Improved Metal Cation Optosensing Membranes through the Incorporation of Sulphated Polysaccharides
Abstract
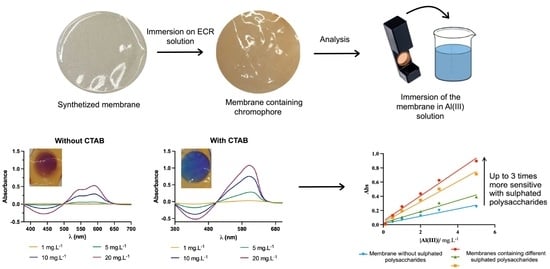
:1. Introduction
2. Results and Discussion
2.1. Membrane Synthesis
2.2. Evaluation of Membrane Composition
2.3. Study of the Incorporation of ECR
2.4. Study of Immersion Time on the Cation Solution
2.5. Evaluation of the Membrane Performance
2.6. Study of Surfactant Incorporation
2.7. Study of the Effect of Interfering Species on the Selectivity of Sensing Membranes
3. Materials and Methods
3.1. Materials and Reagents
3.2. Methods
3.2.1. Synthesis of the Membranes
- Synthesis of Chitosan–Polysiloxane Membranes (CH-PSil)
- Synthesis of Membranes Containing Sulphated Polysaccharide
3.2.2. Membrane Characterization
- Degree of Swelling
- Malleability
- Thickness
- Fourier Transform Infrared Spectroscopy Analysis (FTIR)
- Thermogravimetric Analysis (TGA)
3.2.3. Development of Sensing Membranes for Al(III)
- Analysis on the UV-Vis Spectrophotometer
- Optimization of ECR Concentration
- Optimization of Immersion Time in Metal ion Solutions
- Sensing Membrane Calibration
- Study of the Effect of Surfactants
- Detection Limit Determination
- Study on the Effect of Interfering Species
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Poly. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 1–20. [Google Scholar] [CrossRef]
- Ferreira, V.R.A.; Azenha, M.A.; Pereira, C.M.; Fernando Silva, A. Preparation and evaluation of Pb(II)-imprinted fucoidan-based sorbents. React. Funct. Polym. 2017, 115, 53–62. [Google Scholar] [CrossRef]
- Ferreira, V.R.; Azenha, M.A.; Mêna, M.T.; Moura, C.; Pereira, C.M.; Pérez-Martín, R.I.; Vázquez, J.A.; Silva, A.F. Cationic imprinting of Pb (II) within composite networks based on bovine or fish chondroitin sulfate. J. Mol. Recognit. 2018, 31, e2614. [Google Scholar] [CrossRef]
- Ferreira, V.R.; Azenha, M.A.; Bustamante, A.G.; Mêna, M.T.; Moura, C.; Pereira, C.M.; Silva, A.F. Metal cation sorption ability of immobilized and reticulated chondroitin sulfate or fucoidan through a sol-gel crosslinking scheme. Mater. Today Commun. 2016, 8, 172–182. [Google Scholar] [CrossRef]
- Kanno, K.; Fujita, Y.; Honda, S.; Takahashi, S.; Kato, S. Urethane foam of sulphated polysaccharide ulvan derived from green-tide-forming chlorophyta: Synthesis and application in the removal of heavy metal ions from aqueous solutions. Poly. J. 2014, 46, 813–818. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.v.; Olaniran, A.O.; Okoh, A.I. Chemical characterization of sulphated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Azmi, N.A.; Low, S.C. Investigating film structure of membrane-based colorimetric sensor for heavy metal detection. J. Water Process Eng. 2017, 15, 37–42. [Google Scholar] [CrossRef]
- Sombatsri, S.; Wittayakun, J.; Sanai, K.; Kajsanthia, K.; Prayoonpokarach, S. An optical sensing film for the determination of Co(II) based on disodium-1-nitroso-2-naphthol-3,6-disulfonate immobilized in chitosan film. Sens. Actuators, B 2012, 166–167, 772–776. [Google Scholar] [CrossRef]
- Nur, Y.; Rohaeti, E.; Darusman, L.K. Optical Sensor for the Determination of Lead(II) Based On Immobilization of Dithizone onto Chitosan-Silica Membrane. Indones. J. Chem. 2017, 17, 7. [Google Scholar] [CrossRef] [Green Version]
- Azeman, N.H.; Arsad, N.; Bakar, A.A.A. Polysaccharides as the Sensing Material for Metal Ion Detection-Based Optical Sensor Applications. Sensors 2020, 20, 3924. [Google Scholar] [CrossRef] [PubMed]
- Fen, Y. Optical Properties of Crosslinked Chitosan Thin Film with Glutaraldehyde Using Surface Plasmon Resonance Technique. Am. J. Eng. Appl. Sci. 2011, 4, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix[4]arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators B 2012, 171–172, 287–293. [Google Scholar] [CrossRef]
- Fen, Y.; Yunus, W.; Yusof, N. Immobilization of Tetrabutyl Thiuram Disulfide in Chitosan Thin Film for Sensing Zinc Ion Using Surface Plasmon Resonance Spectroscopy. Sens. Mater. 2013, 25, 99. [Google Scholar]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Ab Rahman, W.B.W.; Huang, N.M.; Yaacob, M.H. Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb (II) ions. Sens. Actuators B 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric (III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Uygun, Z.O.; Ertugrul Uygun, H.D.; Ermis, N.; Canbay, E. Molecularly Imprinted Sensors — New Sensing Technologies. In Biosensors-Micro and Nanoscale Applications; IntechOpen: London, UK, 2015; pp. 85–108. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.N.; Oliveira, M.B.; Costa, R.R.; Mano, J.F. Chitosan/Chondroitin Sulfate Membranes Produced by Polyelectrolyte Complexation for Cartilage Engineering. Biomacromolecules 2016, 17, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Bakker, E. Optical determination of ionophore diffusion coefficients in plasticized poly(vinyl chloride) sensing films. Anal. Chim. Acta 2004, 511, 91–95. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Crouzier, T.; Picart, C. Ion Pairing and Hydration in Polyelectrolyte Multilayer Films Containing Polysaccharides. Biomacromolecules 2009, 10, 433–442. [Google Scholar] [CrossRef]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Klapiszewski, L.; Szalaty, T. Activated lignin and aminosilane- grafted silica as precursors in hybrid material production. Physicochem. Probl. Miner. Process. 2016, 52, 459–478. [Google Scholar]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Hawke, D.J.; Powell, H.K.J.; Simpson, S.L. Equilibrium modelling of interferences in the visible spectrophotometric determination of aluminium(III): Comparison of the chromophores chrome azurol S, eriochrome cyanine R and pyrocatechol violet, and stability constants for eriochrome cyanine R-aluminium complexes. Anal. Chim. Acta 1996, 319, 305–314. [Google Scholar] [CrossRef]
- Marczenko, Z.; Jarosz, M. Formation of ternary complexes of aluminium with some triphenylmethane reagents and cationic surfactants. Analyst 1982, 107, 1431. [Google Scholar] [CrossRef]
- Watsaka, S.; Sopa, T.; Piyanete, C. Quantitation of Aluminium Content in Waters and Soft Drinks by Spectrophotometry Using Erichrome Cyanine R. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1154–1161. [Google Scholar]
- Shokrollahi, A.; Ghaedi, M.; Niband, M.S.; Rajabi, H.R. Selective and sensitive spectrophotometric method for determination of sub-micro-molar amounts of aluminium ion. J. Hazard. Mater. 2008, 151, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Pearson, Description of test kit for Eriochrome Cyanine R method 8326 for Aluminium, 9; Software Engineering Hach Company: Boston, MA, USA, 2018.
- Sommerdijk, N.A.J.M.; Poppe, A.; Gibson, C.A.; Wright, J.D. Unexpected complexation behaviour of a sol–gel immobilised dye: The development of an optical copper(II) sensor. J. Mater. Chem. 1998, 8, 565–567. [Google Scholar] [CrossRef]
- Novoa-Carballal, R.; Pérez-Martín, R.; Blanco, M.; Sotelo, C.G.; Fassini, D.; Nunes, C.; Coimbra, M.A.; Silva, T.H.; Reis, R.L.; Vázquez, J.A. By-products of Scyliorhinus canicula, Prionace glauca and Raja clavata: A valuable source of predominantly 6S sulfated chondroitin sulfate. Carbohydr. Polym. 2017, 157, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carvallal, R.; Reis, R.L.; Antelo, L.T.; Pérez-Martín, R.I.; Valcarcel, J. Isolation and chemical characterization of chondroitin sulfate from cartilage by-products of blackmouth catshark (Galeus melastomus). Mar. Drugs 2018, 16, 344. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carballal, R.; Reis, R.L.; Pérez-Martín, R.I.; Valcarcel, J. Optimal isolation and characterisation of chondroitin sulfate from rabbit fish (Chimaera monstrosa). Carbohydr. Polym. 2019, 210, 302–313. [Google Scholar] [CrossRef] [Green Version]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.R.M.; Johny, A.; Silva, C.Q.; Azenha, M.A.; Vázquez, J.A.; Valcarcel, J.; Pereira, C.M.; Silva, A.F. Improved Metal Cation Optosensing Membranes through the Incorporation of Sulphated Polysaccharides. Molecules 2022, 27, 5026. https://doi.org/10.3390/molecules27155026
Santos PRM, Johny A, Silva CQ, Azenha MA, Vázquez JA, Valcarcel J, Pereira CM, Silva AF. Improved Metal Cation Optosensing Membranes through the Incorporation of Sulphated Polysaccharides. Molecules. 2022; 27(15):5026. https://doi.org/10.3390/molecules27155026
Chicago/Turabian StyleSantos, P. R. M., A. Johny, C. Q. Silva, M. A. Azenha, J. A. Vázquez, J. Valcarcel, C. M. Pereira, and A. F. Silva. 2022. "Improved Metal Cation Optosensing Membranes through the Incorporation of Sulphated Polysaccharides" Molecules 27, no. 15: 5026. https://doi.org/10.3390/molecules27155026
APA StyleSantos, P. R. M., Johny, A., Silva, C. Q., Azenha, M. A., Vázquez, J. A., Valcarcel, J., Pereira, C. M., & Silva, A. F. (2022). Improved Metal Cation Optosensing Membranes through the Incorporation of Sulphated Polysaccharides. Molecules, 27(15), 5026. https://doi.org/10.3390/molecules27155026