Abstract
In silico evaluation of various regioisomeric 5- and 3-hydroxy-substituted alkyl 1-aryl-1H-pyrazole-4-carboxylates and their acyclic precursors yielded promising results with respect to their binding in the active site of dihydroorotate dehydrogenase of Plasmodium falciparum (PfDHODH). Consequently, four ethyl 1-aryl-5-hydroxy-1H-pyrazole-4-carboxylates and their 3-hydroxy regioisomers were prepared by two-step syntheses via enaminone-type reagents or key intermediates. The synthesis of 5-hydroxy-1H-pyrazoles was carried out using the literature protocol comprising acid-catalyzed transamination of diethyl [(dimethylamino)methylene]malonate with arylhydrazines followed by base-catalyzed cyclization of the intermediate hydrazones. For the synthesis of isomeric methyl 1-aryl-3-hydroxy-1H-pyrazole-4-carboxylates, a novel two-step synthesis was developed. It comprises acylation of hydrazines with methyl malonyl chloride followed by cyclization of the hydrazines with tert-butoxy-bis(dimethylamino)methane. Testing the pyrazole derivatives for the inhibition of PfDHODH showed that 1-(naphthalene-2-yl)-5-hydroxy-1H-pyrazole-4-carboxylate and 1-(naphthalene-2-yl)-, 1-(2,4,6-trichlorophenyl)-, and 1-[4-(trifluoromethyl)phenyl]-3-hydroxy-1H-pyrazole-4-carboxylates (~30% inhibition) were slightly more potent than a known inhibitor, diethyl α-{[(1H-indazol-5-yl)amino]methylidene}malonate (19% inhibition).
1. Introduction
Malaria is a serious infectious disease that is endemic in many countries, especially in tropical regions. It threatens a significant portion of the world’s population, with over three billion people at risk of infection in 2013. It is also an important public health problem, with over 200 million clinical cases and 500,000 deaths per year [1]. The emergence of drug-resistant strains of Plasmodium falciparum (P. falciparum) has intensified the research and development of new drugs and therapies for malaria treatment [1,2,3,4,5,6]. Antimalarials with artemisinin-based combination therapies (ACTs) have been successful in the majority of malaria cases for some time, but artemisinin efficacy has been shown to decline in Southeast Asia, jeopardizing recent successes [7]. In some areas, P. falciparum has become resistant to all clinically approved antimalarials. As current trends clearly indicate that malarial disease will continue to impact global health, the discovery and development of new, effective antimalarial drugs is urgently needed.
Targeting the mitochondrial functions of P. falciparum has been successful for enzymes within the mitochondrial electron transport chain. Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) is the central enzyme in the de novo synthesis of pyrimidine nucleotides and thus key to parasite survival. This makes PfDHODH an attractive target for drug development to treat malaria [8,9,10,11]. Several types of selective inhibitors of PfDHODH are known, which are much less active compared to human DHODH. These include malonate (A) [12], an analogue of brequinar (B) [13], an analogue of leflunomide (C) [14], 5-methyl-7-{[4-(trifluoromethyl)phenyl]amino}[1,2,4]triazolo[1,5-a]pyrimidine (D) [15], 3-(benzimidazolyl)thiophene derivative E [16], and others [8] (Figure 1).

Figure 1.
Compounds A–E as examples of known inhibitors of PfDHODH.
Heterocyclic building blocks are useful scaffolds for applications in medicinal chemistry, catalysis, and materials science [17,18,19,20]. In medicinal chemistry, heterocyclic systems are commonly used to restrict the conformational flexibility of small molecules in order to increase their biological activity through enhanced interactions with the active site of the target macromolecule. Using malonate A, a known selective inhibitor of PfDHODH, as the lead compound [12] (cf. Figure 1), we reasoned that its conformationally constrained pyrazole analogues might also be selective inhibitors of PfDHODH, also because of the known examples of pyrazole derivatives with antimalarial activity [21,22,23,24,25]. As shown in Figure 2, formal cyclization of dialkyl 3-anilinomalonate leads to two isomeric alkyl pyrazolone carboxylates 1 and 2, which in turn are accessible by cyclocondensation methods with monosubstituted hydrazines 4 and α-alkoxymethylidene- or α-[(dimethylamino)methylidene]malonates 3 [26].
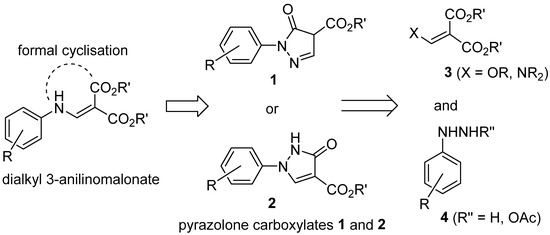
Figure 2.
Dialkyl 3-anilinomalonate and its conformationally constrained pyrazolone analogues 1 and 2.
As shown in Scheme 1, 1-aryl-5-hydroxy-1H-pyrazole-4-carboxylates 1 are readily available by condensation of arylhydrazines 4 with diethyl α-(ethoxymethylidene)malonate (3a) [27] or diethyl α-[(dimethylamino)methylidene]malonate (3b) [28] via enhydrazine intermediates 5 (Scheme 1A). In contrast, the synthesis of isomeric 1-aryl-3-hydroxy-1H-pyrazole-4-carboxylates 2 from methylidene malonates 3 is difficult because the less nucleophilic amino group of 4 must first react with the electrophile 3. So far, two general methods for the synthesis of 2 have been reported. The first method is a three-step synthesis in which N-acetyl-N′-arylhydrazine 4 is treated with 3a in PCl3 to give N-acetyl-N′-arylpyrazolone 7, followed by hydrolysis to give the carboxylic acid 8 [29] (Scheme 1B). The second approach is one-step condensation of 4 with 3a in the presence of a strong base, which deprotonates the more acidic amino group of 4 and makes it more reactive. Thus, the regioisomeric compounds 2 are obtained. However, despite the simplicity of the latter approach, the scope and the yields are usually only moderate [27] (Scheme 1B). Since the known methods for the synthesis of 2 are not optimal, there is a need for the development of improved syntheses of 2. We concluded that perhaps a 4 + 1 approach via cyclization of a C–C–N–N-type 1,4-dinucleophile 10 with a C1-electrophile could provide an elegant route to obtaining compounds 2. The arylhydrazines 4 are first acylated with methyl malonyl chloride (9) to give hydrazides 10. Subsequent treatment of 10 with tert-butoxy-bis(dimethylamino)methane (TBDMAM, Bredereck’s reagent) leads to the corresponding enaminone intermediates 11, which cyclize to the desired 3-pyrazolone-4-carboxylates 2 (Scheme 1C).

Scheme 1.
(A,B): Known syntheses of alkyl 1-aryl-1H-pyrazole-4-carboxylates 1 [27,28] and 2 [27,29]. (C): A novel synthesis of 2 reported in this paper.
Continuing our studies on the development of synthetic methods for the preparation of pyrazole derivatives and their evaluation as potential inhibitors of PfDHODH, we focused on the synthesis of pyrazolone esters 1 and 2 as potential lead structures. In the following, we report the results of this study, including the synthesis of 1-aryl-5-hydroxy-1H-pyrazole-4-carboxylates 1a–d, the development of a new synthetic method, the preparation of isomeric 1-aryl-3-hydroxy-1H-pyrazole-4-carboxylates 2a–d, and the evaluation of products 1a–d and 2a–d for the inhibition of PfDHODH.
2. Results and Discussion
2.1. In Silico Evaluation of Compounds 3a, 1a–d, 2a–d, 5a–d, and 10a–d
We began this study with the in silico evaluation of compounds with general structures 1 and 2 (cf. Figure 2) as possible inhibitors of PfDHODH. These compounds were subjected to molecular docking into the active site of PfDHODH [30,31] (Figure 3A). The active center contains the FMN prosthetic group and binding sites for dihydroorotate and the ubiquinone cofactor. The latter is shaped as a hydrophobic tunnel. It is much larger than the dihydroorotate binding site and exhibits comparatively higher interspecies variability, allowing for the higher selectivity of inhibitors for PfDHODH over HsDHODH. Therefore, this is the usual target site of choice for inhibitor development, for example, 1TV5 [31]. The dihydroorotate binding site has a suitable size for compounds such as the known inhibitors (2Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]but-2-enamide (Teriflunomide) (12) [30,31] and diethyl 2-{([1H-indazol-5-yl)amino]methylidene}malonate (3c) [12]. Accordingly, this binding site could also harbor the conformationally constrained pyrazolone analogues 1 and 2 and their precursors 5 and 10 (Figure 3B, cf. Figure 2), which also follow Lipinski’s rule of five [32,33,34]. Four characteristic aryl residues, 4-chlorophenyl (a), 2-naphthyl (b), 2,4,6-trichlorophenyl (c), and 4-trifluoromethylphenyl (d), were selected for the design of the target compounds 1a–d and 2a–d and their precursors (intermediates) 5a–d and 10a–d (Figure 3B). The selection of substituents a–d was based on the assumption that the N-aryl residues must be para-substituted to ensure lipophilic interactions with the hydrophobic tunnel. In addition, we chose the 2-naphthyl residue b because it represents a larger aromatic system and the 2,4,6-trichlorophenyl residue c due to its larger steric bulk, halogen bonding, and orthogonal bonding potential [35,36,37]. Both structural differences could affect hydrogen, halogen, and orthogonal bonding potential and/or hydrophobic and π–π interactions. For comparison with compounds 1, 2, 5, and 10, we decided to also investigate the known inhibitors 3c [12], 12 [31] (Figure 3B), A, C, and D [12,14,15] (cf. Figure 1) in this part of the study.
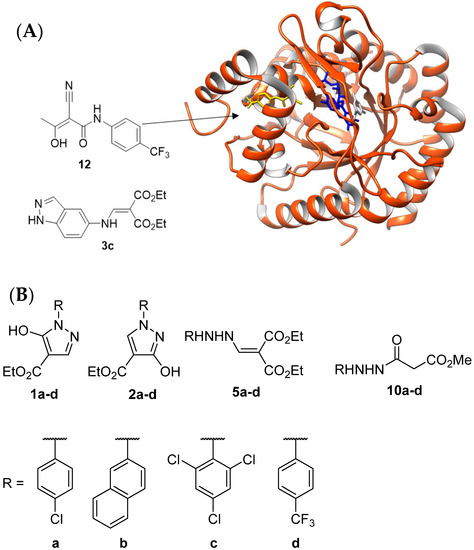
Figure 3.
(A): Three-dimensional structure of PfDHODH (PDB accession code 1TV5) highlighting positions of the binding sites in the active site. The protein is shown as ribbons, the prosthetic group FMN is shown as blue sticks, orotate is shown as gray sticks, and the known inhibitor Teriflunomide (12), bound to the quinone binding site, is shown as yellow sticks. (B): The structures of the in silico investigated compounds 1a–d, 2a–d, 5a–d, and 10a–d.
The binding affinities of compounds 1a–d, 2a–d, 5a–d, and 10a–d and known inhibitors 3c, 12 (cf. Figure 3), A, C, and D (cf. Figure 1) to PfDHODH were evaluated using Glide molecular docking software. The results are presented in the Supplementary Materials (Table S1), whereas the selected results for 1a–d, 2a–d, and known inhibitors 3a and 12 are shown in Table 1. In general, the pyrazole derivatives 1 and 2 had better docking scores than their open-chain hydrazine precursors 5 and 10 (Table S1). As expected, the known inhibitor 12 had the best overall score of about –10.5, whereas the score of the known inhibitor 3c was much lower at about –6.5 (Table 1, entries 9 and 10). Encouragingly, all synthesized compounds 1a–d (Dscores –5.52 to –7.80) and 2a–d (Dscores –6.85 to –8.39) performed better than the known inhibitor 3 (Dscores around –6.5) (Table 1, entries 1–9), with 3-hydroxypyrazole 2c consistently achieving a Dscore of around –8 with both docking methods (Table 1, entry 7).

Table 1.
Scores for docking of compounds 1a–d, 2a–d, 3c, and 12 to PfDHODH.
2.2. Synthesis of Compounds 1a–d, 2a–d, 3c, 5a–d, and 10a–d
Encouraged by the molecular docking results, we proceeded with the synthesis of target compounds 1, 2, and 3c. First, the reference inhibitor 3c and enhydrazines 5a–d were prepared in 70–94% yields by acid-catalyzed transamination of diethyl 2-[(dimethylamino)methylene]malonate (3b) [38] with 5-amino-1H-indazole (13) and arylhydrazines 4a–d, respectively. Subsequently, the enhydrazines 5a–d were cyclized in a 3:3:1 mixture of water, methanol, and triethylamine according to the literature procedure for the synthesis of closely related alkyl 1-aryl-5-hydroxy-1H-pyrazole-4-carboxylates [28] to afford the desired ethyl 1-aryl-5-hydroxy-1H-pyrazole-4-carboxylates 1a–d in 60–86% yields. The reaction pathway is explainable by the initial 1,4-addition of hydrazine 4 to the protonated enaminone 3′b, giving adduct 14, followed by the elimination of dimethylamine to give enhydrazine 5. The base-catalyzed intramolecular cyclocondensation of 5 then yields the 5-hydroxypyrazole derivative 1 (Scheme 2, Table 2).
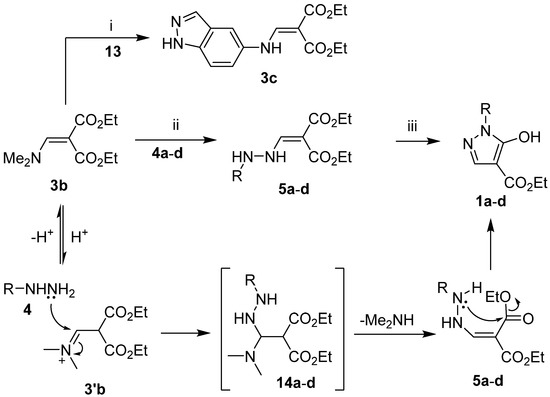
Scheme 2.
Synthesis of compounds 1a–d, 3c, and 5a–d. Reaction conditions: (i) 1H-indazol-5-amine (13) (1 equiv.), 37% aq. HCl (1 equiv.), EtOH, r.t., 24 h; (ii) hydrazine 4a–d hydrochloride (1 equiv.), EtOH, r.t., 24 h; (iii) H2O–EtOH–Et3N (3:3:1), reflux, 1.5 h.

Table 2.
Experimental data of compounds 1a–d, 3c, and 5a–d.
We then proceeded with the synthesis of the isomeric methyl 1-aryl-3-hydroxy-1H-pyrazole-4-carboxylates 2a–d according to the proposed two-step synthetic method (cf. Scheme 1). The acylation of arylhydrazines 4a–d with methyl 3-chloro-3-oxopropanoate (9) was carried out in anhydrous dichloromethane in the presence of one equivalent of triethylamine and gave the corresponding hydrazides 10a–d in 47–53% yields. The anhydrous conditions were essential for the successful performance of the first step. Subsequent treatment of hydrazides 10a–d with an equivalent amount of TBDMAM in anh. toluene at 40–110 °C gave the desired pyrazole derivatives 2a–d in 48–74% yields. It is noteworthy that mixtures of products were formed, and the yield of 2 decreased significantly unless heating was carried out first at 40 °C for half an hour and then at 110 °C. This observation is consistent with the proposed cyclization reaction pathway, in which the first selective formation of the enaminone intermediate 11 occurs at a slightly elevated temperature. After increasing the temperature to 110 °C, cyclization of the anilino group to the enaminone residue occurs via a 1,4-addition–elimination process to form the pyrazolone 2′, which tautomerizes to the desired 3-hydroxypyrazole derivative 2 (Scheme 3, Table 3).
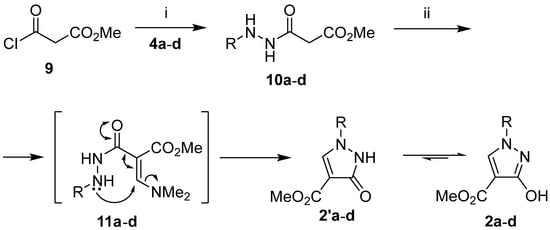
Scheme 3.
Synthesis of compounds 2a–d and 10a–d. Reaction conditions: (i) arylhydrazine 4a–d (1 equiv.), CH2Cl2, Et3N (2 equiv.), 0 °C, 20 min., then r.t., 2 h; (ii) TBDMAM (1 equiv.), toluene, 40 °C, 30 min., then reflux, 2 h.

Table 3.
Experimental data of compounds 8a–d and 10a–d.
2.3. Structure Determination
The structures of compounds 1a–d, 2a–d, 3c, 5a–d, and 10a–d were determined by spectroscopic methods (IR, 1H and 13C NMR, and MS-HRMS) and by elemental analyses for C, H, and N. The NMR data for compounds 1a–d, 2a–d, 3c, 5a–d, and 10a–d are in agreement with the literature data for related compounds [12,28,38,39,40,41]. Tautomerism of compounds 1 and 2 in the solid state was determined by IR spectroscopy (Figure 4). The IR spectra of compounds 1 and 2 exhibited typical C=O absorption bands at about 1700 cm−1, corresponding to the α,β-unsaturated ester carbonyl group, while compounds 1a,c,d and 2c also exhibited less intense vibrations at about 1640 cm−1, corresponding to the lactam carbonyl group of NH-tautomers 1′a,c,d and 2′c, respectively. Typical aliphatic ester vibrations around 1740 cm−1 characteristic of the CH-tautomeric forms 1′′ were not observed. Accordingly, compounds 1b and 2a,b,d exist in the solid state as OH-tautomers, while compounds 1a,c,d and 2c exist in the solid state as the NH-tautomers 1′a,c,d and 2′c, respectively. Due to possible intra- and intermolecular hydrogen bonding, the 1a–d and 2a–d tautomers in the solid state may also be a hybrid of OH- and NH-tautomers, which has already been observed in related pyrazolone derivatives (Figure 4) [42,43,44]. In the 1H NMR spectra of compounds 2a–d in DMSO-d6, the signal for the OH/NH proton appeared at about 11 ppm, while it was absent (exchanged) in the 1H NMR spectra of compounds 1a–d. This observation is consistent with a faster proton exchange in compounds 1a–d, which can be explained by stronger hydrogen bonding. The CH-tautomers 1′′ were not present in the 1H NMR spectra of compounds 1a–d, as evidenced by the absence of signals for the methine proton H–C(4). In the 1H and 13C NMR spectra of 5-hydroxypyrazoles 1a–d, the chemical shifts of the 3–H and 3–C nuclei were around 7.9 ppm and 155 ppm, respectively, whereas in the spectra of 3-hydroxypyrazoles 2a–d, the chemical shifts of the 5–H and 5–C nuclei were around 8.9 ppm and 162 ppm, respectively (Figure 4). These spectral data are in agreement with the typical spectral data of the related isomeric pyrazoles 1 and 2 [26,42,45].
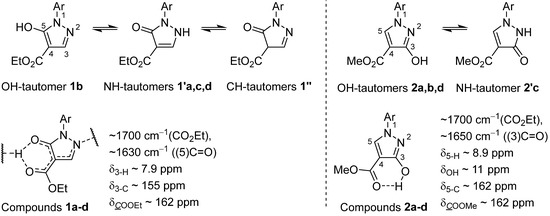
Figure 4.
The structure and tautomerism of pyrazoles 1a–d and 2a–d.
3. Inhibition of PfDHODH
The synthesized pyrazole derivatives 1a–d and 2a–d and the known inhibitor 3c were tested for their inhibition of PfDHODH. A colorimetric assay measuring the reduction of the redox dye 2,6-dichlorophenolindophenol (DCIP) coupled with the re-oxidation of the coenzyme was used. The results are shown in Figure 5 and Table 4. Compounds 1b and 2a–d showed weak inhibitory activity at a concentration of 50 μM. Their relative potencies were calculated as a percentage of inhibition compared to the uninhibited control (Table 4). All compounds studied were at best weak inhibitors of PfDHODH. The strongest potency was found for compounds 1b and 2b–d with approx. 30% inhibition of PfDHODH activity (Table 4, entries 2, 6–8). Compound 2a showed about 15% inhibition (Table 4, entry 5), while compounds 1a, 1c, and 1d did not show statistically significant inhibition of PfDHODH activity (Table 4, entries 1, 3, and 4). However, our assays also showed only weak activity of the known inhibitor 3c (19% inhibition; Table 4, entry 9). Thus, most of the synthesized compounds were equally active (2a) or even more active (1b and 2b–d) than the known inhibitor 3c (Table 4).
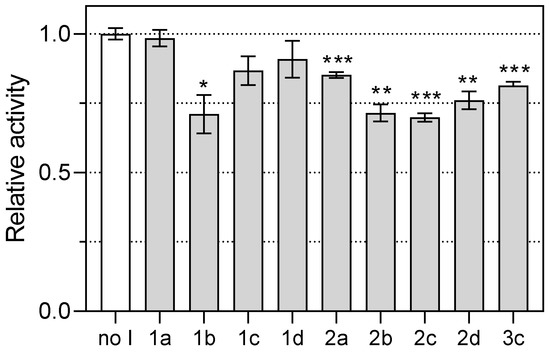
Figure 5.
Effect of compounds 1a–d, 2a–d, and 3c on PfDHODH activity. Asterisks denote statistically significant pairwise differences between the samples and uninhibited control (* p < 0.05, ** p < 0.01, *** p < 0.001) calculated with Welch’s t-test. The plot was drawn with GraphPad Prism 9.4 Software (GraphPad Inc., San Diego, CA, USA), which was also used for statistical comparison of data.

Table 4.
PfDHODH enzyme inhibition data for compounds 1a–d, 2a–d, and 3c.
Since the docking results showed quite solid complementarity between the binding site and the designed ligands (cf. Table 1, Section 2.1.), we expected encouraging results in the in vitro assays, but unfortunately, all ligands proved to be weakly bound to the receptor position. Ligand 2c had the best affinity for PfDHODH, with 30% inhibition at a concentration of 50 μM of the inhibitor (cf. Table 4, entry 7). The proposed docking of the selected compounds 3c, 2c, and 2d to the quinone binding site of the active site of PfDHODH is shown in Figure 6. The known inhibitor 3c shows hydrogen bonding interactions between the imine nitrogen and the histidine residue HIP185 and between the ester carbonyl oxygen and the arginine residue ARG265, as well as a π–π interaction between the indazole residue and the phenyl ring of the phenylalanine residue PHE188 (Figure 5A). In addition to the π–π interaction and hydrogen bonding of the ester group to the tyrosine residue TYR528, compounds 2c and 2d also exhibited hydrogen bonding of the 3-hydroxy group with two amino acid residues of PfDHODH, ARG265 and GLY181 (Figure 5B,C).
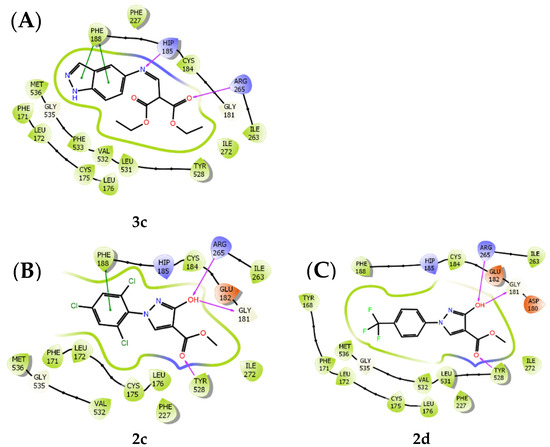
Figure 6.
Molecular docking of compounds 3c (A), 2c (B), and 2d (C) into the coenzyme-binding site of PfDHODH.
4. Experimental Section
4.1. General Methods
All solvents and reagents were used as received. Melting points were determined using the SRS OptiMelt MPA100—Automated Melting Point System (Stanford Research Systems, Sunnyvale, CA, USA). The 1H NMR and 13C NMR spectra were recorded in CDCl3 and DMSO-d6 as solvents using Me4Si as the internal standard on a Bruker Avance DPX 300 and Bruker Avance III UltraShield 500 plus instrument (Bruker, Billerica, MA, USA) at 300 and 500 MHz for 1 H and at 75.5 and 126 MHz for the 13 C nucleus, respectively. IR spectra were recorded on a Bruker FTIR Alpha Platinum spectrophotometer (Bruker, Billerica, MA, USA). Microanalyses were performed by combustion analysis on a Perkin-Elmer CHN Analyzer 2400 II (PerkinElmer, Waltham, MA, USA). Mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA). Column chromatography was performed on silica gel (Silica gel 60, particle size: 0.035–0.070 mm, Sigma-Aldrich, St. Louis, MO, USA).
1H-Indazol-5-amine (13), hydrazines 4a–d, methyl 3-chloro-3-oxopropanoate (9), and tert-butoxy-bis(dimethylamino)methane (TBDMAM, Bredereck’s reagent) are commercially available (Sigma-Aldrich). Compound 3b was prepared following the literature procedure [38].
Molecular docking was performed with Glide, a part of the Schrödinger suite software [46]. The crystal structure of PfDHODH retrieved from the Protein Data Bank under accession code 1TV5 was used as the receptor molecule [31]. The docking protocol consisted of four phases. In the first phase, Schrödinger’s Protein Preparation Wizard (PPW) [47] was used to prepare the protein. The preparation of the protein using PPW is a semi-automatic process including several steps. In the first step, the protein structure (PDB-ID: 1TV5) was downloaded from the Protein Data Bank (PDB) and placed into the workspace of the Maestro GUI. Then, PPW was used to correct bond orders of the ligand and cofactor. Then, hydrogens were applied to all atoms of the model. This step was followed by the neutralization of side chains, which are not close to the binding site and do not participate in salt bridges, and finally, by the restraint minimization of the protein structure. In the next phase, the Receptor Grid Generator generated the interaction grid. Finally, the ligand library was prepared using the original SDF representation of the ligands with LigPrep. LigPrep is a Schrödinger’s utility that combines tools for generating 3D structures from linear SMILES code or 2D SDF. In our case, Schrödinger’s Glide in XP (extra precision) mode was used to perform molecular docking [48].
4.2. Biochemistry
4.2.1. Expression and Purification of Recombinant DHODH
The expression plasmid pRSETb was a kind gift from Prof. Jon Clardy (Harvard Medical School, Boston, MA, USA). The plasmid contains a codon-optimized sequence for an N-truncated variant of PfDHODH (amino acids 159–565). The sequence was further optimized using overlap extension PCR to remove a segment encoding amino acids 384–413, which form a disordered loop, and the product was subcloned into a second expression plasmid, pET28, in-frame with a C-terminal His6-tag. This construct was transformed into E. coli strain BL21 (DE3), and the cells were then grown at 37 °C to an OD600 value of approx. 0.8. Expression of the recombinant protein was induced via autoinduction, and cells were grown overnight at 16 °C. Cells were then collected by centrifugation, resuspended in Ni-affinity chromatography binding buffer (20 mM HEPES, pH 7.5, 500 mM NaCl, and 20 mM imidazole), and lysed by sonication. The resulting homogenate was cleared by centrifugation, and the supernatant was filtered and applied to a HisTrap FF 1 mL column (Cytiva). The column was washed with 10 column volumes of binding buffer, and bound proteins were then eluted with elution buffer (20 mM HEPES, pH 7.5, 500 mM NaCl, and 500 mM imidazole). Fractions containing eluted proteins (approx. sample volumes 500 μL) were pooled, diluted in cation exchange binding buffer (50 mM phosphate, pH 6.7), and applied to a HiTrap SP HP 1 mL column (Cytiva), which was then washed with 10 column volumes of binding buffer. The remaining proteins were eluted in a 20 min linear gradient of 0–100% with elution buffer (50 mM phosphate, pH 6.7, and 1 M NaCl). Fractions containing the desired protein were then pooled, concentrated to a volume of 500 μL, and injected onto a Superdex 75 10/300 GL column to be purified by size exclusion chromatography using the appropriate buffer (20 mM HEPES, pH 7.4, and 500 mM NaCl). The resulting fractions were concentrated and stored at −80 °C until use.
4.2.2. Enzyme Assays
PfDHODH activity was determined with a colorimetric assay measuring the reduction of the redox dye 2,6-dichlorophenolindophenol (DCIP) at 600 nm coupled with the re-oxidation of the coenzyme decylubiquinone [49]. Assays were performed in 200 μL reaction mixtures in 96-well microtiter plates containing 200 μM L-dihydroorotic acid, 20 μM decylubiquinone, 120 μM DCIP, ~50 nM enzyme, and each of the tested compounds at 50 μM. All compounds were dissolved in DMSO. The total concentration of DMSO in the reaction mixtures did not exceed 5% (v/v). Reaction mixtures were incubated for 25 min. at 25 ± 1 °C, and the absorption of the samples was then read at 600 nm in a Tecan infinite 200 Pro spectrophotometer. Appropriate controls without enzyme were performed in parallel to account for background reduction of DCIP, along with positive controls containing the enzyme but with none of the tested compounds. The percentage of inhibition was determined as:
where Ai and Az are absorption values of reactions in the presence and absence of an inhibitor, and Ablank is the absorption value of the appropriate negative control without the enzyme. All experiments were performed in three parallels.
4.3. General Procedure for the Synthesis of Enamine 3c and Enhydrazines 5a–d
A mixture of enaminone 3b (1 mmol), amine 13 or hydrazine 4 (1 mmol), ethanol (2 mL), and 37% aq. HCl (0.1 mL ~3 drops, ~1 mmol) was stirred at room temperature for 24 h. The addition of 37% aq. HCl was omitted when the hydrochloride salt of amine 13 or hydrazine 4 was used. Water (3 mL) was added, and stirring at room temperature was continued for 30 min. The precipitate was collected by filtration, washed with water (3 mL), and dried in vacuo over NaOH pellets to give 3c or 5. The following compounds were prepared in this manner:
4.3.1. Diethyl 2-{[(1H-Indazol-5-yl)amino]methylene}malonate (3c)
Prepared from 1 (215 mg, 1 mmol) and 5-amino-1H-indazole (13) (133 mg, 1 mmol). Yield: 250 mg (83%) of light pink solid; mp 164–167 °C, [12] mp 167–169 °C (found: C, 58.99; H, 5.42; N, 14.11. C15H17N3O4 requires C, 59.40; H, 5.65; N, 13.85%); δH (300 MHz, DMSO-d6) 1.24 and 1.27 (6H, 2t, 1:1, J = 7.2 Hz), 4.13 (2H, q, J = 7.1 Hz), 4.21 (q, J = 7.1 Hz, 2H), 7.40 (dd, J = 8.9, 2.2 Hz, 1H), 7.57 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 2.1 Hz, 1H), 8.06 (s, 1H), 8.43 (d, J = 14.0 Hz, 1H), 10.83 (d, J = 14.0 Hz, 1H), and 13.11 (s, 1H); HRMS (ESI): MH+, found 304.1298. [C15H18N3O4]+ requires 304.1292. Spectral data are in agreement with the literature data [12].
4.3.2. Diethyl 2-{[2-(4-Chlorophenyl)hydrazinyl]methylene}malonate (5a)
Prepared from 3b (215 mg, 1 mmol) and 4-chlorophenylhydrazine hydrochloride (4a) (176 mg, 1 mmol). Yield: 220 mg (70%) of yellow solid; mp 115–116 °C, [39] mp 116 °C (found: C, 54.07; H, 5.25; N, 8.68. C14H17ClN2O4 requires C, 53.77; H, 5.48; N, 8.96%); νmax (ATR) 3264, 2977, 1674, 1645, 1610, 1492, 1427, 1273, 1246, 1222, 1075, 821, and 688 cm−1; δH (500 MHz, DMSO-d6) 1.17 (t, J = 7.1 Hz, 3H), 1.22 (t, J = 7.1 Hz, 3H), 4.04 (q, J = 7.1 Hz, 2H), 4.15 (q, J = 7.0 Hz, 2H), 6.71 (d, J = 8.8 Hz, 2H), 7.25 (s, 2H), 7.92 (d, J = 11.9 Hz, 1H), 8.66 (s, 1H), and 10.07 (d, J = 12.0 Hz, 1H); δC (126 MHz, DMSO-d6) 14.27, 14.33, 59.08, 59.18, 89.04, 114.25, 123.46, 128.84, 147.56, 160.51, 164.62, and 166.61; HRMS (ESI): MH+, found 313.0946. [C14H1835ClN2O4]+ requires 313.095.
4.3.3. Diethyl 2-{[2-(Naphthalene-2-yl)hydrazinyl]methylene}malonate (5b)
Prepared from 3b (215 mg, 1 mmol) and 2-naphthylhydrazine hydrochloride (4b) (195 mg, 1 mmol). Yield: 282 mg (86%) of orange solid; mp 86–87 °C (found: C, 65.67; H, 6.06; N, 8.46. C18H20N2O4 requires C, 65.84; H, 6.14; N, 8.53%); νmax (ATR) 3284, 2975, 1678, 1635, 1600, 1425, 1279, 1226, 1213, 1186, 1077, 1038, 840, 791, 736, and 694 cm−1; δH (500 MHz, DMSO-d6) 1.19 (t, J = 7.1 Hz, 3H), 1.26 (t, J = 7.0 Hz, 3H), 4.06 (q, J = 7.1 Hz, 2H), 4.19 (q, J = 7.1 Hz, 2H), 6.98 (d, J = 2.1 Hz, 1H), 7.05 (dd, J = 8.9, 2.3 Hz, 1H), 7.27 (t, J = 8.0, 6.9, 1.2 Hz, 1H), 7.40 (t, J = 8.1, 6.8, 1.3 Hz, 1H), 7.70–7.82 (m, 3H), 8.03 (d, J = 12.0 Hz, 1H), 8.79 (s, 1H), and 10.19 (d, J = 12.0 Hz, 1H); δC (126 MHz, DMSO-d6) 14.30, 14.36, 59.08, 59.21, 89.01, 105.83, 115.95, 123.06, 126.29, 126.50, 127.55, 128.43, 129.00, 134.03, 146.35, 160.64, 164.67, and 166.77; HRMS (ESI): MH+, found 329.1496. [C18H21N2O4]+ requires 329.1496.
4.3.4. Diethyl 2-{[2-(2,4,6-Trichlorophenyl)hydrazinyl]methylene}malonate (5c)
Prepared from 3b (215 mg, 1 mmol) and 2,4,6-trichlorophenylhydrazine hydrochloride (4c) (211 mg, 1 mmol). Yield: 300 mg (79%) of white solid; mp 90–92 °C, [40] mp 92 °C (found: C, 44.07; H, 3.85; N, 7.20. C14H25Cl3N2O4 requires C, 44.06; H, 3.96; N, 7.34%); νmax (ATR) 3300, 2973, 1688, 1633, 1419, 1379, 1262, 1226, 1059, 1032, 882, 793, 689, and 640 cm−1; δH (500 MHz, DMSO-d6) 1.20 (6H, t, J = 7.1 Hz), 4.06 (2H, q, J =, 7.1 Hz), 4.11 (2H, q, J =, 7.1 Hz), 7.61 (2H, s), 8.12 (1H, d, J = 11.9 Hz), 8.25 (1H, s), and 10.11 (1H, d, J = 11.9 Hz); δC (126 MHz, DMSO-d6) 14.28, 59.07, 59.11, 88.51, 126.61, 127.37, 128.74, 139.08, 160.42, 164.75, and 166.58; HRMS (ESI): MH+, found 381.017. [C14H1635Cl3N2O4]+ requires 381.017.
4.3.5. Diethyl 2-{[2-(4-Trifluoromethylphenyl)hydrazinyl]methylene}malonate (5d)
Prepared from 1 (215 mg, 1 mmol) and 4-trifluoromethylphenylhydrazine hydrochloride (4d) (176 mg, 1 mmol). Yield: 324 mg (94%) of yellowish solid; mp 126–127 °C (found: C, 51.88; H, 4.81; N, 8.01. C15H17F3N2O4 requires C, 52.02; H, 4.95; N, 8.09%); νmax (ATR) 3260, 2985, 1674, 1635, 1616, 1424, 1326, 1275, 1253, 1222, 1157, 1098, 1064, 1033, 1010, 834, 792, and 694 cm−1; δH (500 MHz, DMSO-d6) 1.18 (t, J = 7.1 Hz, 3H), 1.23 (t, J = 7.1 Hz, 3H), 4.04 (q, J = 7.1 Hz, 2H), 4.16 (q, J = 7.1 Hz, 2H), 6.82 (d, J = 8.4 Hz, 2H), 7.55 (d, J = 8.5 Hz, 2H), 7.91 (d, J = 12.0 Hz, 1H), 9.09 (s, 1H), and 10.14 (d, J = 12.0 Hz, 1H); δC (126 MHz, DMSO-d6) 14.75, 14.81, 59.62, 59.72, 90.07, 112.62, 120.18 (q), 125.29 (q), 126.93, 152.29, 160.86, 165.06, and 166.94; HRMS (ESI): MH+, found 347.1214. [C15H18F3N2O4]+ requires 347.1213.
4.4. General Procedure for the Synthesis of 1-Aryl-5-hydroxy-1H-pyrazole-4-carboxylates 1a–d
Compounds 1a–d were obtained following the literature procedure for the preparation of closely related compounds [28]. A mixture of enhydrazine 5 (0.5 mmol) and H2O–MeOH–Et3N (3:3:1, 5 mL) was stirred under reflux for 1.5 h. Volatile components were evaporated in vacuo, and the residue was triturated with 10% aq. HCl (4 mL). The precipitate was collected by filtration, washed with water (2 mL), and triturated again with a mixture of ethanol and water (1:1, 2 mL). The precipitate was collected by filtration and dried in vacuo over NaOH pellets to give 1. The following compounds were prepared in this manner:
4.4.1. Ethyl 1-(4-Chlorophenyl)-5-hydroxy-1H-pyrazole-4-carboxylate (1a)
Prepared from 5a (156 mg, 0.5 mmol). Yield: 80 mg (60%) of white solid; mp 147–149 °C, [39] mp 149 °C (found: C, 54.19; H, 4.00; N, 10.09. C12H11ClN2O3 requires C, 54.05; H, 4.16; N, 10.50%); νmax (ATR) 2987, 1711, 1524, 1495, 1402, 1343, 1241, 1198, 1077, 818, 774, and 732; δH (500 MHz, DMSO-d6) 1.27 (t, J = 7.1 Hz, 3H), 4.22 (q, J = 7.1 Hz, 2H), 7.56 (d, 2H), 7.74 (d, 2H), and 7.83 (s, 1H), OH exchanged; δC (126 MHz, DMSO-d6) 14.40, 59.23, 96.40, 123.53, 129.03, 130.96, 136.57, 140.57, 154.66, and 162.28; HRMS (ESI): MH+, found 267.0527. [C12H1235ClN2O3]+ requires 267.0531.
4.4.2. Ethyl 1-(Naphthalen-2-yl)-5-hydroxy-1H-pyrazole-4-carboxylate (1b)
Prepared from 5b (164 mg, 0.5 mmol). Yield: 110 mg (78%) of white solid; mp 149–151 °C (found: C, 67.90; H, 4.88; N, 9.48. C16H14N2O3 requires C, 68.08; H, 5.00; N, 9.92%); νmax (ATR) 3275, 2980, 1681, 1570, 1535, 1416, 1313, 1277, 1134, 1101, 975, 937, 894, 850, 818, 780, 745, and 636; δH (500 MHz, DMSO-d6) 1.29 (t, J = 7.1 Hz, 3H), 4.25 (q, J = 7.1 Hz, 2H), 7.517.63 (m, 2H), 7.86–7.89 (m, 2H), 7.96–8.02 (m, 2H), 8.05 (d, J = 8.9 Hz, 1H), and 8.23 (d, J = 2.1 Hz, 1H), OH exchanged; δC (126 MHz, DMSO-d6) 14.40, 59.22, 96.41, 119.91, 121.06, 126.36, 126.89, 127.61, 128.03, 128.77, 131.36, 132.69, 135.15, 140.42, 154.62, and 162.36; HRMS (ESI): MH+, found 283.1075. [C16H15N2O3]+ requires 283.1077.
4.4.3. Ethyl 1-(2,4,6-Trichlorophenyl)-5-hydroxy-1H-pyrazole-4-carboxylate (1c)
Prepared from 5c (191 mg, 0.5 mmol). Yield: 145 mg (86%) of white solid; mp 223–225 °C, [40] mp 228 °C (found: C, 42.66; H, 2.28; N, 8.31. C12H9Cl3N2O3 requires C, 42.95; H, 2.70; N, 8.35%); νmax (ATR) 3067, 1691, 1638, 1548, 1461, 1400, 1375, 1341, 1262, 1177, 1148, 1042, 924, 863, 804, 788, 753, and 657; δH (500 MHz, DMSO-d6) 1.27 (t, J = 7.1 Hz, 3H), 4.21 (q, J = 7.1 Hz, 2H), 7.88 (s, 1H), and 7.96 (s, 2H), OH exchanged; δC (126 MHz, DMSO-d6) 14.90, 59.70, 95.43, 129.33, 132.19, 135.62, 136.38, 142.27, 156.18, and 162.60; HRMS (ESI): MH+, found 334.9746. [C12H1035Cl3N2O3]+ requires 334.9752.
4.4.4. Ethyl 1-(4-Trifluoromethylphenyl)-5-hydroxy-1H-pyrazole-4-carboxylate (1d)
Prepared from 5d (173 mg, 0.5 mmol). Yield: 120 mg (80%) of white solid; mp 174–177 °C, [41] mp not given (found: C, 51.86; H, 3.47; N, 9.28. C13H11F3N2O3 requires C, 52.01; H, 3.69; N, 9.33%); νmax (ATR) 3164, 2903, 2752, 1708, 1628, 1560, 1333, 1191, 1168, 1119, 1099, 1059, 927, 828, 786, 752, and 704; δH (500 MHz, DMSO-d6) 1.28 (t, J = 7.1 Hz, 3H), 4.23 (q, J = 7.1 Hz, 2H), 7.88 (d, J = 9.3 Hz, 3H), and 7.99 (d, J = 8.4 Hz, 2H), OH exchanged; δC (126 MHz, DMSO-d6) 14.37, 59.29, 96.68, 121.76, 124.03 (q), 126.31 (d), 126.72 (q), 140.92, 141.14, 155.26, and 162.21; HRMS (ESI): MH+, found 301.0797. [C13H12F3N2O3]+ requires 301.0795.
4.5. General Procedure for the Synthesis of Hydrazides 10a–d
Under argon, a solution of methyl 3-chloro-3-oxopropanoate (9) (325 μL, 3.0 mmol, 1.15 equiv.) in anh. CH2Cl2 (12 mL) was added slowly at 0 °C (ice-bath) to a stirred mixture of hydrazine 4 hydrochloride (2.6 mmol), anh. CH2Cl2 (12 mL), and Et3N (1.1 mL, 7.8 mmol, 3 equiv.). Only 2 equiv. of Et3N (730 μL, 5.2 mmol) were added when free hydrazine 4 was used. Upon addition of 7, the mixture was stirred at 0 °C for 20 min. and then at room temperature for 2 h. The reaction mixture was transferred into a separatory funnel and washed with water (2 × 10 mL) and brine (2 × 10 mL). The organic phase was dried over anh. Na2SO4 and filtered, the filtrate was evaporated in vacuo, and the residue was purified by CC (EtOAc). Fractions containing the product were combined and evaporated in vacuo to give 10. The following compounds were prepared in this manner:
4.5.1. Methyl 3-[2-(4-Chlorophenyl)hydrazinyl]-3-oxopropanoate (10a)
Prepared from 9 (370 μL, 3.34 mmol), hydrazine 4a hydrochloride (528 mg, 2.9 mmol), and Et3N (1.25 mL, 8.7 mmol). Yield: 330 mg (47%) of white solid; mp 143–145 °C (found: C, 49.53; H, 4.59; N, 11.62. C10H11ClN2O3 requires C, 49.50; H, 4.57; N, 11.54%); νmax (ATR) 3320, 2952, 1729, 1645, 1597, 1494, 1432, 1352, 1203, 1173, 1088, 1007, 971, 823, and 693; δH (300 MHz, DMSO-d6) 3.34 (s, 2H), 3.65 (s, 3H), 6.73 (d, J = 8.9 Hz, 1H), 7.17 (d, J = 8.9 Hz, 1H), 8.00 (d, J = 2.5 Hz, 1H), and 9.88 (d, J = 2.5 Hz, 1H); δC (126 MHz, DMSO-d6) 40.6, 52.0, 113.6, 121.8, 128.5, 147.9, 165.1, and 168.1; HRMS (ESI): MH+, found 243.0536. [C10H1235ClN2O3]+ requires 243.0531.
4.5.2. Methyl 3-[2-(Naphthalene-2-yl)hydrazinyl]-3-oxopropanoate (10b)
Prepared from 9 (325 μL, 3 mmol), hydrazine 4b hydrochloride (500 mg, 2.6 mmol), and Et3N (1.12 mL, 7.8 mmol). Yield: 320 mg (48%) of pinkish solid; mp 142–146 °C (found: C, 64.51; H, 5.34; N, 10.66. C14H14N2O3 requires C, 65.11; H, 5.46; N, 10.85%); νmax (ATR) 3289, 3203, 2953, 1735, 1694, 1659, 1633, 1508, 1413, 1342, 1202, 1153, 1014, 820, 743, and 675; δH (500 MHz, DMSO-d6) 3.42 (s, 2H), 3.69 (s, 3H), 7.00 (d, J = 2.0 Hz, 1H), 7.08 (dd, J = 8.8, 2.3 Hz, 1H), 7.19–7.23 (m, 1H), 7.33–7.40 (m, 1H), 7.61 (d, J = 8.2 Hz, 1H), 7.69–7.74 (m, 2H), 8.15 (d, J = 2.5 Hz, 1H), and 10.00 (d, J = 2.5 Hz, 1H); δC (126 MHz, DMSO-d6) 52.0, 104.8, 116.0, 122.2, 125.8, 126.2, 127.5, 127.8, 128.4, 134.3, 146.7, 165.1, 168.1, and 170.8; HRMS (ESI): MH+, found 259.1078. [C14H15N2O3]+ requires 259.1077.
4.5.3. Methyl 3-[2-(2,4,6-Trichlorophenyl)hydrazinyl]-3-oxopropanoate (10c)
Prepared from 9 (165 μL, 1.47 mmol), hydrazine 4c (270 mg, 1.28 mmol), and Et3N (360 μL, 2.56 mmol). Yield: 210 mg (53%) of white solid; mp 172–174 °C (found: C, 38.36; H, 2.62; N, 8.86. C10H9Cl3N2O3 requires C, 38.80; H, 2.28; N, 9.05%); νmax (ATR) 3307, 3201, 3016, 1741, 1663, 1552, 1434, 1288, 1150, 1006, 977, 927, 852, 721, and 610; δH (300 MHz, DMSO-d6) 3.25 (s, 2H), 3.59 (s, 3H), 7.35 (s, 1H), 7.49 (s, 2H), and 10.19 (s, 1H); δC (126 MHz, DMSO-d6) 52.3, 125.0, 125.7, 128.9, 129.4, 141.1, 165.1, and 168.0; HRMS (ESI): MH+, found 310.9755. [C10H1035Cl3N2O3]+ requires 310.9752.
4.5.4. Methyl 3-[2-(4-Trifluoromethylphenyl)hydrazinyl]-3-oxopropanoate (10d)
Prepared from 9 (165 μL, 1.47 mmol), hydrazine 4d (225 mg, 2.84 mmol), and Et3N (360 μL, 2.56 mmol). Yield: 370 mg (47%) of white solid; mp 136–137 °C (found: C, 47.61; H, 3.87; N, 10.11. C11H11F3N2O3 requires C, 47.49; H, 4.01; N, 10.07%); νmax (ATR) 3342, 1731, 1650, 1615, 1320, 1157, 1094, 1063, 833, and 700; δH (500 MHz, DMSO-d6) 3.37 (s, 2H), 3.66 (s, 3H), 6.84 (d, J = 8.4 Hz, 2H), 7.47 (d, J = 8.5 Hz, 2H), 8.50 (d, J = 1.6 Hz, 1H), 10.04 (d, J = 1.7 Hz, 1H); δC (126 MHz, DMSO-d6) 52.0, 111.4, 118.3 (q, J = 31.7 Hz), 125.1 (q, J = 269.2 Hz), 126.2 (d, J = 4.2 Hz), 152.1, 165.2, 168.0, and 170.8; HRMS (ESI): MH+, found 277.0751. [C11H12F3N2O3]+ requires 277.0755.
4.6. General Procedure for the Synthesis of Methyl 1-Aryl-3-hydroxy-1H-pyrazole-4-carboxylates 2a–d
Under argon, TBDMAM (232 μL, 1.125 mmol, 1.5 equiv.) was added to a mixture of hydrazide 10 (0.75 mmol) and anh. toluene (5 mL), and the mixture was stirred at 40 °C for 30 min. and then under reflux for 2 h. Volatile components were evaporated in vacuo. The residue was triturated with methanol (2 mL) for 10 min., and the precipitate was collected by filtration and washed with water (2 mL) to give 2. The following compounds were prepared in this manner:
4.6.1. Methyl 1-(4-Chlorophenyl)-3-hydroxy-1H-pyrazole-4-carboxylate (2a)
Prepared from 10a (182 mg, 0.75 mmol), TBDMAM (232 μL, 1.125 mmol), and anh. toluene (5 mL). Yield: 140 mg (74%) of pinkish solid; mp 206–208 °C (found: C, 52.41; H, 3.72; N, 10.99. C11H9ClN2O3 requires C, 52.29; H, 3.59; N, 11.09%); νmax (ATR) 3119, 2948, 2722, 2626, 1691, 1606, 1587, 1542, 1504, 1452, 1431, 1320, 1294, 1252, 1229, 1188, 1124, 1096, 1057, 1011, 952, 891, 823, and 761; δH (300 MHz, DMSO-d6) 3.74 (s, 3H), 7.53 (ddd, J = 9.0, 3.1, 2.2 Hz, 2H), 7.83 (ddd, J = 9.0, 3.1, 2.2 Hz, 2H), 8.84 (s, 1H), and 10.97 (s, 1H); δC (126 MHz, DMSO-d6) 51.4, 101.8, 120.0, 129.9, 130.8, 132.7, 138.1, 162.0, and 162.7; HRMS (ESI): MH+, found 253.0372. [C11H1035ClN2O3]+ requires 253.0374.
4.6.2. Methyl 1-(Naphthalen-2-yl)-3-hydroxy-1H-pyrazole-4-carboxylate (2b)
Prepared from 10b (220 mg, 0.85 mmol), TBDMAM (263 μL, 1.28 mmol), and anh. toluene (5 mL). Yield: 130 mg (57%) of yellowish solid; mp 168–172 °C (found: C, 67.58; H, 4.52; N, 10.14. C15H12N2O3 requires C, 67.16; H, 4.51; N, 10.44%); νmax (ATR) 3099, 2945, 2631, 1696, 1601, 1516, 1292, 1218, 1192, 1119, 892, 857, 813, 770, 745, and 731; δH (500 MHz, DMSO-d6) 3.76 (s, 3H), 7.51 (ddd, J = 8.1, 6.8, 1.3 Hz, 1H), 7.57 (ddd, J = 8.1, 6.8, 1.3 Hz, 1H), 7.95 (dt, J = 8.1, 1.4, 1.4 Hz, 2H), 8.03 (d, J = 1.4 Hz, 2H), 8.32 (s, 1H), 8.96 (s, 1H), 11.03 (s, 1H); δC (126 MHz, DMSO-d6) 50.9, 101.2, 115.0, 117.3, 125.9, 127.1, 127.7, 127.8, 129.4, 131.2, 132.1, 133.0, 136.3, 161.6, and 162.3; HRMS (ESI): MH+, found 269.0916. [C15H13N2O3]+ requires 269.0921.
4.6.3. Methyl 1-(2,4,6-Trichlorophenyl)-3-hydroxy-1H-pyrazole-4-carboxylate (2c)
Prepared from 10c (420 mg, 1.35 mmol), TBDMAM (420 μL, 2 mmol), and anh. toluene (5 mL). Yield: 210 mg (48%) of white solid; mp 163–166 °C (found: C, 41.18; H, 2.46; N, 8.50. C11H7Cl3N2O3 requires C, 41.09; H, 2.19; N, 8.71%); νmax (ATR) 3290, 3039, 2946, 1699, 1650, 1600, 1557, 1483, 1275, 1209, 1172, 1121, 1090, 1024, 874, 784, and 662; δH (500 MHz, DMSO-d6) 3.72 (s, 3H), 7.96 (s, 2H), 8.39 (s, 1H), 10.93 (s, 1H); δC (126 MHz, DMSO-d6) 51.4, 100.9, 129.3, 134.9, 134.9, 135.9, 138.5, 162.0, and 162.7; HRMS (ESI): MH+, found 320.9596. [C11H835Cl3N2O3]+ requires 320.9595.
4.6.4. Methyl 1-(4-Trifluoromethylphenyl)-3-hydroxy-1H-pyrazole-4-carboxylate (2d)
Prepared from 10d (130 μL, 0.47 mmol), TBDMAM (145 μL, 0.7 mmol), and anh. toluene (5 mL). Yield: 75 mg (48%) of white solid; mp 198–200 °C (found: C, 50.73; H, 2.99; N, 9.66. C12H9F3N2O3 requires C, 50.36; H, 3.17; N, 9.78%); νmax (ATR) 3112, 2951, 1694, 1608, 1542, 1339, 1296, 1229, 1151, 1108, 1076, 951, 839, 768, and 654; δH (500 MHz, DMSO-d6) 33.75 (s, 3H), 7.84 (d, J = 8.5 Hz, 2H), 8.03 (d, J = 8.5 Hz, 2H), 9.00 (s, 1H), and 11.17 (s, 1H); δC (126 MHz, DMSO-d6) 51.1, 102.2, 118.2, 124.1 (q, J = 271.8 Hz), 126.2 (q, J = 32.7 Hz), 126.8 (d, J = 4.3 Hz), 132.9, 141.6, 161.8, and 162.2; HRMS (ESI): MH+, found 287.0644. [C12H10F3N2O3]+ requires 287.0638.
5. Conclusions
Ethyl 1-aryl 5-hydroxypyrazole-4-carboxylates 1 are readily obtainable by cyclization of arylhydrazines 4 with diethyl α-[(dimethylamino)methylene]malonate (3b). On the other hand, the preparation of isomeric methyl 3-hydroxypyrazole-4-carboxylates 2 is difficult. Therefore, a novel two-step synthesis of 2 from arylhydrazines 4 was developed. The synthetic method involves acylation of hydrazines 4 with methyl 3-chloro-3-oxopropanoate (9) to afford the corresponding hydrazides 10, followed by cyclization of 10 with Bredereck’s reagent to afford 3-hydroxypyrazoles 2. Testing compounds 1a–d and 2a–d for the inhibition of PfDHODH revealed only weak activities (15–30% inhibition) for compounds 1b and 2a–d, while compounds 1a, 1c, and 1d showed no significant inhibition of PfDHODH. In general, 3-hydroxy isomers 2 were more effective than 5-hydroxy isomers 1. Encouragingly, compounds 2b–d were more effective (~30% inhibition) than the known malonate-type inhibitor 3c (19% inhibition). In summary, isomeric 5- (1) and 3-hydroxy-1H-pyrazole-4-carboxylates 2 can be prepared in two steps from commercial precursors 3, 4, and 9. Since compounds 1 and 2 showed similar or better inhibition of PfDHODH than the known inhibitor 3c, this could serve as a starting point for further development of new pyrazolone-based inhibitors of PfDHODH.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27154764/s1. Copies of 1H and 13C NMR spectra of new compounds 3c, 1a–d, 2a–d, 5a–d, and 10a–d, copies of IR spectra of compounds 3c, 1a–d, 2a–d, 5a–d, and 10a–d; Table S1: Scores for evaluation of binding affinities compounds 3c, 1a–d, 2a–d, 5a–d, 10a–d, A–E, and 12 by molecular docking.
Author Contributions
Individual contributions of the authors are the following: conceptualization J.W., M.N., and J.S.; methodology L.V., T.M., U.G., M.K., Č.P., B.Š., J.W., M.N., and J.S.; software, L.V., T.M., and Č.P.; validation, M.K. and J.S.; formal analysis, L.V., T.M., M.K. Č.P., and J.S.; investigation, L.V., T.M., M.K., Č.P., and J.S.; resources, M.N. and J.S.; data curation, L.V., T.M., M.K., Č.P., M.N., and J.S..; writing—original draft preparation, L.V., T.M., U.G., M.K., Č.P., B.Š., M.N., and J.S.; writing—review and editing, U.G., M.K., Č.P., B.Š., M.N., and J.S.; visualization, T.M., Č.P., M.K., M.N., and J.S.; supervision, M.K., M.N., and J.S.; project administration, M.K., M.N., and J.S.; funding acquisition, M.K., M.N., and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency (ARRS), research core funding Nos. P1-0179, P1-0140, and P1-0201.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available in the Supplementary Materials.
Acknowledgments
We thank EN-FIST Centre of Excellence, Trg Osvobodilne fronte 13, 1000 Ljubljana, Slovenia, for use of the BX FTIR spectrophotometer.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1a–d, 3c, 2a–d, 5a–d, and 10a–d are available from the authors.
References
- Morag, D. History of malaria and its treatment. In Antimalarial Agents Design and Mechanism of Action, 1st ed.; Patrick, G.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–48. ISBN 9780081012109. [Google Scholar]
- Patrick, G.L. (Ed.) Antimalarial Agents Design and Mechanism of Action, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–604. ISBN 9780081012109. [Google Scholar]
- Fidock, D.A.; Rosenthal, P.J.; Croft, S.L.; Brun, R.; Nwaka, S. Antimalarial drug discovery: Efficacy models for compound screening. Nat. Rev. Drug Disc. 2004, 3, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Su, X.Z. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti-Infect. Ther. 2009, 7, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Pohlit, A.M.; Lima, R.B.; Frausin, G.; Silva, L.F.; Lopes, S.C.; Moraes, C.B.; Cravo, P.; Lacerda, M.V.; Siqueira, A.M.; Freitas-Junior, L.H.; et al. Amazonian Plant Natural Products: Perspectives for Discovery of New Antimalarial Drug Leads. Molecules 2013, 18, 9219–9240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.; Brown, G.V.; Genton, B.; Moorthy, V.S. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar. J. 2012, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, T.; Dao, A.; Yaro, A.S.; Huestis, D.L.; Diallo, M.; Timbiné, S.; Kassogué, Y.; Adamou, A.; Traoré, A.I.; Samaké, D. Phenotypic divergence among the members of the African malaria mosquitoes and strategies of persistence throughout the dry season. In Challenges in Malaria Research: Core Science and Innovation. Malar. J. 2014, 13 (Suppl. S1), O2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnell, E.S. Drugs targeting mitochondrial functions. In Antimalarial Agents Design and Mechanism of Action, 1st ed.; Patrick, G.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–402. ISBN 9780081012109. [Google Scholar]
- Vyas, V.K.; Ghate, M. Recent Developments in the Medicinal Chemistry and Therapeutic Potential of Dihydroorotate Dehydrogenase (DHODH) Inhibitors. Mini-Rev. Med. Chem. 2011, 11, 1039–1055. [Google Scholar] [CrossRef]
- Nixon, G.L.; Pidathala, C.; Shone, A.E.; Antoine, T.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Targeting the mitochondrial electron transport chain of Plasmodium falciparum: New strategies towards the development of improved antimalarials for the elimination era. Future Med. Chem. 2013, 5, 1573–1591. [Google Scholar] [CrossRef]
- Hoelz, L.V.B.; Calil, F.A.; Nonato, M.C.; Pinheiro, L.C.S.; Boechat, N. Plasmodium falciparum dihydroorotate dehydrogenase: A drug target against malaria. Future Med. Chem. 2018, 10, 1853–1874. [Google Scholar] [CrossRef]
- Heikkilä, T.; Ramsey, C.; Davies, M.; Galtier, C.; Stead, A.M.W.; Johnson, A.P.; Fishwick, C.W.G.; Boa, A.N.; McConke, G.A. Design and Synthesis of Potent Inhibitors of the Malaria Parasite Dihydroorotate Dehydrogenase. J. Med. Chem. 2007, 50, 186–191. [Google Scholar] [CrossRef]
- Boa, A.N.; Canavan, S.P.; Hirst, P.R.; Ramsey, C.; Stead, A.M.W.; McConkey, G.A. Synthesis of brequinar analogue inhibitors of malaria parasite dihydroorotate dehydrogenase. Bioorg. Med. Chem. 2005, 13, 1945–1967. [Google Scholar] [CrossRef]
- Davies, M.; Heikka, T.; McConkey, G.A.; Fishwick, C.W.G.; Parsons, M.R.; Johnson, A.P. Structure-based design, synthesis and characterization of inhibitors of human and Plasmodium falciparum dihydroorotate dehydrogenase. J. Med. Chem. 2009, 52, 2683–2693. [Google Scholar] [CrossRef]
- Gujjar, R.; Marwaha, A.; Mazouni, F.E.; White, J.; White, K.; Creason, S.; Shackleford, D.; Baldwin, J.; Charman, W.N.; Buckner, F.S.; et al. Identification of a Metabolically Stable Triazolopyrimidine-Based Dihydroorotate Dehydrogenase Inhibitor with Antimalarial Activity in Mice. J. Med. Chem. 2009, 52, 1864–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skerlj, R.T.; Bastos, C.M.; Booker, M.L.; Kramer, M.L.; Barker, R.H.; Celatka, C.A.; O’Shea, T.J.; Munoz, B.; Sidhu, A.B.; Coretes, J.F.; et al. Optimization of Potent Inhibitors of P. falciparum Dihydroorotate Dehydrogenase for the Treatment of Malaria. ACS Med. Chem. Lett. 2011, 2, 708–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Wiley-Blackwell: New York, NY, USA, 2010; pp. 1–718. ISBN 978-1-405-13300-5. [Google Scholar]
- Patrick, G.L. An Introduction to Medicinal Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2013; pp. 1–816. ISBN 9780199697397. [Google Scholar]
- Pernerstorfer, J. Molecular Design and Combinatorial Compound Libraries. In Handbook of Combinatorial Chemistry. Drugs, Catalysts, Materials; Nicolaou, K.C., Hanko, R., Hartwig, W., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Volume 2, pp. 725–742. ISBN 0-471-49726-6. [Google Scholar]
- Petek, N.; Štefane, B.; Novinec, M.; Svete, J. Synthesis and biological evaluation of 7-(aminoalkyl)pyrazolo [1,5-a]pyrimidine derivatives as cathepsin K inhibitors. Bioorg. Chem. 2019, 84, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Prasher, P. An epigrammatic status of the ‘azole’-based antimalarial drugs. RSC Med. Chem. 2020, 11, 184–211. [Google Scholar] [CrossRef]
- Shamsuddin, M.A.; Ali, A.H.; Zakaria, N.H.; Mohammat, M.F.; Hamzah, A.S.; Shaameri, Z.; Lam, K.W.; Mark-Lee, W.F.; Agustar, H.K.; Razak, M.R.H.A.; et al. Synthesis, Molecular Docking, and Antimalarial Activity of Hybrid 4-Aminoquinoline-pyrano[2,3-c]pyrazole Derivatives. Pharmaceuticals 2021, 14, 1174. [Google Scholar] [CrossRef]
- Kumar, G.; Tanwar, O.; Kumar, J.; Akhter, M.; Sharma, S.; Pillai, C.R.; Alam, M.M.; Zama, M.S. Pyrazole-pyrazoline as promising novel antimalarial agents: A mechanistic study. Eur. J. Med. Chem. 2018, 149, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Nasralla, S.N.; Bekhit, S.A.; Bekhit, A.E.-D.A. Novel Dual Acting Antimalarial Antileishmanial Agents Derived from Pyrazole Moiety. Biointerface Res. Appl. Chem. 2022, 12, 6225–6233. [Google Scholar] [CrossRef]
- Aggarwal, S.; Paliwal, D.; Kaushik, D.; Kumar Gupta, G.; Kumar, A. Pyrazole Schiff Base Hybrids as Anti-Malarial Agents: Synthesis, In Vitro Screening and Computational Study. Comb. Chem. High Throughput Screen. 2018, 21, 194–203. [Google Scholar] [CrossRef]
- Stanovnik, B.; Svete, J. Pyrazoles. In Science of Synthesis, Houben-Weyl Methods of Molecular Transformations; Neier, R., Bellus, D., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2002; Volume 12, pp. 15–225. ISBN 3-13-112271-4. [Google Scholar]
- Taylor, A.W.; Cook, R.T. A direct preparation of 2-aryl-4-ethoxycarbonyl-3-pyrazolin-5-ones from aryl hydrazines. Tetrahedron 1987, 43, 607–616. [Google Scholar] [CrossRef]
- Kralj, D.; Mecinović, J.; Bevk, D.; Grošelj, U.; Stanovnik, B.; Svete, J. 3-(Dimethylamino)propenoate-based regioselective synthesis of 1,4-disubstituted 5-hydroxy-1H-pyrazoles. Heterocycles 2006, 68, 897–914. [Google Scholar] [CrossRef]
- Michaelis, A.; Remy, E. Über die Darstellung des 1-Phenyl-3-pyrazolons. Chem. Ber. 1907, 40, 1020–1021. [Google Scholar] [CrossRef]
- Downloaded from RSCB PDB Protein Data Bank. Available online: https://www.rcsb.org/structure/1TV5 (accessed on 27 April 2021).
- Hurt, D.E.; Widom, J.; Clardy, J. Structure of Plasmodium falciparum dihydroorotate dehydrogenase with a bound inhibitor. Acta Cryst. 2006, D62, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswandhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Nadin, A.; Hattotuwagama, C.; Churcher, I. Lead-oriented synthesis: A new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. 2012, 51, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Hardegger, L.A.; Kuhn, B.; Spinnler, B.; Anselm, L.; Ecabert, R.; Stihle, M.; Gsell, B.; Thoma, R.; Diez, J.; Benz, J.; et al. Systematic Investigation of Halogen Bonding in Protein–Ligand Interactions. Angew. Chem. Int. Ed. 2011, 50, 314–318. [Google Scholar] [CrossRef]
- Paulini, R.; Müller, K.; Diedrich, F. Orthogonal Multipolar Interactions in Structural Chemistry and Biology. Angew. Chem. Int. Ed. 2011, 50, 314–318. [Google Scholar] [CrossRef]
- Roehrig, S.; Straub, A.; Pohlmann, J.; Lampe, T.; Pernerstorfer, J.; Schlemmer, K.-H.; Reinemer, P.; Perzborn, E.J. Discovery of the Novel Antithrombotic Agent 5-Chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): An Oral, Direct Factor Xa Inhibitor. Med. Chem. 2005, 48, 5900–5908. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.D. A New Reagent for the Synthesis of Diethyl Arylaminomethylenemalonates. Synthesis 1971, 1971, 220. [Google Scholar] [CrossRef]
- Desimoni, G.; Righetti, P.P.; Selva, E.; Tacconi, G.; Riganti, V.; Specchiarello, M. Heterodiene syntheses–XIX1: Correlation of the kinetic data with lumo energies in the reaction between 1-aryl-4-benzylidene-5-pyrazolones and isopropyl vinyl ether. Tetrahedron 1977, 33, 2829–2836. [Google Scholar] [CrossRef]
- Gehring, R.; Schallner, O.; Stetter, J.; Santel, H.J.; Schmidt, R.R. 1-Aryl-4-nitropyrazole. DE 3501323 A1 19860717 (1986). Chem. Abstr. 1986, 105, 191074. [Google Scholar]
- Kundu, M.; Nadkarni, S.M.; Gullapalli, S.; Joshi, N.K.; Karnik, P.V. Preparation of pyrazole amides as cannabinoid receptor ligands. WO 2007026215A1 20070308 (2007). Chem. Abstr. 2007, 146, 316911. [Google Scholar]
- Kralj, D.; Grošelj, U.; Meden, A.; Dahmann, G.; Stanovnik, B.; Svete, J. A simple synthesis of 4-(2-aminoethyl)-5-hydroxy-1H-pyrazoles. Tetrahedron 2007, 63, 11213–11222. [Google Scholar] [CrossRef]
- Dorn, H. Tautomerie und Nomenklatur der “Pyrazolone” und Aminopyrazole. J. Prakt. Chem. 1973, 315, 382–418. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Karelson, M.; Harris, P.A. Prototropic tautomerism of heteroaromatic compounds. Heterocyles 1991, 32, 329–369. [Google Scholar] [CrossRef]
- Yet, L. Pyrazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Joule, J., Eds.; Elsevier: Oxford, UK, 2008; Volume 4, pp. 1–141. ISBN 9780080449913. [Google Scholar]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shaw, D.E.; Shelley, M.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, J.; Michnoff, C.H.; Malmquist, N.A.; White, J.; Roth, M.G.; Rathod, P.K.; Phillips, M.A. High-throughput Screening for Potent and Selective Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase. J. Biol. Chem. 2005, 280, 21847–21853. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).