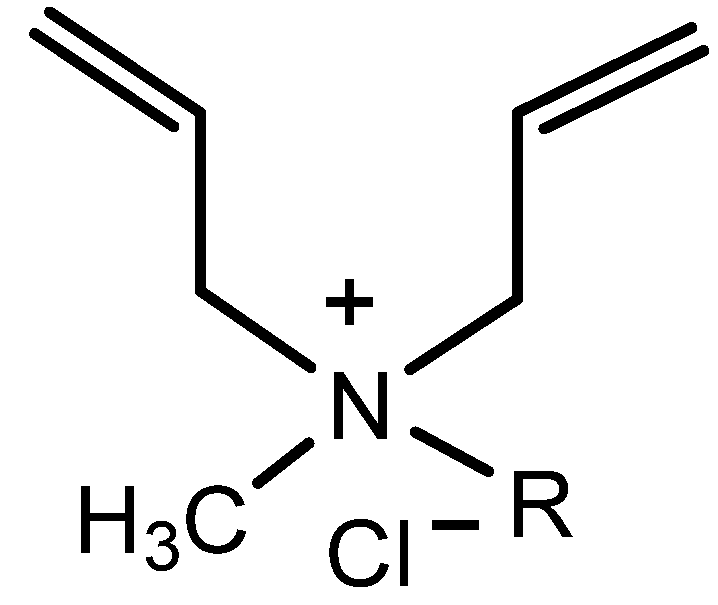
Effect of Substituents on the Homopolymerization Activity of Methyl Alkyl Diallyl Ammonium Chloride
Abstract
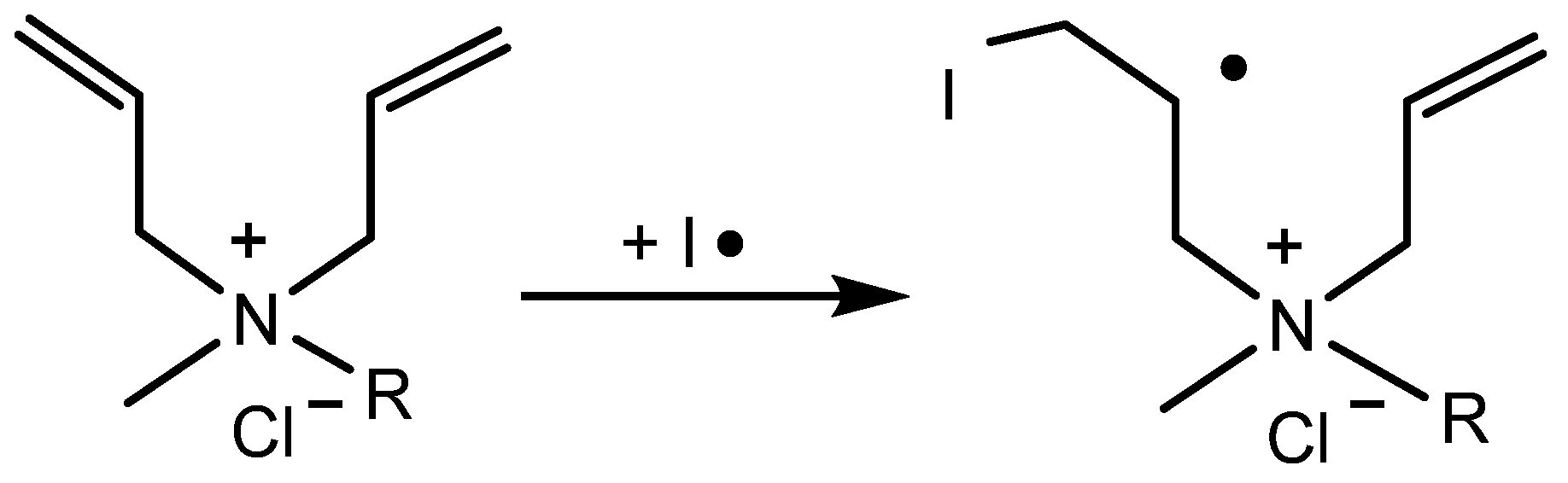
1. Introduction
2. Results
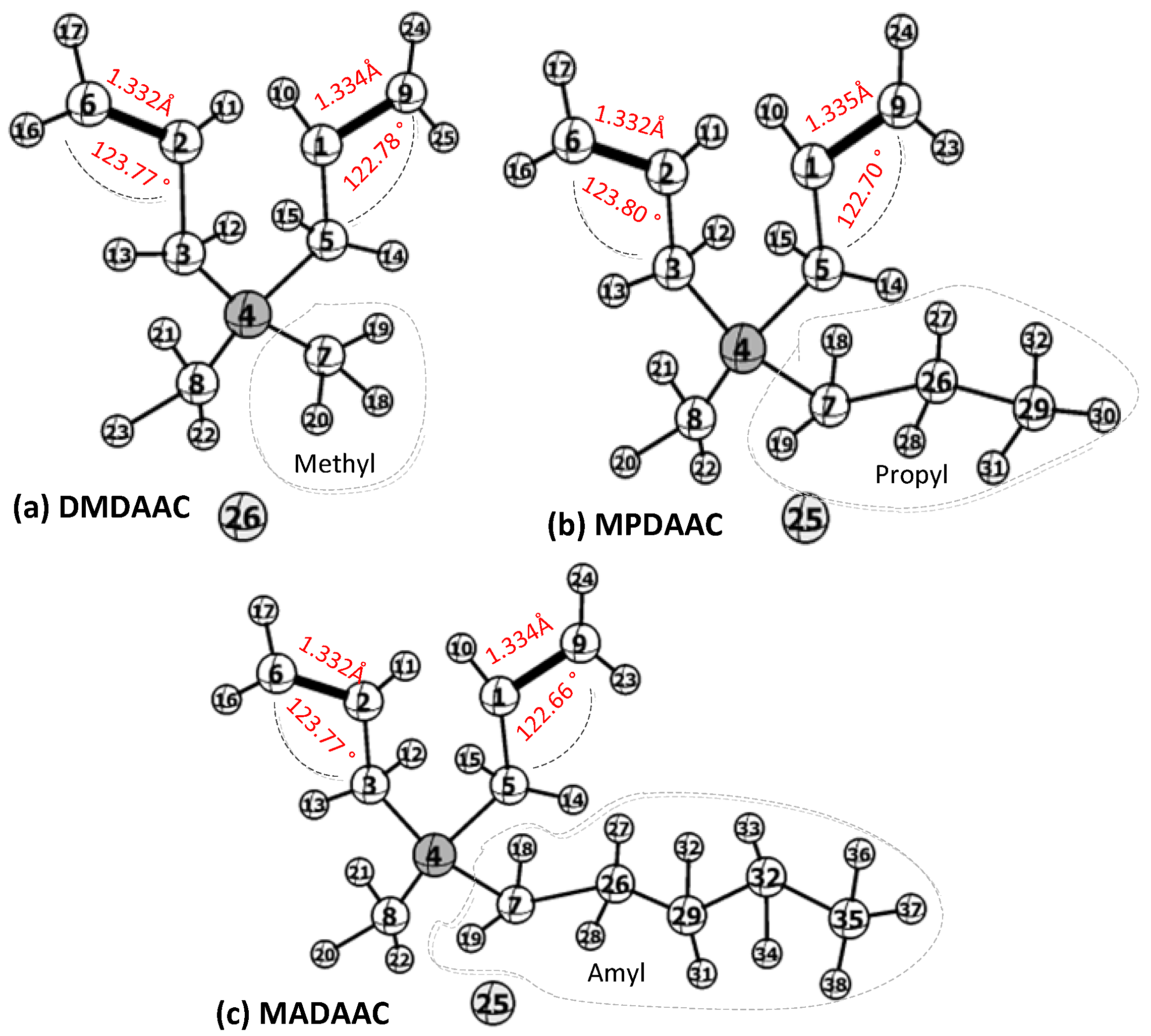
2.1. Monomer Conformation Optimization
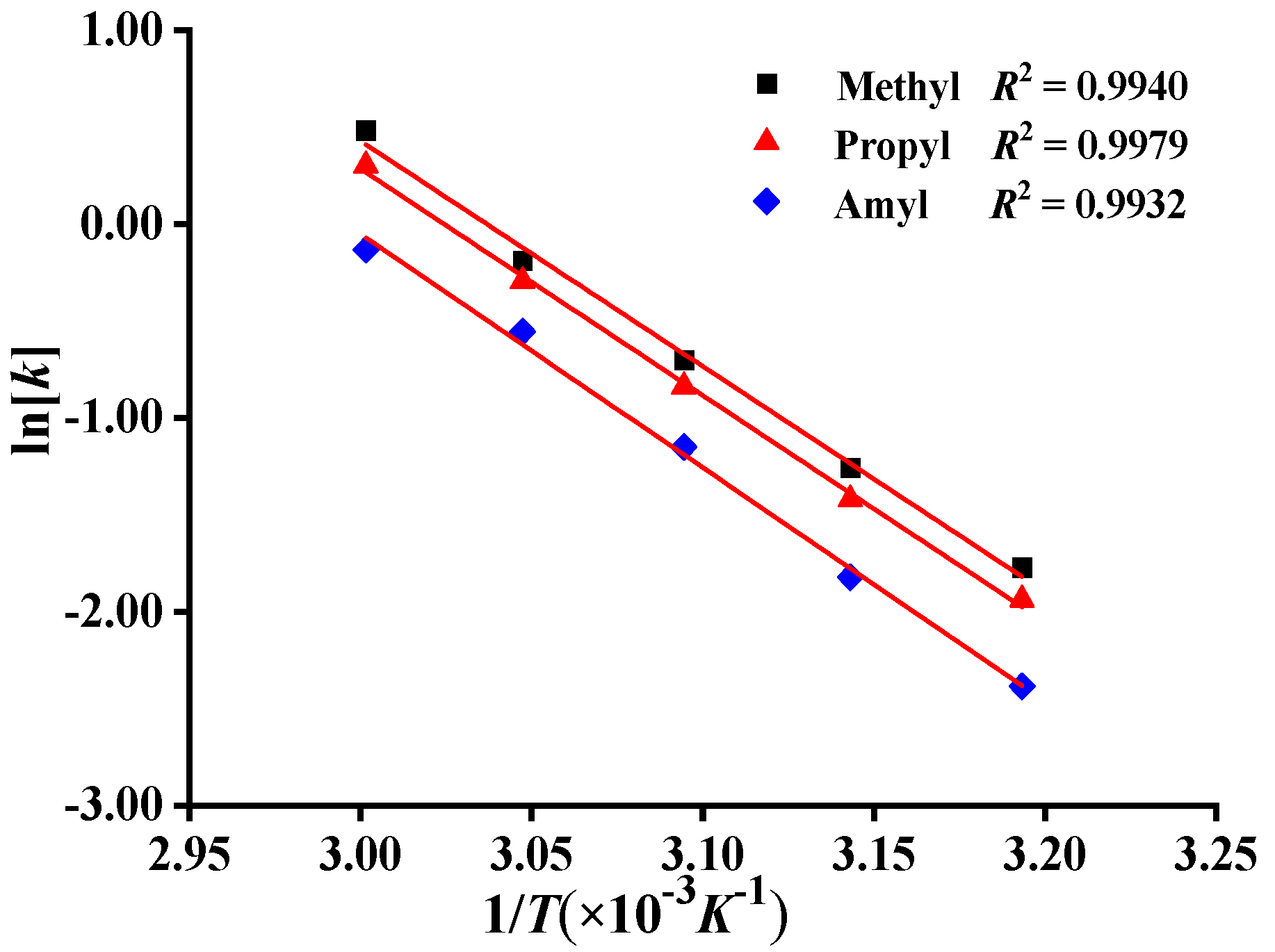
2.2. Determination of Activation Energy of Polymerization of Monomers
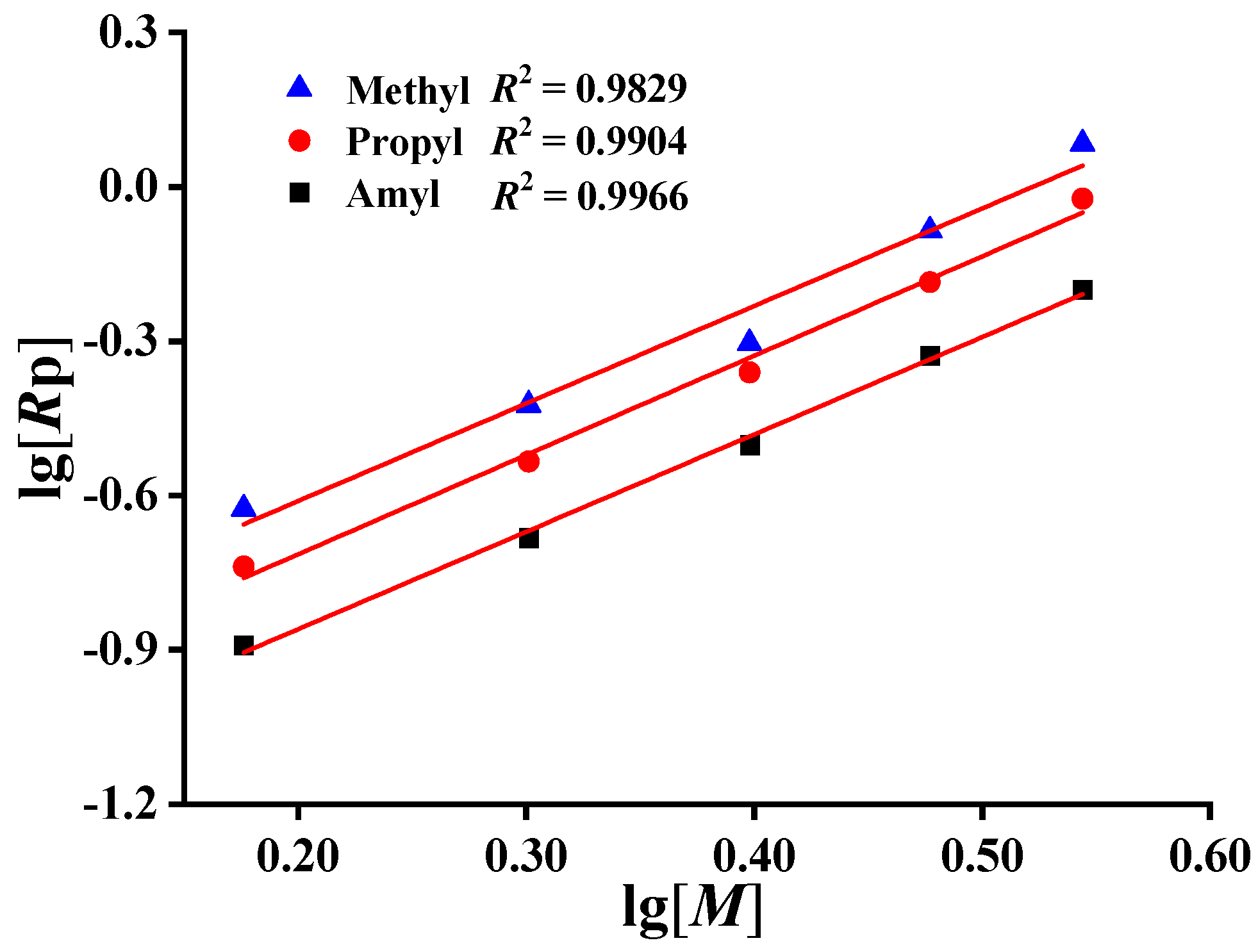
2.3. Determination of Monomer Concentration Iindices
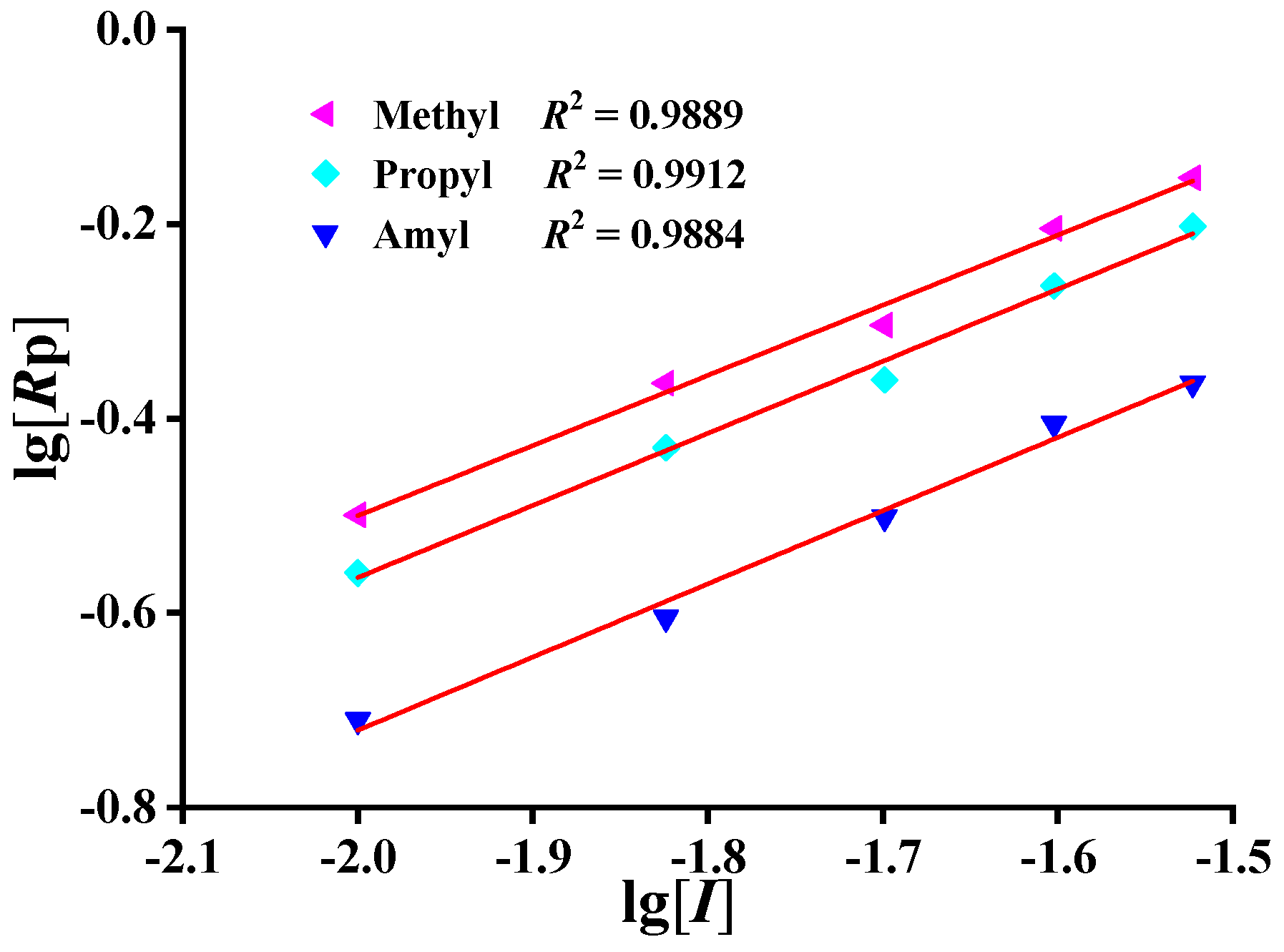
2.4. Determination of Concentration Indices of Initiator
3. Discussion
3.1. The Effect of Substituents on the Conformation Parameters of Monomer Molecules
3.2. Effect of Substituents on the Activity of Monomer Polymerization
3.2.1. Polymerization Reaction Rate Rp Comparison of Monomer Polymerization Activity
3.2.2. Calculation of kp/kt1/2 and Comparison of Polymerization Activities of Different Monomers
3.2.3. Comparison of Polymerization Activities of Different Monomers by Activation Energies Ea
3.3. Effect of Micelle Formation on the Activity of Monomer Polymerization
4. Materials and Methods
4.1. Materials
4.2. Quantum Chemical Calculation Results
4.3. Kinetics of Homopolymerization
4.3.1. Experimental Methods
4.3.2. Determination of Polymerization Rate Equation
4.3.3. Determination of Apparent Activation Energy Ea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhao, X.L.; Zhang, Y.J. Algae-removing and algicidal efficiencies of polydiallyldimethylammonium chloride composite coagulants in enhanced coagulation treatment of algae-containing raw water. Chem. Eng. J. 2011, 173, 164–170. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, X.Y.; Zhang, Z.Q.; Xu, B.C.; Sun, Y.J.; Liu, Y.Z.; Zheng, H.L. Ultrasound-assisted polymerization of P(AM-DMDAAC): Synthesis, characterization and sludge dewatering performance. J. Environ. Chem. Eng. 2017, 5, 5439–5447. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, Q.L.; Chen, Y.Z.; Li, Z.H. Poly(dimethyldiallylammonium chloride) (polyDADMAC) assisted cellulase pretreatment for microfibrillated cellulose (MFC) preparation and MFC analysis. Holzforschung 2018, 72, 531–538. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, L.; Feng, R.Q.; Ma, H.M.; Fan, D.W.; Yan, T.; Feng, R.; Du, B.; Wei, Q. MnCO3 as a New Electrochemiluminescence Emitter for Ultrasensitive Bioanalysis of β-Amyloid1-42 Oligomers Based on Site-Directed Immobilization of Antibody. ACS Appl. Mater. Interfaces 2019, 11, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Kong, F.P.; Chen, G.Y.; Du, C.Y.; Gao, Y.Z.; Yin, G.P. A review of applications of poly(diallyldimethyl ammonium chloride) in polymer membrane fuel cells:From nanoparticles to support materials. Chin. J. Catal. 2016, 37, 1025–1036. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yang, H.; Yang, L.; Li, W.; Xu, L.; Wang, J.W. Improved sludge dewaterability by combining SDS pre-treatment conditioning with PDMDAAC flocculation. Desalination Water Treat. 2019, 158, 59–66. [Google Scholar] [CrossRef]
- Li, X.X.; Zhang, Y.J.; Zhao, X.L.; Liu, C. The performance of several enhanced treatment processes for treatment of algae-containing raw water in typical seasons. Desalination Water Treat. 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Zhou, J.H.; Wu, C.H.; Cheng, G.F.; Hong, Q.K.; Li, Y.Z.; Wang, H.Y. Impact of poly dimethyldiallylammonium chloride on membrane fouling mitigation in a membrane bioreactor. Environ. Technol. 2018, 24, 1043–1049. [Google Scholar] [CrossRef]
- Li, N.; Yue, Q.Y.; Gao, B.Y.; Xu, X.; Kan, Y.J.; Zhao, P. Magnetic graphene oxide functionalized by poly dimethyl diallyl ammonium chloride for efficient removal of Cr(VI). J. Taiwan Inst. Chem. Eng. 2018, 91, 499–506. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Balakaeva, I.V.; Lileev, A.S.; Lyashchenko, A.K. General trends in concentration and temperature behavior of equivalent conductance of N, N-diallylammonium polymers aqueous solutions: Effect of counterion nature and amine structure. Polym. Sci. Ser. C 2017, 59, 141–148. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Kleshcheva, N.A.; Shleeva, M.O.; Filatova, M.P.; Simonova, Y.A.; Ermakov, Y.A.; Kaprelyants, A.S. Nonquaternary poly(diallylammonium) polymers with different amine structure and their biocidal effect on Mycobacterium tuberculosis and Mycobacterium smegmatis. Appl. Microbiol. Biotechnol. 2015, 99, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, W.; Bohrisch, J.; Laschewsky, A. Synthetic polymers with quaternary nitrogen atoms—Synthesis and structure of the most used type of cationic polyelectrolytes. Prog. Polym. Sci. 2010, 35, 511–577. [Google Scholar] [CrossRef]
- Daugulis, O.; Brookhart, M.; Janeta, M.; Heidlas, J.X. 2,4,6-Triphenylpyridinium: A Bulky, Highly Electron-Withdrawing Substituent Which Enhances Properties of Nickel(II) Ethylene Polymerization Catalysts. Angew. Chem. Int. Ed. 2021, 60, 4566–4569. [Google Scholar]
- Zhang, Y.X.; Wang, C.Q.; Mecking, S.; Jian, Z.B. Ultrahighly Branched Main-Chain-Functionalized Polyethylenes via Inverted Insertion Selectivity. Angew. Chem. Int. Ed. 2020, 59, 14296–14302. [Google Scholar] [CrossRef]
- Losada, R.; Wandrey, C. Copolymerization of a Cationic Double-Charged Monomer and Electrochemical Properties of the Copolymers. Macromolecules 2009, 42, 3285–3293. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(N,N-diallylazacycloalkane)s for Anion-Exchange Membranes Functionalized with N-Spirocyclic Quaternary Ammonium Cations. Macromolecules 2017, 50, 2784–2793. [Google Scholar] [CrossRef]
- Jin, L.; Howlett, P.; Efthimiadis, J.; Kar, M.; MacFarlane, D.; Forsyth, M. Lithium doped N,N-dimethyl pyrrolidinium tetrafluoroborate organic ionic plastic crystal electrolytes for solid state lithium batteries. J. Mater. Chem. 2011, 21, 10171–10178. [Google Scholar] [CrossRef]
- Huval, C.C.; Holmes-Farley, S.R.; Mandeville, W.H.; Sacchiero, R.; Dhal, P. Syntheses of hydrophobically modified cationic hydrogels by copolymerization of alkyl substituted diallylamine monomers and their use as bile acid sequestrants. Eur. Polym. J. 2004, 40, 693–701. [Google Scholar] [CrossRef]
- Wandrey, C.; Barajas, H.; Hunkeler, D. Diallyldimethylammonium Chloride and its Polymers. Adv. Polym. Sci. 1999, 145, 123–182. [Google Scholar]
- Gao, D.G.; Ma, J.Z.; Guo, H.Q. Swelling properties of pH-responsive hydrophobically modified hydrogels of poly(methacrylic acid-co-diallylammonium salt) in aqueous solution. Polym. Adv. Technol. 2012, 23, 1494–1499. [Google Scholar] [CrossRef]
- Wang, G.J.; Engberts, J. Synthesis and catalytic properties of hydrophobically modified poly(alkyl methyldiallyl ammonium chlorides). Eur. Polym. J. 1995, 31, 409–417. [Google Scholar] [CrossRef][Green Version]
- Mahdavi, H.; Mahdi, M. Synthesis and applications of cross-linked poly(diallyldimethyl ammonium chloride) and its derivative copolymers as efficient phase transfer catalyst for nucleophilic substitution reactions. Chin. J. Polym. Sci. 2011, 29, 165–172. [Google Scholar]
- Coote, M.L.; Davis, T.P. Propagation Kinetics of Para-Substituted Styrenes: A Test of the Applicability of the Hammett Relationship to Free-Radical Polymerization. Macromolecules 1999, 32, 4290–4298. [Google Scholar] [CrossRef]
- Beckel, E.R.; Nie, J.; Stansbury, J.W.; Bowman, C.N. Effect of aryl substituents on the reactivity of phenyl carbamate acrylate monomers. Macromolecules 2004, 37, 4062–4069. [Google Scholar] [CrossRef]
- Degirmenci, I.; Avci, D.; Aviyente, V.; Van Cauter, K.; Van Speybroeck, V.; Waroquier, M. Density functional theory study of free-radical polymerization of acrylates and methacrylates: Structure-reactivity relationship. Macromolecules 2007, 40, 9590–9602. [Google Scholar] [CrossRef]
- Novikova, A.A.; Vlasov, P.S.; Lin, S.Y.; Sedlakova, Z.; Noskov, B.A. Dynamic surface properties of poly(methylalkyldiallylammonium chloride) solutions. J. Taiwan Inst. Chem. Eng. 2017, 80, 122–127. [Google Scholar] [CrossRef]
- Umar, Y.; Al-Muallem, H.A.; Abu-Sharkh, B.F.; Ali, S. Synthesis and solution properties of hydrophobically associating ionic polymers made from diallylammonium salts/sulfur dioxide cyclocopolymerization. Polymer 2004, 45, 3651–3661. [Google Scholar] [CrossRef]
- Thomas, D.B.; Vasilieva, Y.A.; Armentrout, R.S.; McCormick, C.L. Synthesis, Characterization, and Aqueous Solution Behavior of Electrolyte-and pH-Responsive Carboxybetaine-Containing Cyclocopolymers. Macromolecules 2003, 36, 9710–9715. [Google Scholar] [CrossRef]
- Zhanpeisov, N.U.; Tsujimaru, K.; Anpo, M. Intrinsic band gap in semiconductor oxides and Ti-silicalite: Ab initio and DFT study. Res. Chem. Intermed. 2004, 30, 121–132. [Google Scholar] [CrossRef]
- Brand, F.; Dautzenberg, H.; Jaeger, W.; Hahn, M. Polyelectrolytes with various charge densities. Synthesis and characterization of diallyldimethylammonium chloride-acrylamide copolymers. Angew. Makromol. Chem. 1997, 248, 41–71. [Google Scholar] [CrossRef]
- Yamazaki, H.; Matsumoto, A.; Otsu, T. Effect of N-substituents on polymerization reactivity of N-alkylitaconimides in radical polymerization. Eur. Polym. J. 1997, 33, 157–162. [Google Scholar] [CrossRef]
- Terada, S.; Matsumoto, A. Role of N-substituents of maleimides on penultimate unit effect for sequence control during radical copolymerization. Polym. J. 2019, 51, 1137–1146. [Google Scholar] [CrossRef]
- Losada, R.; Wandrey, C. Non-ideal polymerization kinetics of a cationic double charged acryl monomer and solution behavior of the resulting polyelectrolytes. Macromol. Rapid Commun. 2008, 29, 252–257. [Google Scholar] [CrossRef]
- Otsu, T.; Matsumoto, A.; Kubota, T.; Mori, S. Reactivity in radical polymerization of N-substituted maleimides and thermal stability of the resulting polymers. Polym. Bull. 1990, 23, 43–50. [Google Scholar] [CrossRef]
- Peng, W.H.; Zhang, X.J.; Jia, X.; Xu, X.; Wang, Y.J.; Zhang, Y.J. Synthesis, characterization and demulsification performance of polymethylamyldiallylammonium chloride. J. Macromol. Sci. B Phys. 2021, 10, 309–323. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Quantum density oscillations in an inhomogeneous electron gas. Phys. Rev. 1965, 137, 1697–1705. [Google Scholar] [CrossRef]
- Wang, G.X.; Gong, X.D.; Du, H.C.; Liu, Y.; Xiao, H.M. Theoretical prediction of properties of aliphatic polynitrates. J. Phys. Chem. A 2011, 115, 795–804. [Google Scholar] [CrossRef]
- Wang, G.X.; Gong, X.D.; Liu, Y.; Du, H.C.; Xiao, H.M. Looking for high energy density compounds applicable for propellant among the derivatives of DPO with -N3, -ONO2, and -NNO2 groups. J. Comput. Chem. 2011, 32, 943–952. [Google Scholar] [CrossRef]
- Tuerker, L.; Erkoc, S. Density functional theory calculations for [C2H4N2O6](n)(n = 0, +1, −1). J. Hazard. Mater. 2006, 136, 164–169. [Google Scholar] [CrossRef]
- Yoon, D.H.; Jang, J.W.; Cheong, I.W. Synthesis of cationic polyacrylamide/silica nanocomposites from inverse emulsion polymerization and their flocculation property for papermaking. Colloids Surf. A 2012, 411, 18–23. [Google Scholar] [CrossRef]
- Zeng, X.L.; Chen, W.H.; Liu, J.C.; Kan, J.L. A theoretical study of five nitrates: Electronic structure and bond dissociation energies. J. Mol. Struct. Theochem. 2007, 810, 47–51. [Google Scholar] [CrossRef]
- Hele, T.J.; Althorpe, S.C. Derivation of a true (t→0+) quantum transition-state theory. I. Uniqueness and equivalence to ring-polymer molecular dynamics transition-state-theory. J. Chem. Phys. 2013, 138, 084108/1–084108/13. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.L.; Chen, W.H.; Liu, J.C.; Kan, J.L. Experimental and theoretical studying the thermolysis of nitrate. J. Therm. Anal. Calorim. 2008, 91, 359–363. [Google Scholar] [CrossRef]
- Bisht, H.S.; Ray, S.S.; Chatterjee, A.K. Resonance, polar, and steric effects of substituent on monomer reactivity in radical polymerization of alkyl 4-vinylbenzoate and butylacrylate. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1864–1866. [Google Scholar] [CrossRef]
- Jaeger, W.; Hahn, M.; Wandrey, C.; Seehaus, F.; Reinisch, G. Cyclopolymerization kinetics of dimethyldiallylammonium chloride. J. Macromol. Sci. Chem. 1984, 21, 599–620. [Google Scholar] [CrossRef]
| Monomer | Substituent | E(R) (kJ·mol−1) | Zero-Point Energies (R) | E(TS) (kJ·mol−1) | Zero-Point Energies (TS) | Ea (kJ·mol−1) |
|---|---|---|---|---|---|---|
| DMDAAC | Methy | −1528.4278 | 0.2463 | −1528.4141 | 0.2483 | 42.53 |
| MPDAAC | Propyl | −1607.0537 | 0.3039 | −1607.0351 | 0.3051 | 51.98 |
| MADAAC | Amyl | −1685.6829 | 0.3623 | −1685.6673 | 0.3629 | 59.59 |
| Monomer | Substituent | Slope | R2 | Ea (kJ·mol−1) |
|---|---|---|---|---|
| DMDAAC | Methyl/R1 | −11.629 ± 0.450 | 0.9940 | 96.70 ± 3.74 |
| MPDAAC | Propyl/R2 | −11.696 ± 0.268 | 0.9979 | 97.25 ± 2.23 |
| MADAAC | Amyl/R3 | −12.054 ± 0.413 | 0.9932 | 100.23 ± 3.43 |
| Monomer | Rp | Slope | R2 |
|---|---|---|---|
| DMDAAC | Rp1 | 1.89 ± 0.17 | 0.9829 |
| MPDAAC | Rp2 | 1.93 ± 0.09 | 0.9904 |
| MADAAC | Rp3 | 1.89 ± 0.06 | 0.9966 |
| Monomer | Rp | Slope | R2 |
|---|---|---|---|
| DMDAAC | Rp1 | 0.72 ± 0.04 | 0.9889 |
| MPDAAC | Rp2 | 0.74 ± 0.04 | 0.9912 |
| MADAAC | Rp3 | 0.75 ± 0.04 | 0.9884 |
| Monomer | Ea (kJ·mol−1) | C(9)-C(1)/C(6)-C(2) | C(5)-C(1)-C(9)/C(3)-C(2)-C(6) | |
|---|---|---|---|---|
| Length (Å) | Charge Density | Angle (°) | ||
| DMDAAC | 42.53 | 1.33/1.33 | −0.338, −0.051/−0.313, −0.068 | 123.77/122.78 |
| MPDAAC | 51.98 | 1.33/1.33 | −0.339, −0.051/−0.315, −0.068 | 123.80/122.71 |
| MADAAC | 59.59 | 1.33/1.33 | −0.339, −0.051/−0.314, −0.068 | 123.77/122.66 |
| Monomer | Substituent | Polymerization Rate Equation | Ea (kJ·mol−1) |
|---|---|---|---|
| DMDAAC | Methyl | Rp1 = k1 [I]0.72 [M]1.92 | 96.70 ± 3.74 |
| MPDAAC | Propyl | Rp2 = k2 [I]0.74 [M]1.93 | 97.25 ± 2.23 |
| MADAAC | Amyl | Rp3 = k3 [I]0.75 [M]1.93 | 100.23 ± 3.43 |
| No. | Monomer | [M] (mol·L−1) | [I] (mol·L−1) | T (℃) | Polymerization Rate |
|---|---|---|---|---|---|
| Rp1 | DMDAAC | 2.5 | 0.02 | 50 | Rp1 > Rp2 > Rp3 |
| Rp2 | MPDAAC | 2.5 | 0.02 | 50 | Rp1 > Rp2 > Rp3 |
| Rp3 | MADAAC | 2.5 | 0.02 | 50 | Rp1 > Rp2 > Rp3 |
| No. | Monomer | [M] (mol·L−1) | [I] (mol·L−1) | k | Polymerization Rate |
|---|---|---|---|---|---|
| k1 | DMDAAC | 1.92 | 0.72 | 0.036 | 0.0124 |
| k2 | MPDAAC | 1.93 | 0.74 | 0.034 | 0.0108 |
| k3 | MADAAC | 1.89 | 0.75 | 0.026 | 0.0079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Zhang, X.; Peng, W.; Yang, K.; Xu, X.; Zhang, Y.; Wang, G.; Tao, X. Effect of Substituents on the Homopolymerization Activity of Methyl Alkyl Diallyl Ammonium Chloride. Molecules 2022, 27, 4677. https://doi.org/10.3390/molecules27154677
Jia X, Zhang X, Peng W, Yang K, Xu X, Zhang Y, Wang G, Tao X. Effect of Substituents on the Homopolymerization Activity of Methyl Alkyl Diallyl Ammonium Chloride. Molecules. 2022; 27(15):4677. https://doi.org/10.3390/molecules27154677
Chicago/Turabian StyleJia, Xu, Xiujuan Zhang, Wenhui Peng, Kui Yang, Xiao Xu, Yuejun Zhang, Guixiang Wang, and Xianping Tao. 2022. "Effect of Substituents on the Homopolymerization Activity of Methyl Alkyl Diallyl Ammonium Chloride" Molecules 27, no. 15: 4677. https://doi.org/10.3390/molecules27154677
APA StyleJia, X., Zhang, X., Peng, W., Yang, K., Xu, X., Zhang, Y., Wang, G., & Tao, X. (2022). Effect of Substituents on the Homopolymerization Activity of Methyl Alkyl Diallyl Ammonium Chloride. Molecules, 27(15), 4677. https://doi.org/10.3390/molecules27154677






