Comparative Characterization and Immunomodulatory Activities of Polysaccharides Extracted from the Radix of Platycodon grandiflorum with Different Extraction Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield and Carbohydrate Content of PGs
2.2. Characteristics of PGs
2.2.1. FT-IR and UV Spectroscopy Analyses of PGs
2.2.2. Molecular Weights of PGs
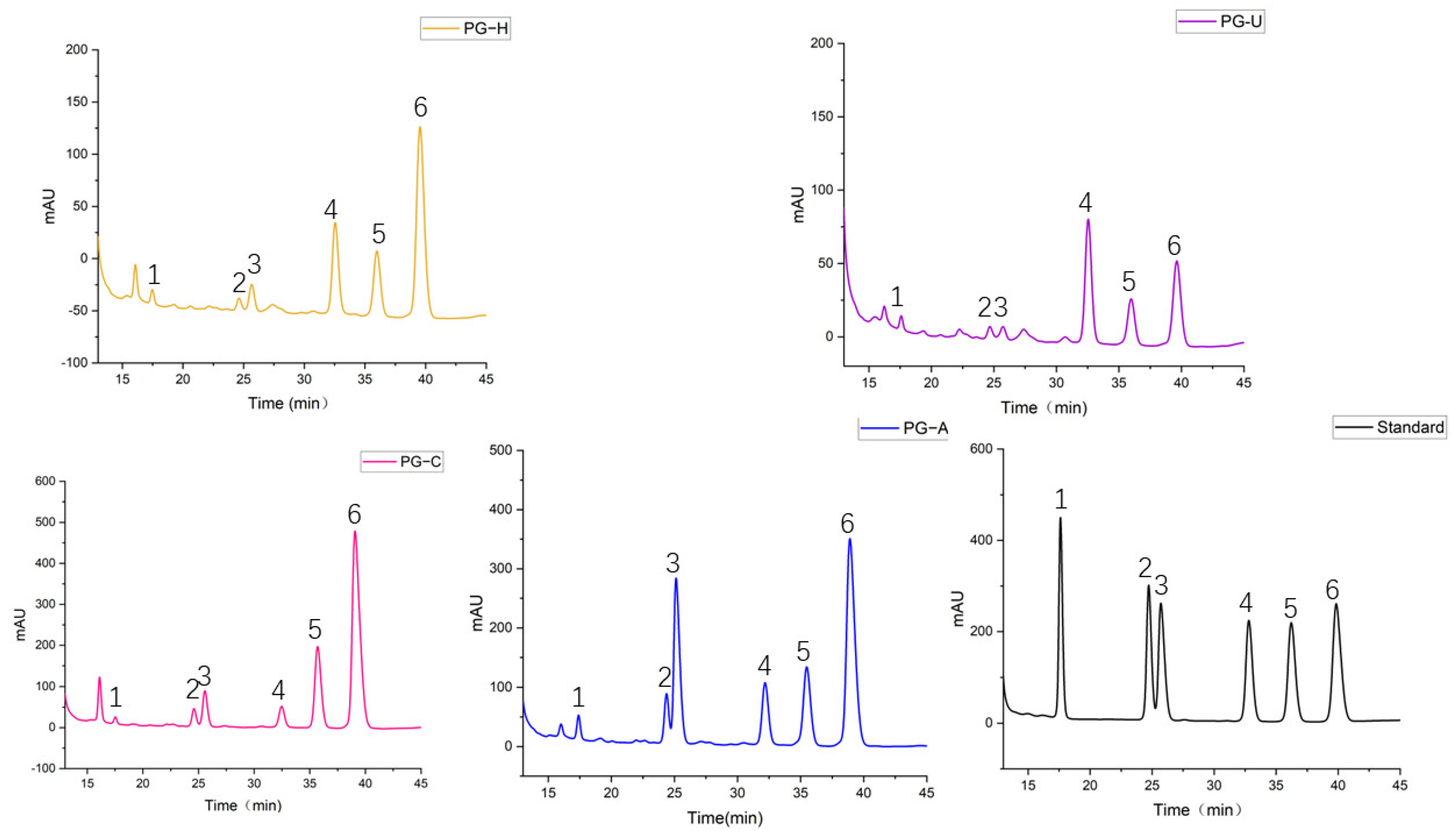
2.2.3. Monosaccharide Composition of PGs
2.2.4. Particle Size and Zeta-Potential of PGs
2.3. Immunomodulatory Effects of PGs
2.3.1. Effect of PGs on Macrophage Cells Proliferation
2.3.2. Effect of PGs on NO Production in Macrophage Cells
2.3.3. Effects of PGs on the Expression of TNF-α and IL-6 in Macrophage Cells
2.3.4. Effect of PGs on Phagocytosis in Macrophage Cells
3. Materials and Method
3.1. Materials
3.2. Extraction of Polysaccharides
3.3. Determination of Extraction Yield
3.4. Determination of Carbohydrate Contents
3.5. Structural Characteristics of PGs
3.5.1. Fourier Transform Infrared (FT-IR) Analysis
3.5.2. UV Analysis
3.5.3. Molecular Weight Determination
3.5.4. Monosaccharide Composition Determination
3.5.5. Molecular Particle Size and Zeta-Potential Analysis
3.6. Measurement of Immunomodulatory Activity
3.6.1. Macrophage Proliferation Assay
3.6.2. Measurement of NO
3.6.3. Measurements of TNF-α and IL-6
3.6.4. Phagocytosis Assay for Macrophages
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zou, Y.; Chen, M.; Fu, Y.; Zhu, Z.; Zhang, Y.; Paulsen, B.S.; Rise, F.; Chen, Y.; Yang, Y.; Jia, R.; et al. Characterization of an antioxidant pectic polysaccharide from Platycodon grandiflorus. Int. J. Biol. Macromol. 2021, 175, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandifloras—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Lei, L. Effects of polysaccharides from Platycodon on tumor growth and immune function in S180 tumor-bearing mice. Hyundai Immunol. 2021, 41, 462–467. [Google Scholar]
- Dong, Z.; Cao, W.G.; Duan, H.; Zhang, X.T.; Cheng, J.; Zhan, K. Platycodon polysaccharide extraction, isolation and purification, and biological activity studies. Genom. Appl. Biol. 2018, 37, 3534–3539. [Google Scholar]
- Han, S.B.; Park, S.H.; Lee, K.H.; Lee, C.W.; Lee, S.H.; Kim, H.C.; Kim, Y.S.; Lee, H.S.; Kim, H.M. Polysaccharide isolated from the radix of Platycodon grandiflorum selectively activates B cells and macrophages but not T cells. Int. Immunopharmacol. 2001, 1, 1969–1978. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, X.; Zhu, L.; Xu, Y.; Cui, W.; He, X.; Wei, K.; Zhu, R. A polysaccharide found in Paulownia fortunei flowers can enhance cellular and humoral immunity in chickens. Int. J. Biol. Macromol. 2019, 130, 213–219. [Google Scholar] [CrossRef]
- Chinen, J.; Shearer, W.T. Secondary immunodeficiencies, including HIV infection. J. Allergy Clin. Immunol. 2010, 125, 195–203. [Google Scholar] [CrossRef]
- Zhan, Q.; Wang, Q.; Lin, R.; He, P.; Lai, F.; Zhang, M.; Wu, H. Structural characterization and immunomodulatory activity of a novel acid polysaccharide isolated from the pulp of Rosa laevigata Michx fruit. Int. J. Biol. Macromol. 2020, 145, 1080–1090. [Google Scholar] [CrossRef]
- Fang, C.; Gangliang, H. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar]
- Song, Y.R.; Ah-Ram, H.; Lim, T.G.; Lee, E.J.; Hong, H.D. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019, 128, 546–555. [Google Scholar] [CrossRef]
- Yang, L.C.; Hsieh, C.C.; Wen, C.L.; Chiu, C.H.; Lin, W.C. Structural characterization of an immunostimulating polysaccharide from the stems of a new medicinal Dendrobium species: Dendrobium Taiseed Tosnobile. Int. J. Biol. Macromol. 2017, 103, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, A.; Masoud, R.; Mehdi, T.; Massimo, R.; Manuela, D.; Francesco, M.; SangGuan, Y.; David, L.; Giancarlo, C. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2018, 124, 131–137. [Google Scholar]
- Chen, C.; Wang, P.; Huang, Q.; You, L.; Liu, R.; Zhao, M.; Fu, X.; Luo, Z. A comparison study on polysaccharides extracted from Fructus Mori using different methods: Structural characterization and glucose entrapment. Food Funct. 2019, 10, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.H.; Wen, L.; Hong, Y.L.; Jing, L.H.; Huan, G.; Shang, L.; Li, Z.; Hong, C.; Yao, W.L.; Ding, T.; et al. Extraction Optimization, Physicochemical Characteristics, and Antioxidant Activities of Polysaccharides from Kiwifruit (Actinidia chinensis Planch.). Molecules 2019, 3, 461. [Google Scholar]
- Fang, C.; Chen, G.; Kan, J. Comparison on characterization and biological activities of Mentha haplocalyx polysaccharides at different solvent extractions. Int. J. Biol. Macromol. 2020, 154, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W. Advances in polysaccharides extraction from medical plants. Mod. Work 2010, 30, 32–36. [Google Scholar]
- Al-Dhabi, N.A.; Ponmurugan, K. Microwave assisted extraction and characterization of polysaccharide from waste jamun fruit seeds. Int. J. Biol. Macromol. 2020, 152, 1157–1163. [Google Scholar] [CrossRef]
- Wu, D.T.; Liu, W.; Han, Q.H.; Wang, P.; Xiang, X.R.; Ding, Y.; Zhao, L.; Zhang, Q.; Li, S.Q.; Qin, W. Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia). Molecules 2019, 24, 2817. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Cui, M.; Wang, Y.; Zhang, M.; Li, F.; Liu, K. Isolation, structure identification and anti-inflammatory activity of a polysaccharide from Phragmites rhizoma. Int. J. Biol. Macromol. 2020, 161, 810–817. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Kong, X.; Li, H. Characterization and Immunological Activities of Polysaccharides from Polygonatum sibiricum. Biol. Pharm. Bulletin. 2020, 43, 959–967. [Google Scholar] [CrossRef]
- Kong, L.; Yu, L.; Feng, T.; Yin, X.; Liu, T.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. J. Technol. 2015, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jalaleldeen, K.M.; Amer, A.M.; Mohamed, I.A.; Mengjiao, M.; Hongxin, W. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from Medemia argun fruit. Int. J. Biol. Macromol. 2020, 155, 919–926. [Google Scholar]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, D.; Zhang, Y.; Zeng, X.A. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhu, P.; Wang, M.; Ma, S.; Wei, Z. Optimization of water-soluble polysaccharides from stem lettuce by response surface methodology and study on its characterization and bioactivities. Int. J. Biol. Macromol. 2017, 105, 912–923. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548–1559. [Google Scholar] [CrossRef]
- Li, M.; Yan, D.; Hu, X.; Ren, G.; Zhu, X.; Wang, Z.; Teng, Z.; Wu, R.; Wu, J. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int. J. Biol. Macromol. 2021, 183, 1774–1783. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef]
- Fan, Y.; Xiaozhou, L.; Ye, Y.; Selina, M.A.; Feihe, W.; Hong, L.; Guiyun, W. A polysaccharide isolated from the fruits of Physalis alkekengi L. induces RAW264.7 macrophages activation via TLR2 and TLR4-mediated MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 140, 895–906. [Google Scholar]
- Ya, S.; Minqian, Z.; Huili, H.; Jie, D.; Meiying, L.; Yuanming, S.; Ruili, Y.; Hong, W.; Riming, H. Structure characterization of a novel polysaccharide from Chinese wild fruits (Passiflora foetida) and its immune-enhancing activity. Int. J. Biol. Macromol. 2019, 136, 324–331. [Google Scholar]
- Tang, C.; Sun, J.; Liu, J.; Jin, C.; Wu, X.; Zhang, X.; Chen, H.; Gou, Y.; Kan, J.; Qian, C.; et al. Immune-enhancing effects of polysaccharides from purple sweet potato. Int. J. Biol. Macromol. 2019, 123, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jing, H.Y.; Li, Y.L.; Dong, X.X.; Zhang, K.; Mang, S.C. Immunomodulatory effect of different molecular weight polysaccharides from Lycium barbarum on RAW264.7 macrophages. Chin. J. New Drugs 2021, 30, 1079–1086. [Google Scholar]
- Gu, J.; Zhang, H.; Wen, C.; Zhang, J.; He, Y.; Ma, H.; Duan, Y. Purification, characterization, antioxidant and immunological activity of polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020, 136, 109345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Nan, C.X.; Zhang, L.X. Study on the extraction of polysaccharides from Platycodon and their antioxidant effects. Food Mach. 2008, 3, 60–63. [Google Scholar]
- Zhao, Y.-H.; Ma, J.; Li, J.-Q.; Wang, L. Active ingredients and active function of extracts from Heracleum Moellendorffii Hance by different solvents. Mod. Food Technol. 2018, 34, 39–45. [Google Scholar]
- De-long, L. Study on ultrasonic extraction of Polysaccharides from Platycodon grandiflorum. J. Heilongjiang Inst. Technol. (Compr. Ed.) 2019, 19, 63–66. [Google Scholar]
- Chen, G.T.; Yuan, B.; Wang, H.X.; Qi, G.H.; Cheng, S.J. Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int. J. Biol. Macromol. 2019, 139, 801–809. [Google Scholar] [CrossRef]
- Bai, R.B.; Ma, Y.L.; Zhang, P.; Wang, Y.P.; Li, Y.D.; Hu, F.D. Content Determination of Saccharide in Polysaccharides Containing Galacturonic Acid by Phenol-sulfuric Acid Method Combined with Calibration Factor Method. China Pharm. 2017, 28, 2974–2978. [Google Scholar]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Novel polysaccharide from Chaenomeles speciosa seeds: Structural characterization, α-amylase and α-glucosidase inhibitory activity evaluation. Int. J. Biol. Macromol. 2020, 153, 757–766. [Google Scholar] [CrossRef]
- Li, Y.; Qin, G.; Cheng, C.; Yuan, B.; Huang, D.; Cheng, S.; Cao, C.; Chen, G. Purification, characterization and anti-tumor activities of polysaccharides from Ecklonia kurome obtained by three different extraction methods. Int. J. Biol. Macromol. 2020, 150, 1000–1010. [Google Scholar] [CrossRef]
- Zhao, S.; Han, Z.; Yang, L.; Hong, B.; Zhu, H. Extraction characterization and antioxidant activity evaluation of polysaccharides from Smilacina japonica. Int. J. Biol. Macromol. 2020, 151, 76–583. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Xie, Z.; Huang, S.; Tai, Y.; Cai, Q.; Jiang, W.; Sun, J.; Yuan, Y. Immune-enhancing effects of polysaccharides extracted from Lilium lancifolium Thunb. Int. J. Biol. Macromol. 2017, 52, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Yao, L.; Zhang, X.; Lin, S. Isolation, purification, characterization, and immunomodulatory effects of polysaccharide from Auricularia auricula on RAW264.7 macrophages. J. Food Biochem. 2020, 42, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hao, L.; Kang, Q.; Cui, Y.; Jiang, H.; Liu, X.; Lu, J. Purification, characterization and biological activities of a polysaccharide from Lepidium meyenii leaves. Int. J. Biol. Macromol. 2017, 103, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, S.; Zhao, J.; Li, S. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 373–382. [Google Scholar] [CrossRef] [PubMed]





| Samples | PG-H | PG-U | PG-C | PG-A |
|---|---|---|---|---|
| Yield (%) | 16.6 | 7.1 | 3.8 | 2.8 |
| Carbohydrate content (%) | 80.9 | 94.0 | 94.5 | 92.3 |
| Sample | PG-H | PG-U | PG-C | PG-A |
|---|---|---|---|---|
| Mw of Peak I | >4414.3 | >5141.5 | >3925.5 | >3479.0 |
| Peak area ratio of Peak I (%) | 42.5 | 33.7 | 53.4 | 71.7 |
| Mw of Peak II | 2.8 | 3.0 | 1.3 | 1.3 |
| Peak area ratio of Peak II (%) | 57.5 | 66.3 | 46.6 | 28.3 |
| PG-H | PG-U | PG-C | PG-A | |
|---|---|---|---|---|
| Man (mol %) | 1.9 | 3.6 | 0.9 | 2.2 |
| Rha (mol %) | 2.7 | 3.2 | 4.2 | 7.2 |
| GalA (mol %) | 5.3 | 3.4 | 7.4 | 23.9 |
| Glc (mol %) | 22.8 | 42.2 | 5.8 | 11.2 |
| Gal (mol %) | 17.1 | 17.0 | 22.5 | 15.1 |
| Ara (mol %) | 50.1 | 30.6 | 59.2 | 40.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Zhou, P.; Wang, X.; Zhao, R.; Wang, Y. Comparative Characterization and Immunomodulatory Activities of Polysaccharides Extracted from the Radix of Platycodon grandiflorum with Different Extraction Methods. Molecules 2022, 27, 4759. https://doi.org/10.3390/molecules27154759
Xiao W, Zhou P, Wang X, Zhao R, Wang Y. Comparative Characterization and Immunomodulatory Activities of Polysaccharides Extracted from the Radix of Platycodon grandiflorum with Different Extraction Methods. Molecules. 2022; 27(15):4759. https://doi.org/10.3390/molecules27154759
Chicago/Turabian StyleXiao, Wanwan, Pingfan Zhou, Xiaoshuang Wang, Ruizhi Zhao, and Yan Wang. 2022. "Comparative Characterization and Immunomodulatory Activities of Polysaccharides Extracted from the Radix of Platycodon grandiflorum with Different Extraction Methods" Molecules 27, no. 15: 4759. https://doi.org/10.3390/molecules27154759
APA StyleXiao, W., Zhou, P., Wang, X., Zhao, R., & Wang, Y. (2022). Comparative Characterization and Immunomodulatory Activities of Polysaccharides Extracted from the Radix of Platycodon grandiflorum with Different Extraction Methods. Molecules, 27(15), 4759. https://doi.org/10.3390/molecules27154759





