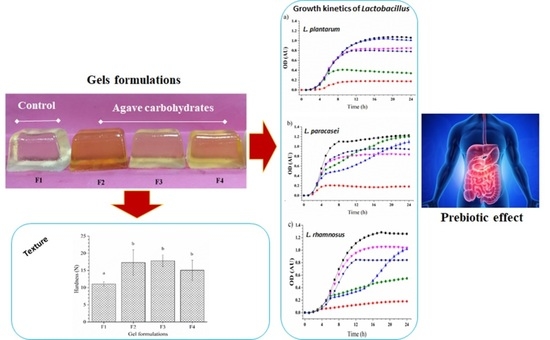
Physical Properties and Prebiotic Activities (Lactobacillus spp.) of Gelatine-Based Gels Formulated with Agave Fructans and Agave Syrups as Sucrose and Glucose Substitutes
Abstract
:1. Introduction
2. Results
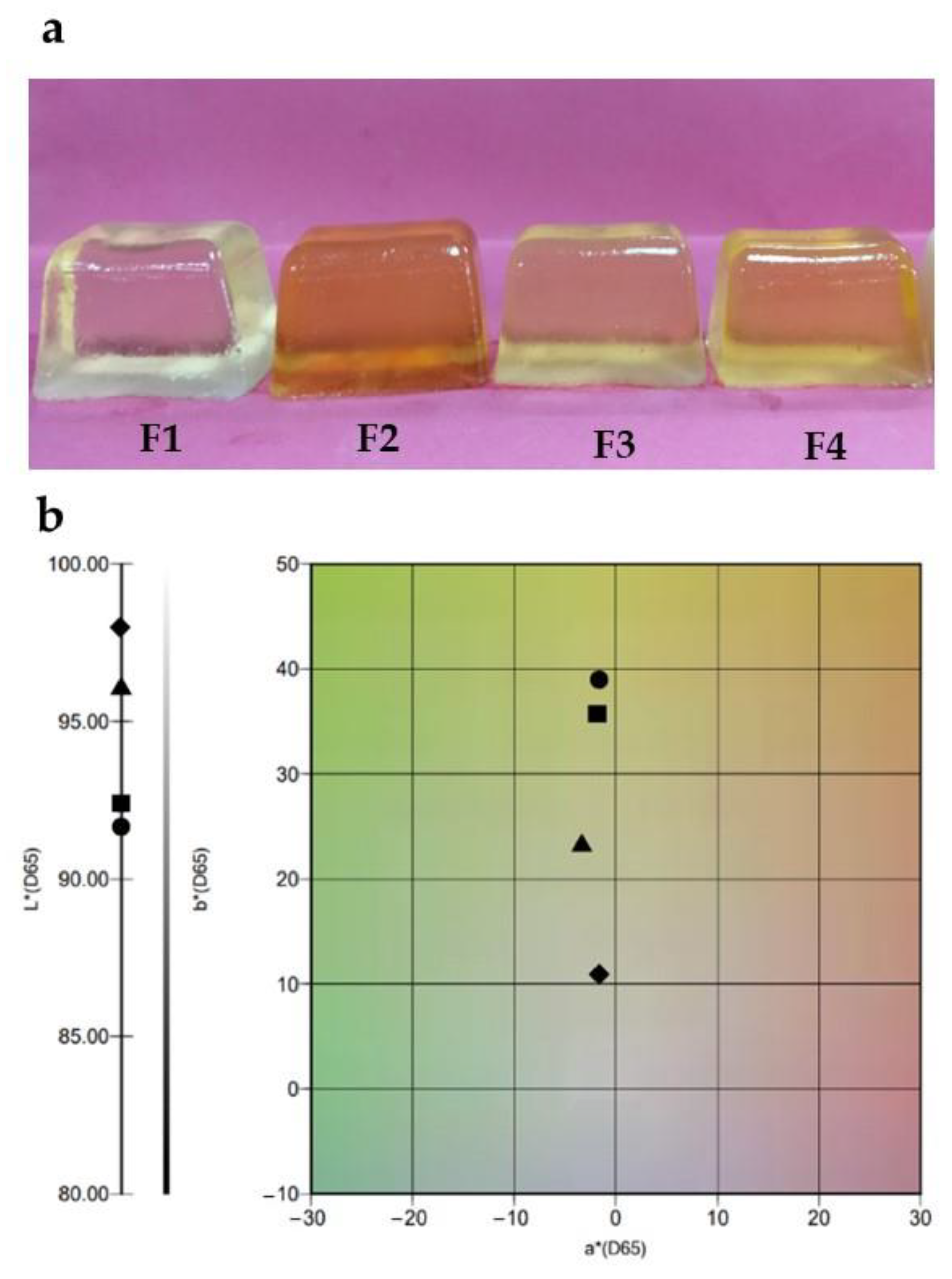
2.1. Physicochemical Properties of Gels
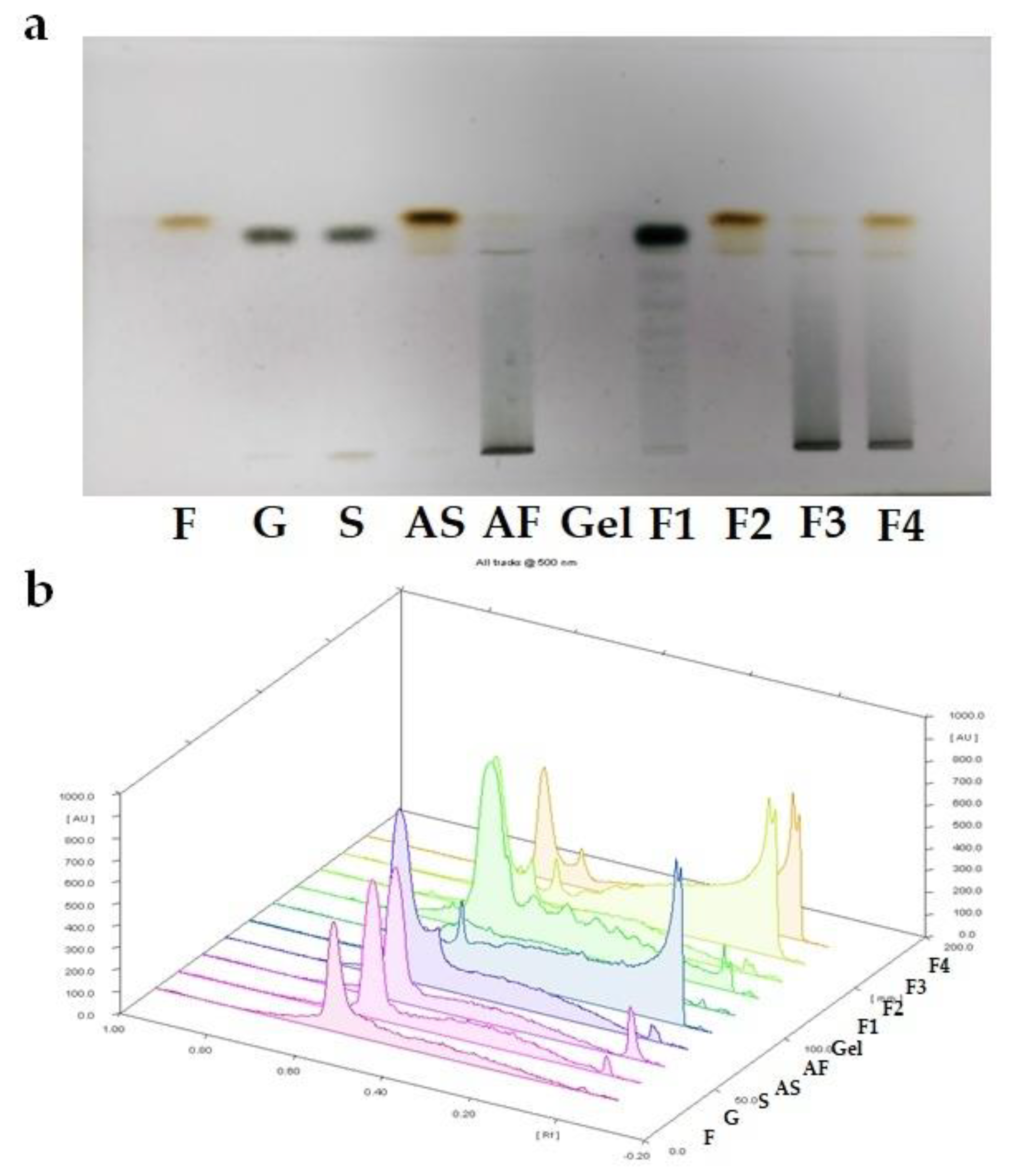
2.2. HP-TLC Analysis
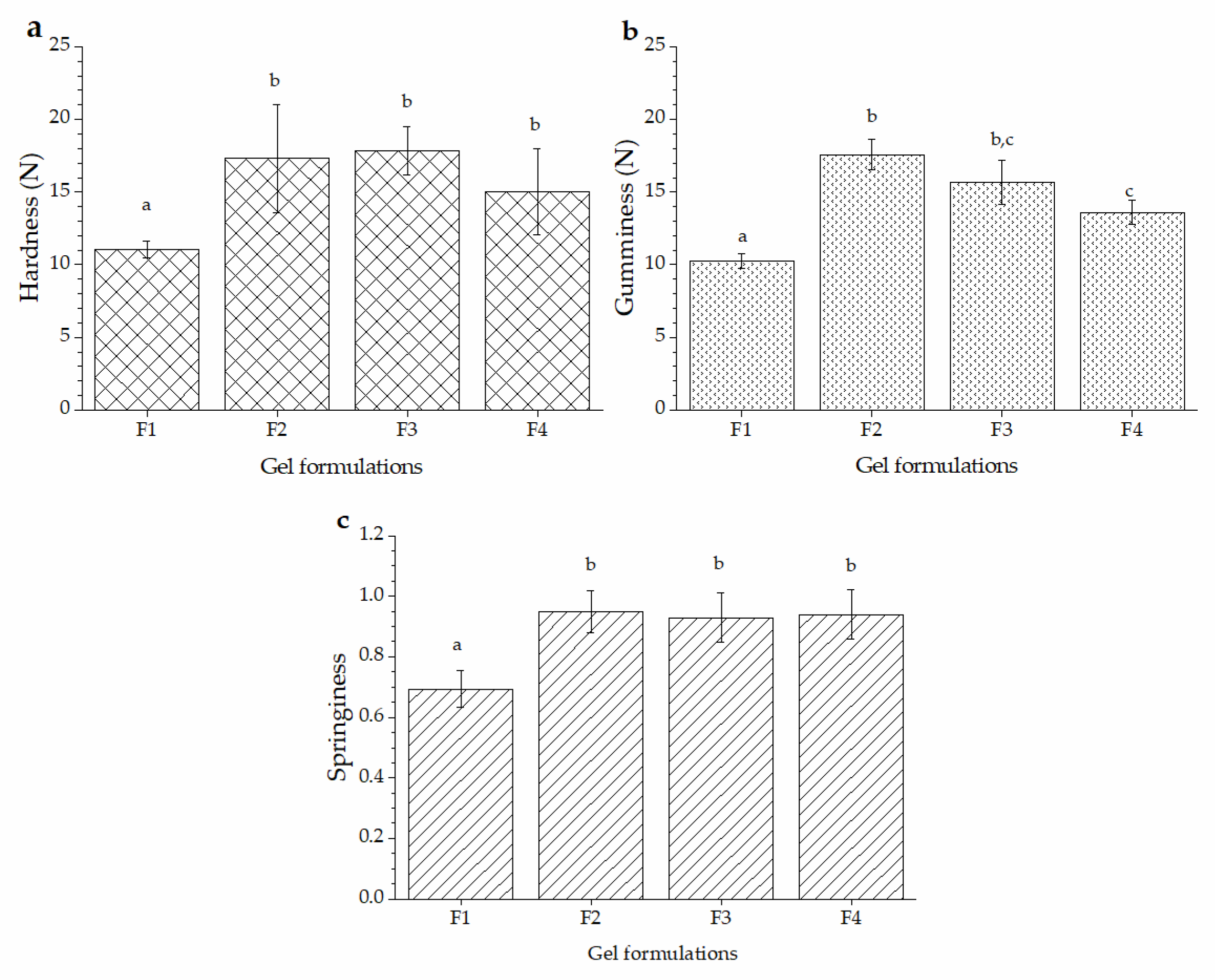
2.3. Texture Profile Analysis
2.4. Probiotic Growth Kinetics
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Physical Properties
Colour, Water Content, Water Activity, and pH of Gels
3.4. HP-TLC Analysis
3.5. Texture Analysis
3.6. Prebiotic Effect
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levaillant, M.; Lièvre, G.; Baert, G. Ending diabetes in Mexico. Lancet 2019, 394, 467–468. [Google Scholar] [CrossRef] [Green Version]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=Globally%2C%20there%20has%20been%3A,of%20transportation%2C%20and%20increasing%20urbanization (accessed on 30 April 2022).
- Belščak-Cvitanović, A.; Komes, D.; Dujmović, M.; Karlović, S.; Biškić, M.; Brnčić, M.; Ježek, D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015, 167, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Santiago-García, P.; Mellado-Mojica, E.; León-Martínez, F.M.; Dzul-Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Fructans (agavins) from Agave angustifolia and Agave potatorum as fat replacement in yogurt: Effects on physicochemical, rheological, and sensory properties. LWT 2021, 140, 110846. [Google Scholar] [CrossRef]
- González-Herrera, S.M.; Rocha-Guzmán, N.E.; Simental-Mendía, L.E.; Rodríguez-Herrera, R.; Aguilar, C.N.; Rutiaga-Quiñones, O.M.; López, M.G.; Gamboa-Gómez, C.I. Dehydrated apple-based snack supplemented with Agave fructans exerts prebiotic effect regulating the production of short-chain fatty acid in mice. J. Food Processing Preserv. 2019, 43, e14026. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Urías-Silva, J.E.; Morales-Hernández, N. The role of agave fructans in health and food applications: A review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; dos Santos Pereira, E.; de Oliveira Raphaelli, C.; Radünz, M.; Camargo, T.M.; da Rocha Concenço, F.I.G.; Cantillano, R.F.F.; Fiorentini, Â.M.; Nora, L. Application of prebiotics in apple products and potential health benefits. J. Food Sci. Technol. 2022, 59, 1249–1262. [Google Scholar] [CrossRef]
- Arellano-Plaza, M.; Paez-Lerma, J.B.; Soto-Cruz, N.O.; Kirchmayr, M.R.; Gschaedler Mathis, A. Mezcal production in Mexico: Between tradition and commercial exploitation. Front. Sustain. Food Syst. 2022, 6, 832532. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Water state diagram and thermal properties of fructans powders. J. Therm. Anal. Calorim. 2018, 132, 197–204. [Google Scholar] [CrossRef]
- García-Gamboa, R.; Ortiz-Basurto, R.I.; Calderón-Santoyo, M.; Bravo-Madrigal, J.; Ruiz-Álvarez, B.E.; González-Avila, M. In vitro evaluation of prebiotic activity, pathogen inhibition and enzymatic metabolism of intestinal bacteria in the presence of fructans extracted from agave: A comparison based on polymerization degree. LWT 2018, 92, 380–387. [Google Scholar] [CrossRef]
- Jiménez-Avalos, J.A.; Arrevillaga-Boni, G.; González-López, L.; García-Carvajal, Z.Y.; González-Avila, M. Classical methods and perspectives for manipulating the human gut microbial ecosystem. Crit. Rev. Food Sci. Nutr. 2021, 61, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barajas-Álvarez, P.; González-Ávila, M.; Espinosa-Andrews, H. Microencapsulation of Lactobacillus rhamnosus HN001 by spray drying and its evaluation under gastrointestinal and storage conditions. LWT 2022, 153, 112485. [Google Scholar] [CrossRef]
- Michel-Barba, M.G.; Espinosa-Andrews, H.; García-Reyes, R.A.; Desjardins, Y.; González-Ávila, M. Effect of blueberry extract, carriers, and combinations on the growth rate of probiotic and pathogenic bacteria. Int. J. Food Sci. Nutr. 2019, 70, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ozuna, C.; Franco-Robles, E. Agave syrup: An alternative to conventional sweeteners? A review of its current technological applications and health effects. LWT 2022, 162, 113434. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of rosemary (Rosmarinus officinalis L.) extract as antioxidant in jelly candies made with fructan fibres and stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Gunes, R.; Palabiyik, I.; Konar, N.; Said Toker, O. Soft confectionery products: Quality parameters, interactions with processing and ingredients. Food Chem. 2022, 385, 132735. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA J. Food 2018, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Čižauskaitė, U.; Jakubaitytė, G.; Žitkevičius, V.; Kasparavičienė, G. Natural ingredients-based gummy bear composition designed according to texture analysis and sensory evaluation in vivo. Molecules 2019, 24, 1442. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Andrews, H.; Páez-Hernández, G. Optimization of ultrasonication curcumin-hydroxylated lecithin nanoemulsions using response surface methodology. J. Food Sci. Technol. 2020, 57, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Luiz-Santos, N.; Prado-Ramírez, R.; Arriola-Guevara, E.; Camacho-Ruiz, R.-M.; Moreno-Vilet, L. Performance evaluation of tight ultrafiltration membrane systems at pilot scale for agave fructans fractionation and purification. Membranes 2020, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Ergun, R.; Lietha, R.; Hartel, R.W. Moisture and shelf life in sugar confections. Crit. Rev. Food Sci. Nutr. 2010, 50, 162–192. [Google Scholar] [CrossRef]
- Efe, N.; Bielejewski, M.; Tritt-Goc, J.; Mert, B.; Oztop, M.H. NMR relaxometry study of gelatin based low-calorie soft candies. Mol. Phys. 2019, 117, 1034–1045. [Google Scholar] [CrossRef]
- Zhang, Y.; Barringer, S. Effect of hydrocolloids, sugar, and citric acid on strawberry volatiles in a gummy candy. J. Food Process. Preserv. 2018, 42, e13327. [Google Scholar] [CrossRef]
- Michel-Cuello, C.; Gallegos Fonseca, G.; Maldonado Cervantes, E.; Aguilar Rivera, N. Effect of temperature and pH environment on the hydrolysis of maguey fructans to obtain fructose syrup. Rev. Mex. Ing. Quim. 2015, 14, 615–622. [Google Scholar]
- Espinosa-Andrews, H.; Urias-Silvas, J.E. Thermal properties of agave fructans (Agave tequilana Weber var. Azul). Carbohydr. Polym. 2012, 87, 2671–2676. [Google Scholar] [CrossRef]
- Jardines, A.P.; Arjona-Román, J.L.; Severiano-Pérez, P.; Totosaus-Sánchez, A.; Fiszman, S.; Escalona-Buendía, H.B. Agave fructans as fat and sugar replacers in ice cream: Sensory, thermal and texture properties. Food Hydrocoll. 2020, 108, 106032. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Yu, J.-H.; Yu, H. Effect of gelatin content and oral processing ability on vitamin C release in gummy jelly. J. Food Sci. Technol. 2021, 59, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hartel, W. Confectionery gels: Gelling behavior and gel properties of gelatin in concentrated sugar solutions. Food Hydrocoll. 2022, 124, 107132. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Katopo, L.; Pang, E.; Mantri, N.; Kasapis, S. Structural variation in gelatin networks from low to high-solid systems effected by honey addition. Food Res. Int. 2019, 121, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Richard, W. Citric acid and heating on gelatin hydrolysis and gelation in confectionery gels. Food Hydrocoll. 2022, 129, 107642. [Google Scholar] [CrossRef]
- González-Herrera, S.M.; Simental-Mendía, L.E.; López, M.G.; Rocha-Guzmán, N.E.; Rutiaga-Quiñones, O.M.; Rodríguez-Herrera, R.; Gamboa-Gómez, C.I. Effect of agave fructans on the production of short chain fatty acid in mice. Food Sci. Biotechnol. 2019, 28, 1493–1498. [Google Scholar] [CrossRef]
- Lee, Y.K.; Margolles, A.; Mayo, B.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilán, C.; Chapman, T.A.; Chin, J.J.; Crittenden, R.; Donohue, D.; et al. Probiotic Microorganisms. In Handbook of Probiotics and Prebiotics; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2008; pp. 1–176. [Google Scholar]
- Gänzle, M.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, D.; Mital, B.K.; Garg, S.K. Utilization of sugars by Lactobacillus acidophilus strains. Int. J. Food Microbiol. 1990, 10, 51–57. [Google Scholar] [CrossRef]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of selected probiotic strains with fructans from different sources relating to degree of polymerization and structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Yang, F.; Sun, H.; Zhou, X.; Guo, Y.; Wang, X.; Zhang, M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, H.; Zhang, R.; Dong, L.; Liu, L.; Ma, Y.; Jia, X.; Wang, G.; Zhang, M. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef]
- Alvarado, C.; Camacho, R.M.; Cejas, R.; Rodríguez, J.A. Profiling of commmercial agave fructooligosaccharides using ultrafiltration and high performance thin layer cromatography. Rev. Mex. Ing. Quim. 2014, 13, 417–427. [Google Scholar]

| Gel Formulations | ||||
|---|---|---|---|---|
| Parameter | F1 | F2 | F3 | F4 |
| L* | 97.98 ± 0.05 a | 92.27 ± 1.18 b | 96.06 ± 0.06 c | 91.73 ± 0.51 d |
| a* | −1.73 ± 0.04 a | −1.90 ± 0.12 b | −3.41 ± 0.07 c | −1.58 ± 0.23 d |
| b* | 10.86 ± 0.32 b | 35.86 ± 0.13 b | 23.08 ± 0.10 c | 39.09 ± 0.52 d |
| Water content (g/100 g) | 38.98 ± 0.14 a | 41.86 ± 0.28 b | 40.37 ± 0.38 c | 40.25 ± 0.06 c |
| Water activity | 0.890 ± 0.005 a,b | 0.823 ± 0.001 c | 0.929 ± 0.001 a | 0.855 ± 0.019 b,c |
| pH | 3.50 ± 0.01 a,b | 3.51 ± 0.01 a | 3.43 ± 0.01 c | 3.45 ± 0.01 b,c |
| Maximum Growth Rates, µmax (h−1) | |||
|---|---|---|---|
| Sample | L. plantarum | L. paracasei | L. rhamnosus |
| MRS | 0.155 ± 0.001 ab | 0.280 ± 0.013 a | 0.176 ± 0.001 a |
| MRSm | 0.042 ± 0.001 e | 0.076 ± 0.006 e | 0.024 ± 0.000 f |
| F1 | 0.152 ± 0.002 b | 0.049 ± 0.005 f 0.146 ± 0.009 d,* | 0.068 ± 0.001 e 0.084 ± 0.003 d,* |
| F2 | 0.162 ± 0.002 c | 0.199 ± 0.007 b,c | 0.152 ± 0.002 b |
| F3 | 0.117 ± 0.001 d | 0.054 ± 0.002 f 0.184 ± 0.002 c,* | 0.024 ± 0.001 f 0.085 ± 0.002 d,* |
| F4 | 0.157 ± 0.002 a | 0.205 ± 0.005 b | 0.137 ± 0.002 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rodríguez, R.; Barajas-Álvarez, P.; Morales-Hernández, N.; Camacho-Ruíz, R.M.; Espinosa-Andrews, H. Physical Properties and Prebiotic Activities (Lactobacillus spp.) of Gelatine-Based Gels Formulated with Agave Fructans and Agave Syrups as Sucrose and Glucose Substitutes. Molecules 2022, 27, 4902. https://doi.org/10.3390/molecules27154902
Rodríguez-Rodríguez R, Barajas-Álvarez P, Morales-Hernández N, Camacho-Ruíz RM, Espinosa-Andrews H. Physical Properties and Prebiotic Activities (Lactobacillus spp.) of Gelatine-Based Gels Formulated with Agave Fructans and Agave Syrups as Sucrose and Glucose Substitutes. Molecules. 2022; 27(15):4902. https://doi.org/10.3390/molecules27154902
Chicago/Turabian StyleRodríguez-Rodríguez, Rogelio, Paloma Barajas-Álvarez, Norma Morales-Hernández, Rosa María Camacho-Ruíz, and Hugo Espinosa-Andrews. 2022. "Physical Properties and Prebiotic Activities (Lactobacillus spp.) of Gelatine-Based Gels Formulated with Agave Fructans and Agave Syrups as Sucrose and Glucose Substitutes" Molecules 27, no. 15: 4902. https://doi.org/10.3390/molecules27154902
APA StyleRodríguez-Rodríguez, R., Barajas-Álvarez, P., Morales-Hernández, N., Camacho-Ruíz, R. M., & Espinosa-Andrews, H. (2022). Physical Properties and Prebiotic Activities (Lactobacillus spp.) of Gelatine-Based Gels Formulated with Agave Fructans and Agave Syrups as Sucrose and Glucose Substitutes. Molecules, 27(15), 4902. https://doi.org/10.3390/molecules27154902