Highly Effective Proton-Conduction Matrix-Mixed Membrane Derived from an -SO3H Functionalized Polyamide
Abstract
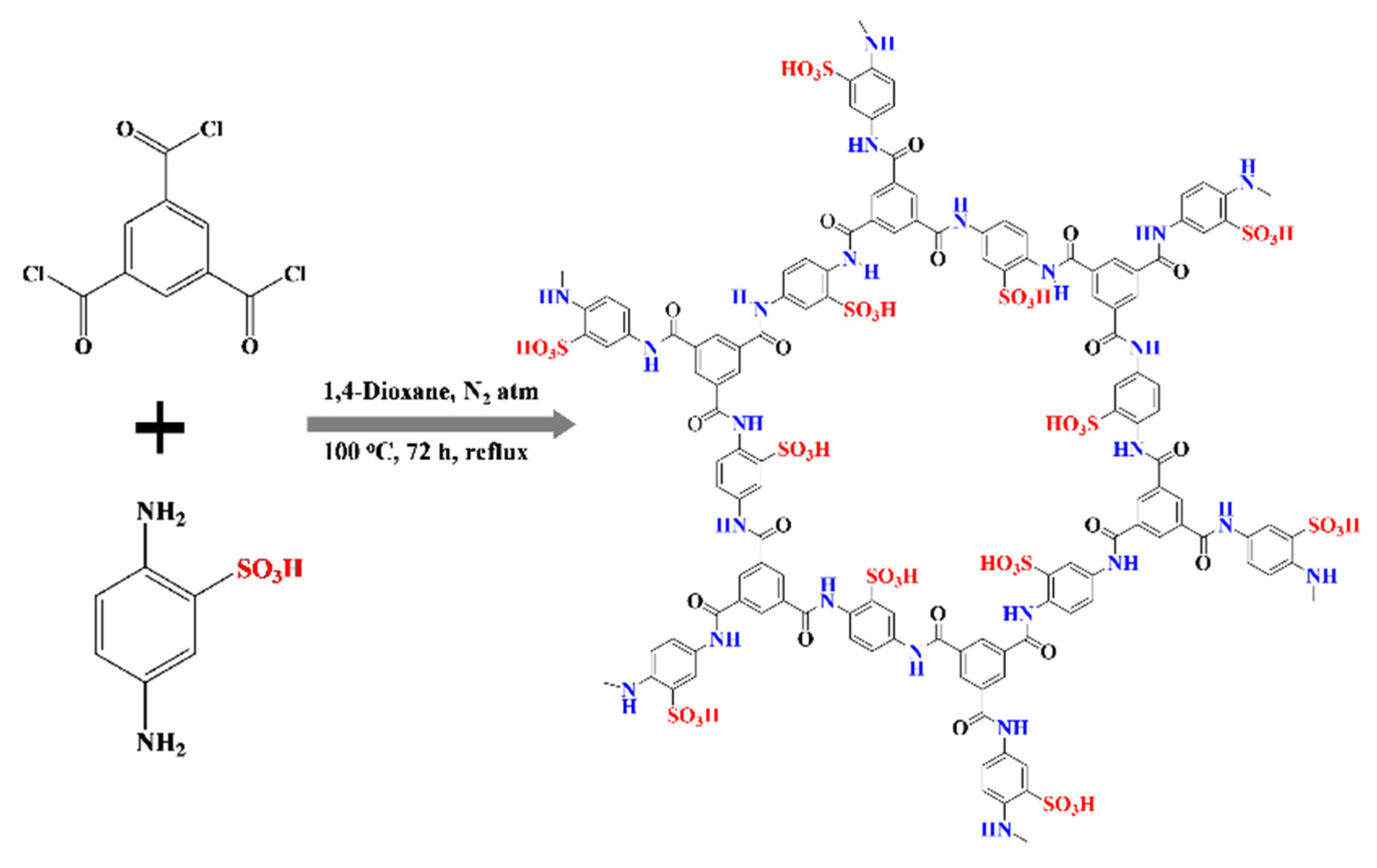
:1. Introduction
2. Results and Discussion
2.1. Morphology and Structural Analyses
2.2. Porosity and Thermal Stability Analyses
2.3. Impedance Analysis and Proton Conductivity of the Polyamides
2.4. Matrix-Mixed Membrane Fabrication and Characterizations
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PA-PhSO3H
3.3. Synthesis of PA-Ph
3.4. Fabrication of Matrix-Mixed Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Li, H.; Qiu, C.; Ren, S.; Dong, Q.; Zhang, S.; Zhou, F.; Liang, X.; Wang, J.; Li, S.; Yu, M. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 2020, 367, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Gao, W.; Guo, J.; Chen, P. Emerging Materials and Methods toward Ammonia-Based Energy Storage and Conversion. Adv. Mater. 2021, 33, 2005721. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Fu, F.; Astruc, D. Hydrogen Generation upon Nanocatalyzed Hydrolysis of Hydrogen-Rich Boron Derivatives: Recent Developments. Acc. Chem. Res. 2020, 53, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Kibria, M.G.; Mullins, C.B. Metal-free photocatalysts for hydrogen evolution. Chem. Soc. Rev. 2020, 49, 1887–1931. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, Y.; Liu, Y.; Song, C.; Li, Q.; Wang, D.; Li, Y. Designing Atomic Active Centers for Hydrogen Evolution Electrocatalysts. Angew. Chem. Int. Ed. 2020, 59, 20794–20812. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in Proton Conductors for Fuel-Cell Applications: Simulations, Elementary Reactions, and Phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [Green Version]
- Kraytsberg, A.; Ein-Eli, Y. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells-A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Zhu, M.; Iwano, T.; Tan, M.; Akutsu, D.; Uchida, S.; Chen, G.; Fang, X. Macrocyclic Polyoxometalates: Selective Polyanion Binding and Ultrahigh Proton Conduction. Angew. Chem. Int. Ed. 2022, 61, e202200666. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Knoll, S.; Liu, R.; Li, G.; Peng, Q.; Qiu, P.; He, D.; Streb, C.; Chen, X. High Proton-Conductivity in Covalently Linked Polyoxometalate-Organoboronic Acid-Polymers. Angew. Chem. Int. Ed. 2021, 60, 16953–16957. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xu, A.; Pan, D.; Zhao, T. Aqueous proton-selective conduction across two-dimensional graphyne. Nat. Commun. 2019, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, H.; Shen, Y.; Li, X.-Z.; Wang, E.G.; Meng, S. Transparent proton transport through a two-dimensional nanomesh material. Nat. Commun. 2019, 10, 3971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-S.M.; Otake, K.-I.; Kitagawa, S. Transport properties in porous coordination polymers. Coord. Chem. Rev. 2020, 421, 213447. [Google Scholar] [CrossRef]
- Lim, D.-W.; Kitagawa, H. Proton Transport in Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef]
- Ye, Y.; Gong, L.; Xiang, S.; Zhang, Z.; Chen, B. Metal-Organic Frameworks as a Versatile Platform for Proton Conductors. Adv. Mater. 2020, 32, 1907090. [Google Scholar] [CrossRef]
- Karmakar, A.; Illathvalappil, R.; Anothumakkool, B.; Sen, A.; Samanta, P.; Desai, A.V.; Kurungot, S.; Ghosh, S.K. Hydrogen-Bonded Organic Frameworks (HOFs): A New Class of Porous Crystalline Proton-Conducting Materials. Angew. Chem. Int. Ed. 2016, 55, 10667–10671. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; He, G.; Benziger, J. Differences in Water Sorption and Proton Conductivity Between Nafion and SPEEK. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1437–1445. [Google Scholar] [CrossRef]
- Paul, D.K.; McCreery, R.; Karan, K. Proton Transport Property in Supported Nafion Nanothin Films by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2014, 161, F1395–F1402. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yin, L.; Cheng, D.; Zhao, S.; Zang, H.-Y.; Zhang, N.; Zhu, G. Surface-Mediated Construction of an Ultrathin Free-Standing Covalent Organic Framework Membrane for Efficient Proton Conduction. Angew. Chem. Int. Ed. 2021, 60, 14875–14880. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wu, H.; Cao, Y.; Fan, C.; Zhao, R.; He, X.; Yang, P.; Shi, B.; You, X.; Jiang, Z. Weakly Humidity-Dependent Proton-Conducting COF Membranes. Adv. Mater. 2020, 32, 2005565. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xu, G.; Hu, Z.; Cheng, Y.; Chi, C.; Yuan, D.; Cheng, H.; Zhao, D. Mechanoassisted Synthesis of Sulfonated Covalent Organic Frameworks with High Intrinsic Proton Conductivity. ACS Appl. Mater. Interfaces 2016, 8, 18505–18512. [Google Scholar] [CrossRef]
- Chandra, S.; Kundu, T.; Dey, K.; Addicoat, M.; Heine, T.; Banerjee, R. Interplaying Intrinsic and Extrinsic Proton Conductivities in Covalent Organic Frameworks. Chem. Mater. 2016, 28, 1489–1494. [Google Scholar] [CrossRef]
- Yang, S.-J.; Ding, X.; Han, B.-H. Conjugated Microporous Polymers with Dense Sulfonic Acid Groups as Efficient Proton Conductors. Langmuir 2018, 34, 7640–7646. [Google Scholar] [CrossRef] [PubMed]
- Furtmair, M.; Timm, J.; Marschall, R. Sulfonation of porous materials and their proton conductivity. Micropor. Mesopor. Mater. 2021, 312, 110745. [Google Scholar] [CrossRef]
- Bhanja, P.; Palui, A.; Chatterjee, S.; Kaneti, Y.V.; Na, J.; Sugahara, Y.; Bhaumik, A.; Yamauchi, Y. Crystalline Porous Organic Polymer Bearing −SO3H Functionality for High Proton Conductivity. ACS Sustain. Chem. Eng. 2020, 8, 2423–2432. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Y.; Luan, T.-X.; Cheng, K.; Li, C.; Li, P.-Z. Facile construction of a click-based robust porous organic polymer and its in-situ sulfonation for proton conduction. Micropor. Mesopor. Mater. 2021, 325, 111348. [Google Scholar] [CrossRef]
- Zou, X.-N.; Zhang, D.; Xie, Y.; Luan, T.-X.; Li, W.; Li, L.; Li, P.-Z. High Enhancement in Proton Conductivity by Incorporating Sulfonic Acids into a Zirconium-Based Metal-Organic Framework via “Click” Reaction. Inorg. Chem. 2021, 60, 10089–10094. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Zhao, Y. Click chemistry as a versatile reaction for construction and modification of metal-organic frameworks. Coord. Chem. Rev. 2019, 380, 484–518. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y. A Triazole-Containing Metal−Organic Framework as a Highly Effective and Substrate Size-Dependent Catalyst for CO2 Conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Z.; Wang, X.-J.; Tan, S.Y.; Ang, C.Y.; Chen, H.; Liu, J.; Zou, R.; Zhao, Y. Clicked Isoreticular Metal–Organic Frameworks and Their High Performance in the Selective Capture and Separation of Large Organic Molecules. Angew. Chem. Int. Ed. 2015, 54, 12748–12752. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, J.; Guo, B.-B.; Agarwal, S.; Greiner, A.; Xu, Z.-K. Interfacial Polymerization at the Alkane/Ionic Liquid Interface. Angew. Chem. Int. Ed. 2021, 60, 14636–14643. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Guo, Z.-X.; Qiu, T.; Tuo, X. Construction of Aramid Engineering Materials via Polymerization-Induced Para-Aramid Nanofiber Hydrogel. Adv. Mater. 2021, 33, 2101280. [Google Scholar] [CrossRef]
- Zeng, Y.; Gordiichuk, P.; Ichihara, T.; Zhang, G.; Sandoz-Rosado, E.; Wetzel, E.D.; Tresback, J.; Yang, J.; Kozawa, D.; Yang, Z.; et al. Irreversible synthesis of an ultrastrong two-dimensional polymeric material. Nature 2022, 602, 91–95. [Google Scholar] [CrossRef]
- García, J.M.; García, F.C.; Serna, F.; de la Pena, J.L. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, P.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Meng, X.; Wang, H.; Song, S.; Zhang, H. Proton-conducting crystalline porous materials. Chem. Soc. Rev. 2017, 46, 464–480. [Google Scholar] [CrossRef]
- Hou, G.-L.; Wang, X.-B. Molecular specificity and proton transfer mechanisms in aerosol prenucleation clusters relevant to new particle formation. Acc. Chem. Res. 2020, 53, 2816–2827. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P.; Hao, L.; Cheng, P.; Chen, Y.; Zhang, Z. Grotthuss Proton-Conductive Covalent Organic Frameworks for Efficient Proton Pseudocapacitors. Angew. Chem. Int. Ed. 2021, 60, 21838–21845. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Liu, Y.; Coppens, M.-O.; Jiang, Z. Mixed-dimensional membranes: Chemistry and structure-property relationships. Chem. Soc. Rev. 2021, 50, 11747–11765. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Khraisheh, M.; AlMomani, F. Innovative BPPO Anion Exchange Membranes Formulation Using Diffusion Dialysis-Enhanced Acid Regeneration System. Membranes 2021, 11, 311. [Google Scholar] [CrossRef]
- Ali, F.; Saeed, S.; Shah, S.S.; Rahim, F.; Duclaux, L.; Levêque, J.-M.; Reinert, L. Sulfonated Polyimide-Clay Thin Films for Energy Application. Recent Pat. Nanotechnol. 2016, 10, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, B.; Zhou, Y.; Xu, C.; Chen, W.; Zhao, X.; Li, J. Sulfonated aromatic polyamides containing nitrile groups as proton exchange fuel cell membranes. Int. J. Hydrogen Energy 2015, 40, 6422–6429. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Shin, D.W.; Lee, S.Y.; Kang, N.R.; Lee, Y.M.; Guiver, M.D. Fluorene-Based Poly(arylene ether sulfone)s Containing Clustered Flexible Pendant Sulfonic Acids as Proton Exchange Membranes. Macromolecules 2011, 44, 7296–7306. [Google Scholar] [CrossRef] [Green Version]





| Temperature | 98%RH | 85%RH | 75%RH | 63%RH | 53%RH | 43%RH | 33%RH |
|---|---|---|---|---|---|---|---|
| 80 °C | 8.85 × 10−2 | 7.63 × 10−2 | 1.09 × 10−2 | 3.64 × 10−4 | 5.76 × 10−6 | 3.18 × 10−6 | 2.60 × 10−6 |
| 70 °C | 5.32 × 10−2 | 4.55 × 10−2 | 3.86 × 10−3 | 1.30 × 10−4 | 3.66 × 10−6 | 2.69 × 10−6 | 2.23 × 10−6 |
| 60 °C | 4.50 × 10−2 | 3.37 × 10−2 | 2.32 × 10−3 | 8.42 × 10−6 | 3.32 × 10−6 | 2.10 × 10−6 | 2.02 × 10−6 |
| 50 °C | 1.17 × 10−2 | 1.05 × 10−2 | 1.54 × 10−3 | 6.48 × 10−6 | 2.13 × 10−6 | 1.86 × 10−6 | 1.80 × 10−6 |
| 40 °C | 5.83 × 10−3 | 4.27 × 10−3 | 7.66 × 10−4 | 4.68 × 10−6 | 1.83 × 10−6 | 1.70 × 10−6 | 1.64 × 10−6 |
| 30 °C | 2.18 × 10−3 | 2.07 × 10−3 | 5.58 × 10−4 | 3.31 × 10−6 | 1.68 × 10−6 | 1.55 × 10−6 | 1.17 × 10−6 |
| Membranes | IEC (mmol/g) | WU (%) | Dimensional Stability (%) | Swelling Ratio (%) | Chemical Stability | Hydrolytic Stability | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (h) | T (h) | ||||||||||||
| IECT | IECExp. | 30 °C | 80 °C | 30 °C | 80 °C | 30 °C | 80 °C | 30 °C | 80 °C | 30 °C | 80 °C | 50 °C | |
| PA-PhSO3H-PAN (3:1) | 2.95 | 2.89 | 16.22 | 28.13 | 3.32 | 4.21 | 4.08 | 5.62 | 4.66 | 6.80 | 18 | 18 | 120 |
| PA-PhSO3H-PAN (1:1) | 2.60 | 2.52 | 8.14 | 15.33 | 2.54 | 2.87 | 2.14 | 4.46 | 2.54 | 3.89 | 18 | 18 | 120 |
| PA-PhSO3H-PAN (0.4:1) | 1.91 | 1.85 | 4.76 | 9.20 | 0.95 | 1.18 | 1.24 | 1.98 | 1.47 | 3.03 | 18 | 12 | 96 |
| PA-PhSO3H-PAN (0.1:1) | 1.62 | 1.57 | 2.17 | 4.13 | 0.45 | 0.96 | 0.72 | 1.6 | 1.28 | 1.91 | 18 | 6 | 84 |
| Temperature | PA-PhSO3H-PAN (0.1:1) | PA-PhSO3H-PAN (0.4:1) | PA-PhSO3H-PAN (1:1) | PA-PhSO3H-PAN (3:1) |
|---|---|---|---|---|
| 80 °C | 7.61 × 10−3 | 1.18 × 10−2 | 2.89 × 10−2 | 4.90 × 10−2 |
| 70 °C | 7.20 × 10−3 | 1.02 × 10−2 | 2.68 × 10−2 | 4.56 × 10−2 |
| 60 °C | 6.73 × 10−3 | 9.54 × 10−3 | 2.35 × 10−2 | 4.13 × 10−2 |
| 50 °C | 6.40 × 10−3 | 9.21 × 10−3 | 2.02 × 10−2 | 3.51 × 10−2 |
| 40 °C | 5.90 × 10−3 | 8.86 × 10−3 | 1.82 × 10−2 | 2.99 × 10−2 |
| 30 °C | 5.34 × 10−3 | 8.35 × 10−3 | 1.61 × 10−2 | 2.57 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, J.; Fu, Y.; Luan, T.-X.; Su, Z.; Li, P.-Z. Highly Effective Proton-Conduction Matrix-Mixed Membrane Derived from an -SO3H Functionalized Polyamide. Molecules 2022, 27, 4110. https://doi.org/10.3390/molecules27134110
Afzal J, Fu Y, Luan T-X, Su Z, Li P-Z. Highly Effective Proton-Conduction Matrix-Mixed Membrane Derived from an -SO3H Functionalized Polyamide. Molecules. 2022; 27(13):4110. https://doi.org/10.3390/molecules27134110
Chicago/Turabian StyleAfzal, Jamal, Yaomei Fu, Tian-Xiang Luan, Zhongmin Su, and Pei-Zhou Li. 2022. "Highly Effective Proton-Conduction Matrix-Mixed Membrane Derived from an -SO3H Functionalized Polyamide" Molecules 27, no. 13: 4110. https://doi.org/10.3390/molecules27134110
APA StyleAfzal, J., Fu, Y., Luan, T.-X., Su, Z., & Li, P.-Z. (2022). Highly Effective Proton-Conduction Matrix-Mixed Membrane Derived from an -SO3H Functionalized Polyamide. Molecules, 27(13), 4110. https://doi.org/10.3390/molecules27134110







