Abstract
A general method for the synthesis of pyrrolizidine derivatives using an intramolecular hydroaminomethylation protocol (HAM) under microwave (MW) dielectric heating is reported. Starting from a 3,4-bis(benzyloxy)-2-[(benzyloxy)methyl]-5-vinylpyrrolidine, MW-assisted intramolecular HAM in the presence of gaseous H2 and CO gave the natural alkaloid hyacinthacine A2 protected as benzyl ether. The same approach gave a lentiginosine analogue starting from the corresponding vinyl N-hydroxypyrrolidine. The nature of the reaction products and the yields were strongly influenced by the relative stereochemistry of the starting pyrrolidines, as well as by the catalyst/ligand employed. The use of ethanol as a solvent provides environmentally friendly conditions, while the ligand/catalyst system can be recovered by separating the alkaloid product with an SCX column and recycling the ethanolic solution. HAM worked up to three times with the recycled catalyst solution without any significant impact on yield.
1. Introduction
The pyrrolizidine nucleus can be considered as a privileged scaffold, since in nature more than 6000 animal or plant species hold pyrrolizidine alkaloids (Figure 1) [1,2], which are responsible for a wide range of biological activities depending on substituents and stereochemistry [3] Highly polyhydroxylated pyrrolizidine alkaloids can act as sugar mimics that inhibit glycosidases [4,5] The main pharmaceutical application of pyrrolizidine-based compounds is in traditional medicine (with the plant extracts used as ingredients), although several concerns have been posed regarding their safety on humans [3] In addition, some pyrrolizidine alkaloids (necine bases) and analogues can be used as pesticides thanks to their toxicity and natural deterrent effect on insects [6,7].
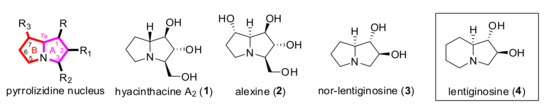
Figure 1.
Representative biologically active natural pyrrolizidine alkaloids and analogues of indolizidine ones.
Among the various methods developed for their synthesis [8,9], the chiral pool strategy is one of the most effective approaches for the preparation of hyacinthacine A2 (1), alexine (2), of the natural indolizidine alkaloid lentiginosine (4) and pyrrolizidine analogues such as nor-lentiginosine (3). Natural proline, tartaric acid, and various sugars have been explored as enantiomerically pure starting materials, but the proposed multistep syntheses often suffer from lack of general applicability and in some cases scarce diastereoselectivity. Cycloadditions and sigmatropic rearrangements are probably the most used reactions for the synthesis of natural pyrrolizidines and their derivatives, along with condensations and metal-catalysed reactions [9]. A chemoenzymatic approach, starting from prolynal, has also been reported [10] to build up the polyhydroxylated part of the molecule (ring A). However, this method suffers from poor atomic economy, the use of non-environmentally friendly DMF and requires the separation of the diastereoisomers obtained. When sugars are used as starting material, the second cycle (ring B) is usually built up by ring-closing metathesis or amidation/alkylation reactions over the N atom, but variable stereoselectivity in positions 7a is commonly observed. The development of flexible and effective methods for the preparation of non-natural pyrrolizidine analogues is therefore an important topic, especially to investigate the structure-activity relationships of this class of molecules. Our previous studies have established nitrones derived from l-tartaric acid and d-arabinose or l-xylose [11] (A and B in Scheme 1) as suitable substrates for efficient access to pyrrolizidine alkaloids by means of cycloaddition reactions [12] or additions of Grignard reagents followed by proper elaboration of adducts. Particularly, the addition of vinylMgBr to these nitrones occurred with nearly complete stereoselectivity[13] for steric and electronic reasons [14] and resulted amenable to the synthesis of hyacinthacine A2 [15] or nor-lentiginosine derivatives [16]after several synthetic steps.
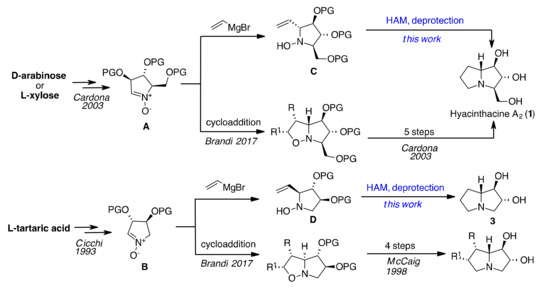
Scheme 1.
Different approaches to the synthesis of pyrrolizidine alkaloids starting from nitrones of general structures A and B.
We envisaged that a much more direct access to pyrrolizidine alkaloids such as hyacinthacine A2 (1) and nor-lentiginosine (3) might be provided by a hydroaminomethylation reaction (HAM) carried out on the same intermediates C [15] and D [17], respectively (Scheme 1).[11,15,17,18,19,20,21,22,23,24] This could be an elegant approach for the preparation of polycyclic derivatives with promising large-scale applications, as the HAM process is based on the hydroformylation reaction (HF), the most commonly used catalytic transformation in the industry [25].
Although characterized by high atom economy, HF and HAM are featured by harsh reaction conditions in terms of temperature (>100 °C), syngas pressure (50 bar) and time (24 h) [26,27,28,29,30]. However, many reactions that require very harsh conditions, such as aminocarbonylation, hydrogen borrowing, reductive amination, HF, and HAM itself, can benefit from MW assistance [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. We report here a generally relevant, mild, and sustainable method for the synthesis of pyrrolizidine nuclei via an intramolecular HAM process under MW dielectric heating.
2. Results and Discussion
Our retrosynthetic strategy for the synthesis of generic pyrrolizidine derivatives is shown in Scheme 2. The pyrrolizidine skeleton can be obtained by the HAM of olefin E or F derived from the reduction of hydroxylamine C or D, which in turn was obtained by the stereoselective addition of vinylmagnesium bromide to enantiomerically pure nitrones A or B.
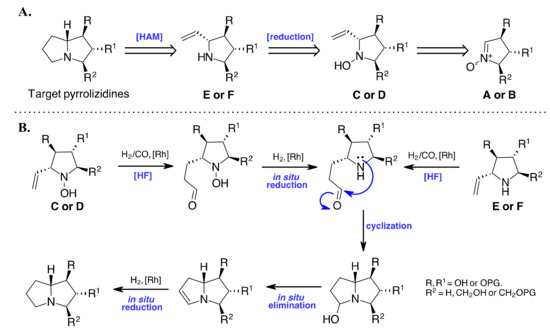
Scheme 2.
(A). Retrosynthesis for target pyrrolizidines. (B). A putative mechanism of the HAM approach.
In the intramolecular HAM process, we expected the aldehyde to form an iminium/enamine intermediate after HF on the olefin [28,29,46]. Nucleophilic binding of the amine, followed by reduction catalyzed by the Rh catalysts, would give the expected bicyclic pyrrolizidine product. To set up the process, we investigated the use of both hydroxylamine C and D and the corresponding amine E and F, possibly formed by the in-situ reduction in the reaction conditions.
We started from nitrone 5 [47], an intermediate suitable for the synthesis of the nor-lentiginosine (3). A highly diastereoselective addition of vinylmagnesium bromide 6 gave hydroxylamine 7 in 96% yield and allowed the incorporation of a new (S)-configured stereogenic center in C-2 position (Scheme 3) [17]. Compound 7 and the corresponding amine 9, obtained by reduction with catalytic In and Zn in NH4Cl solution [48,49], served as model substrates for the HAM process under MW dielectric heating. Different conditions were tested for the reactions, varying the catalyst/ligand, temperature, and solvent used, and the results are shown in Table 1.
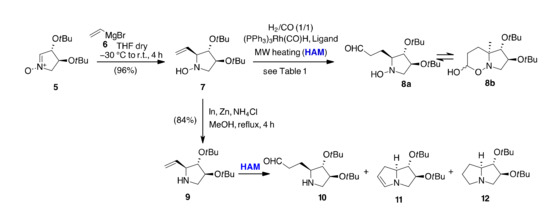
Scheme 3.
HAM of hydroxylamine 7 and amine 9.

Table 1.
HAM optimisation for the synthesis of nor-lentiginosine.
Under standard MW-assisted conditions for HF (H2/CO, 1/1, Xantphos/(PPh3)3Rh(CO)H in toluene containing [bmim][BF4], 110 °C, 30 min) [45], 7 gave the aldehyde 10 as the main reaction product (44%) together with a 10% of the desired bicyclic product 12 and traces of enamine 11 (Table 1, entry 1).
Under the same reaction conditions, the amine 9 furnished solely the enamine 11, but with a poor 27% conversion. Better results were obtained with EtOH as solvent, especially starting from hydroxylamine 7 that gave the aldehyde 8a, in equilibrium with its cyclic hemiacetal form 8b, as the major reaction product in 65% isolated yield, while 9 showed almost no reactivity (Table 1, entry 2). In toluene alone, at lower temperatures (80 °C), no conversion was observed starting from 7, while starting from 9 only the hydrogenation by-product 13b was formed (Table 1, entry 3). At higher temperatures (130 °C), a relatively small amount of the enamine 11 was obtained from the two vinylpyrrolidines 7 and 9 (Table 1, entry 4). HAM with Biphephos or PPh3 as ligands resulted in the hydrogenation of the C=C double bond and product 13 was obtained as the major component of the reaction mixture (40–90%) regardless of the starting material (Table 1, entries 5–6). Other variations in conditions, such as [RhCl(COD)]2 as catalysts, pressure of syngas (3–14 bar) and reaction times (40–60 min) were tested without improvement in conversion or selectivity. The better result was then the conversion of hydroxylamine 7 into the mixture of 8a and 8b using EtOH as solvent. Treatment of this mixture with PTSA in refluxing toluene (30 min, MW) reversed the equilibrium towards aldehyde 8a, which could be hydrogenated (H2, 1 bar, MeOH MW, 40 °C, 15 min) to give compound 12 that, upon deprotection with trifluoroacetic acid, ref. [17] afforded nor-lentiginosine (3) (Scheme 4).
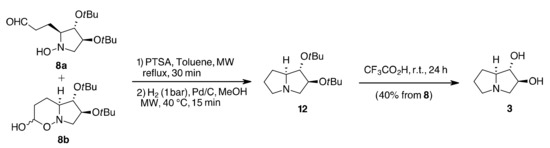
Scheme 4.
Synthesis of nor-lentiginosine 3.
However, when we explored the possible application of this methodology to a differently protected 2-vinyl-N-hydroxypyrrolidine hydroxylamine (14, Scheme 5), we were able to obtain only the intermediate aldehyde mixture 15a/15b in just 37% isolated yields. The following transformation into the corresponding bicyclic derivative 16 by reducing the hydroxylamine and subjecting the product to intramolecular reductive amination conditions did not occur using H2 and Pd/C under both traditional heating or MW dielectric heating.
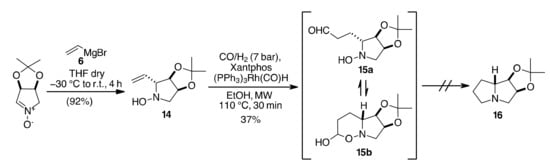
Scheme 5.
HAM of 14.
For the synthesis of hyacinthacine A2 (1), we started from the known hydroxylamine 17 obtained from the corresponding nitrone and vinylmagnesium bromide [15]. Under the previously established conditions for HF, 7 bar syngas in the presence of Xantphos/(PPh3)3Rh(CO)H in toluene and [bmim][BF4] at 110 °C for 30 min, only a small amount of hydrogenation product 18 was obtained (Table 2, entry 1), while most of the starting material remained unreacted. Increasing the temperature to 130 °C resulted in only 27% conversion to the desired compound 22 together with 2% of the enamine 21, but 56% of the starting material 17 was still present in the final reaction mixture (Scheme 6 and Table 2, entry 2).

Table 2.
HAM optimisation for Hyacintacine A2 synthesis.
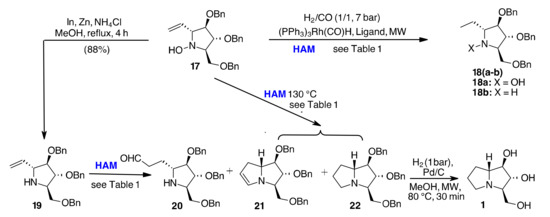
Scheme 6.
HAM for the synthesis of Hyacintacine A2.
With EtOH as solvent instead of toluene, compound 17 did not react at all (Table 2, entry 3). Increasing the syngas pressure had no effect, while using Biphephos as a ligand gave a complete conversion to the hydrogenation compound 18a (Table 2, entry 4). Pyrrolidine 19 was then prepared following our well-established protocol that employs catalytic indium and stoichiometric Zn as reducing agent [48,50], and HAM was investigated under different conditions (Scheme 5). The unsaturated pyrrolizidine 21 was isolated from 19 in toluene at 110 °C in 43 % yield (Table 2, entry 5). At higher temperature (130 °C), a mixture of products 20, 21, and 22 was observed (Table 2, entry 6). However, the use of EtOH as a solvent allowed almost complete conversion of 19 into 22 (Table 2, entry 7). When different ligands (Biphephos, PPh3) were used, a reduction of the C=C bond with a concomitant reduction of the N-O bond was the only reaction observed (Table 2, entries 8 and 9). Lower or higher syngas pressures always led to worse results in terms of yield of compound 22. Hydrogenolysis of 21 or 22 over Pd/C has been previously reported to give hyacinthacine A2 (1) [15,51,52,53] and is here used together with MW irradiation. Moreover, as we worked in a very small scale, the final product was obtained in such a low amount that good quality NMR spectra could not be recorded. Furthermore, both elemental analysis and [α]D are in agreement with data reported in the literature.
To investigate the possibility of reusing both the catalyst and the solvent used, the reaction was carried out on a 100-mg scale and the crude product filtered directly through an SCX column. Pyrrolizidine 22 was retained by the column and further recovered by washing the column with NH3 in EtOH. The first ethanolic fraction containing the ligand and catalyst was then recycled by addition of amine 19 and the solution was subjected to a new HAM cycle under the conditions given in entry 7 of Table 2. The expected product 22 was obtained with 80% yield. The recycle was repeated 3 times without any effect on the reaction yield, proving that the catalyst can be recycled efficiently. It is worth noting that, for all substrates studied, the CO/H2 addition to the C=C bond occurs regioselectively at the terminal position, as no branched aldehydes or the corresponding hemiacetals were observed.
In summary, we have shown that intramolecular HAM is a transformation that can be applied to the synthesis of pyrrolizidine alkaloids and their derivatives with high atom economy. We investigated both the use of hydroxylamines obtained from the reaction of nitrones with vinylmagnesium bromide and the corresponding amines. The use of the hydroxylamine as a substrate for HAM gave an intermediate aldehyde that was directly reduced to pyrrolizidine derivatives. In our experiments with different substrates, we found that the decoration of the A ring and/or the configuration at the different stereogenic centres have an influence on the reaction outcome. The hydroxylamine proved to be effective for our purposes only in the synthesis of the nor-lentiginosine 3 by a two-step process. Indeed, the intermediate aldehyde (8a) formed by HF remains in the corresponding unreactive hemiacetal form (8b), which requires further treatment and subsequent reducing conditions to afford 3. However, once a substituent was introduced at C-3 of the pyrrolidine ring, the amine performed better than the corresponding hydroxylamine and afforded the pyrrolizidine derivative in a one-pot reaction with 82% isolated yield. In all cases, intermediate enamine derivatives, potentially useful for other synthetic aims, were formed in different ratios, depending on the reaction conditions and catalyst/ligand used.
3. Materials and Methods
All reagents were used as purchased from commercial suppliers without further purification. Flash column chromatography was performed in glass columns using Merk silica gel 60 Å, 230–400 mesh particle size. For analytical thin-layer chromatography, Merck aluminium-backed plates pre-coated with silica gel 60 (UV254) were used and visualised by staining with a solution of p-anisaldehyde in EtOH or a KMnO4 solution. 1H NMR and 13C NMR spectra were recorded using a 400 MHz Brucker Advance NMR spectrometer (Bruker BioSpin AG, Fällanden, Switzerland). Deuterated chloroform and methanol were used as solvents. Chemical shift (δ) values are given in parts per million (ppm) and refer to the residual signals of the deuterated solvent (δ 7.26 for 1H and δ 77.6 for 13C in CDCl3, δ 3.34 for 1H and δ 49.00 for 13C in CD3OD). The data are presented as follows: chemical shift, multiplicity (s = singlet, d = doublet, dd = doublet of doublets, dt = doublet of triplets, t = triplet, q = quartet, m = multiplet or multiple resonances, bs = broad singlet), coupling constant (J) in hertz and the integration in ppm. Mass spectrometry data were collected using an Agilent 1100 LC /MSD VL system.
MW assisted reactions are performed in a CEM Discover MW (CEM s.r.l., Cologno Al Serio (BG), Italy) oven equipped with a 10 mL tube for reactions under pressure and an external IR sensor to record the reaction temperature during irradiation (CEM s.r.l., Cologno Al Serio (BG), Italy). This glass vial, tested to withstand pressures up to 250 psi (17 bar, 1723 KPa), is equipped with a tubing connection to an external pressure control system that includes a valve and output tubing to vent the vial at the end of the reaction. The output tubing was connected via a three-way connector to a cylinder containing CO/H2 (1:1) equipped with two taps to pre-purge the system prior to MW irradiation.
The nitrone derivatives were synthesised as previously reported [47,51,54].
General method for the preparation of 1-hydroxy-2-vynylpyrrolidine derivatives 7, 14, 17 [17]: To a stirred solution of the proper nitrone (4.37 mmol) in dry Et2O (40 mL), a 1 M solution of vinyl magnesium bromide in THF (5.5 mL, 5.5 mmol), was slowly added under nitrogen atmosphere at 20 °C. After stirring at 20 °C for 1 h and 45 min, 15 mL of saturated aqueous NaHCO3 were added. The precipitate was filtered and the mixture extracted with diethyl ether (3 × 15 mL). The combined organic extracts were dried over dry Na2SO4, and the solvent evaporated under vacuum and directly used in the next step. Single 1H-NMR spectra in Supplementary Materials.
General procedure for the synthesis of 2-vinylpirrolidine 9 and 19 [17]: To a stirred solution of the proper 1-hydroxy-2vinylpirrolidine (0.83 mmol) in dry methanol (10 mL), a saturated solution of NH4Cl (15 mL), powdered Zn (218 mg, 3.33 mmol) and a catalytic amount of indium dust (1.7 mg, 0.015 mmol) were added at 20 °C. The mixture was reflux overnight under N2. The solvent was evaporated under vacuum and a saturated aqueous solution of Na2CO3 (15 mL) was added. The mixture was extracted with Et2O (3 × 5 mL) and the combined organic phases were dried over dry Na2SO4, and the solvent evaporated under vacuum. The brownish oil (was used in the next step. Single 1H-NMR spectra in Supplementary Materials.
3-[(2S,3S,4S)-3,4-Di-tert-butoxy-1-hydroxy-2-pyrrolidinyl]propionaldehyde (8a): To a solution of 7 (30 mg, 0.16 mmol) in EtOH (500 μL), (PPh3)3Rh(CO)H (18 mg, 0.02 mmol) and Xantphos (46 mg, 0.08 mmol) were added. The yellow solution obtained was submitted to pressurized syngas at 100 psi (7 bar) and heated for 30 min at 110 °C by MW dielectric heating at 150 W (value previously settled on the MW oven). The flask was cooled down to rt and the internal gas released. The reaction mixture was evaporated in vacuo and the yellow oil obtained was purified by flash chromatography (CHCl3/MeOH: 95/5). Expected product 8 was obtained as a pale brown oil in 65% yields. ES-MS: 288 [M + H]+, 310 [M + Na]+. 1H-NMR (400 MHz, CDCl3): δ 9.77 (s, 1H, CHO), 4.37–4.25 (m, 1H, 4-H), 3.94–3.87 (m, 1H, 3-H), 3.17–3.11 (m, 1H, 5-Ha), 3.00 (t, J = 8.28 Hz, 1H, 5-Hb), 2.76–2.66 (m, 1H, 2-H), 2.46–20142.28 (m, 4H, CH2CH2), 1.25 (s, 9H, CH3 × 3), 1.23 (s, 9H, CH3 × 3) ppm. 13C-NMR (100 MHz, CDCl3, 308.15 K): δ 203.8, 81.4, 78.6, 77.8, 77.1, 41.1, 28.9, 19.7 ppm. Elemental Analysis for C15H29NO4: calcd. C-62.69, H-10.17, N-4.87, O-22.27; found C-62.45; H-10.43; N-4.92.
(1S,2S,7aS)-Hexahydro-1H-pyrrolizine-1,2-diol (3): A solution of 8 (16 mg, 0.06 mmol) in toluene (1 mL) in the presence of p-toluenesulfonic acid (1 mg, 0.0056 mmol) was submitted to MWs in open vessel conditions at 120 °C for 30 min at 300 W (value previously settled on the MW oven). The reaction mixture was evaporated in vacuo and the oil obtained was dissolved in MeOH (1 mL). Pd/C 10% wt (5.9 mg, 0.0056 mmol), and HCl 12 N (1 μL) was added and suspension obtained was submitted to two consecutive vacuum/H2 cycles, then pressurized with 15 psi (1 bar) of H2 and heated for 30 min at 40 °C by MW dielectric heating at 50 W (value previously settled on the MW oven). The flask was cooled to rt and the internal gas released. The reaction mixture was filtered over a Celite pad with MeOH (2 × 5 mL) and the solution passed through an SCX column washing with EtOH (2 × 10 mL). The column was washed with a 30% v/v NH3 solution in EtOH (2 × 10 mL): the solution obtained was evaporated in vacuo obtaining 12 that was directly treated with a 10% solution of CF3COOH in CH2Cl2 (2 mL) at r.t. for 3 h. The mixture was treated with a solution of NH3 in EtOH and passed through an SCX colum. After evaporation in vacuo 3 was obtained (4 mg) in 40 % yield. ES-MS: 144 [M + H]+, 166 [M + Na]+, 309 [2M + Na]+. 1H-NMR (400 MHz, D2O): δ 4.21 (q, J = 5.3 Hz, 1H), 3.92–3.99 (m, 1H), 3.48–3.66 (m, 2H), 3.24–3.32 (m, 1H), 2.83-3.01 (m, 2H), 1.73–17 (m, 4H) ppm. 13C-NMR (100 MHz, D2O): δ 82.09, 77.88, 71.74, 58.89, 58.03, 29.57, 26.27 ppm. Elemental Analysis for C7H13NO2: calcd. C-58.72; H-9.15; N-9.78; O-22.35; found C-58.37; H-9.24; N-9.83.
(1R,2R,3R,7aR)-1,2-Bis(benzyloxy)-3-[(benzyloxy)methyl]hexahydro-1H-pyrrolizine (22): To a solution of 19 (30 mg, 0.067 mmol) in EtOH (500 μL), (PPh3)3Rh(CO)H (18 mg, 0.02 mmol) and Xantphos (46 mg, 0.08 mmol) were added. The yellow solution obtained was submitted to pressurized syngas at 100 psi (7 bar) and heated for 30 min at 110 °C by MW dielectric heating at 150 W (value previously settled on the MW oven). The flask was cooled to rt and the internal gas released. The reaction mixture was passed through an SCX column washing with EtOH (2 × 10 mL). The column was then washed with a 30% v/v NH3 solution in EtOH (2 × 10 mL): the solution obtained was evaporated in vacuo obtaining 2 (24 mg) as a pale yellow solid in 82 % yield. M.p.: 49–51 °C Lit. [52] 47.5 °C ES-MS: 444 [M+H]+. 1H-NMR (400 MHz, CDCl3): δ 7.30–7.25 (m, 15H, ArH), 4.66 (d, J = 11.6 Hz, 1H, OCH2Ph); 4.67–4.49 (m, 5H, OCH2Ph), 4.04 (t, J = 7.2 Hz, 1H, 3-CH), 3.78 (t, J = 7.2 Hz, 1H, 3-CH), 3.56–3.45 (m, 3H, 1-H, 2-H, 7a-H), 3.10–3.03 (m, 1H, 3-CH), 3.00–2.92 (m, 1H, 5-CH), 2.81–2.72 (m, 1H, 5-H), 1.99–1.57 (m, 4H, 6-CH2, 7-CH2) ppm. [α]25D -4.7 (c 1, CHCl3) Lit. [52] [α]25D -5 (c 1, CHCl3). Lit. [21] [α]24D -5.1 (c 0.6, CHCl3). Elemental Analysis for C29H33NO3: calcd. C-78.52; H-7.50; N-3.16; O-10.82; found C-78.79; H-7.34; N-3.02.
(1R,2R,3R,7aR)-3-(Hydroxymethyl)hexahydro-1H-pyrrolizine-1,2-diol (1): A suspension of 17 (24 mg, 0.05 mmol), Pd/C 10% wt (5.3 mg, 0.005 mmol), and HCl 12 N (3 μL) in MeOH was submitted to 2 vacuum/H2 cycles, then pressurized with 15 psi (1 bar) of H2, and heated for 30 min at 80 °C by MW irradiation at 50W (value previously settled on the MW oven). The flask was cooled down to r.t. and the internal gas released. The reaction mixture was filtered over a Celite pad with MeOH (2 × 5 mL) and the solution passed through an SCX column washing with EtOH (2 × 10 mL). The column was washed with a 30% v/v NH3 solution in EtOH (2 × 10 mL): the solution obtained was evaporated in vacuo obtaining 1 in quantitative yields. [α]25D +12.1 (c 0.4, D2O) Lit. [53] [α]25D +12.5 (c 0.4, H2O). Lit. [21] [α]24D +12.4 (c 0.2, H2O). Elemental Analysis for C8H15NO3: calcd. C-55.47, H-8.73, N-8.09, O-27.71; found C 55.58, H 7.87, N 8.38.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27154762/s1. Single 1H-NMR spectra.
Author Contributions
Conceptualization, E.P., F.C., A.G. and M.T.; methodology, E.P. and F.C.; formal analysis, S.M.; investigation, S.Z., E.P., S.M., C.M. and F.C.; resources, E.P.; data curation, S.M.; writing—original draft preparation, E.P.; writing—review and editing, F.C., A.G. and M.T.; supervision, E.P., F.C. and M.T.; funding acquisition, E.P. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
“Il fondo di Ateneo per il supporto alla pubblicazione in OA” of the University of Siena, and Fondo di beneficenza Intesa San Paolo 2021 project SFORA.
Data Availability Statement
Not applicable.
Acknowledgments
We than Marco Bonanni, Dipartimento di Chimica “Ugo Schiff” (DICUS), Università degli studi di Firenze, for the preparation of some synthetic intermediates.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Smith, L.W.; Culvenor, C.C.J. Plant Sources of Hepatotoxic Pyrrolizidine Alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Ziarani, G.; Jamasbi, N.; Mohajer, F. Recent Advances on the Synthesis of Natural Pyrrolizidine Alkaloids: Alexine, and Its Stereoisomers. Nat. Prod. Bioprospect. 2022, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ruan, W.; Vrieling, K. Current Knowledge and Perspectives of Pyrrolizidine Alkaloids in Pharmacological Applications: A Mini-Review. Molecules 2021, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tropea, J.E.; Molyneux, R.J.; Kaushal, G.P.; Pan, Y.T.; Mitchell, M.; Elbein, A.D. Australine, a Pyrrolizidine Alkaloid That Inhibits Amyloglucosidase and Glycoprotein Processing. Biochemistry 1989, 28, 2027–2034. [Google Scholar] [CrossRef]
- Asano, N.; Ikeda, K.; Kasahara, M.; Arai, Y.; Kizu, H. Glycosidase-Inhibiting Pyrrolidines and Pyrrolizidines with a Long Side Chain in Scilla Peruviana. J. Nat. Prod. 2004, 67, 846–850. [Google Scholar] [CrossRef]
- Rousseaux, C.G.; Schachter, H. Regulatory Issues Concerning the Safety, Efficacy and Quality of Herbal Remedies. Birth Defects Res. Part B-Dev. Reprod. Toxicol. 2003, 68, 505–510. [Google Scholar] [CrossRef]
- EMA European Medicines Agency. Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrrolizidine Alkaloids (PAs); EMA: Amsterdam, The Netherlands, 2014; Volume 44. [Google Scholar]
- Stocker, B.L.; Dangerfield, E.M.; Win-Mason, A.L.; Haslett, G.W.; Timmer, M.S.M. Recent Developments in the Synthesis of Pyrrolidine-Containing Iminosugars. Eur. J. Org. Chem. 2010, 2010, 1615–1637. [Google Scholar] [CrossRef]
- Ratmanova, N.K.; Andreev, I.A.; Leontiev, A.V.; Momotova, D.; Novoselov, A.M.; Ivanova, O.A.; Trushkov, I.V. Strategic Approaches to the Synthesis of Pyrrolizidine and Indolizidine Alkaloids. Tetrahedron 2020, 76, 131031. [Google Scholar] [CrossRef]
- Calveras, J.; Casas, J.; Parella, T.; Joglar, J.; Clapés, P. Chemoenzymatic Synthesis and Inhibitory Activities of Hyacinthacines A1 and A2 Stereoisomers. Adv. Synth. Catal. 2007, 349, 1661–1666. [Google Scholar] [CrossRef]
- Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F.M.; Goti, A. Stereocontrolled Cyclic Nitrone Cycloaddition Strategy for the Synthesis of Pyrrolizidine and Indolizidine Alkaloids. Chem.A Eur. J. 2009, 15, 7808–7821. [Google Scholar] [CrossRef]
- Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F.M.; Goti, A. [3 + 2] Dipolar Cycloadditions of Cyclic Nitrones with Alkenes. In Organic Reactions; Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 94. [Google Scholar]
- Delso, I.; Marca, E.; Mannucci, V.; Tejero, T.; Goti, A.; Merino, P. Tunable Diastereoselection of Biased Rigid Systems by Lewis Acid Induced Conformational Effects: A Rationalization of the Vinylation of Cyclic Nitrones En Route to Polyhydroxylated Pyrrolidines. Chem.-A Eur. J. 2010, 16, 9901–9919. [Google Scholar] [CrossRef] [Green Version]
- Merino, P.; Revuelta, J.; Tejero, T.; Cicchi, S.; Goti, A. Fully Stereoselective Nucleophilic Addition to a Novel Chiral Pyrroline N-Oxide: Total Syntheses of (2S,3R)-3-Hydroxy-3-Methylproline and Its (2R)-Epimer. Eur. J. Org. Chem. 2004, 2004, 776–782. [Google Scholar] [CrossRef]
- Delso, I.; Tejero, T.; Goti, A.; Merino, P. Synthesis of D-Arabinose-Derived Polyhydroxylated Pyrrolidine, Indolizidine and Pyrrolizidine Alkaloids. Total Synthesis of Hyacinthacine A2. Tetrahedron 2010, 66, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- McCaig, A.E.; Meldrum, K.P.; Wightman, R.H. Synthesis of Trihydroxylated Pyrrolizidines and Indolizidines Using Cycloaddition Reactions of Functionalized Cyclic Nitrones, and the Synthesis of (+)- and (−)-Lentiginosine. Tetrahedron 1998, 54, 9429–9446. [Google Scholar] [CrossRef]
- Cardona, F.; Moreno, G.; Guarna, F.; Vogel, P.; Schuetz, C.; Merino, P.; Goti, A. New Concise Total Synthesis of (+)-Lentiginosine and Some Structural Analogues. J. Org. Chem. 2005, 70, 6552–6555. [Google Scholar] [CrossRef]
- Cardona, F.; Goti, A.; Picasso, S.; Vogel, P.; Brandi, A. Polyhydroxypyrrolidine Glycosidase Inhibitors Related to (+)-Lentiginosine1. J. Carbohydr. Chem. 2000, 19, 585–601. [Google Scholar] [CrossRef]
- Martella, D.; Cardona, F.; Parmeggiani, C.; Franco, F.; Tamayo, J.A.; Robina, I.; Moreno-Clavijo, E.; Moreno-Vargas, A.J.; Goti, A. Synthesis and Glycosidase Inhibition Studies of 5-Methyl-Substituted Tetrahydroxyindolizidines and -Pyrrolizidines Related to Natural Hyacinthacines B. Eur. J. Org. Chem. 2013, 2013, 4047–4056. [Google Scholar] [CrossRef]
- D’Adamio, G.; Sgambato, A.; Forcella, M.; Caccia, S.; Parmeggiani, C.; Casartelli, M.; Parenti, P.; Bini, D.; Cipolla, L.; Fusi, P.; et al. New Synthesis and Biological Evaluation of Uniflorine A Derivatives: Towards Specific Insect Trehalase Inhibitors. Org. Biomol. Chem. 2015, 13, 886–892. [Google Scholar] [CrossRef]
- Bonaccini, C.; Chioccioli, M.; Parmeggiani, C.; Cardona, F.; lo Re, D.; Soldaini, G.; Vogel, P.; Bello, C.; Goti, A.; Gratteri, P. Synthesis, Biological Evaluation and Docking Studies of Casuarine Analogues: Effects of Structural Modifications at Ring B on Inhibitory Activity towards Glucoamylase. Eur. J. Org. Chem. 2010, 2010, 5547–5585. [Google Scholar] [CrossRef]
- D’Adamio, G.; Parmeggiani, C.; Goti, A.; Moreno-Vargas, A.J.; Moreno-Clavijo, E.; Robina, I.; Cardona, F. 6-Azido Hyacinthacine A2 Gives a Straightforward Access to the First Multivalent Pyrrolizidine Architectures. Org. Biomol. Chem. 2014, 12, 6250–6266. [Google Scholar] [CrossRef]
- D’Adamio, G.; Goti, A.; Parmeggiani, C.; Moreno-Clavijo, E.; Robina, I.; Cardona, F. Total Synthesis of (+)-Hyacinthacine A1, (+)-7a-Epi- Hyacinthacine A1, (6R)-6-Hydroxyhyacinthacine A1 and (6S)-6-Hydroxy-7a-Epi-Hyacinthacine A1. Eur. J. Org. Chem. 2011, 2011, 7155–7162. [Google Scholar] [CrossRef]
- Pecchioli, T.; Cardona, F.; Reissig, H.U.; Zimmer, R.; Goti, A. Alkoxyallene-Based Stereodivergent Syntheses of (−)-Hyacinthacine B4 and of Putative Hyacinthacine C5 Epimers: Proposal of Hyacinthacine C5 Structure. J. Org. Chem. 2017, 82, 5835–5844. [Google Scholar] [CrossRef]
- Cornils, B.; Herrmann, W.A.; Rasch, M. Otto Roelen, Pioneer in Industrial Homogeneous Catalysis. Angew. Chem. Int. Ed. 1994, 33, 2144–2163. [Google Scholar] [CrossRef]
- Raoufmoghaddam, S. Recent Advances in Catalytic C-N Bond Formation: A Comparison of Cascade Hydroaminomethylation and Reductive Amination Reactions with the Corresponding Hydroamidomethylation and Reductive Amidation Reactions. Org. Biomol. Chem. 2014, 12, 7179–7193. [Google Scholar] [CrossRef]
- Yang, J.; Delolo, F.G.; Spannenberg, A.; Jackstell, R.; Beller, M. A Selective and General Cobalt-Catalyzed Hydroaminomethylation of Olefins to Amines. Angew. Chem. Int. Ed. 2022, 61, e202112597. [Google Scholar] [CrossRef]
- Chen, C.; Dong, X.Q.; Zhang, X. Recent Progress in Rhodium-Catalyzed Hydroaminomethylation. Org. Chem. Front. 2016, 3, 1359–1370. [Google Scholar] [CrossRef]
- Kalck, P.; Urrutigoïty, M. Tandem Hydroaminomethylation Reaction to Synthesize Amines from Alkenes. Chem. Rev. 2018, 118, 3833–3861. [Google Scholar] [CrossRef]
- An, J.; Gao, Z.; Wang, Y.; Zhang, Z.; Zhang, J.; Li, L.; Tang, B.; Wang, F. Heterogeneous Ru/TiO2 for Hydroaminomethylation of Olefins: Multicomponent Synthesis of Amines. Green Chem. 2021, 23, 2722–2728. [Google Scholar] [CrossRef]
- Pizzetti, M.; Russo, A.; Petricci, E. Microwave-Assisted Aminocarbonylation of Ynamides by Using Catalytic [Fe3(CO)12] at Low Pressures of Carbon Monoxide. Chem.-A Eur. J. 2011, 17, 4523–4528. [Google Scholar] [CrossRef]
- Cardullo, F.; Donati, D.; Merlo, G.; Paio, A.; Petricci, E.; Taddei, M. Microwave-Assisted Aminocarbonylation of Aryl Bromides at Low Carbon Monoxide Pressure. Synlett 2009, 2009, 47–50. [Google Scholar] [CrossRef]
- Risi, C.; Cini, E.; Petricci, E.; Saponaro, S.; Taddei, M. In Water Markovnikov Hydration and One-Pot Reductive Hydroamination of Terminal Alkynes under Ruthenium Nanoparticle Catalysis. Eur. J. Inorg. Chem. 2020, 2020, 1000–1003. [Google Scholar] [CrossRef]
- Petricci, E.; Risi, C.; Ferlin, F.; Lanari, D.; Vaccaro, L. Avoiding Hot-Spots in Microwave-Assisted Pd/C Catalysed Reactions by Using the Biomass Derived Solvent γ-Valerolactone. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumde, V.R.; Petricci, E.; Petrucci, C.; Santillo, N.; Taddei, M.; Vaccaro, L. Domino Hydrogenation-Reductive Amination of Phenols, a Simple Process to Access Substituted Cyclohexylamines. Org. Lett. 2015, 17, 3990–3993. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Santillo, N.; Petrucci, C.; Lanari, D.; Petricci, E.; Taddei, M.; Vaccaro, L. Continuous-Flow Palladium-Catalyzed Synthesis of Cyclohexanones from Phenols Using Sodium Formate as a Safe Hydrogen Source. ChemCatChem 2018, 10, 1277–1281. [Google Scholar] [CrossRef]
- Verheyen, T.; Santillo, N.; Marinelli, D.; Petricci, E.; de Borggraeve, W.M.; Vaccaro, L.; Smet, M. An Effective and Reusable Hyperbranched Polymer Immobilized Rhodium Catalyst for the Hydroformylation of Olefins. ACS Appl. Polym. Mater. 2019, 1, 1496–1504. [Google Scholar] [CrossRef]
- Airiau, E.; Chemin, C.; Girard, N.; Lonzi, G.; Mann, A.; Petricci, E.; Salvadori, J.; Taddei, M. Microwave-Assisted Domino Hydroformylation/Cyclization Reactions: Scope and Limitations. Synthesis 2010, 2010, 2901–2914. [Google Scholar] [CrossRef]
- Pizzetti, M.; de Luca, E.; Petricci, E.; Porcheddu, A.; Taddei, M. A General Approach to Substituted Benzimidazoles and Benzoxazoles via Heterogeneous Palladium-Catalyzed Hydrogen-Transfer with Primary Amines. Adv. Synth. Catal. 2012, 354, 2453–2464. [Google Scholar] [CrossRef]
- Arena, G.; Cini, E.; Petricci, E.; Randino, R.; Taddei, M. A Highly Stereo-Controlled Protocol to Prepare Pipecolic Acids Based on Heck and Cyclohydrocarbonylation Reactions. Org. Chem. Front. 2015, 2, 526–530. [Google Scholar] [CrossRef]
- Balducci, E.; Bellucci, L.; Petricci, E.; Taddei, M.; Tafi, A. Microwave-Assisted Intramolecular Huisgen Cycloaddition of Azido Alkynes Derived from α-Amino Acids. J. Org. Chem. 2009, 74, 1314–1321. [Google Scholar] [CrossRef]
- Salvadori, J.; Balducci, E.; Zaza, S.; Petricci, E.; Taddei, M. Microwave-Assisted Carbonylation and Cyclocarbonylation of Aryl Iodides under Ligand Free Heterogeneous Catalysis. J. Org. Chem. 2010, 75, 1841–1847. [Google Scholar] [CrossRef]
- Migliorini, F.; Dei, F.; Calamante, M.; Maramai, S.; Petricci, E. Micellar Catalysis for Sustainable Hydroformylation. ChemCatChem 2021, 13, 2794–2806. [Google Scholar] [CrossRef]
- Petricci, E.; Santillo, N.; Castagnolo, D.; Cini, E.; Taddei, M. Iron-Catalyzed Reductive Amination of Aldehydes in Isopropyl Alcohol/Water Media as Hydrogen Sources. Adv. Synth. Catal. 2018, 360, 2560–2565. [Google Scholar] [CrossRef]
- Petricci, E.; Mann, A.; Schoenfelder, A.; Rota, A.; Taddei, M. Microwaves Make Hydroformylation a Rapid and Easy Process. Org. Lett. 2006, 8, 3725–3727. [Google Scholar] [CrossRef]
- Petricci, E.; Mann, A.; Salvadori, J.; Taddei, M. Microwave Assisted Hydroaminomethylation of Alkenes. Tetrahedron Lett. 2007, 48, 8501–8504. [Google Scholar] [CrossRef]
- Cicchi, S.; Höld, I.; Brandi, A. New Synthesis of Five-Membered Cyclic Nitrones from Tartaric Acid. J. Org. Chem. 1993, 58, 5274–5275. [Google Scholar] [CrossRef]
- Matassini, C.; Bonanni, M.; Marradi, M.; Cicchi, S.; Goti, A. On the Virtue of Indium in Reduction Reactions. A Comparison of Reductions Mediated by Indium and Zinc: Is Indium Metal an Effective Catalyst for Zinc Induced Reductions? Eur. J. Inorg. Chem. 2020, 2020, 1106–1113. [Google Scholar] [CrossRef] [Green Version]
- Bini, D.; Forcella, M.; Cipolla, L.; Fusi, P.; Matassini, C.; Cardona, F. Synthesis of Novel Iminosugar-Based Trehalase Inhibitors by Cross-Metathesis Reactions. Eur. J. Org. Chem. 2011, 2011, 3995–4000. [Google Scholar] [CrossRef]
- Cicchi, S.; Bonanni, M.; Cardona, F.; Revuelta, J.; Goti, A. Indium-Mediated Reduction of Hydroxylamines to Amines. Org. Lett. 2003, 5, 1773–1776. [Google Scholar] [CrossRef]
- Cardona, F.; Faggi, E.; Liguori, F.; Cacciarini, M.; Goti, A. Total Syntheses of Hyacinthacine A2 and 7-Deoxycasuarine by Cycloaddition to a Carbohydrate Derived Nitrone. Tetrahedron Lett. 2003, 44, 2315–2318. [Google Scholar] [CrossRef]
- Desvergnes, S.; Py, S.; Vallee, Y. Total Synthesis of (+)-Hyacinthacine A2 (I) Based on SmI2-Induced Nitrone Umpolung. ChemInform 2005, 36. [Google Scholar] [CrossRef]
- Rambaud, L.; Compain, P.; Martin, O.R. First Total Synthesis of (+)-Hyacinthacine A2. Tetrahedron Asymmetry 2001, 12, 1807–1809. [Google Scholar] [CrossRef]
- Martella, D.; D’Adamio, G.; Parmeggiani, C.; Cardona, F.; Moreno-Clavijo, E.; Robina, I.; Goti, A. Cycloadditions of Sugar-Derived Nitrones Targeting Polyhydroxylated Indolizidines. Eur. J. Org. Chem. 2016, 2016, 1588–1598. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).