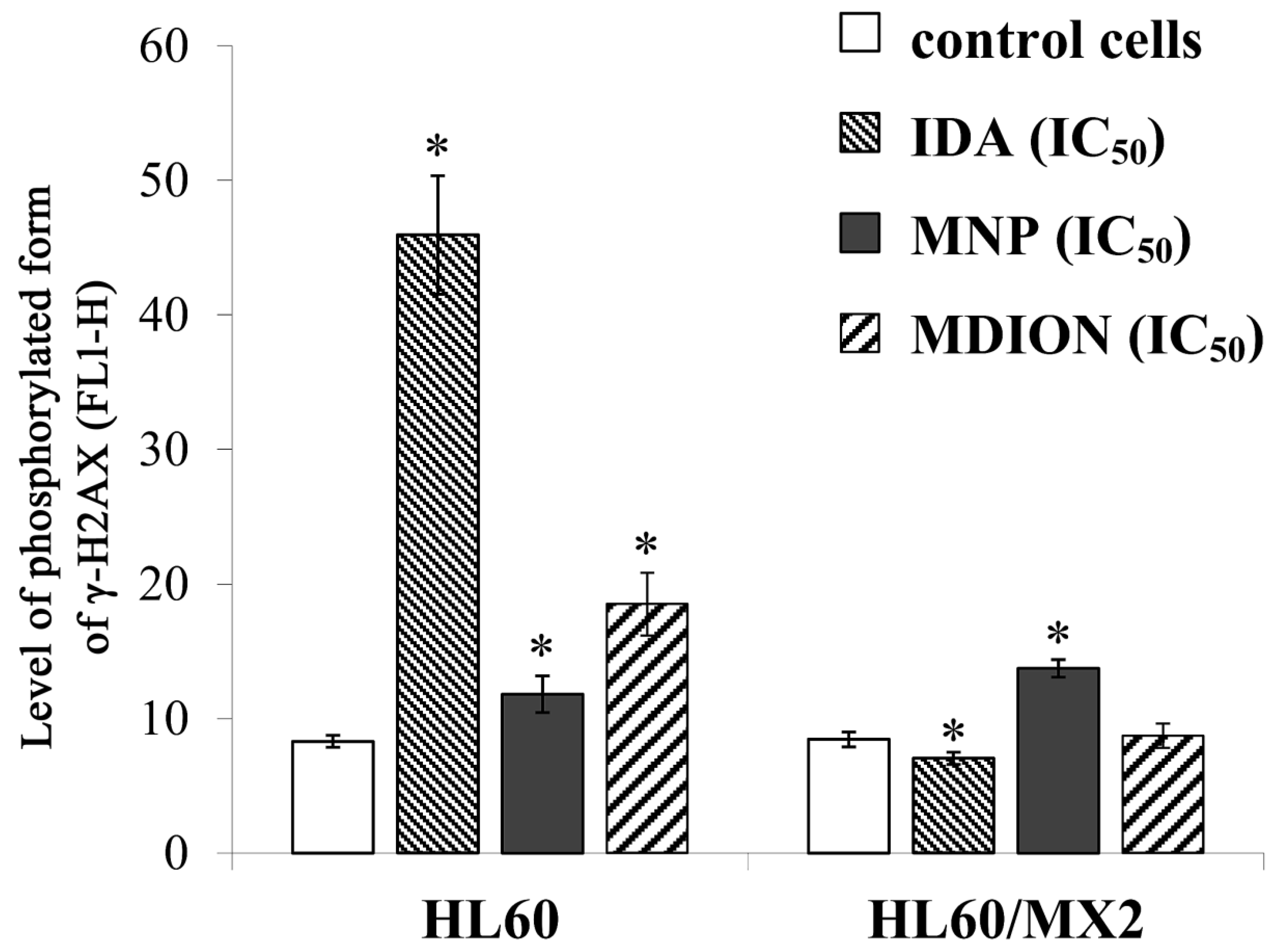
2.4. The Effect of MNP and MDION on Cellular DNA Damage in Sensitive HL60 and Resistant HL60/MX2 Leukaemia Cells
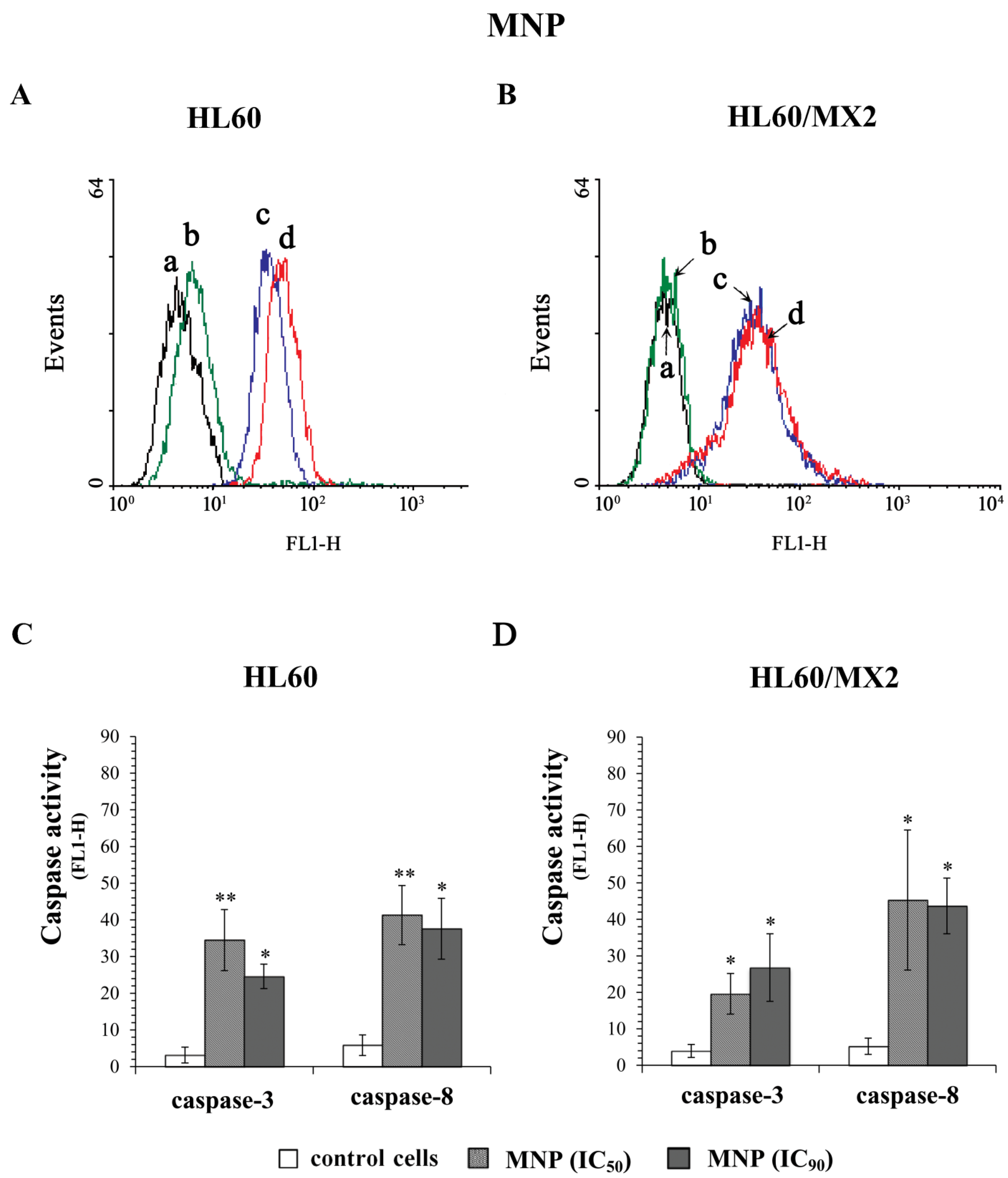
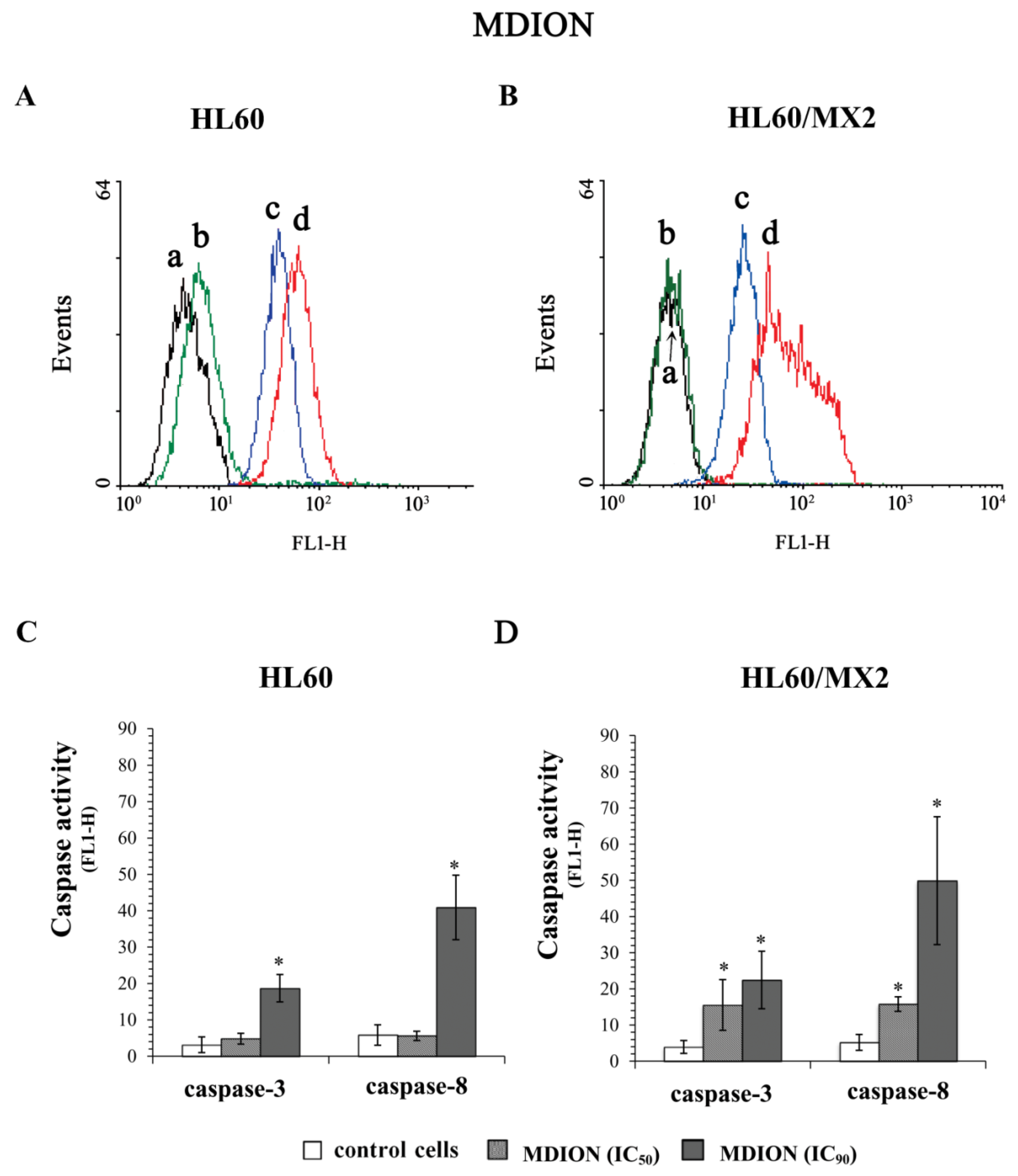
2.5. The Effect of MNP and MDION on Caspase-3 and Caspase-8 Activation in Sensitive HL60 and Resistant HL60/MX2 Leukaemia Cells
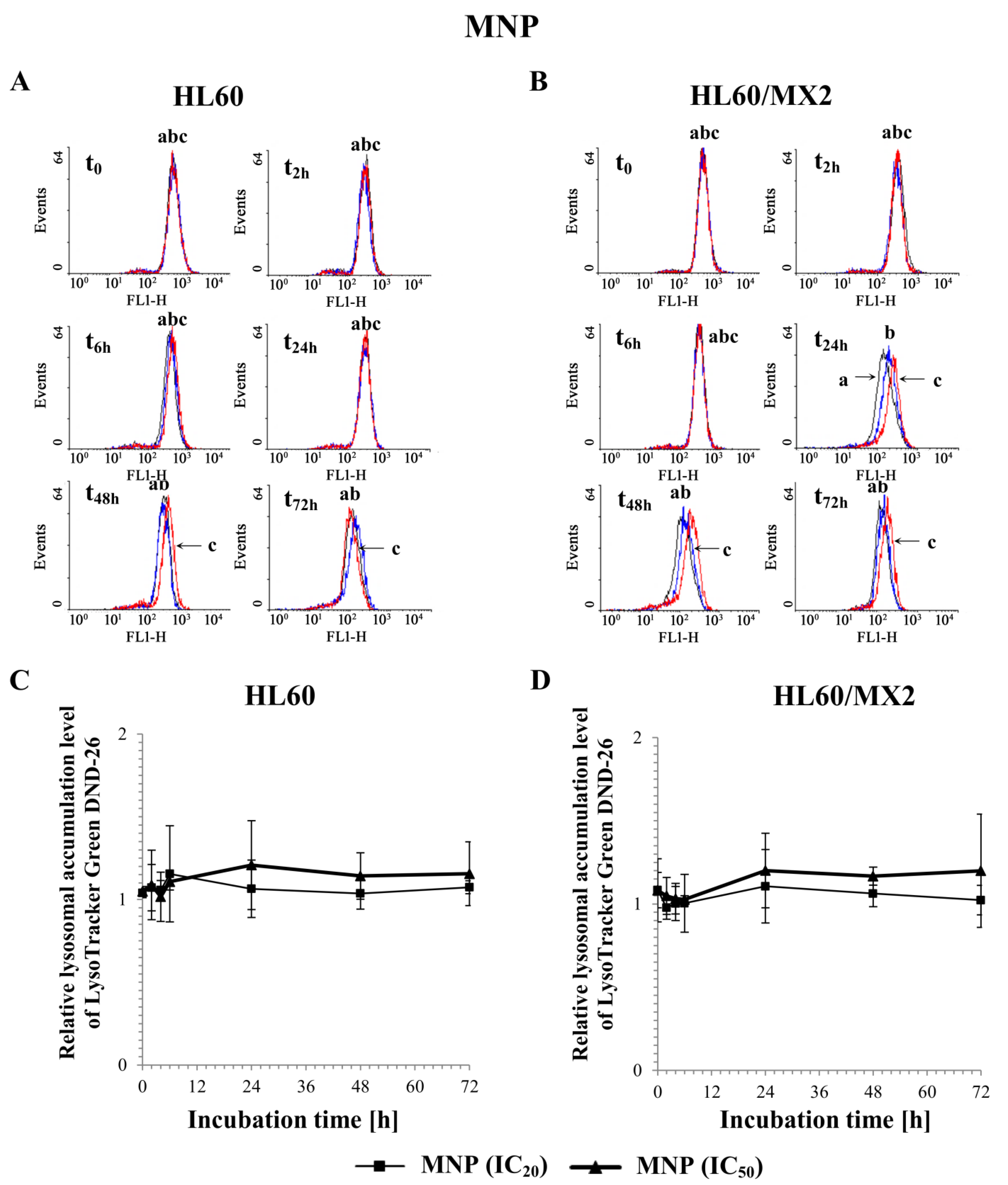
2.6. The Effect of MNP and MDION on Lysosomal Membrane Integrity in Sensitive HL60 and Resistant Leukaemia HL60/MX2 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Growth Inhibition Assay
4.4. Intracellular ROS Level Assay
4.5. Cell Cycle Distribution Analysis
4.6. γ-H2AX Histone Phosphorylation Assay
4.7. Caspase-3 and Caspase-8 Activity Assays
4.8. Lysosomal Integrity Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Cao, H.; Qi, X.; Li, H.; Ye, P.; Wang, Z.; Wang, D.; Sun, M.; Guo, H.C.Q. Research progress in reversal of tumor multi-drug resistance via natural products. Anticancer Agents Med. Chem. 2017, 17, 1466–1476. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hoti, S.L. Development of fourth generation ABC inhibitors from natural products: A novel approach to overcome cancer multidrug resistance. Anticancer Agents Med. Chem. 2015, 15, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updat. 2019, 46, 100645. [Google Scholar] [CrossRef] [PubMed]
- Paleari, L.; Trombino, S.; Falugi, C.; Gallus, L.; Carlone, S.; Angelini, C.; Sepcic, K.; Turk, T.; Faimali, M.; Noonan, U.M.; et al. Marine sponge-derived polymeric alkylpyridinium salts as a novel tumor chemotherapeutic targeting the cholinergic system in lung tumors. Int. J. Oncol. 2006, 29, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Dasari, V.R.; Muthyala, M.K.; Nikku, M.Y.; Donthireddy, S.R. Novel pyridinium compound from marine actinomycete, amycolatopsis alba var. nov. DVR D4 showing antimicrobial and cytotoxic activities in vitro. Microbiol. Res. 2012, 167, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Zovko, A.; Viktorsson, K.; Lewensohn, R.; Kološa, K.; Filipič, M.; Xing, H.; Kem, W.R.; Paleari, L.; Turk, T. APS8, a polymeric alkylpyridinium salt blocks α7 nAChR and induces apoptosis in non-small cell lung carcinoma. Mar. Drugs 2013, 11, 2574–2594. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Al-Sodies, S.A.; Aouad, M.R.; Bardaweel, S.; Messali, M.; El Ashry, E.S.H. An eco-friendly ultrasound-assisted synthesis of novel fluorinated pyridinium salts-based hydrazones and antimicrobial and antitumor screening. Int. J. Mol. Sci. 2016, 17, 766. [Google Scholar] [CrossRef] [PubMed]
- El-Mekawy, R.E.; Fadda, A.A. Synthesis of some novel dimethine, bis-dimethine cyanine dyes and octacosamethine cyanine dyes endowed with promising biological potency against (HepG2), (Hela), (MCF-7), (MIA), (SN12C) and (H358) cell lines. Bioorg. Med. Chem. Lett. 2018, 28, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Onyango, E.O.; Williams, C.R.; Royce, D.B.; Gribble, G.W.; Sporn, M.B.; Liby, K.T. Novel synthetic pyridyl analogues of CDDO-Imidazolide are useful new tools in cancer prevention. Pharmacol. Res. 2015, 100, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Prencipe, F.; Lopez-Cara, L.C.; Oliva, P.; Baraldi, P.; Baraldi, P.G.; Estévez-Sarmiento, F.; Quintana, J.; Estévez, F. Synthesis and biological evaluation of alpha-bromoacryloylamido indolyl pyridinyl propenones as potent apoptotic inducers in human leukaemia cells. J. Enzym. Inhib. Med. Chem. 2018, 33, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Asghari, S.; Rahnamay, M.; Dehghan, G.; Feizi, M.; Balalaie, S. Cytotoxicity, oxidative stress, and apoptosis in K562 leukemia cells induced by an active compound from pyrano-pyridine derivatives. Hum. Exp. Toxicol. 2018, 37, 1105–1116. [Google Scholar] [CrossRef]
- Ananda, H.; Sharath Kumar, K.S.; Nishana, M.; Hegde, M.; Srivastava, M.; Byregowda, R.; Choudhary, B.; Raghavan, S.C.; Rangappa, K.S. Regioselective synthesis and biological studies of novel 1-aryl-3, 5-bis (het) aryl pyrazole derivatives as potential antiproliferative agents. Mol. Cell Biochem. 2017, 426, 149–160. [Google Scholar] [CrossRef]
- Xu, J.; He, H.; Zhou, L.J.; Liu, Y.Z.; Li, D.W.; Jiang, F.L.; Liu, Y. Pyridinium and indole orientation determines the mitochondrial uncoupling and anti-cancer efficiency of F16. Eur. J. Med. Chem. 2018, 154, 305–313. [Google Scholar] [CrossRef]
- Beckham, T.H.; Lu, P.; Jones, E.E.; Marrison, T.; Lewis, C.S.; Cheng, J.C.; Ramshesh, V.K.; Beeson, G.; Beeson, C.C.; Drake, R.R.; et al. LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. J. Pharmacol. Exp. Ther. 2013, 344, 167–178. [Google Scholar] [CrossRef]
- Ogata, S.; Takeuchi, M.; Teradaira, S.; Yamamoto, N.; Iwata, K.; Okumura, K.; Taguchi, H. Radical scavenging activities of niacin-related compounds. Biosci. Biotechnol. Biochem. 2002, 66, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, R.; Bernabeu, M.C.; Brillas, E.; Carranco, I.; Díaz, J.L.; Llorente, N.; Rayo, M.; Spada, A. Productive trapping of NAD-type radicals. Non-biomimetic reduction of pyridinium salts. Chem. Commun. 2002, 8, 850–885. [Google Scholar] [CrossRef]
- Olek, R.A.; Ziolkowski, W.; Kaczor, J.J.; Greci, L.; Popinigis, J.; Antosiewicz, J. Antioxidant activity of NADH and its analogue—An in vitro study. J. Biochem. Mol. Biol. 2004, 37, 416–421. [Google Scholar] [CrossRef]
- Fahs, S.; Rowther, F.B.; Dennison, S.R.; Patil-Sen, Y.; Warr, T.; Snape, T.J. Development of a novel, multifunctional, membrane-interactive pyridinium salt with potent anticancer activity. Bioorg. Med. Chem. Lett. 2014, 24, 3430–3433. [Google Scholar] [CrossRef]
- Parlar, S.; Erzurumlu, Y.; Ilhan, R.; Ballar Kırmızıbayrak, P.; Alptüzün, V.; Erciyas, E. Synthesis and evaluation of pyridinium-hydrazone derivatives as potential antitumoral agents. Chem. Biol. Drug Des. 2018, 92, 1198–1205. [Google Scholar] [CrossRef]
- Yun, M.; Lee, D.; Park, M.N.; Kim, E.O.; Sohn, E.J.; Kwon, B.M. Cinnamaldehyde derivative (CB-PIC) sensitizes chemo-resistant cancer cells to drug-induced apoptosis via suppression of MDR1 and its upstream STAT3 and AKT signalling. Cell Physiol. Biochem. 2015, 35, 1821–1830. [Google Scholar]
- Wieczorkowska, M.; Zielonka, J.; Celinska, J.; Adamus, J.; Ciesielska, E.; Marcinek, A.; Szmigiero, L.; Gebicki, J. 1-Methyl-3-nitropyridine: An efficient oxidant of NADH in non-enzymatic and enzyme-mediated processes. Free Radic Res. 2003, 37, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Maruszewska, A.; Kostrzewa-Nowak, D.; Adamus, J.; Czupryńska, K.; Maryniak, D.; Gebicki, J.; Tarasiuk, J. The ability of selected pyridinium salts to increase the cytotoxic activity of vincristine but not doxorubicin towards sensitive and multidrug resistant promyelocytic leukaemia HL60 cells. J. Pharm. Pharmacol. 2008, 60, 647–653. [Google Scholar] [CrossRef]
- Wieczorkowska, M.; Szajerski, P.; Michalski, R.; Adamus, J.; Marciniak, A.; Gebicki, J.; Ciesielska, E.; Szmigiero, L.; Lech-Marańda, E.; Szmigielska-Kapłon, A.; et al. Cytotoxic activity of the selected pyridinium salts against murine leukemia L1210. Pharmacol. Rep. 2007, 59, 216–223. [Google Scholar]
- Marcinek, A.; Adamus, J.; Gębicki, J.; Platz, M.S.; Bednarek, P. Hydrogen-transferred radical cations of NADH model compounds. 3. 1,8-Acridinediones. J. Phys. Chem. A 2000, 104, 724–728. [Google Scholar] [CrossRef]
- Żwierełło, W.; Maruszewska, A.; Nowak, R.; Kostrzewa-Nowak, D.; Tarasiuk, J. DNA damage induced by NADPH cytochrome P450 reductase-activated idarubicin in sensitive and multidrug resistant MCF7 breast cancer cells. Pharmacol. Rep. 2017, 69, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxidative Med. Cell Longev. 2016, 17, 6475624. [Google Scholar] [CrossRef]
- Lin, K.T.; Xue, J.Y.; Sun, F.F.; Wong, P.Y.K. Reactive oxygen species participate in peroxynitrite-induced apoptosis in HL-60 cells. BioChem. Biophys. Res. Commun. 1997, 230, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sadhukhan, R.; Mondal, J.; Khuda-Bukhsh, A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 2013, 46, 153–163. [Google Scholar] [CrossRef]
- Wolf, D.; Rotter, V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc. Natl. Acad. Sci. USA 1985, 82, 790–794. [Google Scholar] [CrossRef]
- Olivier, M.; Eeles, R.; Hollstein, M.; Khan, M.A.; Harris, C.C.; Hainaut, P. The IARC TP53 database: New online mutation analysis and recommendations to users. Hum. Mutat. 2002, 19, 607–614. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Jäättelä, M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta 2009, 1793, 746–754. [Google Scholar] [CrossRef]
- Groth-Pedersen, L.; Jäättelä, M. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer Lett. 2013, 332, 265–274. [Google Scholar] [CrossRef]
- Serrano-Puebla, A.; Boya, P. Lysosomal membrane permeabilization as a cell death mechanism in cancer cells. Biochem. Soc. Trans. 2018, 46, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Di Yang, N.; Zhou, F.; Shen, T.; Duan, T.; Zhou, J.; Shi, Y.; Zhu, X.-Q.; Shen, H.-M. (−)-Epigallocatechin-3-gallate induces non-apoptotic cell death in human cancer cells via ROS-mediated lysosomal membrane permeabilization. PLoS ONE 2012, 7, e46749. [Google Scholar]
- Nowak, R.; Tarasiuk, J. Retaining cytotoxic activity of anthrapyridone CO1 against multidrug resistant cells is related to the ability to induce concomitantly apoptosis and lysosomal death of leukaemia HL60/VINC and HL60/DOX cells. J. Pharm. Pharmacol. 2013, 65, 855–867. [Google Scholar] [CrossRef]
- Maruszewska, A.; Tarasiuk, J. Antitumour effects of selected plant polyphenols, gallic acid and ellagic acid, on sensitive and multidrug-resistant leukaemia HL60 cells. Phytother. Res. 2019, 33, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Paquet, C.; Sané, A.T.; Beauchemin, M.; Bertrand, R. Caspase- and mitochondrial dysfunction-dependent mechanisms of lysosomal leakage and cathepsin B activation in DNA damage-induced apoptosis. Leukemia 2005, 19, 784–791. [Google Scholar] [CrossRef]
- Johansson, A.C.; Appelqvist, H.; Nilsson, C.; Kågedal, K.; Roberg, K.; Ollinger, K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 2010, 15, 527–540. [Google Scholar] [CrossRef] [PubMed]






| Compound | HL60 Cells | HL60/MX2 Cells | |||||
|---|---|---|---|---|---|---|---|
| IC20 {µM} | IC50 {µM} | IC90 {µM} | IC20 {µM} | IC50 {µM} | RF | IC90 {µM} | |
| MNP | 7.5 ± 0.9 | 24.3 ± 7.2 | 82.1 ± 25.3 | 7.2 ± 1.4 | 20.5 ± 5.2 | 0.84 | 77.3 ± 12.8 |
| MDION | 15.0 ± 1.9 | 80.5 ± 10.5 | 573.0 ± 171.4 | 17.0 ± 3.4 | 95.5 ± 19.9 | 1.19 | 591.2 ± 164.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasiuk, J.; Kostrzewa-Nowak, D.; Żwierełło, W. Antitumour Effects of Selected Pyridinium Salts on Sensitive Leukaemia HL60 Cells and Their Multidrug Resistant Topoisomerase II-Defective HL60/MX2 Counterparts. Molecules 2022, 27, 5138. https://doi.org/10.3390/molecules27165138
Tarasiuk J, Kostrzewa-Nowak D, Żwierełło W. Antitumour Effects of Selected Pyridinium Salts on Sensitive Leukaemia HL60 Cells and Their Multidrug Resistant Topoisomerase II-Defective HL60/MX2 Counterparts. Molecules. 2022; 27(16):5138. https://doi.org/10.3390/molecules27165138
Chicago/Turabian StyleTarasiuk, Jolanta, Dorota Kostrzewa-Nowak, and Wojciech Żwierełło. 2022. "Antitumour Effects of Selected Pyridinium Salts on Sensitive Leukaemia HL60 Cells and Their Multidrug Resistant Topoisomerase II-Defective HL60/MX2 Counterparts" Molecules 27, no. 16: 5138. https://doi.org/10.3390/molecules27165138
APA StyleTarasiuk, J., Kostrzewa-Nowak, D., & Żwierełło, W. (2022). Antitumour Effects of Selected Pyridinium Salts on Sensitive Leukaemia HL60 Cells and Their Multidrug Resistant Topoisomerase II-Defective HL60/MX2 Counterparts. Molecules, 27(16), 5138. https://doi.org/10.3390/molecules27165138








