Benzothiazole and Chromone Derivatives as Potential ATR Kinase Inhibitors and Anticancer Agents
Abstract
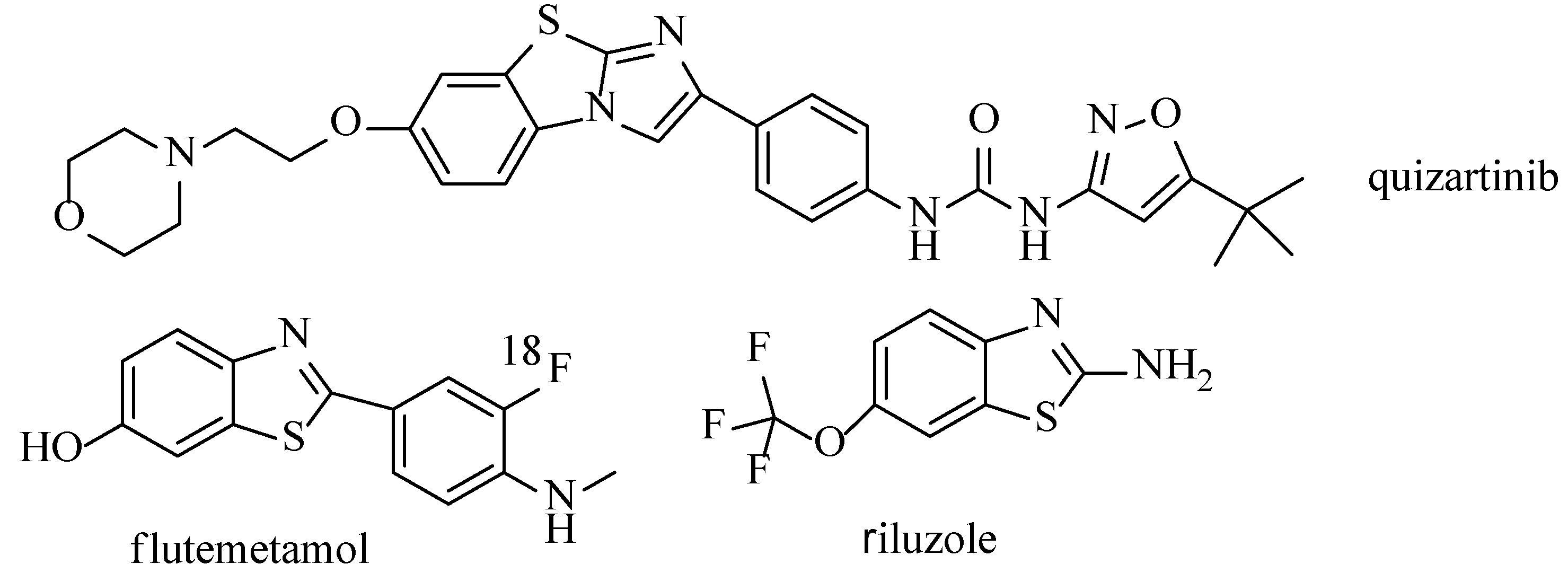
:1. Introduction
2. Results and Discussion
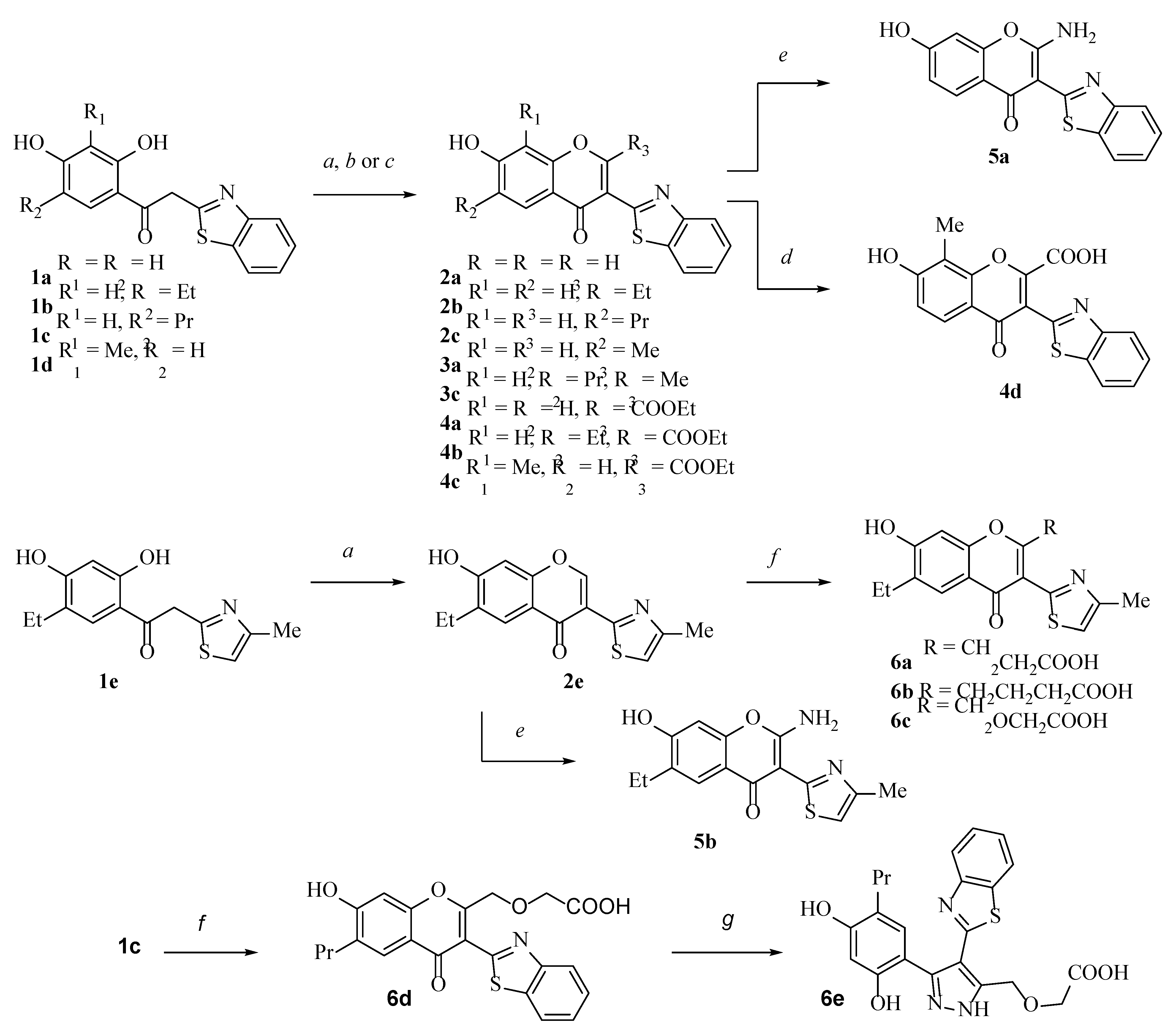
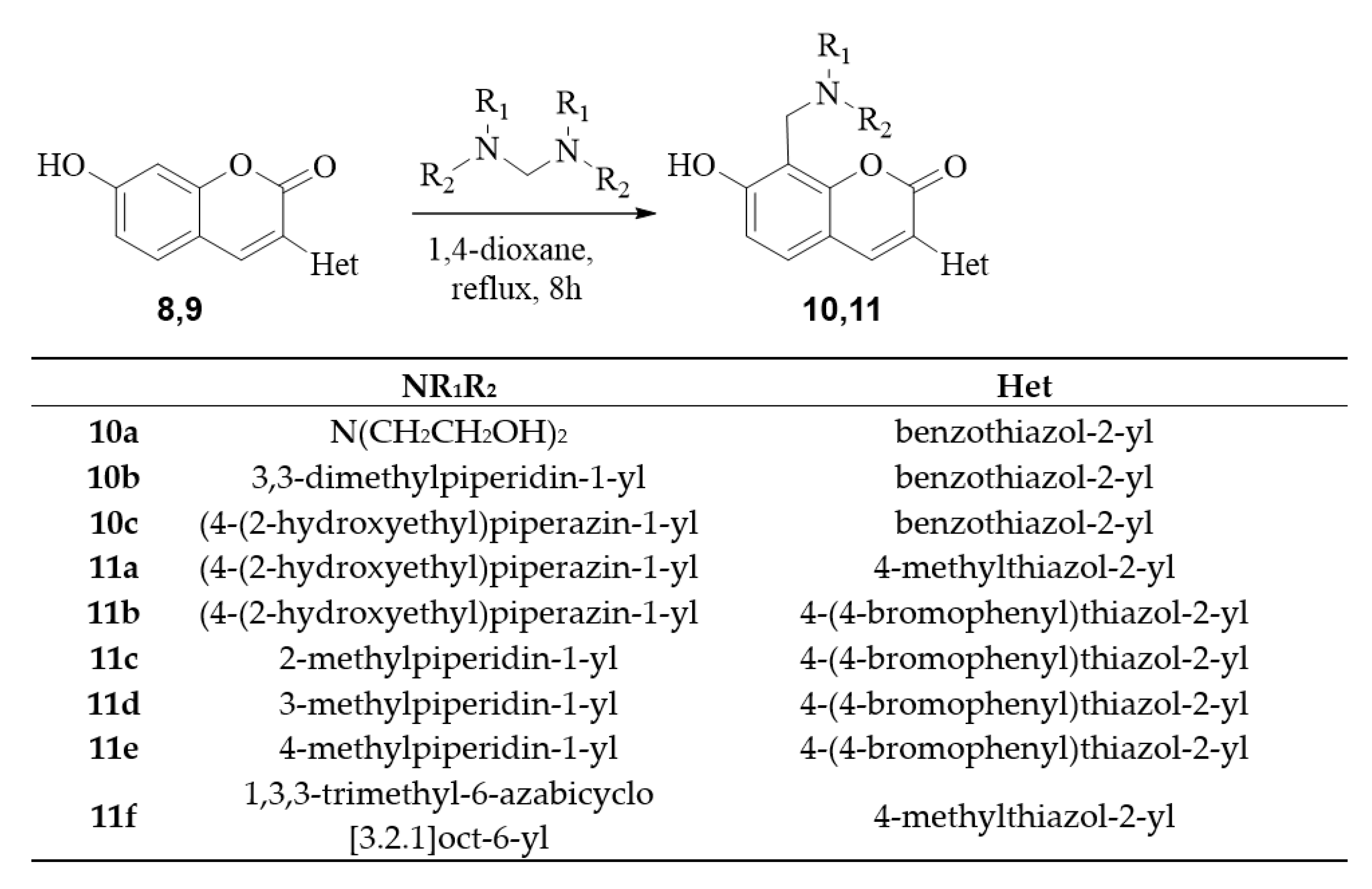
2.1. Chemistry
2.2. Biological Evaluation
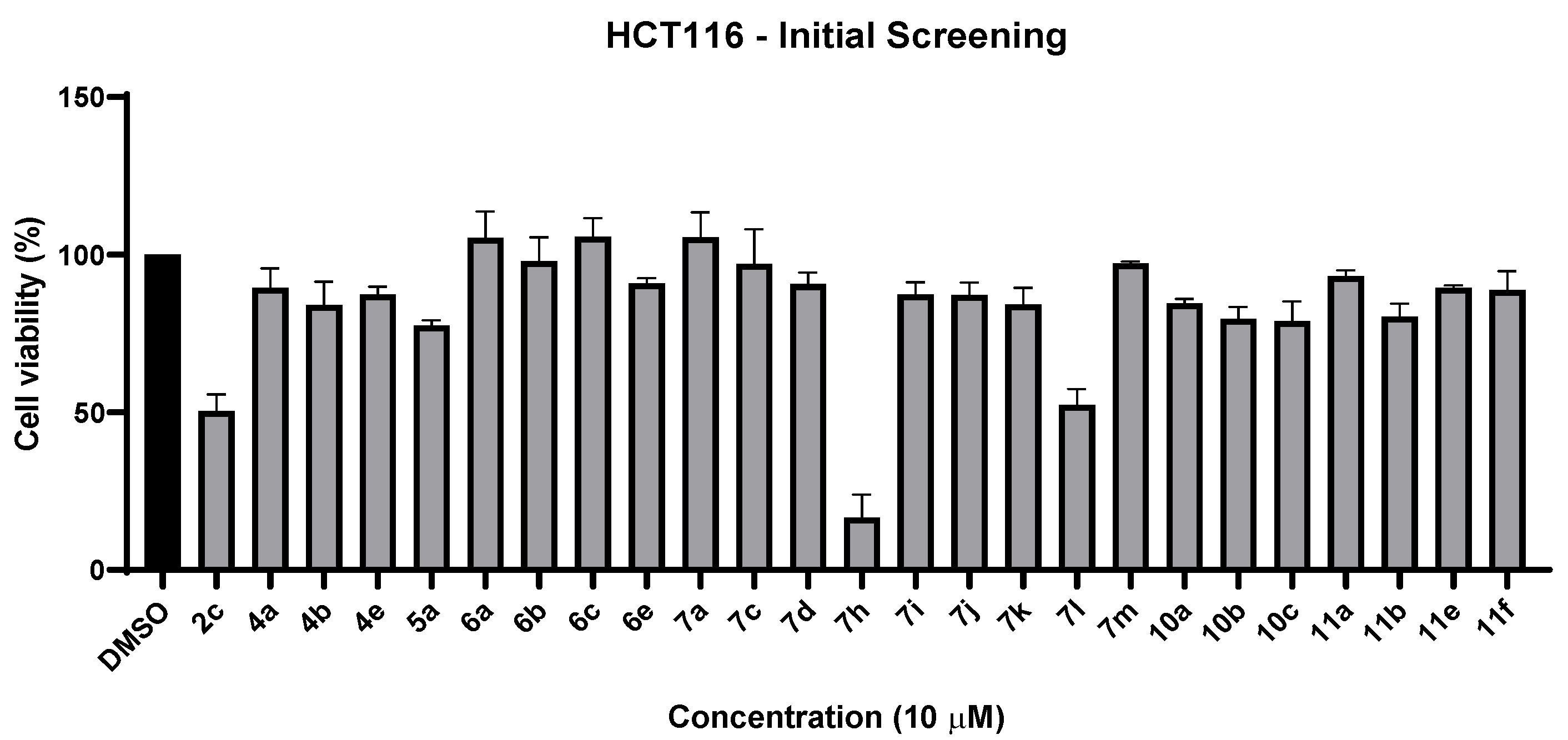
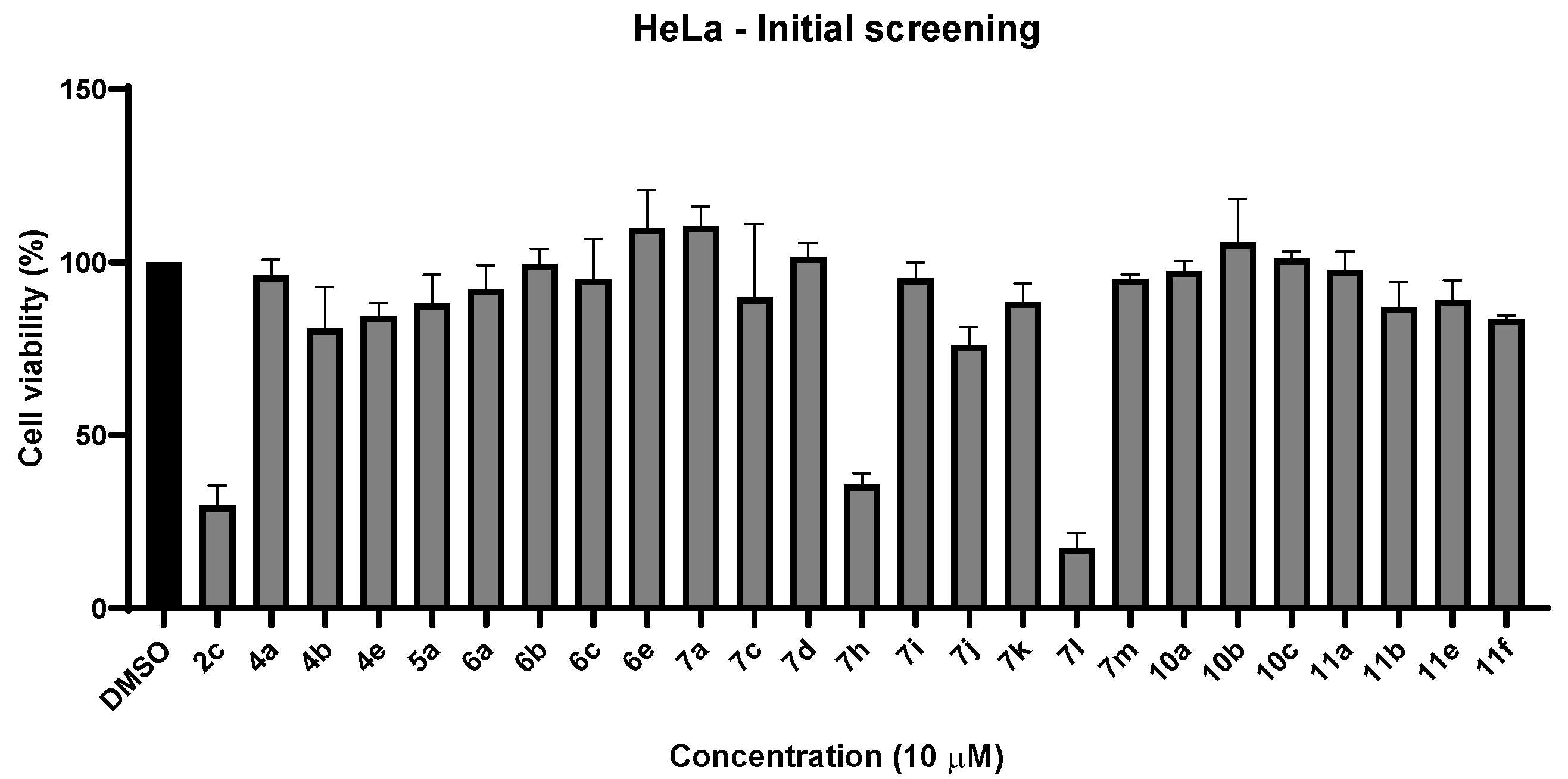
2.2.1. Cell Viability Studies—Initial Screening
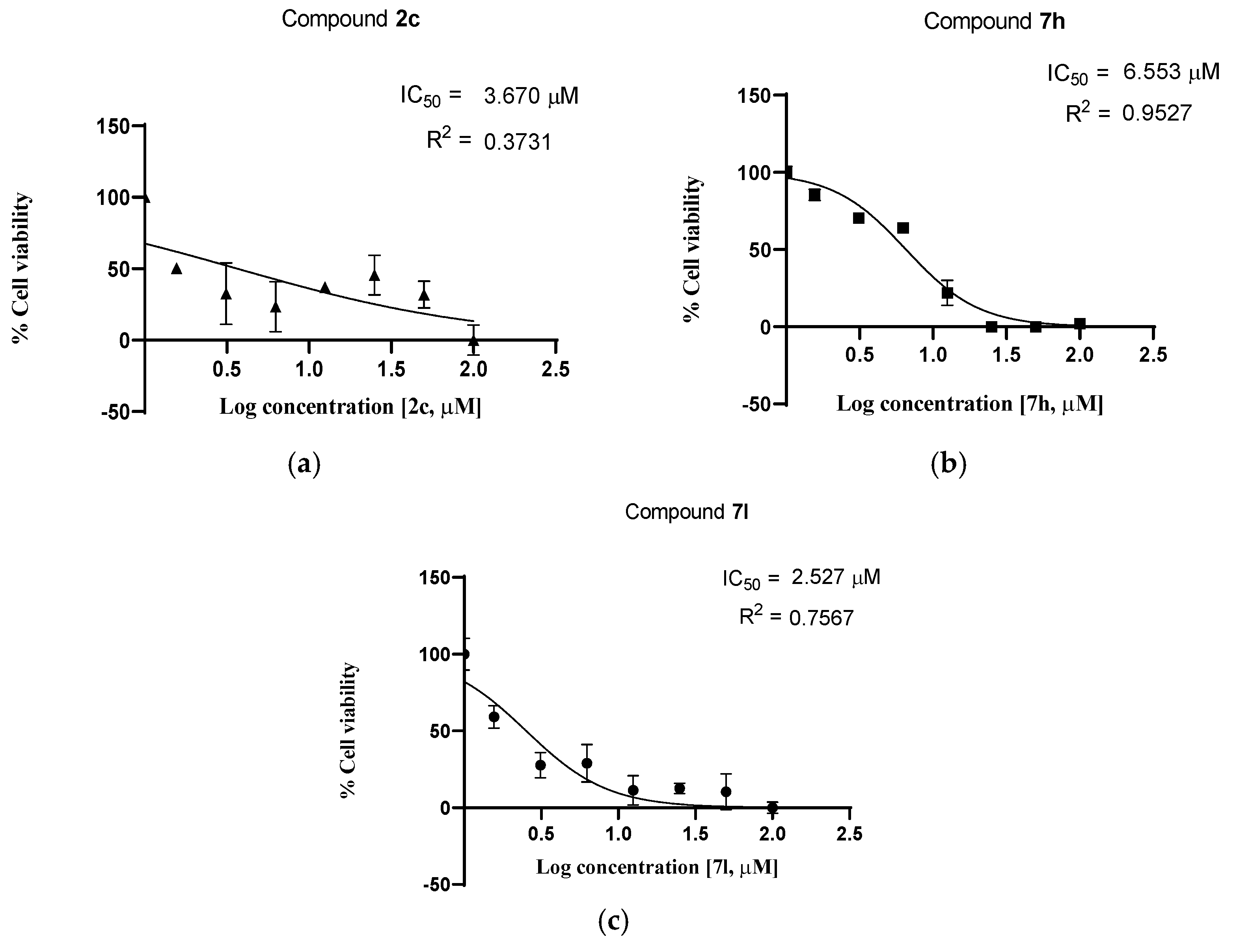
2.2.2. Cell Viability Studies—Dose Response
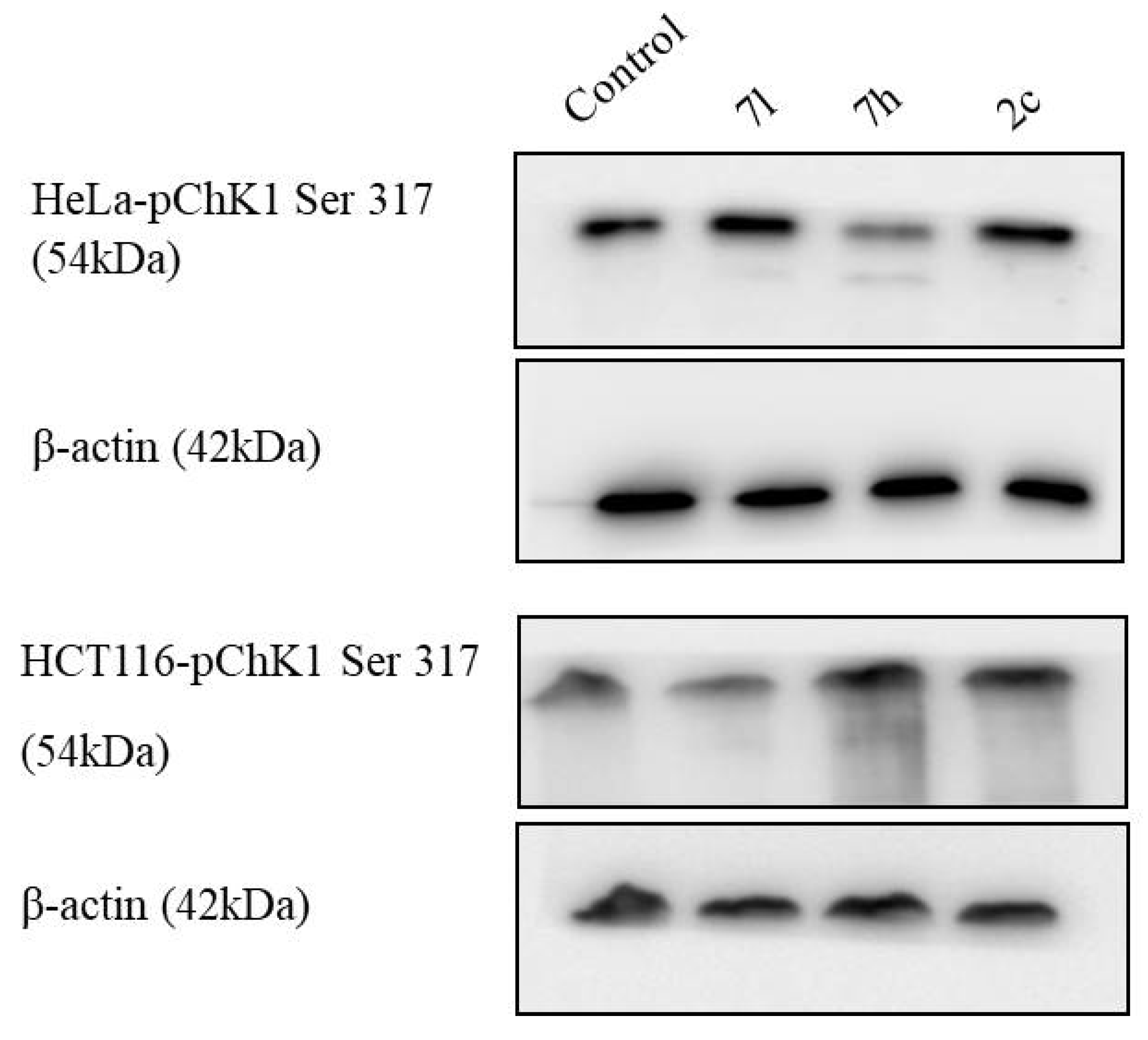
2.2.3. Immunoblot Assay
2.3. Molecular Docking
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of 2-Ethoxycarbonyl Chromones 4a–4c
3.2.2. General Procedure for the Synthesis of 2-Aminochromones 5a,b
3.2.3. General Procedure for Synthesis of 8-Aminomethyl Derivatives 7
3.2.4. General Procedure for Synthesis of Coumarin Aminomethyl Derivatives 10,11
4. Biological Evaluation
4.1. Cell Viability Studies
4.2. Immunoblot Assay
4.3. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Bhakuni, R.; Shaik, A.; Priya, B.; Kirubakaran, S. Characterization of SPK 98, a Torin2 analog, as ATR and mTOR dual kinase inhibitor. Bioorg. Med. Chem. Lett. 2020, 30, 127517. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, F.; Thelen, L.; Reinhardt, H.C. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends Genet. 2014, 30, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Prevo, R.; Hammond, E.M.; Brunner, T.B.; McKenna, W.G.; Muschel, R.J. Targeting ATR in DNA damage response and cancer therapeutics. Cancer Treat. Rev. 2014, 40, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cilibrasi, V.; Spanò, V.; Bortolozzi, R.; Barreca, M.; Raimondi, M.V.; Rocca, R.; Maruca, A.; Montalbano, A.; Alcaro, S.; Ronca, R.; et al. Synthesis of 2H-Imidazo[2′,1′:2,3] [1,3]thiazolo[4,5-e]isoindol-8-yl-phenylureas with promising therapeutic features for the treatment of acute myeloid leukemia (AML) with FLT3/ITD mutations. Eur. J. Med. Chem. 2022, 235, 114292. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Ali, E.M.; Kandeel, M.; Venugopala, K.N.; Nair, A.B.; Greish, H.; El-Daly, M. Screening and Molecular Docking of Novel Benzothiazole Derivatives as Potential Antimicrobial Agents. Antibiotics 2020, 9, 221. [Google Scholar] [CrossRef]
- Haroun, M.; Tratrat, C.; Petrou, A.; Geronikaki, A.; Ivanov, M.; Ciric, A.; Soković, M.; Nagaraja, S.; Venugopala, K.N.; Nair, A.B.; et al. Exploration of the Antimicrobial Effects of Benzothiazolylthiazolidin-4-One and In Silico Mechanistic Investigation. Molecules 2021, 26, 4061. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, N.P. Synthesis, anti-inflammatory and analgesic evaluation of thiazole/oxazole substituted benzothiazole derivatives. Bioorg. Chem. 2021, 107, 104608. [Google Scholar] [CrossRef]
- Ugwu, D.I.; Okoro, U.C.; Ukoha, P.O.; Gupta, A.; Okafor, S.N. Novel anti-inflammatory and analgesic agents: Synthesis, molecular docking and in vivo studies. J. Enzym. Inhib. Med. Chem. 2018, 33, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Djuidje, E.N.; Barbari, R.; Baldisserotto, A.; Durini, E.; Sciabica, S.; Balzarini, J.; Liekens, S.; Vertuan, S.; Manfredini, S. Benzothiazole Derivatives as Multifunctional Antioxidant Agents for Skin Damage: Structure–Activity Relationship of a Scaffold Bearing a Five-Membered Ring System. Antioxidants 2022, 11, 407. [Google Scholar] [CrossRef]
- Cabrera-Pérez, L.C.; Padilla-Martínez, I.I.; Cruz, A.; Mendieta-Wejebe, J.E.; Tamay-Cach, F.; Rosales-Hernández, M.C. Evaluation of a new benzothiazole derivative with antioxidant activity in the initial phase of acetaminophen toxicity. Ar. J. Chem. 2019, 12, 3871–3882. [Google Scholar] [CrossRef]
- Kumar, K.R.; Karthik, K.N.S.; Begum, P.R.; Prasada, R.C.M.M. Synthesis, characterization and biological evaluation of benzothiazole derivatives as potential antimicrobial and analgesic agents. Asian J. Res. Pharm. Sci. 2017, 7, 115–119. [Google Scholar] [CrossRef]
- Uremis, N.; Uremis, M.M.; Tolun, F.I.; Ceylan, M.; Doganer, A.; Kurt, A.H. Synthesis of 2-Substituted Benzothiazole Derivatives and Their In Vitro Anticancer Effects and Antioxidant Activities against Pancreatic Cancer Cells. Anticancer Res. 2017, 37, 6381–6389. [Google Scholar] [PubMed]
- Irfan, A.; Batool, F.; Naqvi, S.Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J Enzym. Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.K.; Baek, A.-R.; Sung, B.; Yang, B.-W.; Choi, G.; Park, H.-J.; Kim, Y.-H.; Kim, M.; Ha, S.; Lee, G.-H.; et al. Synthesis, Characterization, and Anticancer Activity of Benzothiazole Aniline Derivatives and Their Platinum (II) Complexes as New Chemotherapy Agents. Pharmaceuticals 2021, 14, 832. [Google Scholar] [CrossRef]
- Osmaniye, D.; Levent, S.; Karaduman, A.B.K.; Ilgın, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of New Benzothiazole Acylhydrazones as Anticancer Agents. Molecules 2018, 23, 1054. [Google Scholar] [CrossRef] [Green Version]
- Asiri, Y.I.; Alsayari, A.; Muhsinah, A.B.; Mabkhot, Y.N.; Hassan, M.Z. Benzothiazoles as potential antiviral agents. J. Pharm. Pharmacol. 2020, 72, 1459–1480. [Google Scholar] [CrossRef]
- Elamin, M.B.; Abd Elaziz, A.A.E.S.; Abdallah, E.M. Benzothiazole moieties and their derivatives as antimicrobial and antiviral agents: A mini-review. Int. J. Res. Pharm. Sci. 2020, 3, 3309–3315. [Google Scholar] [CrossRef]
- Kumar, M.; Chung, S.-M.; Enkhtaivan, G.; Patel, R.V.; Shin, H.-S.; Mistry, B.M. Molecular Docking Studies and Biological Evaluation of Berberine–Benzothiazole Derivatives as an Anti-Influenza Agent via Blocking of Neuraminidase. Int. J. Mol. Sci. 2021, 22, 2368. [Google Scholar] [CrossRef]
- Khyati Bhagdev, K.; Sarkar, S. Benzothiazole Moiety and Its Derivatives as Antiviral Agents. Med. Sci. Forum 2021, 7, 2–7. [Google Scholar]
- Al-Masoudia, N.A.; Jafar, N.N.A.; Abbas, L.J.; Baqir, S.J.; Pannecouque, C. Synthesis and anti-HIV Activity of New Benzimidazole, Benzothiazole and Carbohyrazide Derivatives of the anti-Inflammatory Drug Indomethacin. Z. Naturforsch. 2011, 66b, 953–960. [Google Scholar] [CrossRef]
- Petrou, A.; Eleftheriou, P.; Geronikaki, A.; Akrivou, M.G.; Vizirianakis, I. Novel thiazolidin-4-ones as potential non-nucleoside inhibitors of HIV-1 reverse transcriptase. Molecules 2019, 24, 3821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, S.; Mirza, S.; Salar, U.; Hussain, S.; Javaid, K.; Khan, K.M.; Khalil, R.; Atia-Tul-Wahab; Ul-Haq, Z.; Perveen, S.; et al. 2-Mercapto Benzothiazole Derivatives: As Potential Leads for the Diabetic Management. Med. Chem. 2020, 16, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Shahar, Y.M.; Pathania, S.; Grover, G.; Debnath, B.; Akhtar, M.J. Synthesis and anticonvulsant evaluation of indoline derivatives of functionalized aryloxadiazole amine and benzothiazole acetamide. J. Mol. Struct. 2021, 1228, 129742. [Google Scholar] [CrossRef]
- Khokra, S.L.; Arora, K.; Khan, S.A.; Kaushik, P.; Saini, R.; Husain, A. Synthesis, Computational Studies and Anticonvulsant Activity of Novel Benzothiazole Coupled Sulfonamide Derivatives. Iran. J. Pharm. Res. 2019, 18, 826–840. [Google Scholar]
- Hadanu, R.; Idris, S.; Sutapa, I.W. QSAR analysis of benzothiazole derivatives of antimalarial compounds based on am1 semi-empirical method. Indones. J. Chem. 2015, 15, 86–92. [Google Scholar] [CrossRef]
- Suresh, A.J.; Bharathi, K.; Surya, P.R. Design, Synthesis, Characterization and Biological Evaluation of Some Novel Benzothiazole Derivatives as Anti Tubercular Agents Targeting Glutamine Synthetase-I. And Cyclopropanemycolic acid synthase-2. J. Pharm. Chem. Biol. Sci. 2018, 5, 312–319. [Google Scholar]
- Mehra, R.; Rajput, V.S.; Gupta, M.; Chib, R.; Kumar, A.; Wazir, P.; Ali Khan, I.; Nargotra, A. Benzothiazole Derivative as a Novel Mycobacterium tuberculosis Shikimate Kinase Inhibitor: Identification and Elucidation of Its Allosteric Mode of Inhibition. J. Chem. Inf. Model. 2016, 56, 930–940. [Google Scholar] [CrossRef]
- Shahare, H.V.; Talele, G.S. Designing of benzothiazole derivatives as promising EGFR tyrosine kinase inhibitors: A pharmacoinformatics study. J. Biomol. Struct. Dyn. 2020, 38, 1365–1374. [Google Scholar] [CrossRef]
- Cao, S.; Cao, R.; Liu, X.; Luo, X.; Zhong, W. Design, Synthesis and Biological Evaluation of Novel Benzothiazole Derivatives as Selective PI3KβInhibitors. Molecules 2016, 21, 876. [Google Scholar] [CrossRef] [Green Version]
- Sugita, Y.; Takao, K.; Uesawa, Y.; Nagai, J.; Iijima, Y.; Sano, M.; Sakagami, H. Development of Newly Synthesized Chromone Derivatives with High Tumor Specificity against Human Oral Squamous Cell Carcinoma. Medicines 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Bouhenna, M.M.; Mameri, N.; Pérez, M.V.; Talhi, O.; Bachari, K.; Silva, A.M.S.; Luyten, W. Anticancer Activity Study of Chromone and Coumarin Hybrids using Electrical Impedance Spectroscopy. Anticancer Agents. Med. Chem. 2018, 18, 854–864. [Google Scholar]
- Zhan, Q.; Xu, Y.; Zhan, L.; Wang, B.; Guo, Y.; Wu, X.; Ai, W.; Song, Z.; Yu, F. Chromone Derivatives CM3a Potently Eradicate Staphylococcus aureus Biofilms by Inhibiting Cell Adherence. Infect. Drug Resist. 2021, 14, 979–986. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Chang, C.-H.; Liao, H.-R.; Fu, S.-L.; Chen, J.J. Anti-Cancer and Anti-Inflammatory Activities of Three New Chromone Derivatives from the Marine-Derived Penicillium citrinum. Mar. Drugs 2021, 19, 408–426. [Google Scholar] [CrossRef]
- Matta, A.; Sharma, A.K.; Tomar, S.; Cao, P.; Kumar, S.; Balwani, S.; Ghosh, B.; Prasad, A.K.; Van der Eycken, E.V.; DePass, A.L.; et al. Synthesis and anti-inflammatory activity evaluation of novel chroman derivatives. New J. Chem. 2020, 44, 13716–13727. [Google Scholar]
- Mohsin, N.u.A.; Irfan, M.; Hassan, S.u.; Saleem, U. Current Strategies in Development of New Chromone Derivatives with Diversified Pharmacological Activities: A Review. Pharm. Chem. J. 2020, 54, 241–257. [Google Scholar] [CrossRef]
- Nalla, V.; Shaikh, A.; Bapat, S.; Vyas, R.; Karthikeyan, M.; Yogeeswari, P.; Sriram, D.; Muthukrishnan, M. Identification of potent chromone embedded [1,2,3]-triazoles as novel anti-tubercular agents. R. Soc. Open Sci. 2018, 5, 171750. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Chen, M.; Qiu, J.; Xie, Z.; Cao, A. Synthesis, in vitro α-glucosidase inhibitory activity and docking studies of novel chromone-isatin derivatives. Bioorganic Med. Chem. Lett. 2018, 8, 113–116. [Google Scholar] [CrossRef]
- Sugiyama, T.; Narukawa, Y.; Shibata, S.; Masui, R.; Kiuchi, F. New 2-(2-Phenylethyl)chromone Derivatives and Inhibitors of Phosphodiesterase (PDE) 3A from Agarwood. Nat. Prod. Commun. 2016, 11, 795–797. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.J.; Abhijit, K. Synthesis of Novel Functionalized Pyrano Annulated/Oxazolone Pendent Chromone Derivatives as Potent Anti-Diabetic Agents. Russ. J. Gen. Chem. 2020, 90, 1074–1082. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef] [PubMed]
- Khilya, V.P.; Grishko, L.G.; Sokolova, T.N. Chemistry of heteroanalogs of isoflavones. III. Synthesis of benzimidazole and benzothiazole analogs of isoflavones. Chem. Heterocycl. Compd. 1975, 11, 1353–1355. [Google Scholar] [CrossRef]
- Gorbulenko, N.V.; Frasinyuk, M.S.; Khilya, V.P. Chemistry of heteroanalogs of isoflavones. 16. Benzthiazole analogs of isoflavones. Chem. Heterocycl. Compd. 1994, 30, 405–412. [Google Scholar] [CrossRef]
- Frasinyuk, M.S.; Turov, A.V.; Khilya, V.P. Chemistry of the hetero analogs of isoflavones. 22. Mannich reaction in the benzimidazole and benzothiazole analogs of isoflavones. Chem. Heterocycl. Compd. 1998, 34, 923–928. [Google Scholar] [CrossRef]
- Andrews, S.P.; Mason, J.S.; Hurrell, E.; Congreve, M. Structure-based drug design of chromone antagonists of the adenosine A2A receptor. MedChemComm 2014, 5, 571–575. [Google Scholar] [CrossRef]
- Frasinyuk, M.S.; Bondarenko, S.P.; Gorbulenko, N.V.; Turov, A.V.; Khilya, V.P. Cyclic Carboxylic Anhydrides as New Reagents for Formation of Chromone Ring. J. Heterocycl. Chem. 2014, 51, 768–774. [Google Scholar] [CrossRef]
- Mrug, G.P.; Bondarenko, S.P.; Khilya, V.P.; Frasinyuk, M.S. Synthesis and aminomethylation of 7-hydroxy-5-methoxyisoflavones. Chem. Nat. Compd. 2013, 49, 235–241. [Google Scholar] [CrossRef]
- Khilya, O.V.; Frasinyuk, M.S.; Turov, A.V.; Khilya, V.P. Chemistry of 3-Hetarylcoumarins. 1. 3-(2-Benzazolyl)coumarins. Chem. Heterocycl. Compd. 2001, 37, 1029–1037. [Google Scholar] [CrossRef]
- Khilya, O.V.; Shablykina, O.V.; Frasinyuk, M.S.; Turov, A.V.; Ishchenko, V.V.; Khilya, V.P. Chemistry of 3-hetarylcoumarins. 2. 3-(2-thiazolyl)coumarins. Chem. Heterocycl. Compd. 2004, 40, 1408–1420. [Google Scholar] [CrossRef]
- Katoueezadeh, M.; Pilehvari, N.; Fatemi, A.; Hassanshahi, G.; Torabizadeh, S.A. Inhibition of DNA damage response pathway using combination of DDR pathway inhibitors and radiation in treatment of acute lymphoblastic leukemia cells. Future Oncol. 2021, 17, 2803–2816. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput.-Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [PubMed]
- Shaik, A.; Bhakuni, R.; Kirubakaran, S. Design, Synthesis, and Docking Studies of New Torin2 Analogs as Potential ATR/mTOR Kinase Inhibitors. Molecules 2018, 23, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput.-Aided Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput.-Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]





| Sl. No. | Compound | IC50 Value (µM) | |
|---|---|---|---|
| HCT116 | HeLa | ||
| 1 | 2c | 3.670 | 2.642 |
| 2 | 7h | 6.553 | 3.995 |
| 3 | 7l | 2.527 | 2.659 |
| Sl. No. | Interaction | Point Interaction | Donor Atom | Acceptor Atom | Type of Interaction | Bond Distance (Å) | Binding Energy (Dock Score) (kcal/mol) |
|---|---|---|---|---|---|---|---|
| 1. | 2c | 2c OH–Val 88 | 2c: O | Val 88 | Hydrogen bond | 1.72 | −5.5 |
| 2c aromatic ring–Trp 87 | 2c: aromatic ring | Trp 87 | π-π stacking | 3.37, 3.81, 3.87 | |||
| 2c benzothiazole–Lys 16 | 2c: benzothiazole | Lys 16 | π-Cation interaction | 5.15, 5.37 | |||
| 2. | Torin2 | Lys 100–Torin2 O | Lys 100 | Torin2: O | Hydrogen bond | 1.80 | −5.1 |
| Torin2 NH2–Val 88 | Torin2: N | Val 88 | Hydrogen bond | 2.31 | |||
| Lys 16–Torin2 N | Lys 16 | Torin2: N | Hydrogen bond | 2.01 | |||
| Torin2 pyridine–Trp 87 | Torin2: pyridine | Trp 87 | π-π stacking | 3.47, 3.77 | |||
| 3. | 7h | 7h OH–Asn 89 | 7h: O | Asn 89 | Hydrogen bond | 1.90 | −3.4 |
| 7h NH–Thr 91 | 7h: N | Thr 91 | Hydrogen bond | 1.93 | |||
| 7h benzothiazole–Trp 87 | 2c: benzothiazole | Trp 87 | π-π stacking | 4.00 | |||
| 4. | 7l | Lys 16–7l O | Lys 16 | 7l: O | Hydrogen bond | 1.94 | −4.8 |
| 7l benzothiazole–Trp 87 | 7l: benzothiazole | Trp 87 | π-π stacking | 5.02 | |||
| Lys 16–7l benzothiazole | Lys 16 | 7l: benzothiazole | π-Cation interaction | 5.01 | |||
| Lys 100–7l O | Lys 100 | 7l: O | Salt Bridge | 2.80 |
| Sl. No. | Compound | Concentration for Blot (µM) | |
|---|---|---|---|
| HCT116 | HeLa | ||
| 1 | 2c | 3.6 | 2.6 |
| 2 | 7h | 6.5 | 4.0 |
| 3 | 7l | 2.5 | 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasinyuk, M.; Chhabria, D.; Kartsev, V.; Dilip, H.; Sirakanyan, S.N.; Kirubakaran, S.; Petrou, A.; Geronikaki, A.; Spinelli, D. Benzothiazole and Chromone Derivatives as Potential ATR Kinase Inhibitors and Anticancer Agents. Molecules 2022, 27, 4637. https://doi.org/10.3390/molecules27144637
Frasinyuk M, Chhabria D, Kartsev V, Dilip H, Sirakanyan SN, Kirubakaran S, Petrou A, Geronikaki A, Spinelli D. Benzothiazole and Chromone Derivatives as Potential ATR Kinase Inhibitors and Anticancer Agents. Molecules. 2022; 27(14):4637. https://doi.org/10.3390/molecules27144637
Chicago/Turabian StyleFrasinyuk, Mykhaylo, Dimple Chhabria, Victor Kartsev, Haritha Dilip, Samvel N. Sirakanyan, Sivapriya Kirubakaran, Anthi Petrou, Athina Geronikaki, and Domenico Spinelli. 2022. "Benzothiazole and Chromone Derivatives as Potential ATR Kinase Inhibitors and Anticancer Agents" Molecules 27, no. 14: 4637. https://doi.org/10.3390/molecules27144637
APA StyleFrasinyuk, M., Chhabria, D., Kartsev, V., Dilip, H., Sirakanyan, S. N., Kirubakaran, S., Petrou, A., Geronikaki, A., & Spinelli, D. (2022). Benzothiazole and Chromone Derivatives as Potential ATR Kinase Inhibitors and Anticancer Agents. Molecules, 27(14), 4637. https://doi.org/10.3390/molecules27144637







