A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health
Abstract
:1. Introduction
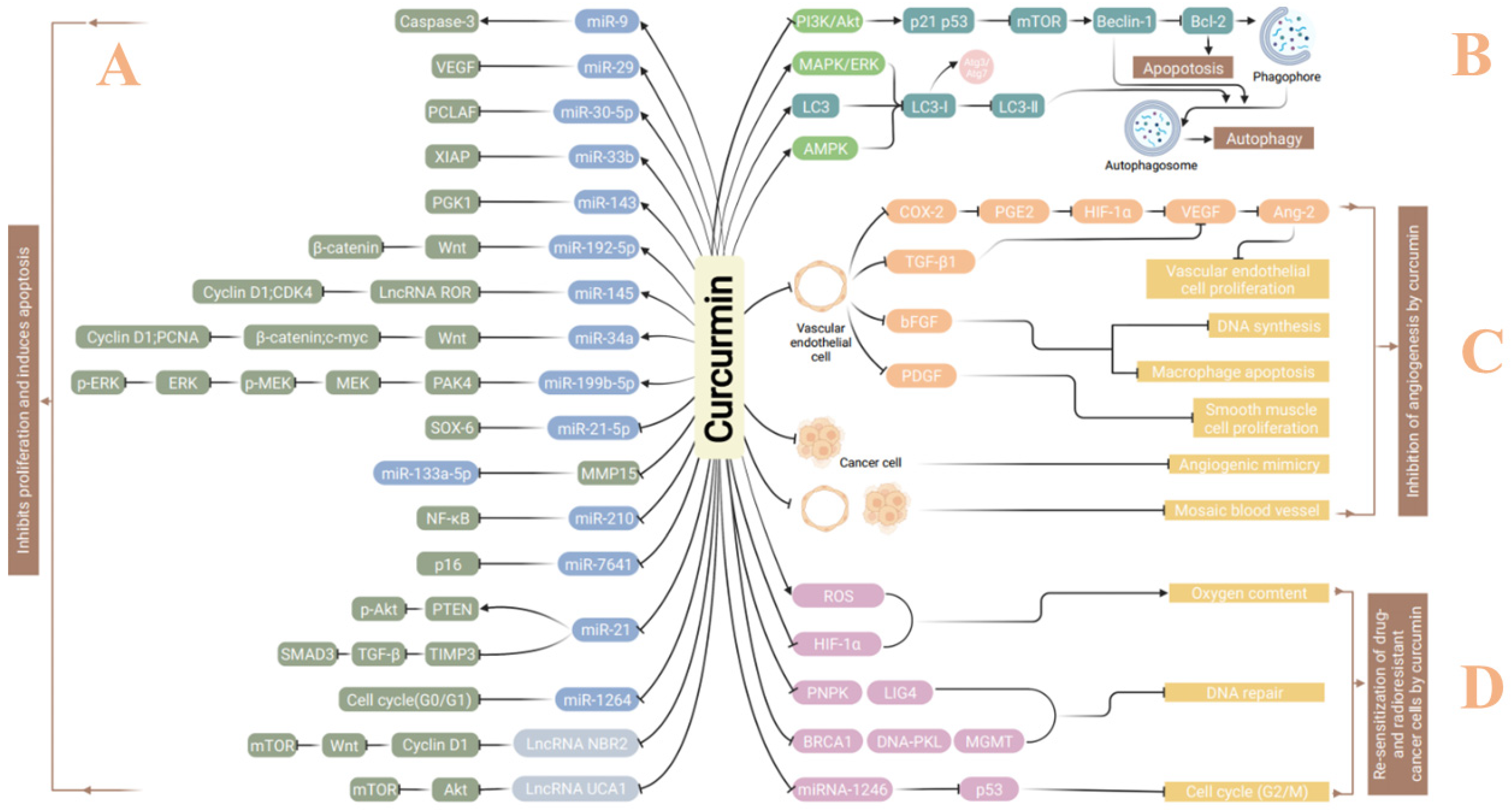
2. Curcumin in Treatment of Malignant Tumors
2.1. Induction of Autophagy
2.2. Inhibition of Invasion and Metastasis
2.3. Inhibition of Tumor Angiogenesis
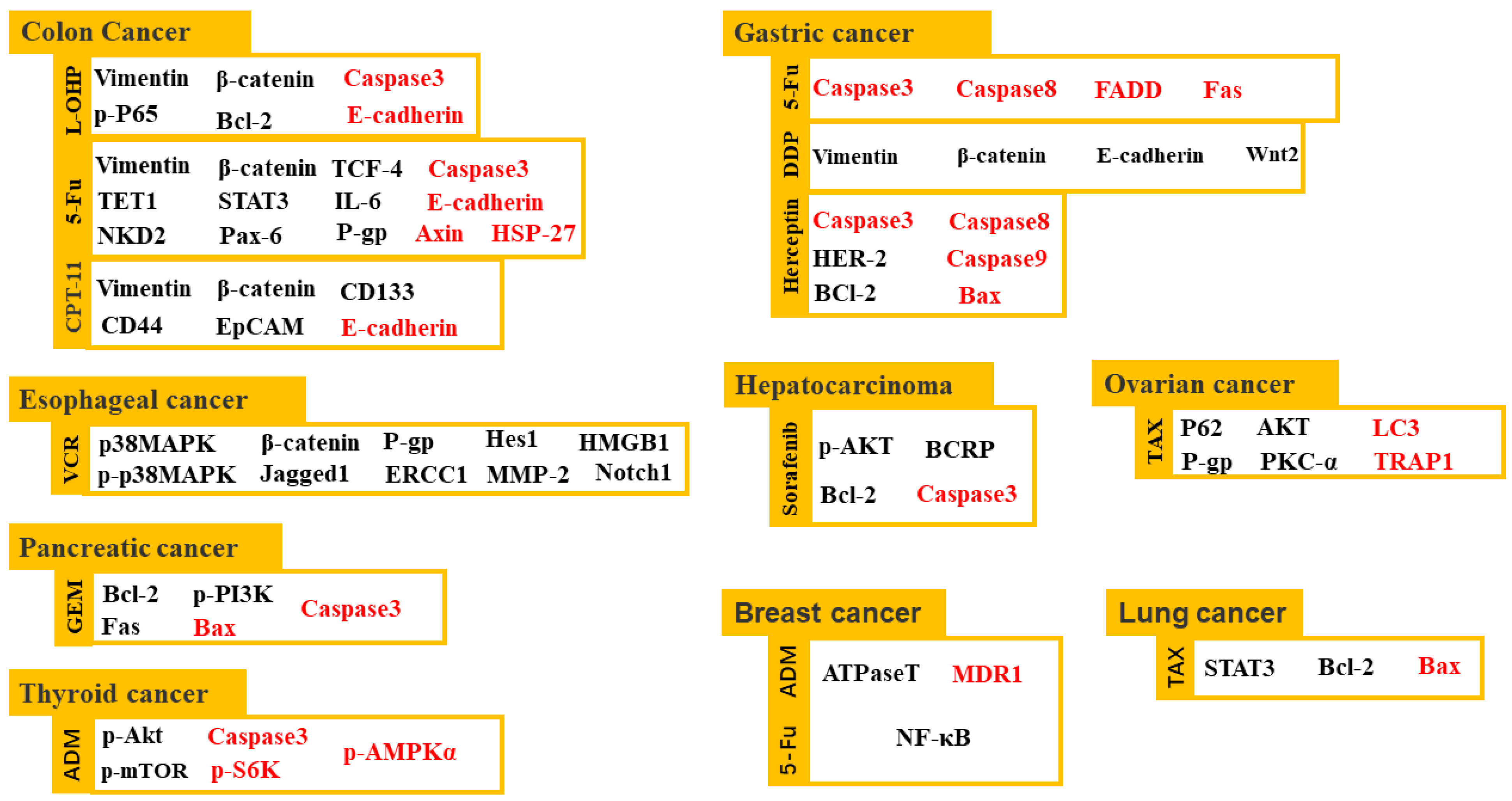
2.4. Sensitization of Cancer Treatment
3. Curcumin in Treatment of AD
3.1. Reduction in Beta-Amyloid Deposition
3.2. Prevention of TAU Protein Deposition
3.3. Regeneration of Synapses
4. Curcumin in Treatment of Abnormal Blood Conditions
4.1. Reduction in Blood Lipids
4.2. Reduction in Blood Sugar
4.3. Anticoagulation and Antiplatelet Aggregation
5. Curcumin in Treatment of Viral Infection
6. Hurdles in the Applications of Curcumin
6.1. Low Bioavailability and Solubility
6.1.1. Combination with Other Drugs
6.1.2. Curcumin Complex
6.2. Controversial in Purity
6.3. Lack of Supported Clinical Reaserch Results
7. Side Effects of Curcumin
7.1. Risk of Kidney Calculi in Susceptible Individuals
7.2. Anemia in Patients with Iron Failure
7.3. Liver Injury
7.4. Abnormal Cardiac Conductions
7.5. Influence on Other Drugs
7.6. Allergic Reactions
7.7. Cancer Induction
7.8. Other Risks
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin--from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, H.B.; Guo, H. Research progress of turmeric and prediction analysis of quality marker (Q-Marker). Chin. Herb. Med. 2021, 52, 4700–4710. [Google Scholar]
- Vallée, A.; Lecarpentier, Y. Curcumin and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Akbar, U.; Mohan, C. Curcumin in Autoimmune and Rheumatic Diseases. Nutrients 2019, 11, 1004. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.D.; Jiao, Z.X.; Song, Y.; Teng, W.-H.; Liu, Z.; Liu, J.-L. Study on the effect and mechanism of curcumin inducing apoptosis of colon cancer LoVo cells. Chin. J. Tradit. Chin. Med. 2013, 38, 2191–2196. [Google Scholar]
- Liu, Y.; Zhang, X.C.; Chen, Q.W. Study on the mechanism of curcumin on the proliferation and apoptosis of colorectal cancer cells. Mod. Dig. Interv. Diagn. Treat. 2020, 25, 1475–1479. [Google Scholar]
- Guo, Y.; Hua, C.; Yu, X.L.; Pei, X.; Li, Y. Curcumin regulates the proliferation, apoptosis, migration and invasion of thyroid cancer cell TPC-1 by miR-152. J. Guangxi Med. Univ. 2021, 38, 1283–1289. [Google Scholar]
- Qin, F.; Qin, S.Y.; Wang, X.S. The mechanism of curcumin inhibiting the proliferation of human non-small cell lung cancer A549 cells and promoting apoptosis by up-regulating the expression of miR-15a/16. Chin. J. Geriatr. 2019, 39, 5615–5620. [Google Scholar]
- Dong, X.D.; Mu, S.H. Curcumin inhibits the proliferation of renal cancer A498 cells and its mechanism. PLA Med. J. 2020, 32, 43–46. [Google Scholar]
- Pan, L.; Sha, J.; Lin, W.; Wang, Y.; Bian, T.; Guo, J. Curcumin inhibits prostate cancer progression by regulating the miR-30a-5p/PCLAF axis. Exp. Ther. Med. 2021, 22, 969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zheng, Z.; Huang, J.; Ma, X.; Huang, C.; Wu, R.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; et al. Modulation of miR-34a in curcumin-induced antiproliferation of prostate cancer cells. J. Cell. Biochem. 2019, 120, 15616–15624. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.W.; Yu, H.J.; Feng, Y.G.; Chen, L.; Liang, F. Curcumin inhibits prostate cancer by targeting PGK1 in the FOXD3/miR-143 axis. Cancer Chemother. Pharmacol. 2017, 79, 985–994. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Liu, Y.; Li, Q.; Guo, H.; Guo, Y.; Chang, Z. Curcumin Inhibits Hepatocellular Carcinoma via Regulating miR-21/TIMP3 Axis. Evid. Based Complement Alternat. Med. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin Suppresses the Colon Cancer Proliferation by Inhibiting Wnt/β-Catenin Pathways via miR-130a. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Li, B.; Shi, C.; Li, B.; Zhao, J.M.; Wang, L. The effects of Curcumin on HCT-116 cells proliferation and apoptosis via the miR-491/PEG10 pathway. J. Cell Biochem. 2018, 119, 3091–3098. [Google Scholar] [CrossRef]
- Ling, Y.L.; Xu, L.; Wu, C. Curcumin inhibits the proliferation, migration and invasion of colon cancer SW1116 cells through miR-199b-5p. Chin. Pharmacol. Bull. 2020, 36, 957–964. [Google Scholar]
- Pan, Y.; Sun, Y.; Liu, Z.; Zhang, C. miR-192-5p upregulation mediates the suppression of curcumin in human NSCLC cell proliferation, migration and invasion by targeting c-Myc and inactivating the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2020, 22, 1594–1604. [Google Scholar] [CrossRef]
- Wang, K.; Tan, S.-L.; Lu, Q.; Xu, R.; Cao, J.; Wu, S.-Q.; Wang, Y.-H.; Zhao, X.-K.; Zhong, Z.-H. Curcumin Suppresses microRNA-7641-Mediated Regulation of p16 Expression in Bladder Cancer. Am. J. Chin. Med. 2018, 46, 1357–1368. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Wu, S.; Qu, J.; Yuan, H.; Zhou, Y.; Lu, Q. MicroRNA-1246 regulates the radio-sensitizing effect of curcumin in bladder cancer cells via activating P53. Int. Urol. Nephrol. 2019, 51, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.Y.; Hoan, T.; Pu, J. Curcumin affects the apoptosis of human prostate cancer PC3 cell line based on the down-regulation of the expression of miR210 and TLR4/NF-κB signaling pathway. Pharmacol. Clin. Tradit. Chin. Med. 2021, 37, 64–68. [Google Scholar]
- Qian, J.R. Role of miR-21/PTEN/Akt Pathway in the Anti-Gastric Cancer Effect of Curcumin and the Synergistic Effect of PD98059 against Gastric Cancer. Ph.D. Thesis, Southern Medical University, Nanjing, China, 2019. [Google Scholar]
- Shao, J. Study on the Effect and Mechanism of Curcumin on the Regulation of miR-133a-3p-Targeted MMP15 in the Intervention of Gastric Cancer Proliferation and Metastasis. Ph.D. Thesis, Yangzhou University, Yangzhou, China, 2017. [Google Scholar]
- Sun, Q.Q. Effect of Curcumin on the Proliferation and Apoptosis of Gastric Cancer Cells Induced by miR-33b. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2018. [Google Scholar]
- Zhao, S.-F.; Zhang, X.-J.; Shi, X.-Q.; Yu, Z.-J.; Kan, Q.-C. Induction of microRNA-9 mediates cytotoxicity of curcumin against SKOV3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 3363–3368. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Xu, Q.; Zhao, H.F. Intervention of curcumin on the expression of miR-29 and VEGF in liver cancer cells. Zhejiang J. Integr. Tradit. Chin. West. Med. 2020, 30, 785–790. [Google Scholar]
- Guo, Y.; Zhou, L.Y.; You, J.L. Curcumin regulates the expression of long-chain non-coding RNA AK125910 and mediates the apoptosis of liver cancer cells. J. Nanjing Univ. Chin. Med. 2015, 31, 254–257. [Google Scholar]
- Wang, W.; Chen, J.; Zhang, B.; Lu, S.; Wang, F.; Peng, L.; Dai, J.; Sun, Y. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating lncRNA UCA1. Pharmazie 2018, 73, 402–407. [Google Scholar]
- Yu, H.; Xie, Y.; Zhou, Z.; Wu, Z.; Dai, X.; Xu, B. Curcumin Regulates the Progression of Colorectal Cancer via LncRNA NBR2/AMPK Pathway. Technol. Cancer Res. Treat. 2019, 18, 1533033819870781. [Google Scholar] [CrossRef]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a molecular target for cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, T.J.; Woo, B.H.; Choi, K.U.; Lee, C.H.; Ryu, M.H.; Park, H.R. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch. Oral Biol. 2012, 57, 1018–1025. [Google Scholar] [CrossRef]
- Zhu, J. Effects and Mechanisms of Extracts of Two Chinese Herbal Medicines on the Regulation of Autophagy in Colon Cancer Cells. Ph.D. Thesis, Beijing University of Technology, Beijing, China, 2020. [Google Scholar]
- Liu, H.; Ren, P.; Liu, F.L. Effects of curcumin on autophagy of prostate cancer cells. J. Qiannan Natl. Med. Coll. 2021, 34, 1–5. [Google Scholar]
- Liu, L.-D.; Pang, Y.-X.; Zhao, X.-R.; Li, R.; Jin, C.-J.; Xue, J.; Dong, R.-Y.; Liu, P.-S. Curcumin induces apoptotic cell death and protective autophagy by inhibiting AKT/mTOR/p70S6K pathway in human ovarian cancer cells. Arch. Gynecol. Obstet. 2019, 299, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2015, 35, 1065–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, N.-Y.; Suh, Y.-A.; Lee, C. Involvement of ROS in Curcumin-induced Autophagic Cell Death. Korean J. Physiol. Pharmacol. 2011, 15, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Men, Y.; Wang, H.; Chen, R.; Han, X.; Liu, J. Curcumin Inhibits Proliferation and Migration of A549 Lung Cancer Cells Through Activation of ERK1/2 Pathway-induced Autophagy. Nat. Prod. Commun. 2019, 14, 1934578X19848179. [Google Scholar] [CrossRef] [Green Version]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin Induces Autophagy via Activating the AMPK Signaling Pathway in Lung Adenocarcinoma Cells. J. Pharmacol. Sci. 2013, 123, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Chen, C.; Wu, F.; Qiu, L.; Ke, Q.; Sun, R.; Duan, Q.; Luo, M.; Luo, Z. Curcumin Inhibits the Migration and Invasion of Non-Small-Cell Lung Cancer Cells Through Radiation-Induced Suppression of Epithelial-Mesenchymal Transition and Soluble E-Cadherin Expression. Technol. Cancer Res. Treat. 2020, 19, 1533033820947485. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Zhang, Y.; Wang, Z. Curcumin suppresses the malignancy of non-small cell lung cancer by modulating the circ-PRKCA/miR-384/ITGB1 pathway. Biomed. Pharmacother. 2021, 138, 111439. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhao, Y.T.; Wang, J.M. Effects and mechanisms of curcumin on colorectal cancer cell migration, invasion and epithelial-mesenchymal transition. World Chin. Med. 2021, 16, 2596–2599. [Google Scholar]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Kuzhuvelil, H.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef]
- Aedo-Aguilera, V.; Carrillo-Beltrán, D.; Calaf, G.M.; Muñoz, J.P.; Guerrero, N.; Osorio, J.C.; Tapia, J.C.; León, O.; Contreras, H.R.; Aguayo, F. Curcumin decreases epithelial-mesenchymal transition by a Pirin-dependent mechanism in cervical cancer cells. Oncol. Rep. 2019, 42, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Babaei, G.; Aziz, S.G.; Jaghi, N. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother. 2021, 133, 110909. [Google Scholar] [CrossRef] [PubMed]
- Siddhartha, R.; Garg, M. Molecular and clinical insights of matrix metalloproteinases into cancer spread and potential therapeutic interventions. Toxicol. Appl. Pharmacol. 2021, 426, 115593. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kong, D.; Zhang, Y.; Li, S.; Li, Y.; Dong, L.; Zhang, N.; Ma, J. Curcumin inhibits the viability, migration and invasion of papillary thyroid cancer cells by regulating the miR-301a-3p/STAT3 axis. Exp. Ther. Med. 2021, 22, 875. [Google Scholar] [CrossRef]
- Cai, J.; Sun, H.; Zheng, B.; Xie, M.; Xu, C.; Zhang, G.; Huang, X.; Zhuang, J. Curcumin attenuates lncRNA H19‑induced epithelial-mesenchymal transition in tamoxifen-resistant breast cancer cells. Mol. Med. Rep. 2021, 23, 13. [Google Scholar] [CrossRef]
- Yin, S.; Du, W.; Wang, F.; Han, B.; Cui, Y.; Yang, D.; Chen, H.; Liu, D.; Liu, X.; Zhai, X.; et al. MicroRNA-326 sensitizes human glioblastoma cells to curcumin via the SHH/GLI1 signaling pathway. Cancer Biol. Ther. 2018, 19, 260–270. [Google Scholar] [CrossRef]
- Schiavoni, G.; Gabriele, L.; Mattei, F. The tumor microenvironment: A pitch for multiple players. Front. Oncol. 2013, 3, 90. [Google Scholar] [CrossRef] [Green Version]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Park, K.-S.; Kim, K.-T.; Gil, E.Y. The inhibitory effect of curcumin via fascin suppression through JAK/STAT3 pathway on metastasis and recurrence of ovary cancer cells. BMC Womens Health 2020, 20, 256. [Google Scholar] [CrossRef]
- Choe, S.R.; Kim, Y.N.; Park, C.G.; Cho, K.H.; Cho, D.Y.; Lee, H.Y. RCP induces FAK phosphorylation and ovarian cancer cell invasion with inhibition by curcumin. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.Z.Q.; Chen, L.B. Curcumin on sarcoma cells in vitro angiogenesis mimicry and its mechanism. West. Med. 2020, 33, 21–25. [Google Scholar]
- Fang, H.; Chen, L.B. Effects of curcumin combined with endostatin on growth inhibition and angiogenesis of sarcoma in mice. Chin. J. Mod. Appl. Pharm. 2020, 37, 572–576. [Google Scholar]
- Zhang, C.; Hao, Y.; Wu, L.; Dong, X.; Jiang, N.; Cong, B.; Liu, J.; Zhang, W.; Tang, D.; de Perrot, M.; et al. Curcumin induces apoptosis and inhibits angiogenesis in murine malignant mesothelioma. Int. J. Oncol. 2018, 53, 2531–2541. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Xu, Y.; Li, X.; Tie, L.; Pan, Y.; Li, X. Opposite angiogenic outcome of curcuminagainst ischemia and Lewis lung cancer models: In silico, in vitro and in vivo studies. Biochim. Biophys. Acta 2014, 1842, 1742–1754. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-H.; You, H.-Y.; Feng, Y.-J.; Zhang, Z.-T. LncRNA KCNQ1OT1 is a key factor in the reversal effect of curcumin on cisplatin resistance in the colorectal cancer cells. Mol. Cell. Biochem. 2020, 476, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Wang, J.J.; Wang, M.Y. Study on the mechanism of curcumin reversing paclitaxel resistance in ovarian cancer cells. J. Hunan Norm. Univ. 2021, 18, 109–113. [Google Scholar]
- Huang, Y.P.; Zhuang, I.F.; Cheng, J.; Li, Q. Curcumin inhibits Wnt signaling pathway and increases the sensitivity of gastric cancer cells to cisplatin. J. Partial. Surg. 2021, 30, 749–753. [Google Scholar]
- Karthika, C.; Sureshkumar, R.; Sajini, D.V.; Ashraf, G.M.; Rahman, M.H. 5-fluorouracil and curcumin with pectin coating as a treatment regimen for titanium dioxide with dimethylhydrazine-induced colon cancer model. Environ. Sci. Pollut. Res. Int. 2022. [Google Scholar] [CrossRef]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef] [Green Version]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397. [Google Scholar] [CrossRef] [Green Version]
- Vinod, B.S.; Antony, J.; Nair, H.H.; Puliyappadamba, V.T.; Saikia, M.; Narayanan, S.S.; Bevin, A.; Anto, R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013, 4, e505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, I.H.; Wang, L.; Lee, D.; Woo, J.; Kim, T.H.; Jeong, H.Y.; Oh, H.J.; Choi, K.S.; Kim, T.; Hur, H. Curcumin inhibits the cancer-associated fibroblast-derived chemoresistance of gastric cancer through the suppression of the JAK/STAT3 signaling pathway. Int. J. Oncol. 2022, 61, 85. [Google Scholar] [CrossRef] [PubMed]
- de Porras, V.R.; Bystrup, S.; Martínez-Cardús, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci. Rep. 2016, 6, 24675. [Google Scholar] [CrossRef] [Green Version]
- Su, P.; Yang, Y.; Wang, G.; Chen, X.; Ju, Y. Curcumin attenuates resistance to irinotecan via induction of apoptosis of cancer stem cells in chemoresistant colon cancer cells. Int. J. Oncol. 2018, 53, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhang, X.J.; Zhang, R.Z. Curcumin in vitro and in vivo reversal of 5- fluorouracil resistance in colon cancer and explore the mechanism. Chin. J. Mod. Appl. Pharm. 2020, 37, 1793–1800. [Google Scholar]
- Zhang, N.; Gao, M.; Wang, Z.; Zhang, J.; Cui, W.; Li, J.; Zhu, X.; Zhang, H.; Yang, D.-H.; Xu, X. Curcumin reverses doxorubicin resistance in colon cancer cells at the metabolic level. J. Pharm. Biomed. Anal. 2021, 201, 114129. [Google Scholar] [CrossRef]
- Kim, J.H.; Gupta, S.C.; Park, B.; Yadav, V.R.; Aggarwal, B.B. Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-κB and NF-κB-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis. Mol. Nutr. Food Res. 2012, 56, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.D. Curcumin Enhances the Sensitivity of Liver Cancer Cells to Sorafenib by Inhibiting PI3K/AKT Pathway. Master’s Thesis, Henan University, Zhengzhou, China, 2020. [Google Scholar]
- Liu, W.H.; Yuan, J.B.; Yang, L. Study on the mechanism of curcumin reversing the drug resistance of hesperidin in gastric cancer cells. Pharm. J. 2018, 53, 1817–1824. [Google Scholar]
- Liu, Q. Study on the Mechanism of Curcumin Combined with 5-FU Inducing Apoptosis of Gastric Cancer SGC-7901 Cells Based on Fas Signaling Pathway. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2020. [Google Scholar]
- Ren, H.Y.; Sun, J.D.; Li, D.; Niu, S.R.; Liu, H.; Luo, Q.; Zhang, L.X. Study of curcumin reversing multi-drug resistance of Eca-109/VCR cells in esophageal cancer through Wnt2/β-catenin pathway. Chin. Pharmacol. Bulletin. 2018, 34, 1455–1460. [Google Scholar]
- Peng, M.Y.; Qiu, F.; Huang, D.; Qin, X.; Zhang, D. Study on the reversal effect and mechanism of curcumin on nejicitabine of pancreatic cancer SW1990 cells. China Pharm. 2019, 30, 1192–1197. [Google Scholar]
- Wang, Y.S. Curcumin on the MCF-7 Cell Resistance Regulation and Action Mechanism Research. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2015. [Google Scholar]
- Wu, B. Effects of Curcumin on the Proliferation of Adriamycin-Resistant Thyroid Undifferentiated Cancer Cell Line HTh74Rdox and Its Mechanism. Master’s Thesis, Nanjing University of Traditional Chinese Medicine, Nanjing, China, 2018. [Google Scholar]
- Ren, J. Experimental Study of Curcumin Improving the Sensitivity of Paclitaxel to A549 Cells and Reducing Liver Injury. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2019. [Google Scholar]
- Huang, W.P.; Wang, H.Q.; Guo, T.T.; Hu, C.X. Research progress of radiation sensitization of curcumin on malignant tumor. Chin. J. Tradit. Chin. Med. 2017, 35, 2855–2857. [Google Scholar]
- Zhang, S.Q.; Cui, M.; Xiao, H.W.; Fan, S.J.L. Effect of curcumin on radiosensitivity of non-small cell lung cancer cells A549 and H460. Int. J. Radiat. Med. Nucl. Med. 2020, 44, 164–173. [Google Scholar]
- Ospina-Romero, M.; Abdiwahab, E.; Kobayashi, L.; Filshtein, T.; Brenowitz, W.D.; Mayeda, E.R.; Glymour, M.M. Rate of Memory Change Before and After Cancer Diagnosis. JAMA Netw. Open. 2019, 2, e196160. [Google Scholar] [CrossRef]
- Ospina-Romero, M.; Glymour, M.M.; Hayes-Larson, E.; Mayeda, E.M.; Graff, R.E.; Brenowitz, W.D.; Ackley, S.F.; Witte, J.; Kobayashi, L.C. Association Between Alzheimer Disease and Cancer With Evaluation of Study Biases: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020, 3, e2025515. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer disease inverse relationship: An age-associated diverging derailment of shared pathways. Mol. Psychiatry 2021, 26, 280–295. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Koo, B.-B.; Calderazzo, S.; Bowley, B.G.; Kolli, A.; Moss, M.B.; Rosene, D.L.; Moore, T.L. Long-term effects of curcumin in the non-human primate brain. Brain Res. Bull. 2018, 142, 88–95. [Google Scholar] [CrossRef]
- Cordone, S.; Annarumma, L.; Rossini, P.M.; De Gennaro, L. Sleep and β-Amyloid Deposition in Alzheimer Disease: Insights on Mechanisms and Possible Innovative Treatments. Front. Pharmacol. 2019, 10, 695. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, H.; Si, L.; Li, Y. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s Disease. Pharmacol. Rep. 2011, 63, 1101–1108. [Google Scholar]
- den Haan, J.; Morrema, T.H.J.; Rozemuller, A.J.; Bouwman, F.H.; Hoozemans, J.J.M. Different curcumin forms selectively bind fibrillar amyloid beta in post mortem Alzheimer’s disease brains: Implications for in-vivo diagnostics. Acta Neuropathol. Commun. 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.M. Therapeutic effect of curcumin on Alzheimer’s disease mice. Chin. J. Geriatr. 2019, 39, 4577–4580. [Google Scholar]
- Huang, P. Study on the Mechanism of Curcumin Inhibiting the Production of β -Amyloid Protein. Ph.D. Thesis, Wuhan University, Wuhan, China, 2019. [Google Scholar]
- Zhang, X.; Li, Y. In vitro study of curcumin against AD by inhibiting the activity of GSK-3β. Chin. Pharmacol. Bull. 2009, 25, 1507–1512. [Google Scholar]
- Binder, L.I.; Frankfurter, A.; Rebhun, L.I. The distribution of tau in the mammalian central nervous system. J. Cell Biol. 1985, 101, 1371–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimers Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiong, K.; Gong, Y.; Yu, X. Research progress of antioxidant mechanism of curcumin based on signal transduction pathway. Chin. Herb. Med. 2016, 47, 2373–2380. [Google Scholar]
- Xia, X.; Xu, Z.Q.; Peng, X. Effects of curcumin on cognitive function, inflammatory response and synaptophysin expression in hippocampus of APP/PS1 double transgenic mice. China J. Clin. Neurosurg. 2018, 23, 609–612. [Google Scholar]
- Wei, P.; Li, R.S.; Wang, H.; Ren, Y.; Sun, H.Y.; Yang, J.D.; Wang, P.W. Effect of curcumin on the expression of synapse-related proteins in APP/PS1 double transgenic mice. Chin. J. Tradit. Chin. Med. 2012, 37, 1818–1821. [Google Scholar]
- Ying, Z.; Chen, J.; Shu, K.L.; Li, W.; Lu, W.W. The effect of curcumin on PC12 cells against oxidative damage induced by Nrf2 gene. Chin. J. Integr. Tradit. Chin. West. Med. 2022, 42, 61–65. [Google Scholar]
- Neha, A.; Mishra, P.C. Scavenging mechanism of curcumin toward the hydroxyl radical: A theoretical study of reactions producing ferulic acid and vanillin. J. Phys. Chem. A. 2011, 115, 14221–14232. [Google Scholar]
- Qin, S.; Huang, L.; Gong, J.; Huang, J.; Ren, H.; Hu, H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet–fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, A.Y.; Wu, X.J.; Shao, A.M.; Chen, G. Effects of curcumin on blood lipid, inflammatory factors and endothelial function in atherosclerotic rabbits. Sci. Technol. Tradit. Chin. Med. 2020, 27, 373–375. [Google Scholar]
- Zhang, T.; He, Q.; Liu, Y.; Chen, Z.; Hu, H. Efficacy and Safety of Curcumin Supplement on Improvement of Insulin Resistance in People with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid.-Based Complement. Altern. Med. 2021, 2021, 4471944. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Cheng, J.L.; Gao, Z.G.; Cui, X.; Li, X.C.; Chang, B.C. Curcumin promotes skeletal muscle GLUT4 translocation and improves insulin resistance in diabetic rats. Chin. J. Endocrinol. Metab. 2021, 37, 143–148. [Google Scholar]
- Du, G.H.; Liu, B.W.; Yin, F.Z.; Qi, X.M.; Fan, D.M. Effect of liraglutide on the expression and mechanism of liver glycosuria key enzymes PEPCK and G6pase in diabetic mice. Chin. J. Comp. Med. 2020, 30, 98–102. [Google Scholar]
- Qian, W.L.; Gao, X.B.; Yu, Z.W.; Nan, L.H. Study on the improvement effect and mechanism of curcumin central administration on insulin resistance mice. Fujian Tradit. Chin. Med. 2019, 50, 32–34. [Google Scholar]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Boarescu, P.M.; Boarescu, I.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Pop, R.M.; Râjnoveanu, R.-M.; Bolboacă, S.D. Antioxidant and Anti-Inflammatory Effects of Curcumin Nanoparticles on Drug-Induced Acute Myocardial Infarction in Diabetic Rats. Antioxidants 2019, 8, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol. Nutr. Food Res. 2012, 57, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. Biomed Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef] [PubMed]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Maheswaraiah, A.; Rao, L.J.; Naidu, K.A. Anti-platelet activity of water dispersible curcuminoids in rat platelets. Phytother. Res. 2015, 29, 450–458. [Google Scholar] [CrossRef]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Wu, C.H. Inhibition of SARS-CoV-2 Infection by Extracellular Vesicles and the Effect of Curcumin on Extracellular Vesicles. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2021. [Google Scholar]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Liu Ni Meng, Y.R.; Zhang, J.L.; Zhu, Y.T.; Huang, Z.C. Experimental study of curcumin against influenza viruses H1N1 and H3N2 in vitro. Zhejiang J. Integr. Tradit. Chin. West. Med. 2008, 9, 534–535. [Google Scholar]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef] [Green Version]
- Si, X.; Wang, Y.; Wong, J.; Zhang, J.; McManus, B.M.; Luo, H. Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J. Virol. 2007, 81, 3142–3150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Zhang, Y.; Ke, C.; Chen, H.; Ren, P.; He, Y.; Hu, P.; Ma, D.; Luo, J.; Meng, Z. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J. Gastroenterol. 2017, 23, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Lee, M.Y.; Chuang, J.J.; Li, Y.Z.; Ning, S.T.; Chen, J.C.; Liu, Y.W. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int. J. Mol. Med. 2012, 30, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Kumar, R.; Tyagi, A.; Kohaar, I.; Hedau, S.; Bharti, A.C.; Sarker, S.; Dey, D.; Saluja, D.; Das, B. Curcumin modulates cellular AP-1, NF-kB, and HPV16 E6 proteins in oral cancer. Ecancermedicalscience 2015, 9, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šudomová, M.; Hassan, S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; An, Z.; Chen, H.; Wang, Z.; Liu, L. Mechanism of curcumin resistance to human cytomegalovirus in HELF cells. BMC Complement Altern. Med. 2014, 14, 284. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin is an APE1 redox inhibitor and exhibits an antiviral activity against KSHV replication and pathogenesis. Antiviral. Res. 2019, 167, 98–103. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chen, D.-Y.; Wen, H.-W.; Ou, J.-L.; Chiou, S.-S.; Chen, J.-M.; Wong, M.-L.; Hsu, W.-L. Inhibition of enveloped viruses infectivity by curcumin. PLoS ONE 2013, 8, e62482. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; Wang, C.L.; Wang, Y.X.; Qi, X.Q. Research progress of curcumin pharmacokinetics and its anti-breast cancer effect. New Drugs Tradit. Chin. Med. Clin. Pharmacol. 2021, 32, 744–750. [Google Scholar]
- Fang, K.; Gao, W. The combination of lycopene and curcumin on the acute ethanol oxidative damage in mice antioxidant effects. Cap. Med. Univ. 2021, 42, 89–93. [Google Scholar]
- Chang, M.X.; Wu, M.M.; Li, H.M. The inhibitory effect of curcumin combined with glycyrrhetinic acid on the proliferation of hepatoma HepG-2 cells. Drug Eval. Study 2017, 40, 42–47. [Google Scholar]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, L.Q.; Niu, J.; Liu, W.; Peng, S.F.; Liu, C.M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef]
- Saengkrit, N.; Saesoo, S.; Srinuanchai, W.; Phunpee, S.; Ruktanonchai, U.R. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf. B Biointerfaces 2014, 114, 349–356. [Google Scholar] [CrossRef]
- Ye Xl Wang, C.Y.; Ting, L.; Xiong, X.F.; Wu, M.J. Preparation of borneol-modified curcumin cationic liposomes and its brain targeting. Chin. J. Mod. Appl. Pharm. 2021, 38, 1469–1473. [Google Scholar]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and evaluation of alginate-chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Huang, J.; Dai, L.; Du, J.; McClements, D.J.; Mao, L.; Liu, J.; Gao, Y.; Gao, Y. Fabrication and Characterization of Layer-by-Layer Composite Nanoparticles Based on Zein and Hyaluronic Acid for Codelivery of Curcumin and Quercetagetin. ACS Appl. Mater. Interfaces. 2019, 11, 16922–16933. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Bao, H.; Liang, J.; Xu, S.; Cheng, G.; Zhu, Y. Fabrication and Characterization of Silk Fibroin/Curcumin Sustained-Release Film. Materials 2019, 12, 3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Q.; Cai, X.; Zhang, F.; Wang, S. Fabrication of self-assembled Radix Pseudostellariae protein nanoparticles and the entrapment of curcumin. Food Chem. 2019, 274, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Peng Hl Gan, Z.D.; Xiong, H. Self-Assembly of Protein Nanoparticles from Rice Bran Waste and Their Use as Delivery System for Curcumin. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar]
- Arima, H.; Hayashi, Y.; Higashi, T.; Motoyama, K. Recent advances in cyclodextrin delivery techniques. Expert. Opin. Drug Deliv. 2015, 12, 1425–1441. [Google Scholar] [CrossRef]
- Wan, S.L.; Zhong, M.; Yang, M.; Hu, X.Y.; Zhang, J.Q. Pharmacokinetics and Intestinal Absorption of Curcumin Chitosan Hydrochloride Coated Liposome in Rats. Zhong Yao Cai 2021, 12, 63–66. [Google Scholar]
- Han, D.X. Study on the Mechanism of Cyclodextrin Solubilization of Small Molecular Compounds. Master’s Thesis, Shenyang Normal University, Shenyang, China, 2020. [Google Scholar]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Asghar, S.; Hu, Z.; Qiu, Y.; Zhang, J.; Shao, F.; Xiao, Y. Understanding the cellular uptake and biodistribution of a dual-targeting carrier based on redox-sensitive hyaluronic acid-ss-curcumin micelles for treating brain glioma. Int. J. Biol. Macromol. 2019, 136, 143–153. [Google Scholar] [CrossRef]
- Zhao, M.F. Synthesis of Curcumin-Selenium Complex and Its Effect on Liver Injury Induced by Cadmium in Rats. Master’s Thesis, North China University of Science and Technology, Tangshan, China, 2015. [Google Scholar]
- Zuo, X.M. Study on the Preparation of Novel Nano-Drug Combination and Its Preparations. Master’s Thesis, Qingdao University of Science and Technology, Qingdao, China, 2019. [Google Scholar]
- Liang, Y.S. Construction and Evaluation of Ultrasound-Sensitive Tanshinone–Curcumin Eutectic Complex against Otitis Media. Master’s Thesis, Guangdong Pharmaceutical University, Guangzhou, China, 2020. [Google Scholar]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Gandapu, U.; Chaitanya, R.K.; Kishore, G.; Reddy, R.C.; Kondapi, A.K. Curcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication in vitro. PLoS ONE 2011, 6, e23388. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.J.; Li, Z.Y.; Chen, X.T. Pharmacokinetic study of curcumin precursor liposome in rats. Massage Rehabil. Med. 2021, 12, 63–66. [Google Scholar]
- Ren, B.Q.; Tian, X.F.; Chen, Y.; Xu, F. Effects of Curcumin and Curcuma longa extract on coagulation function in thrombus model rats. Int. J. Lab. Med. 2019, 40, 1025–1027+1031. [Google Scholar]
- Kukula-Koch, W.; Grabarska, A.; Łuszczki, J.; Czernicka, L.; Nowosadzka, E.; Gumbarewicz, E.; Jarząb, A.; Audo, G.; Upadhyay, S.; Głowniak, K.; et al. Superior anticancer activity is demonstrated by total extract of Curcuma longa L. as opposed to individual curcuminoids separated by centrifugal partition chromatography. Phytother Res. 2018, 32, 933–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical development plan: Curcumin. J. Cell Biochem. Suppl. 1996, 26, 72–85.
- Deodhar, S.D.; Sethi, R.; Srimal, R.C. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J. Med. Res. 1980, 71, 632–634. [Google Scholar]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Larson-Meyer, D.E.; Liebman, M. Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Baxmann, A.C.; De OG Mendonça, C.; Heilberg, I.P. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003, 63, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Wilkinson, J., IV; Pietsch, C.E.; Buss, J.L.; Wang, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J., IV; Di., X.; Wang, W.; Hatcher, H.; Kock, N.D.; Agostino, R.D., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Chin, D.; Huebbe, P.; Frank, J.; Rimbach, G.; Pallauf, K. Curcumin may impair iron status when fed to mice for six months. Redox. Biol. 2014, 2, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Imam, Z.; Khasawneh, M.; Jomaa, D.; Iftikhar, H.; Sayedahmad, Z. Drug Induced Liver Injury Attributed to a Curcumin Supplement. Case Rep. Gastrointest. Med. 2019, 2019, 6029403. [Google Scholar] [CrossRef] [Green Version]
- Lukefahr, A.L.; McEvoy, S.; Alfafara, C.; Funk, J.L. Drug-induced autoimmune hepatitis associated with turmeric dietary supplement use. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Nah, S.-S.; Byon, J.-S.; Ko, H.J.; Park, S.-H.; Lee, S.-J.; Shin, W.-Y.; Jin, D.-K. Transient complete atrioventricular block associated with curcumin intake. Int. J. Cardiol. 2011, 150, e50–e52. [Google Scholar] [CrossRef] [PubMed]
- Hussaarts, K.G.; Hurkmans, D.P.; Hoop, E.O.-D.; van Harten, L.J.; Berghuis, S.; van Alphen, R.J.; Spierings, L.E.; van Rossum-Schornagel, Q.C.; Vastbinder, M.B.; van Schaik, R.H.; et al. Impact of Curcumin (with or without Piperine) on the Pharmacokinetics of Tamoxifen. Cancers 2019, 11, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, Y.; Arora, A.; Taneja, P. Antimutagenic potential of curcumin on chromosomal aberrations in Wistar rats. Mutat Res. 2002, 515, 197–202. [Google Scholar] [CrossRef]
- Liddle, M.; Hull, C.; Liu, C.; Powell, D. Contact Urticaria from Curcumin. Dermatitis 2006, 17, 196–197. [Google Scholar] [CrossRef]
- Lopez-Villafuerte, L.; Clores, K.H. Contact dermatitis caused by turmeric in a massage oil. Contact Dermat. 2016, 75, 52–53. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S.; Murata, M. Evaluation for safety of antioxidant chemopreventive agents. Antioxid. Redox. Signal. 2005, 7, 1728–1739. [Google Scholar] [CrossRef]
- Dance-Barnes, S.T.; Kock, N.D.; Moore, J.E.; Lin, E.Y.; Mosley, L.J.; D’Agostino, R.B.; McCoy, T.P.; Townsend, A.J.; Miller, M.S. Lung tumor promotion by curcumin. Carcinogenesis 2009, 30, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Chen, J.; Jiang, L. Effect of curcumin combined with glibenclamide on glucose metabolism in type 2 diabetic rats and its mechanism. Chin. Pharm. 2014, 17, 912–915. [Google Scholar]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients With Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Rithaporn, T.; Monga, M.; Rajasekaran, M. Curcumin: A potential vaginal contraceptive. Contraception 2003, 68, 219–223. [Google Scholar] [CrossRef]
| Number | Status | ID | Phase | Study Start Date | Study Completion Date | Sponsor | Condition or Disease | Number of Participants | Intervention/Treatment | Research Endpoint |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Completed | NCT00192842 | 2 | Jul-04 | Sep-10 | Rambam Health Care Campus | Advanced Pancreatic Cancer | 17 | Curcumin 8 g po qd (+gemcitabine) | To assess the efficacy of the standard chemotherapy gemcitabine plus curcumin in patients with advanced pancreatic cancer. |
| 2 | Completed | NCT00094445 | 2 | Nov-04 | Apr-14 | M.D. Anderson Cancer Center | Advanced Pancreatic Cancer | 50 | Curcumin 8 g po qd for 22 weeks | To learn if treatment with curcumin can help slow the growth of pancreatic cancers and to access the safety. |
| 3 | Completed | NCT00113841 | - | Nov-04 | Aug-09 | M.D. Anderson Cancer Center | Patients With Multiple Myeloma | 42 | Curcumin 2 g po bid (+Bioperine 5 mg po bid) | To assess the Quality of life (QOL). |
| 4 | Completed | NCT03211104 | - | Aug-07 | Aug-15 | Samsung Medical Center | Patients With Prostate Cancer Undergoing Intermittent Androgen Deprivation Therapy | 107 | Curcumin 240 mg po tid for 6 month | To assess whether curcumin influences the duration of treatment interruption and rate of prostatic specific antigen (PSA) progression. |
| 5 | Recruiting | NCT00745134 | 2 | Aug-08 | Mar-23 | M.D. Anderson Cancer Center | Rectal Cancer | 45 | Curcumin po bid +(Radiation therapy and capecitabine) for 11.5 weeks | To asscess if curcumin can make tumor cells more sensitive to radiation therapy. |
| 6 | Completed | NCT01160302 | 1 | Jun-10 | Jan-16 | Louisiana State University Health Sciences Center Shreveport | Head and Neck Cancer | 33 | Microgranular Curcumin C3 Complex® 4 g po bid for 21–28 days | To assess the adverse effects. |
| 7 | Completed | NCT01333917 | 1 | Nov-10 | Jan-13 | University of North Carolina, Chapel Hill | People with positive colonoscopy screening | 40 | Curcumin C3 4 g qd for 30 days | To identify genes that are modified by curcumin that could be used as biomarkers in future chemoprevention studies, and also to evaluate the tolerability and toxicity. |
| 8 | Completed | NCT01917890 | - | Mar-11 | Oct-13 | Shahid Beheshti University of Medical Sciences | Prostate Cancer | 40 | Curcumin or placebo (500 mg po qd for 7–8 weeks) | To assess Progression free survival, Time to Disease Progression and Time to treatment failure. |
| 9 | Completed | NCT01490996 | 2 | Feb-12 | May-17 | University of Leicester | Inoperable Colorectal Cancer | 41 | Curcumin C3 2 g po qd (+FOLFOX) | To assess the safety, tolerability, efficacy(measured by response rate with RECIST and overall survival in months). |
| 10 | Completed | NCT01740323 | 2 | May-15 | Jul-18 | Emory University | Chemotherapy-Treated Breast Cancer Patients Undergoing Radiotherapy | 30 | Curcumin or placebo (500 mg po bid) | To assess if curcumin reduces NF-kB DNA binding and its downstream mediator IL-6. |
| 11 | Completed | NCT02439385 | 2 | Aug-15 | Aug-19 | Gachon University Gil Medical Center | Colorectal Cancer Patients With Unresectable Metastasis | 44 | Curcumin 100mg po bid (+Avastin/FOLFIRI) | To assess Progression free survival, Overall survival rate, Overall response rate, safety and fatigue score. |
| 12 | Completed | NCT02321293 | 1 | Aug-15 | Dec-16 | Lady Davis Institute | EGFR -Mutant Advanced NSCLC | 20 | Longvida® Optimized Curcumin 80 mg po qd (+Tyrosine Kinase Inhibitor) last for 8 weeks | To assess the safety and tolerability. |
| 13 | Recruiting | NCT02724202 | 1 | Mar-16 | - | Baylor Research Institute | Colon Cancer | 13 | Curcumin 500 mg po bid for 2 weeks. (Patients will continue on curcumin at same dose for an additional 6 weeks while being treated with 3 cycles of 5-Fu) | To test the safety, effects and find the Response Rate. |
| 14 | Completed | NCT03072992 | 2 | Mar-17 | Jun-19 | National Center of Oncology, Armenia | Advanced Breast Cancer | 150 | Paclitaxel +(curcumin or placebo) (300 mg i.v. once weekly for 12 weeks.) | To assess the adverse effects, Quality of life, Progression free survival, Time to Disease Progression, and Time to treatment failure. |
| 15 | Completed | NCT03534024 | - | Aug-18 | - | National Nutrition and Food Technology Institute | Metabolic Syndrome | 50 | Nanomicielle curcumin or placebo | To determine the effects of nanomicelle curcumin on glycemic control, serum lipid profile, blood pressure and anthropometric measurements |
| 16 | Recruiting | NCT03769766 | 3 | Mar-19 | - | University of Texas Southwestern Medical Center | Prostate Cancer | 291 | Curcumin or placebo (500 mg po bid) | To assess the efficacy. |
| 17 | Recruiting | NCT03980509 | 1 | Jan-20 | - | Medical University of South Carolina | Invasive Breast Cancer | 20 | Curcumin 500 mg po bid. (Curcumin will be given from the time surgical resection is scheduled until the night before surgical resection.) | To determine whether curcumin causes biological changes in primary tumors of breast cancer patients. |
| 18 | Not yet recruiting | NCT04294836 | 2 | Dec-21 | - | Instituto Nacional de Cancerologia, Columbia | Cervical Cancer | 240 | Cisplatin plus concomitant radiation therapy (teletherapy + high or low rate brachytherapy) + Curcugreen (BCM95) or placebo 2000 mg daily (each 6 h) | To assess the efficacy and safety. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules 2022, 27, 4400. https://doi.org/10.3390/molecules27144400
Liu S, Liu J, He L, Liu L, Cheng B, Zhou F, Cao D, He Y. A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules. 2022; 27(14):4400. https://doi.org/10.3390/molecules27144400
Chicago/Turabian StyleLiu, Siyu, Jie Liu, Lan He, Liu Liu, Bo Cheng, Fangliang Zhou, Deliang Cao, and Yingchun He. 2022. "A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health" Molecules 27, no. 14: 4400. https://doi.org/10.3390/molecules27144400
APA StyleLiu, S., Liu, J., He, L., Liu, L., Cheng, B., Zhou, F., Cao, D., & He, Y. (2022). A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health. Molecules, 27(14), 4400. https://doi.org/10.3390/molecules27144400





