Abstract
An efficient continuous-flow nitration process of o-xylene at pilot scale was demonstrated. The effects of parameters such as temperature, ratio of H2SO4 to HNO3, H2SO4 concentration, flow rate, and residence time on the reaction were studied. Under the optimal conditions, the yield of products reached 94.1%, with a product throughput of 800 g/h. The main impurities of this continuous-flow nitration process were also studied in detail. Compared with batch process, phenolic impurity decreased from 2% to 0.1%, which enabled the omission of the alkaline solution washing step and thus reduced the wastewater emission. The method was also successfully applied to the nitrification of p-xylene, toluene, and chlorobenzene with good yields.
1. Introduction
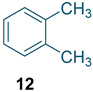
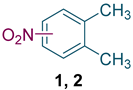
Nitro-o-xylenes are important ingredients and intermediates applied broadly in pharmaceuticals, agricultural chemicals, dyes, and many other areas. For instance, 1,2-dimethyl-3-nitrobenzene (Figure 1, 1) is the raw material of mefenamic acid [1] (Figure 1, 3), an effective nonsteroidal anti-inflammatory drug. Meanwhile, 1,2-dimethyl-4-nitrobenzene (Figure 1, 2) is extensively used to produce medicines such as riboflavin [2] (Figure 1, 4), the herbicide pendimethalin [3] (Figure 1, 5), and the cardiovascular drug tolvaptan [4] (Figure 1, 6).
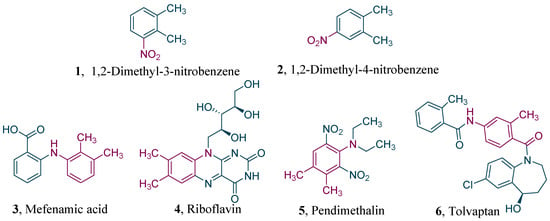
Figure 1.
Structures of nitro-o-xylenes and related downstream products.
Nitro-o-xylenes are primarily synthesized by the nitration of o-xylene. Among the versatile methods [5], nitration with mixed acid [6,7,8,9] is the most commonly used one in the industry due to its economy and maturity. Although scientists have reported several greener strategies, such as using NO2 [10,11,12] and N2O5 [13] as nitrating agents or using acidic solid catalysts [14,15] to replace liquid acids, these methods suffer from problems, such as using expensive catalytic systems, difficulty of scaling up, and ensuring the process safety. Therefore, these new methods remain inferior to mixed-acid nitration as the predominant technique used in industries.
Obviously, nitration with mixed acid has its limitations. First, nitration using this method produces a large amount of waste acid, which causes environmental stress. This problem can be solved to some extent by recycling the waste acid, although extra expenses are incurred. Second, nitration reactions are highly exothermic [16]. The production of nitrocompounds using a traditional tank reactor usually generates large amounts of liquid holdup and has poor heat-transfer efficiency. Nitrocompounds are also usually explosive, and their raw materials are inflammable. These characteristics make nitration reaction extremely hazardous. The safe generation of nitration products in industries is urgently needed, and continuous-flow reactors may serve as powerful tools to achieve this goal.
Over the past decades, continuous-flow reactors have been greatly developed and extensively applied in organic syntheses [17,18,19,20,21]. The characteristic properties of these reactors are their exceptionally fast heat and mass transfer. Thus, the accumulation of heat, formation of hot spots, and dangers of thermal runaways can be prevented. Moreover, the small reactor volumes result in the significantly improved overall safety of the process, even under harsh reaction conditions [22]. Therefore, continuous-flow reactors are suitable for nitration.
The first report about the application of continuous-flow technology to nitrition reactions date back to 1956 [23]. Since then, continuous flow nitrition reactions have been developed rapidly [24,25,26,27,28,29,30,31]. There are also some elegant studies in continuous-flow nitrification of xylenes. In 2015, Kulkarni’s group studied an efficient continuous-flow nitration process of o-xylene using a tubular reactor [32]. In this process, 99% conversion of o-xylene and 7.2% dinitro impurities were observed using only fuming nitric acid (FNA) as the nitrating agent. However, up to six times as much FNA as o-xylene was used, which was a tremendous waste, and the best yield of nitro-o-xylenes was calculated to be 91.8%, which still has room for improvement. In 2021, Watts’s group disclosed a biphasic continuous-flow nitration procedure to generate various nitrobenzenes in a sonicated PTFE tube reactor and Uniqsis chip reactor [33]. Although the nitration in the sonicated PTFE tube reactor gave excellent yields, this method was not easy to scale up. Nitration in a Uniqsis chip reactor is much easier to scale up to decagram scale per hour, but the yield of nitro-o-xylenes is only 88%, which is unsatisfactory. Very recently, Li’s group designed an efficient two-step mononitration method of m-xylene in a microreactor [34]. Under the optimal experimental conditions, the yield of mononitro m-xylenes reached 99% and a throughput of 1 kg/h. However, this method requires the use of CH2Cl2 as a solvent, which is not good for the production of bulk chemicals.
Because o-xylene contains two electron-donating groups, it is highly susceptible to dinitrification side reactions, making it difficult to increase the yield of its mononitrification products beyond 90%. However, mononitro-o-xylenes are bulk chemical raw materials with an annual global demand of more than 100,000 tons. Increasing the yield by just a few percentage points can also significantly reduce production costs. Herein, we demonstrated a nitration process of o-xylene with high yield and high throughput by using a commercially available continuous-flow reactor. The main impurities of the process were researched in detail. Additionally, the method was also applied to the nitrification of p-xylene, toluene, and chlorobenzene.
2. Results and Discussion
2.1. Optimization of Nitration in Continuous-Flow
The initial experiment was performed to investigate the parameters affecting the reaction greatly, such as temperature, ratio of H2SO4 to HNO3, H2SO4 concentration, and flow rate. As shown in Figure 2, H2SO4 was diluted into a certain concentration and mixed with HNO3 to prepare the mixed acid. The mixed acid and o-xylene were then pumped into the continuous reactor (Corning Advanced-Flow Reactor G1 with five standard fluidic modules). The reaction solution was collected in a vessel. The organic phase was separated and washed with water and brine successively, and then the samples were analyzed by gas chromatography.
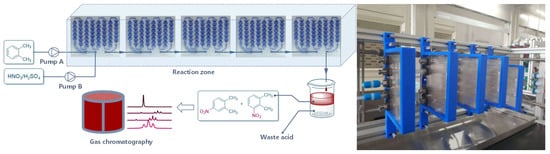
Figure 2.
Continuous-flow reactor setup for condition optimization.
The influence of temperature from 50 °C to 110 ℃ was initially studied with the other parameters fixed as follows: H2SO4 concentration = 70%, H2SO4/HNO3 mole ratio = 1.0, HNO3/o-xylene mole ratio = 1.2, and flow rate of o-xylene = 10 g/min. With increased temperature, the conversion of o-xylene increased. The selectivity of the products peaked at 100 ℃ (Figure 3a). The H2SO4/HNO3 mole ratio was investigated next. Results show that H2SO4/HNO3 = 4.0 gave the best conversion of substrate, and H2SO4/HNO3 = 3.0 gave the best product selectivity (Figure 3b). H2SO4/HNO3 = 3.0 was selected for further study considering that relatively less acid was more favorable for manufacturing. The H2SO4 concentration was subsequently examined. With increased H2SO4 concentration, the conversion obviously increased. However, the selectivity of products sharply decreased when H2SO4 concentration exceeded 70% (Figure 3c). Considering that the nitration is a two-phase reaction, flow rate greatly affected the mass transfer and hence the reaction results [35]. With increased flow rate, conversion and selectivity increased. When the flow rate of o-xylene exceeded 10 g/min, the reaction conversion and selectivity were maintained (Figure 3d).
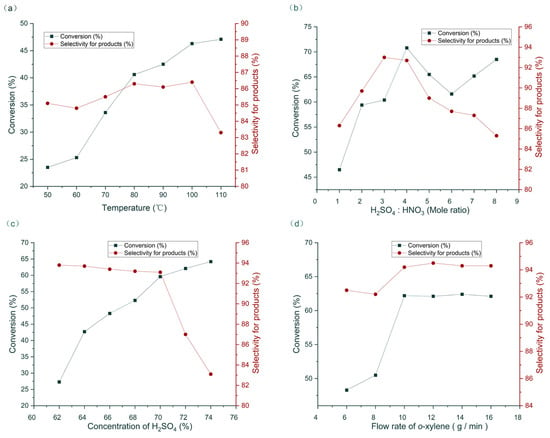
Figure 3.
Optimization of nitration in continuous flow. (a) The influence of temperature; (b) the influence of mole ratio of H2SO4 and HNO3; (c) the influence of H2SO4 concentration; (d) the influence of flow rate.
Under the existing conditions, the conversion remained too low. Accordingly, five more reaction modules were added to this system to prolong the residence time (Figure 4). Residence time (t) was determined as follows Equation where V, Qaq, and Qorg were the microchannel volume, aqueous volumetric flow rate, and organic volumetric flow rate:
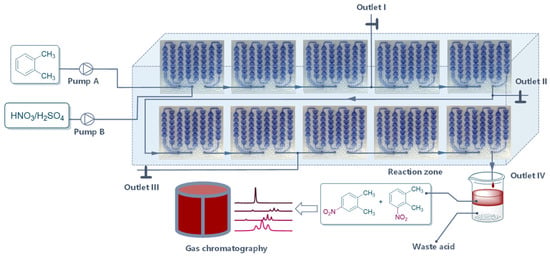
Figure 4.
Continuous-flow reactor setup for the inspection of residence time.
Samples were collected at different outlets, which showed that prolonging the residence time improved the conversion to some certain extent. However, the reaction was nearly balanced because conversion from outlet III was very close to that from outlet IV (Table 1).

Table 1.
Influence of residence time on the reaction a.
To break the balance and achieve high product yield, another flow of mixed acid with different concentrations of H2SO4 and HNO3/o-xylene was pumped into the system at the sixth module (Figure 5). The addition of mixed acid to the continuous-flow system significantly promoted the reaction (Table 2). To our delight, when the initial mixed acid from pump B and supplemental mixed acid from pump C were 70% H2SO4 and HNO3/o-xylene = 1.2, the product yield reached 94.1%, with a throughput of 800 g/h of products (Table 2, entry 3). A comparison experiment with an initial double flow rate of mixed acid was conducted, too. The results show that the direct pumping in double flow rate of mixed acid produced more dinitrification products, resulting in a significant decrease in yield and product selectivity (Table 2, entry 4).
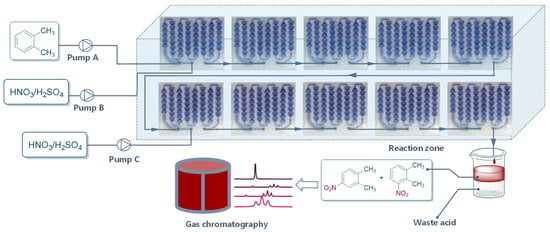
Figure 5.
Continuous-flow reactor setup for the optimal process.

Table 2.
The influence of the added mixed acid on the reaction a.
2.2. Research on Impurities
The impurities of this process were also studied in detail. The main impurities were isolated, and their structures were identified by 1H NMR and/or X-ray crystal-structure determination. Results indicate that they were all dinitroproducts of o-xylene (Figure 6, 7–10). Unlike this continuous process, another phenolic impurity 11 in the batch reaction process amounted to 2%, which seriously affected the product quality (Figure 5, 11). The phenolic impurity 11 may have been generated from the oxidation of impurity 7 by HNO3. To remove it, a large amount of NaOH aqueous solution was necessary for postprocessing, which produced substantial liquid waste. Interestingly, impurity 11 was diminished to about 0.1% in this continuous-flow process, which enabled the omission of the alkaline solution washing step and reduced the wastewater emission. The sharp decrease in impurity 11 may be correlated with the short reaction time in our continuous-flow process.
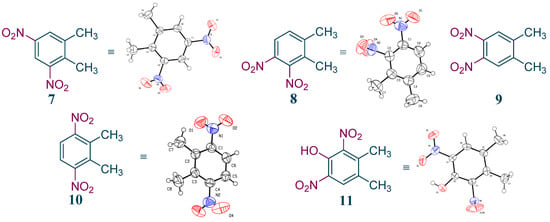
Figure 6.
The structures of the main impurities in o-xylene nitration process.
2.3. Extension to Analogues
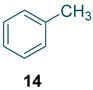
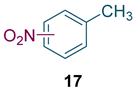
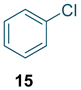
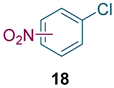
To test the generality of the method, the reaction conditions were subsequently extended to the synthesis of other analogues. p-Xylene was converted to 2-nitro-p-xylene with 93.8% yield under these conditions (Table 3, entry 2). Toluene was converted to nitrotoluenes with 96.0% yield, and its main products were o- and p- nitro products. Similar to o-xylene (Table 3, entry 1), toluene exhibits poor regioselectivity under these conditions (Table 3, entry 3). However, the situation changed a lot in chlorobenzene’s nitration. Chlorobenzene not only obtained a 97.2% mononitration yield, but also obtained >99% para-selectivity (Table 3, entry 4). The slight electron-withdrawing group on the benzene ring rather than the electron-donating group might be the reason for the extremely high regioselectivity.

Table 3.
Synthesis of analogues a.
3. Materials and Methods
3.1. General Information
All the chemicals were purchased from standard commercial vendors and were used without any further purification. Flash column chromatography was performed using silica gel (300–400 mesh). 1H NMR chemical shifts were reported in ppm (δ) relative to tetramethylsilane (TMS) with the solvent resonance employed as the internal standard (CDCl3, 7.26 ppm, DMSO-d6, 2.50 ppm). Gas chromatography was recorded on a Shimadzu GC 2014. Crystallographic data were collected on an Agilent Technologies Germini by using the Cu-Kα radiation (λ = 1.5406 Å) at room temperature. All products and impurities were isolated by flash chromatography on silica gel and characterized by 1H NMR. Impurities 7, 8, 10, and 11 were crystallized in ethanol and confirmed by X-ray crystal structure determination.
3.2. Batch Nitration Procedure A
Into a 250 mL round-bottom flask equipped with a mechanical stirrer was added 53 g (500 mmol) of o-xylene and then cooled to 15–20 °C. 63 g (660 mmol, 1.3 equiv.) Concentrate H2SO4 (98%) was mixed with 7 mL of ice water and 37 g (600 mmol, 1.2 equiv.) of FNA to make the mixed acid. The mixed acid was then added dropwise into the flask in an hour. The reaction was kept at 15–20 °C for additional 30 min. The organic phase was separated and then washed two times with 50 mL water. It was further washed with 50 mL brine to remove residual water. The products were analyzed using gas chromatography with an HP5 capillary column and a flame ionization detector (FID).
3.3. Continuous Flow Reactor Setup
All flow chemistry experiments were conducted using a Corning Advanced-Flow Reactor G1 setup, consisting of plunger pumps (Corning dual pump KP-22-33DC, Hanbang technology NP7010C), heat exchanger, and fluidic modules. Each fluidic module consists of four confined structured hybrid glass layers that yield three zones for the flow: a reaction zone (of 8.3 mL volume) sandwiched by two heat transfer zones (with an area per unit volume of 788.5 m−1). The internal diameter of the reaction channel is 1 mm at its narrowest point and 1 cm at its widest point. Connections between plunger pumps and the microreactor consisted of PTFE tubing (1/4 in o.d.). The recommended flow rates for this device range from 10 to 200 mL/min.
3.4. General Procedure B for Condition Optimization of Continuous Nitration
The concentrate H2SO4 (98%) was mixed with a known quantity of ice-cold water to make the required concentration of H2SO4. The diluted H2SO4 was then mixed with a known quantity of FNA to make the mixed acid with desired mole ratio. The two reactants (o-xylene and mixed acid) were pumped into the continuous reactor through plunger pumps to react under desired flow rate and temperature. Samples were collected at the outlet in a fixed quantity of ice-cold water. A known quantity of CH2Cl2 was used to extract the organic phase from these samples. The extracted organic phase was washed two times with water and separated by gravity. It was further washed with brine to remove residual water. The samples were analyzed using gas chromatography with an HP5 capillary column and a flame ionization detector (FID).
3.5. General Procedure C for the Long Run of Continous Niration of o-xylene and Its Analogues
The concentrate H2SO4 (98%) was mixed with ice-cold water to make the 70% H2SO4. The diluted H2SO4 was then mixed with FNA to make the mixed acid (10.4 mol/L H2SO4, 3.5 mol/L HNO3). The organic phase was pumped in with a flow rate of 11.4 mL/min from pump A through the first fluidic module. The mixed acid was pumped in from pump B and pump C through the first module and the sixth module with a flow rate of 32.2 mL/min, respectively. The reaction temperature was set to 100 °C and the residence time was 90 s. Samples were collected at the outlet in a fixed quantity of ice-cold water. A known quantity of CH2Cl2 was used to extract the organic phase from these samples. The extracted organic phase was washed two times with water and separated by gravity. It was further washed with brine to remove residual water. The samples were analyzed using gas chromatography with an HP5 capillary column and a flame ionization detector (FID).
3.6. Preparation Procedure D for Impurities
Into a 25 mL round-bottom flask equipped with a mechanical stirrer was added 5.3 g (50.0 mmol) of o-xylene and then cooled to 15–20 °C. A total of 6.3 g (65.6 mmol, 1.3 equiv.) of concentrate H2SO4 (98%) was mixed with 3.7 g (60.0 mol, 1.2 equiv.) of FNA (98%) to make the mixed acid. The mixed acid was then added dropwise into the flask in an hour. The reaction was kept at 15–20 °C for an additional 30 min. The organic phase was separated and then washed two times with 50 mL water. It was further washed with 50 mL brine to remove residual water. The residue was purified by flash chromatography on silica gel to give the corresponding impurities.
4. Conclusions
A continuous-flow nitration process of o-xylene and its main impurities was studied in detail. Under the optimal conditions of this continuous-flow nitration process, the yield of products reached 94.1%, with a throughput of 800 g/h. Compared with the batch process, phenolic impurity decreased from 2% to 0.1%, which simplified the postreaction treatment and reduced the wastewater emission. The method was also applied to the nitrification of p-xylene, toluene, and chlorobenzene. The good yield, high throughput, and good control of impurities of the proposed process indicated its important industrial application potential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27165139/s1, Image of continuous flow reactor setup; 1H NMR data and spectrum, character for compound 1, 2, 7–11, 16–18; XRD single crystal data for compound 7, 8, 10 and 11.
Author Contributions
Conceptualization, Q.S.; investigation, X.L., S.Y., S.W., J.W. and J.C.; formal analysis, Y.X. and Q.H.; supervision, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Department of Sichuan Province, grant number 2021YFG0290 and 2021YFG0291; Science and Technology Department of Yibin, grant number 2021YG02 and Xihua University, grant number Z202030.
Data Availability Statement
The data presented in this study are available in the supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Some of the compounds described in this article are available from the authors.
References
- Tu, T.; Fang, W.; Jiang, J. A Highly Efficient Precatalyst for Amination of Aryl Chlorides: Synthesis, Structure and Application of a Robust Acenaphthoimidazolylidene Palladium Complex. Chem. Commun. 2011, 47, 12358–12360. [Google Scholar] [CrossRef]
- Neti, S.S.; Poulter, C.D. Site-Selective Synthesis of 15N- and 13C- Enriched Flavin Mononucleotide Coenzyme Isotopologues. J. Org. Chem. 2016, 81, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Han, M.; Ye, C.; Dang, M.; Chen, G. Safe, Efficient and Selective Synthesis of Dinitro Herbicidesvia a Multifunctional Continuous-Flow Microreactor: One-Step Dinitration with Nitric Acid as Agent. Green Chem 2013, 15, 91–94. [Google Scholar] [CrossRef]
- Corderovargas, A.; Quicletsire, B.; Zard, S. A Flexible Approach for the Preparation of Substituted Benzazepines: Application to the Synthesis of Tolvaptan. Bioorg. Med. Chem. 2006, 14, 6165–6173. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, G. Synthetic Nitrogen Products: A Practical Guide to the Products and Processes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Emerson, O.H.; Smith, L.I. The Chemistry of Vitamin E. XX. 1 The Preparation of o-Xylohydroquinone. J. Am. Chem. Soc. 1940, 62, 141–142. [Google Scholar] [CrossRef]
- Crossley, A.W.; Renouf, N. XXVII.—Nitro-Derivatives of Ortho-Xylene. J. Chem. Soc. Trans. 1909, 95, 202–218. [Google Scholar] [CrossRef]
- Kobe, K.A.; Brennecke, H.M. Mononitration of m-Xylene. Ind. Eng. Chem. 1954, 46, 728–732. [Google Scholar] [CrossRef]
- Kobe, K.A.; Pritchett, P.W. Mononitration of o-Xylene. Ind. Eng. Chem. 1952, 44, 1398–1401. [Google Scholar] [CrossRef]
- Adamiak, J.; Chmielarek, M. Efficient and Selective Nitration of Xylenes over MoO3/SiO2 Supported Phosphoric Acid. J. Ind. Eng. Chem. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Liu, H.; Ji, C.; Dong, X.; Peng, X.; Shi, C. Regioselective Preparation of 4-Nitro-o-Xylene Using Nitrogen Dioxide/Molecular Oxygen over Zeolite Catalysts. Remarkable Enhancement of Para -Selectivity. Chem. Lett. 2014, 43, 817–819. [Google Scholar] [CrossRef]
- Tang, B.; Wei, S.; Peng, X. Acid-Catalyzed Regioselective Nitration of o-Xylene to 4-Nitro-o-Xylene with Nitrogen Dioxide: Brønsted Acid Versus Lewis Acid. Synth. Commun. 2014, 44, 2057–2065. [Google Scholar] [CrossRef]
- Millar, R.; Colclough, M.; Desai, H.; Golding, P.; Honey, P.; Paul, N.; Sanderson, A.; Stewart, M. Novel Syntheses of Energetic Materials Using Dinitrogen Pentoxide; ACS Publications: Washington, DC, USA, 1996; pp. 104–121. [Google Scholar]
- Patil, P.T.; Malshe, K.M.; Dagade, S.P.; Dongare, M.K. Regioselective Nitration of o-Xylene to 4-Nitro-o-Xylene Using Nitric Acid over Solid Acid Catalysts. Catal. Commun. 2003, 4, 429–434. [Google Scholar] [CrossRef]
- Mathew, S.M.; Biradar, A.V.; Umbarkar, S.B.; Dongare, M.K. Regioselective Nitration of Cumene to 4-Nitro Cumene Using Nitric Acid over Solid Acid Catalyst. Catal. Commun. 2006, 7, 394–398. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, P.; Liu, J.; Wang, W.; Yu, C.; Su, W. Continuous-Flow Process for Selective Mononitration of 1-Methyl-4-(Methylsulfonyl)Benzene. Org. Process Res. Dev. 2016, 20, 199–203. [Google Scholar] [CrossRef]
- De Risi, C.; Bortolini, O.; Brandolese, A.; Di Carmine, G.; Ragno, D.; Massi, A. Recent Advances in Continuous-Flow Organocatalysis for Process Intensification. React. Chem. Eng. 2020, 5, 1017–1052. [Google Scholar] [CrossRef]
- Berton, M.; de Souza, J.M.; Abdiaj, I.; McQuade, D.T.; Snead, D.R. Scaling Continuous API Synthesis from Milligram to Kilogram: Extending the Enabling Benefits of Micro to the Plant. J. Flow Chem. 2020, 10, 73–92. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Dombrowski, A.W. Emerging Trends in Flow Chemistry and Applications to the Pharmaceutical Industry. J. Med. Chem. 2019, 62, 6422–6468. [Google Scholar] [CrossRef]
- Donnelly, K.; Baumann, M. Scalability of Photochemical Reactions in Continuous Flow Mode. J. Flow Chem. 2021, 11, 223–241. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, F.; Li, L.; Ge, X.; Zhang, S.; Qiu, T. Scale-up of Microreactor: Effects of Hydrodynamic Diameter on Liquid–Liquid Flow and Mass Transfer. Chem. Eng. Sci. 2020, 226, 115838. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-Flow Technology—A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Brennecke, H.M.; Kobe, K.A. Mixed Acid Nitration of Toluene. Ind. Eng. Chem. 1956, 48, 1298–1304. [Google Scholar] [CrossRef]
- Burns, J.R.; Ramshaw, C. A Microreactor for the Nitration of Benzene and Toluene. Chem. Eng. Commun. 2002, 189, 1611–1628. [Google Scholar] [CrossRef]
- Panke, G.; Schwalbe, T.; Stirner, W.; Taghavi-Moghadam, S.; Wille, G. A Practical Approach of Continuous Processing to High Energetic Nitration Reactions in Microreactors. Synthesis 2003, 2003, 2827–2830. [Google Scholar]
- Ducry, L.; Roberge, D.M. Controlled Autocatalytic Nitration of Phenol in a Microreactor. Angew. Chem. Int. Ed. 2005, 44, 7972–7975. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Nivangune, N.T.; Kalyani, V.S.; Joshi, R.A.; Joshi, R.R. Continuous Flow Nitration of Salicylic Acid. Org. Process Res. Dev. 2008, 12, 995–1000. [Google Scholar] [CrossRef]
- Gage, J.R.; Guo, X.; Tao, J.; Zheng, C. High Output Continuous Nitration. Org. Process Res. Dev. 2012, 16, 930–933. [Google Scholar] [CrossRef]
- Knapkiewicz, P.; Skowerski, K.; Jaskólska, D.E.; Barbasiewicz, M.; Olszewski, T.K. Nitration under Continuous Flow Conditions: Convenient Synthesis of 2-Isopropoxy-5-Nitrobenzaldehyde, an Important Building Block in the Preparation of Nitro-Substituted Hoveyda–Grubbs Metathesis Catalyst. Org. Process Res. Dev. 2012, 16, 1430–1435. [Google Scholar] [CrossRef]
- Kulkarni, A.A. Continuous Flow Nitration in Miniaturized Devices. Beilstein J. Org. Chem. 2014, 10, 405–424. [Google Scholar] [CrossRef]
- Köckinger, M.; Wyler, B.; Aellig, C.; Roberge, D.M.; Hone, C.A.; Kappe, C.O. Optimization and Scale-up of the Continuous Flow Acetylation and Nitration of 4-Fluoro-2-Methoxyaniline to Prepare a Key Building Block of Osimertinib. Org. Process Res. Dev. 2020, 24, 2217–2227. [Google Scholar] [CrossRef]
- Sharma, Y.; Joshi, R.A.; Kulkarni, A.A. Continuous-Flow Nitration of o-Xylene: Effect of Nitrating Agent and Feasibility of Tubular Reactors for Scale-Up. Org. Process Res. Dev. 2015, 19, 1138–1147. [Google Scholar] [CrossRef]
- Sagandira, M.B.; Sagandira, C.R.; Watts, P. Continuous Flow Synthesis of Xylidines via Biphasic Nitration of Xylenes and Nitro-Reduction. J. Flow Chem. 2021, 11, 193–208. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, G.; Zhan, L.; Li, B. Process Design of Two-Step Mononitration of m-Xylene in a Microreactor. J. Flow Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Nieves-Remacha, M.J.; Kulkarni, A.A.; Jensen, K.F. Hydrodynamics of Liquid–Liquid Dispersion in an Advanced-Flow Reactor. Ind. Eng. Chem. Res. 2012, 51, 16251–16262. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).