Optimisation of Glycerol and Itaconic Anhydride Polycondensation
Abstract
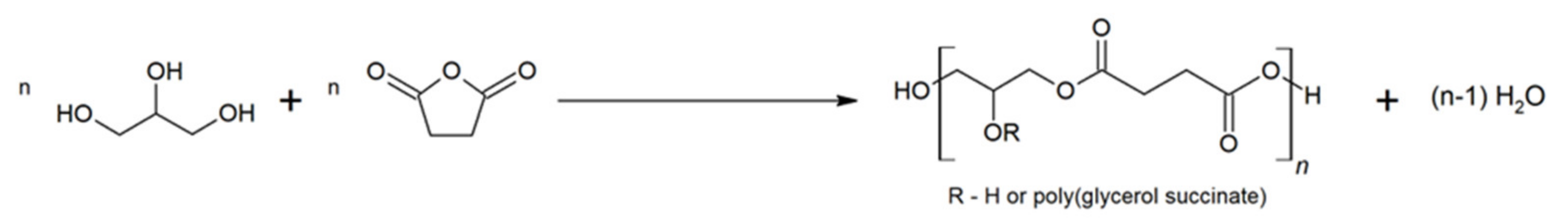
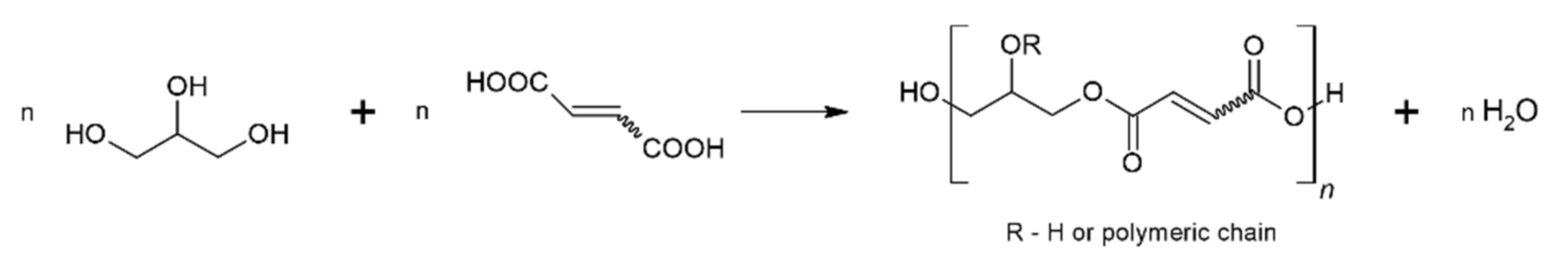
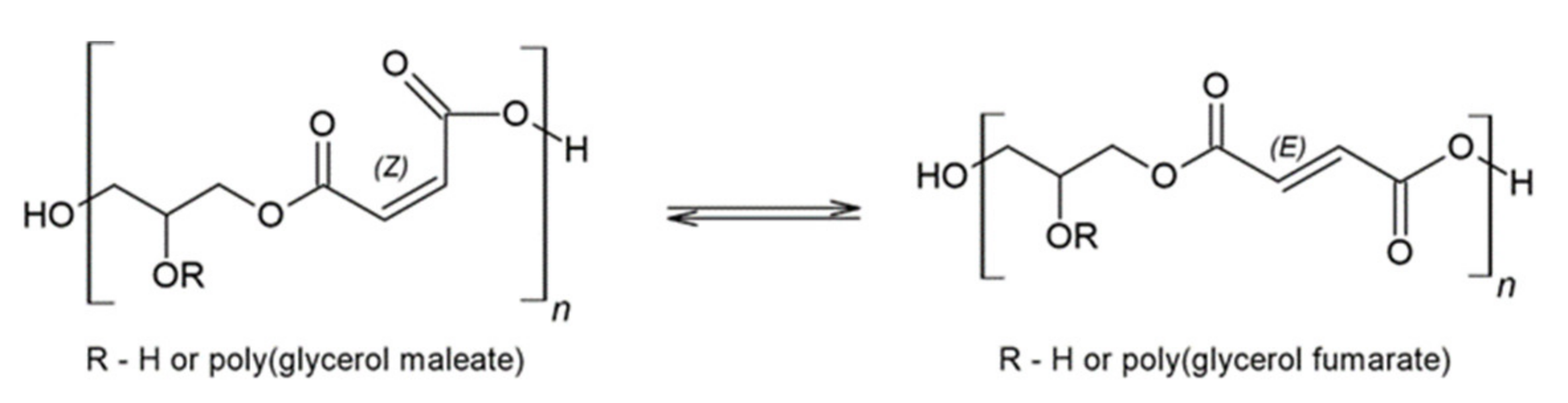
:1. Introduction
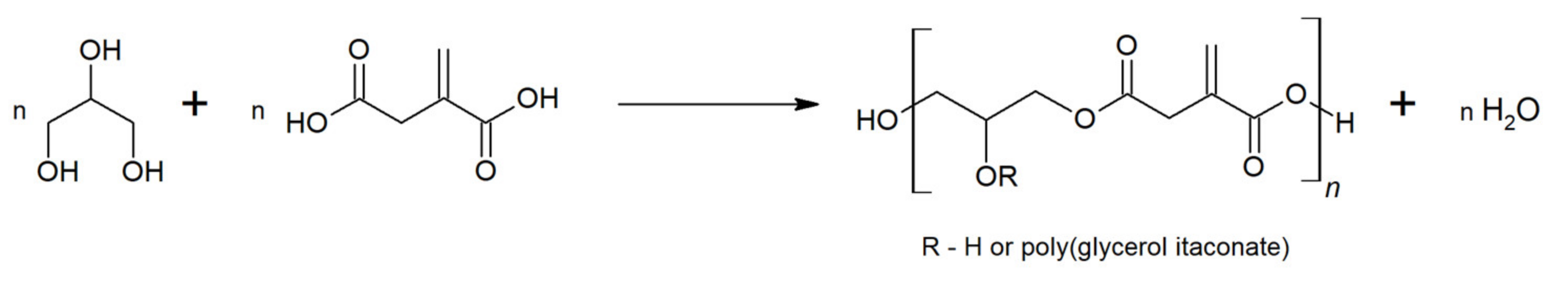
2. Results and Discussion
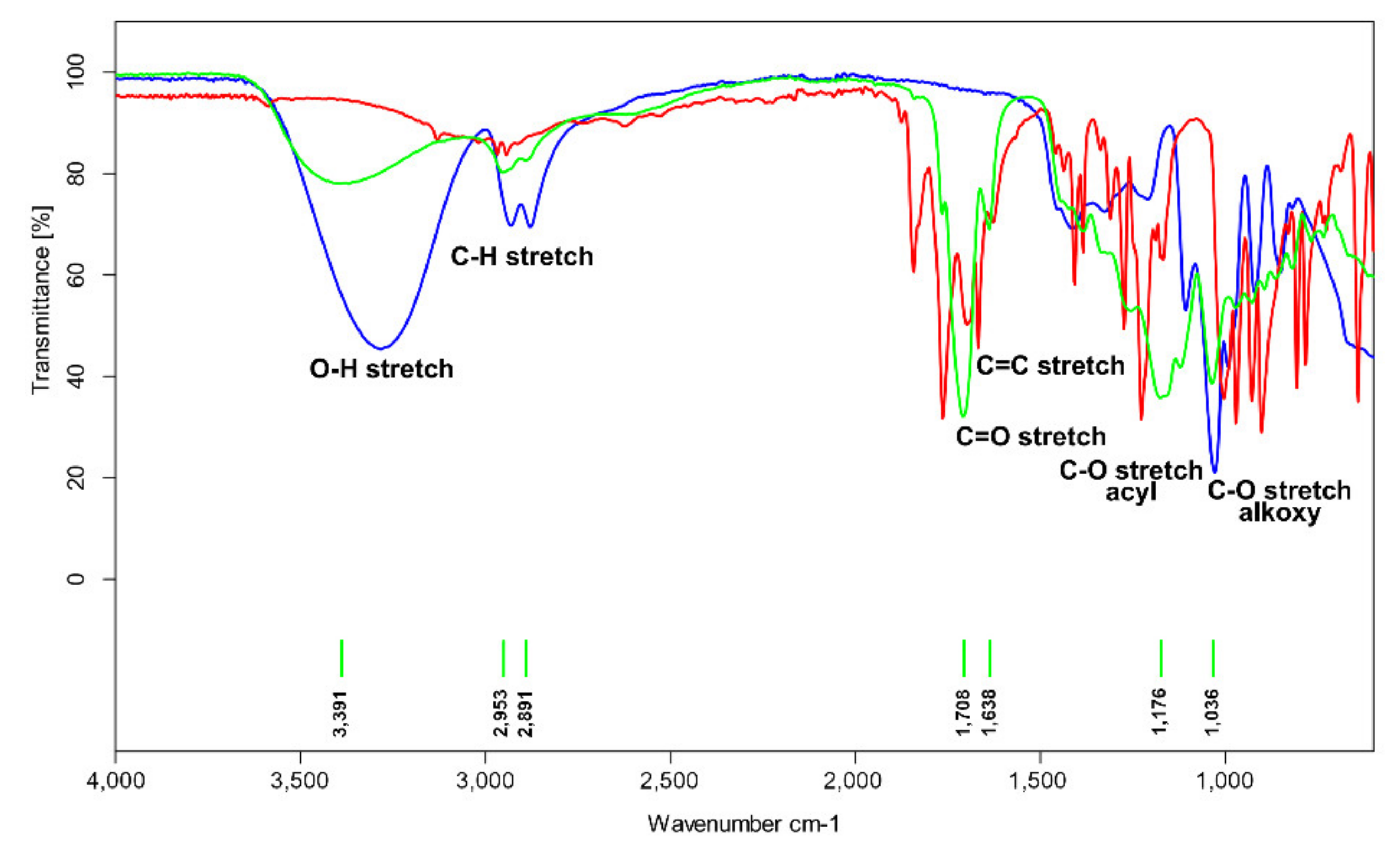
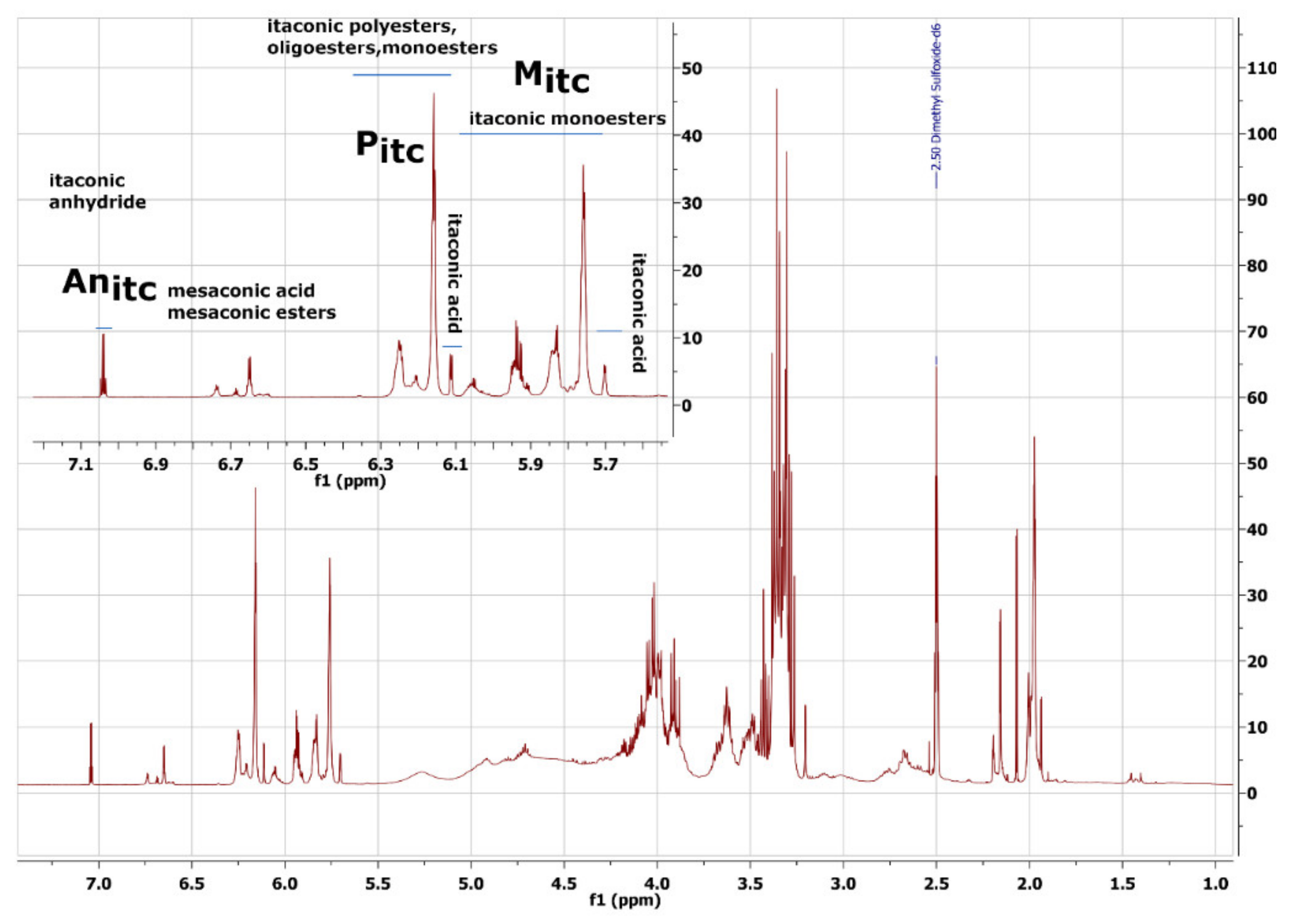
2.1. FTIR and NMR Analysis
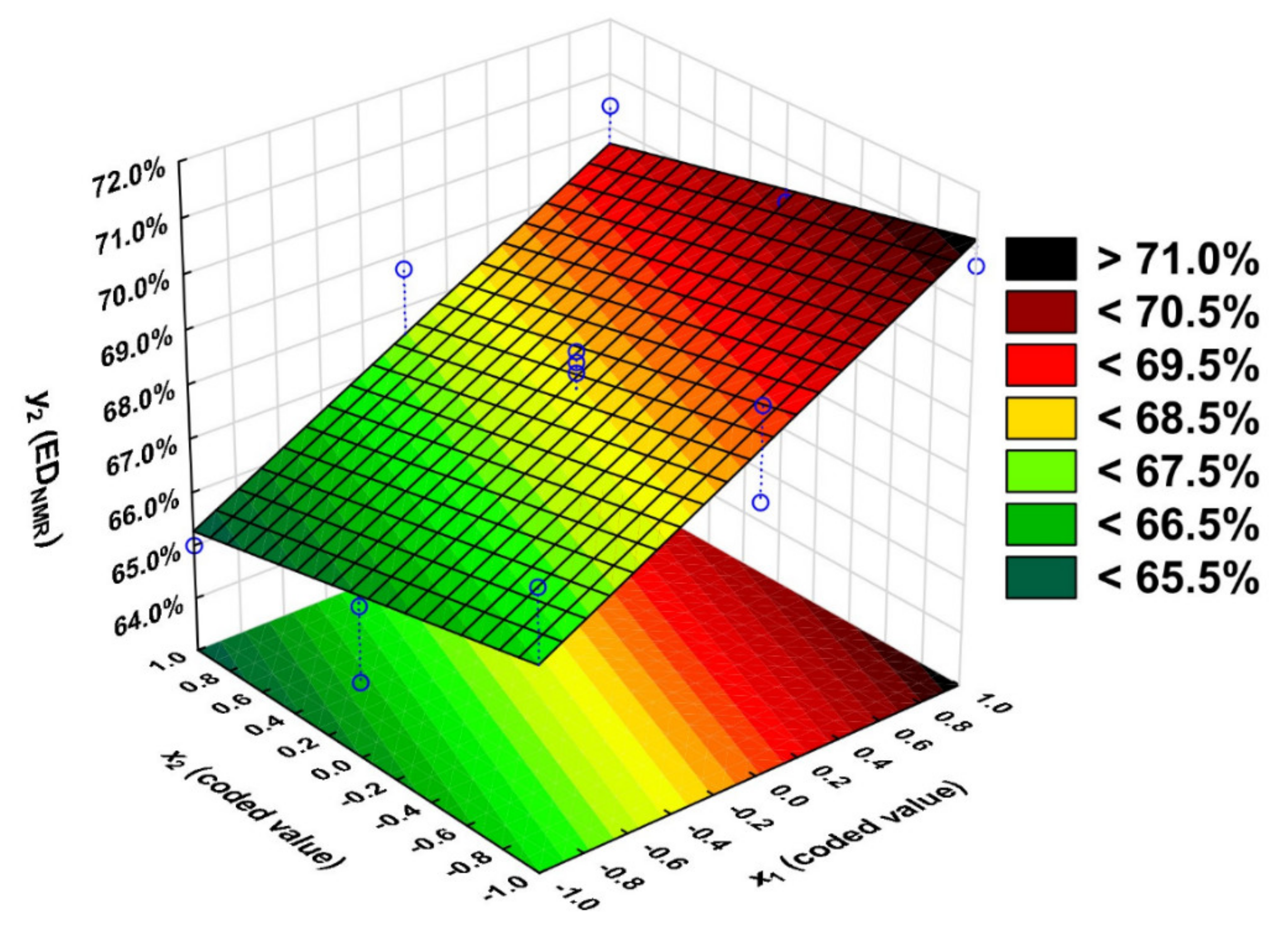
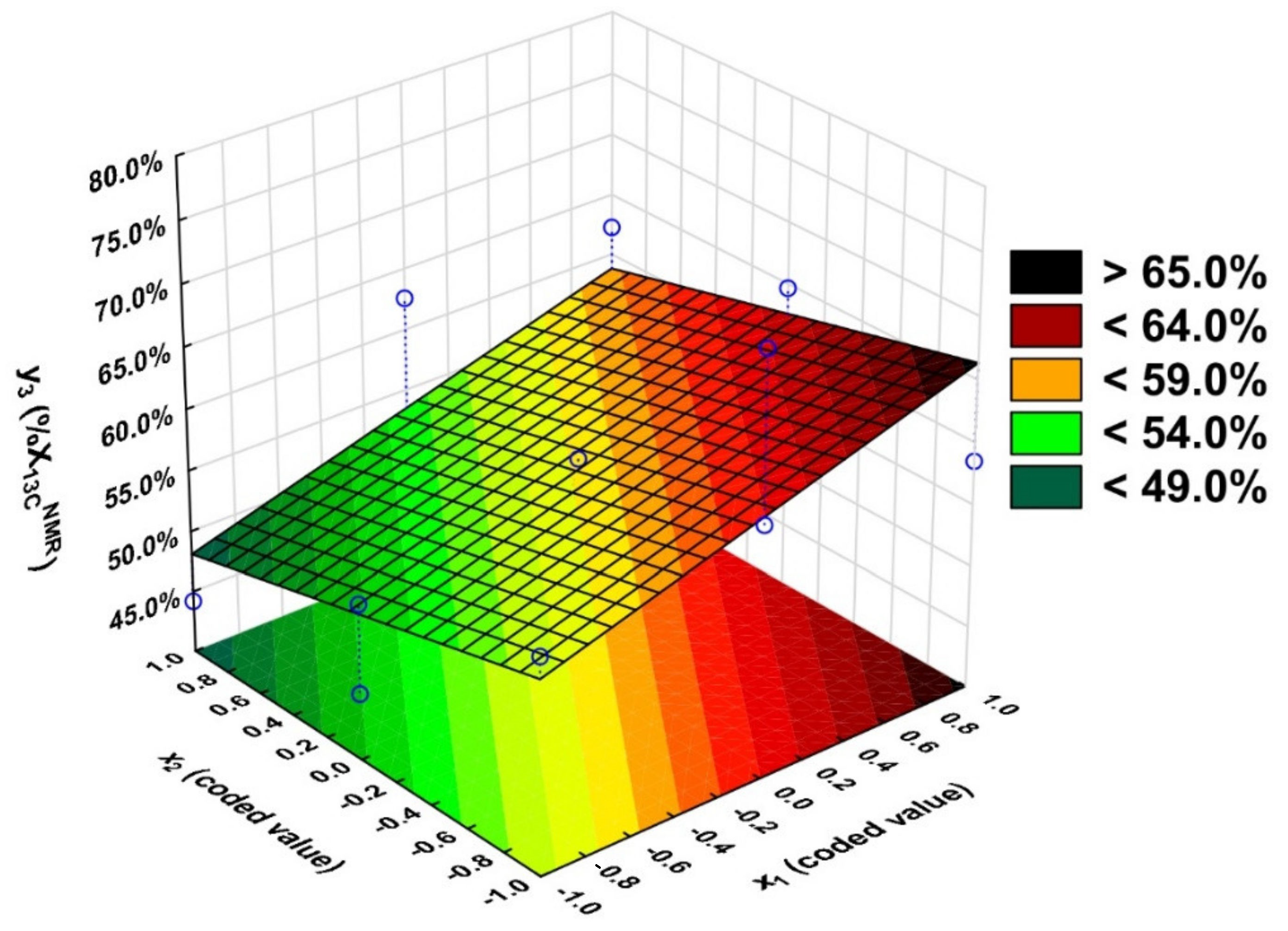
2.2. Statistical Analysis
2.3. Experiment under Optimal Conditions
3. Materials and Methods
3.1. NMR
3.2. FTIR
3.3. Acid Number
3.4. Ester Number
3.5. Esterification Degree
3.6. Statistical Analysis
3.7. Synthesis Procedure
3.8. Optimisation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Grinstaff, M.W. Recent advances in glycerol polymers: Chemistry and biomedical applications. Macromol Rapid. Commun. 2014, 35, 1906–1924. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, R.; Valerio, O.; Mekonnen, T.H. Recent developments in short- and medium-chain- length Polyhydroxyalkanoates: Production, properties, and applications. Int. J. Biol. Macromol. 2021, 187, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Muddu, B.N. Eleven-year results of the ABG I hip replacement. Int. Orthop. 2006, 30, 182–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewend, M.G.; Brem, S.; Gilbert, M.; Goodkin, R.; Penar, P.L.; Varia, M.; Cush, S.; Carey, L.A. Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control. Clin. Cancer Res. 2007, 13, 3637–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundback, C.A.; McFadden, J.; Hart, A.; Kulig, K.M.; Wieland, A.M.; Pereira, M.J.N.; Pomerantseva, I.; Hartnick, C.J.; Masiakos, P.T. Behavior of poly(glycerol sebacate) plugs in chronic tympanic membrane perforations. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2012, 100, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef]
- Cai, M.; Liu, H.; Jiang, Y.; Wang, J.; Zhang, S. A high-strength biodegradable thermoset polymer for internal fixation bone screws: Preparation, in vitro and in vivo evaluation. Colloids Surfaces B Biointerfaces 2019, 183, 110445. [Google Scholar] [CrossRef]
- Hsieh, Y.K.; Hung, P.H.; Huang, C.W.; Chuang, K.C.; Wang, J. Study on the degradation of biodegradable poly (glycerol maleate) (PGM) microbeads. Polym. Degrad. Stab. 2020, 179, 109223. [Google Scholar] [CrossRef]
- Wang, W.H.; Huang, C.W.; Tsou, E.Y.; Ao-Ieong, W.S.; Hsu, H.C.; Wong, D.S.H.; Wang, J. Characterization of degradation behavior of poly(glycerol maleate) films in various aqueous environments. Polym. Degrad. Stab. 2021, 183, 1–8. [Google Scholar] [CrossRef]
- Guarneri, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vassallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of Enzymatically Synthesized Rigid Poly(itaconate)s via Post-Polymerization Aza-Michael Addition of Primary Amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar] [CrossRef] [Green Version]
- Pagliaro, M.; Rossi, M. Glycerol: Properties and Production. The Future of Glycerol; The Royal Society of Chemistry: London, UK, 2010; pp. 1–28. [Google Scholar]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Goyal, S.; Hernández, N.B.; Cochran, E.W. An update on the future prospects of glycerol polymers. Polym. Int. 2021, 70, 911–917. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.C.; Highley, C.B.; Ouyang, L.; Burdick, J.A. 3D printing of photocurable poly(glycerol sebacate) elastomers. Biofabrication 2016, 8, 045004. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. [Google Scholar] [CrossRef]
- Gadomska-Gajadhur, A.; Wrzecionek, M.; Matyszczak, G.; Pietowski, P.; Wiecław, M.; Ruśkowski, P. Optimization of Poly(glycerol sebacate) Synthesis for Biomedical Purposes with the Design of Experiments. Org. Process Res. Dev. 2018, 22, 1793–1800. [Google Scholar] [CrossRef]
- Aydin, H.M.; Salimi, K.; Rzayev, Z.M.O.; Pişkin, E. Microwave-assisted rapid synthesis of poly(glycerol-sebacate) elastomers. Biomater. Sci. 2013, 1, 503–509. [Google Scholar] [CrossRef]
- Louage, B.; Tack, L.; Wang, Y.; De Geest, B.G. Poly(glycerol sebacate) nanoparticles for encapsulation of hydrophobic anti-cancer drugs. Polym. Chem. 2017, 8, 5033–5038. [Google Scholar] [CrossRef]
- Liang, B.; Shi, Q.; Xu, J.; Chai, Y.M.; Xu, J.G. Poly (Glycerol Sebacate)-Based Bio-Artificial Multiporous Matrix for Bone Regeneration. Front. Chem. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Bodakhe, S.; Verma, S.; Garkhal, K.; Samal, S.K.; Sharma, S.S.; Kumar, N. Injectable photocrosslinkable nanocomposite based on poly(glycerol sebacate) fumarate and hydroxyapatite: Development, biocompatibility and bone regeneration in a rat calvarial bone defect model. Nanomedicine 2013, 8, 1777–1795. [Google Scholar] [CrossRef]
- Li, Y.; Cook, W.D.; Moorhoff, C.; Huang, W.C.; Chen, Q.Z. Synthesis, characterization and properties of biocompatible poly(glycerol sebacate) pre-polymer and gel. Polym. Int. 2013, 62, 534–547. [Google Scholar] [CrossRef]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef]
- Yang, B.; Lv, W.; Deng, Y. Drug loaded poly(glycerol sebacate) as a local drug delivery system for the treatment of periodontal disease. RSC Adv. 2017, 7, 37426–37435. [Google Scholar] [CrossRef] [Green Version]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Wrzecionek, M.; Howis, J.; Ruskowski, P.; Gadomska-Gajadhur, A. Optimizing the conditions of pgsu synthesis with simplex method. Chem. Process Eng.-Inz. Chem. Proces. 2020, 41, 119–128. [Google Scholar] [CrossRef]
- Valerio, O.; Horvath, T.; Pond, C.; Misra, M.; Mohanty, A. Improved utilization of crude glycerol from biodiesel industries: Synthesis and characterization of sustainable biobased polyesters. Ind. Crops Prod. 2015, 78, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Carnahan, M.A.; Grinstaff, M.W. Synthesis of generational polyester dendrimers derived from glycerol and succinic or adipic acid. Macromolecules. 2006, 39, 609–616. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Offeman, R.D.; Klamczynski, A.P.; Glenn, G.M.; Mattoso, L.H.C.; Orts, W.J. Synthesis, Characterization and Nanocomposite Formation of Poly(glycerol succinate-co-maleate) with Nanocrystalline Cellulose. J Polym Environ. 2014, 22, 219–226. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Sustainable biobased blends of poly(lactic acid) (PLA) and poly(glycerol succinate-: Co -maleate) (PGSMA) with balanced performance prepared by dynamic vulcanization. RSC Adv. 2017, 7, 38594–38603. [Google Scholar] [CrossRef] [Green Version]
- Kolbuk, D.; Jeznach, O.; Wrzecionek, M.; Gadomska-Gajadhur, A. Poly(glycerol succinate) as an eco-friendly component of PLLA and PLCL fibres towards medical applications. Polymers 2020, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Agach, M.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V. Acyl Poly(Glycerol-Succinic Acid) Oligoesters: Synthesis, Physicochemical and Functional Properties, and Biodegradability. J. Surfactants Deterg. 2016, 19, 933–941. [Google Scholar] [CrossRef]
- Yin, G.Z.; Yang, X.M.; Zhou, Z.; Li, Q.F. A green pathway to adjust the mechanical properties and degradation rate of PCL by blending bio-sourced poly(glycerol-succinate) oligoesters. Mater. Chem. Front. 2018, 2, 544–553. [Google Scholar] [CrossRef]
- Chen, S.W.; Xin, Q.; Kong, W.X.; Min, L.; Li, J.F. Anxiolytic-like effect of succinic acid in mice. Life Sci. 2003, 73, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Nakiou, E.A.; Christodoulou, E.; Bikiaris, D.N.; Kontonasaki, E.; Liverani, L.; Boccaccini, A.R. Polyglycerol hyperbranched polyesters: Synthesis, properties and pharmaceutical and biomedical applications. Int. J. Mol. Sci. 2019, 20, 6210. [Google Scholar] [CrossRef] [Green Version]
- Farmer, T.J.; Castle, R.L.; Clark, J.H.; Macquarrie, D.J. Synthesis of unsaturated polyester resins from various bio-derived platform molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef] [Green Version]
- Tatara, A.M.; Watson, E.; Satish, T.; Scott, D.W.; Kontoyiannis, D.P.; Engel, P.S.; Mikos, A.G. Synthesis and Characterization of Diol-Based Unsaturated Polyesters: Poly(diol fumarate) and Poly(diol fumarate-co-succinate). Biomacromolecules 2017, 18, 1724–1735. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.C. Polyesters based on diacid monomers. Adv. Drug Deliv. Rev. 2003, 55, 585–609. [Google Scholar] [CrossRef]
- Kolankowski, K.; Gadomska-Gajadhur, A.; Wrzecionek, M.; Ruśkowski, P. Mathematically described model of poly(glycerol maleate) cross-linking process using triethylenetetramine addition. Polym. Adv. Technol. 2022, 33, 1298–1306. [Google Scholar] [CrossRef]
- Feuer, S.S.; Bockstahler, T.E.; Brown, C.A.; Rosenthal, I. Maleic-Fumaric Isomerization in Unsaturated Polyesters. Ind. Eng. Chem. 1954, 46, 1643–1645. [Google Scholar] [CrossRef]
- Curtis, L.G.; Edwards, D.L.; Simons, R.M.; Trent, P.J.; Von Bramer, P.T. Investigation of maleate-fumarate isomerization in unsaturated polyesters by nuclear magnetic resonance. Ind. Eng. Chem. Prod. Res. Dev. 1964, 3, 218–221. [Google Scholar] [CrossRef]
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Magalhães, A.I.; Soccol, C.R. Biobased itaconic acid market and research trends-is it really a promising chemical? Chim. Oggi-Chem. Today 2018, 36, 56–58. [Google Scholar]
- Sriariyanun, M.; Heitz, J.H.; Yasurin, P.; Asavasanti, S.; Tantayotai, P. Itaconic acid: A promising and sustainable platform chemical? Appl. Sci. Eng. Prog. 2019, 12, 75–82. [Google Scholar] [CrossRef]
- Sano, M.; Tanaka, T.; Ohara, H.; Aso, Y. Itaconic acid derivatives: Structure, function, biosynthesis, and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 9041–9051. [Google Scholar] [CrossRef]
- Sano, M.; Yada, R.; Nomura, Y.; Kusukawa, T.; Ando, H.; Matsumoto, K.; Wada, K.; Tanaka, T.; Ohara, H.; Aso, Y. Microbial screening based on the mizoroki–heck reaction permits exploration of hydroxyhexylitaconic-acid-producing fungi in soils. Microorganisms 2020, 8, 648. [Google Scholar] [CrossRef]
- Qin, W.; Qin, K.; Zhang, Y.; Jia, W.; Chen, Y.; Cheng, B.; Peng, L.; Chen, N.; Liu, Y.; Zhou, W.; et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat. Chem. Biol. 2019, 15, 983–991. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, Y.; Tang, H.; Liu, D.; Chen, Y.; Liu, Y.; Wang, C. Chemoproteomic Profiling of Itaconation by Bioorthogonal Probes in Inflammatory Macrophages. J. Am. Chem. Soc. 2020, 142, 10894–10898. [Google Scholar] [CrossRef]
- Chanda, S.; Ramakrishnan, S. Poly(alkylene itaconate)s—An interesting class of polyesters with periodically located exo-chain double bonds susceptible to Michael addition. Polym. Chem. 2015, 6, 2108–2114. [Google Scholar] [CrossRef]
- Farmer, T.J.; Macquarrie, D.J.; Comerford, J.W.; Pellis, A.; Clark, J.H. Insights into post-polymerisation modification of bio-based unsaturated itaconate and fumarate polyesters via aza-michael addition: Understanding the effects of C=C isomerisation. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1935–1945. [Google Scholar] [CrossRef] [Green Version]




| No. | Coded Variables | EDtitr [%] | EDNMR [%] | %X13CNMR [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | Exp. | Calc. | Rest | Exp. | Calc. | Rest | Exp. | Calc. | Rest | |
| 1 | −1 | −1 | 0 | 46.7 | 49.4 | −2.7 | 68.1 | 66.7 | 1.4 | 57.4 | 55.6 | 1.8 |
| 2 | 1 | −1 | 0 | 51.1 | 49.4 | 1.7 | 70.7 | 71.2 | −0.5 | 58.5 | 66.3 | −7.8 |
| 3 | −1 | 1 | 0 | 48.4 | 49.4 | −1.0 | 65.0 | 65.3 | −0.3 | 44.2 | 48.1 | −3.9 |
| 4 | 1 | 1 | 0 | 60.9 | 49.4 | 11.5 | 70.4 | 69.7 | 0.7 | 62.2 | 58.8 | 3.4 |
| 5 | −1 | 0 | −1 | 67.8 | 63.8 | 4.0 | 65.8 | 66.0 | −0.2 | 52.7 | 51.8 | 0.9 |
| 6 | 1 | 0 | −1 | 19.9 | 35.0 | −15.1 | 69.7 | 70.4 | −0.7 | 57.0 | 62.6 | −5.6 |
| 7 | −1 | 0 | 1 | 44.3 | 35.0 | 9.3 | 64.4 | 66.0 | −1.6 | 45.4 | 51.9 | −6.5 |
| 8 | 1 | 0 | 1 | 54.0 | 63.8 | −9.8 | 70.2 | 70.4 | −0.2 | 64.7 | 62.6 | 2.1 |
| 9 | 0 | −1 | −1 | 50.1 | 49.4 | 0.7 | 68.0 | 69.0 | −1.0 | 60.3 | 61.0 | −0.7 |
| 10 | 0 | 1 | −1 | 48.9 | 49.4 | −0.5 | 66.5 | 67.5 | −1.0 | 51.0 | 53.5 | −2.5 |
| 11 | 0 | −1 | 1 | 43.5 | 49.4 | −5.9 | 69.7 | 69.0 | 0.7 | 74.0 | 61.0 | 13.0 |
| 12 | 0 | 1 | 1 | 60.0 | 49.4 | 10.6 | 68.7 | 67.5 | 1.2 | 62.6 | 53.5 | 9.1 |
| 13 | 0 | 0 | 0 | 48.7 | 49.4 | −0.7 | 68.5 | 68.2 | 0.3 | 57.3 | 57.2 | 0.1 |
| 14 | 0 | 0 | 0 | 47.9 | 49.4 | −1.5 | 68.9 | 68.2 | 0.7 | 54.7 | 57.2 | −2.5 |
| 15 | 0 | 0 | 0 | 48.5 | 49.4 | −0.9 | 68.7 | 68.2 | 0.5 | 56.2 | 57.2 | −1.0 |
| Results | EDtit [%] | EDNMR [%] | %X13CNMR [%] |
|---|---|---|---|
| Calculated | 52.8 | 69.7 | 63.0 |
| Experimental | 62.3 | 70.4 | 62.8 |
| Parameter | Natural Variable | Coded Value | Step | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| x1 | OH/COOH ratio | 0.5 | 1 | 1.5 | 0.5 |
| x2 | Temperature [°C] | 110 | 125 | 140 | 15 |
| x3 | Time [h] | 3 | 4 | 5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolankowski, K.; Miętus, M.; Ruśkowski, P.; Gadomska-Gajadhur, A. Optimisation of Glycerol and Itaconic Anhydride Polycondensation. Molecules 2022, 27, 4627. https://doi.org/10.3390/molecules27144627
Kolankowski K, Miętus M, Ruśkowski P, Gadomska-Gajadhur A. Optimisation of Glycerol and Itaconic Anhydride Polycondensation. Molecules. 2022; 27(14):4627. https://doi.org/10.3390/molecules27144627
Chicago/Turabian StyleKolankowski, Krzysztof, Magdalena Miętus, Paweł Ruśkowski, and Agnieszka Gadomska-Gajadhur. 2022. "Optimisation of Glycerol and Itaconic Anhydride Polycondensation" Molecules 27, no. 14: 4627. https://doi.org/10.3390/molecules27144627
APA StyleKolankowski, K., Miętus, M., Ruśkowski, P., & Gadomska-Gajadhur, A. (2022). Optimisation of Glycerol and Itaconic Anhydride Polycondensation. Molecules, 27(14), 4627. https://doi.org/10.3390/molecules27144627









