High Expression Level of α2-3-Linked Sialic Acids on Salivary Glycoproteins of Breastfeeding Women May Help to Protect Them from Avian Influenza Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. StudyApprovaland Participants
2.2. Whole Saliva Sample Collection and Treatment
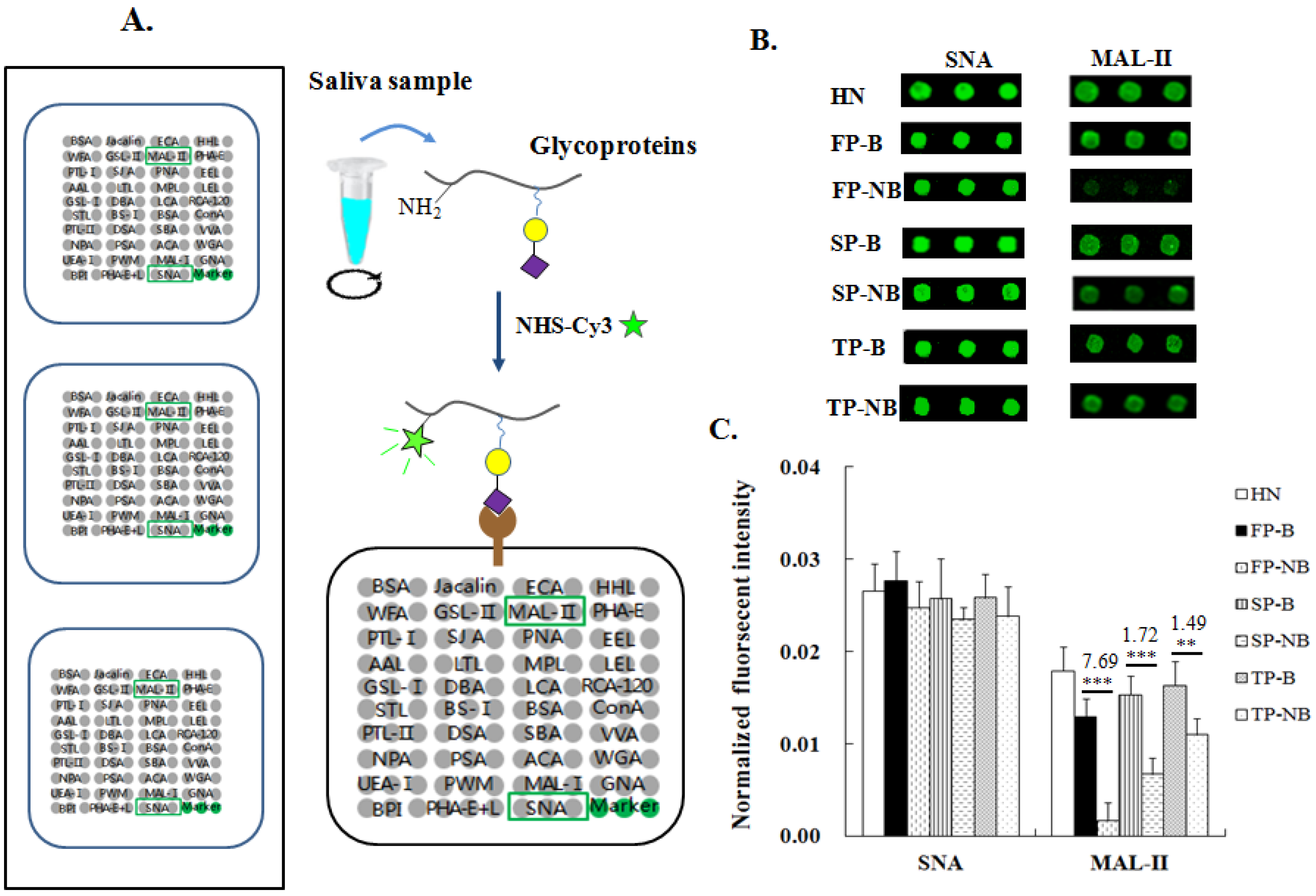
2.3. Lectin Microarrays and Data Analysis
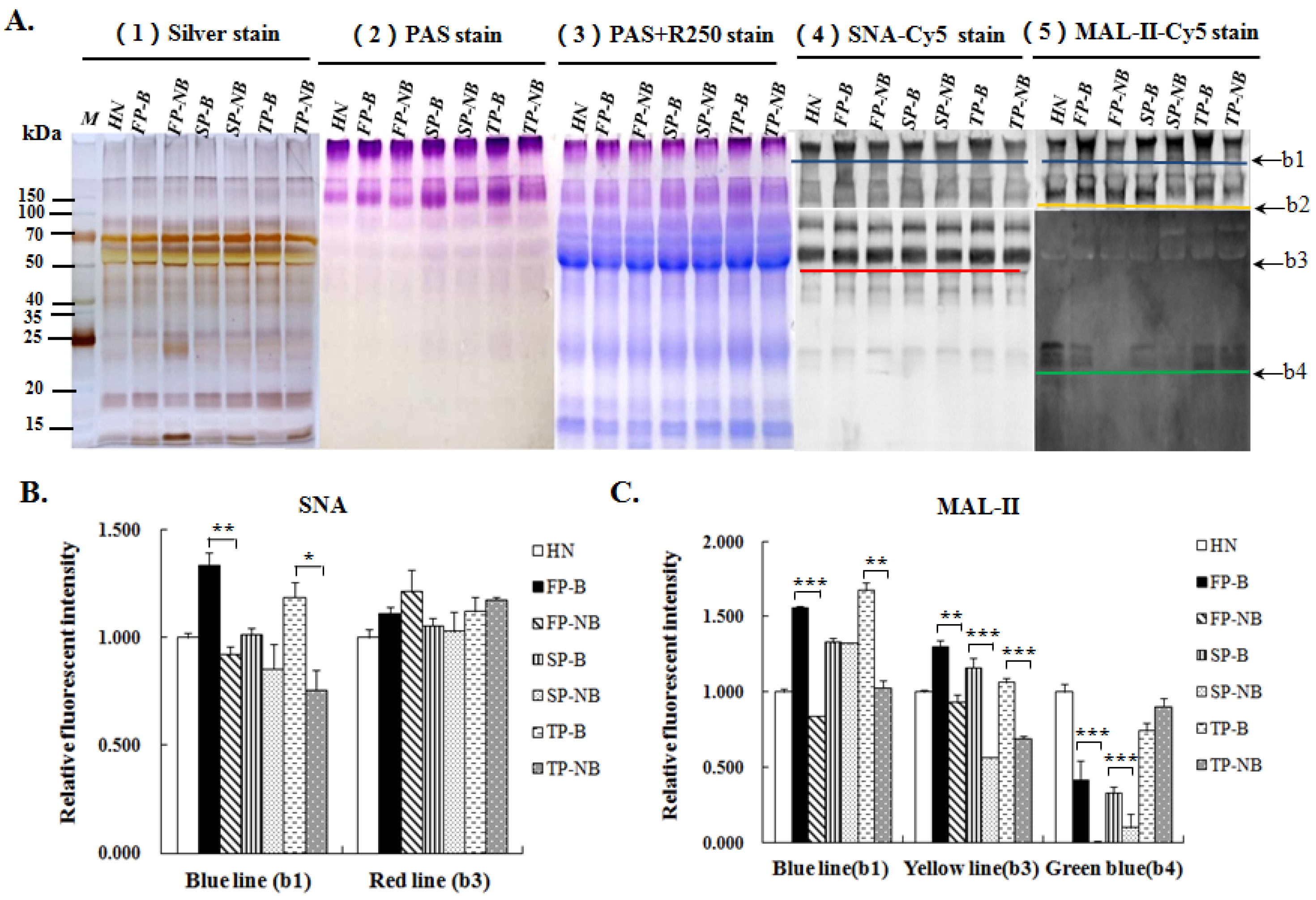
2.4. SDS-PAGE and Lectin Blotting Analysis
2.5. Assessment of Salivary Proteins Binding Ability to AIVs
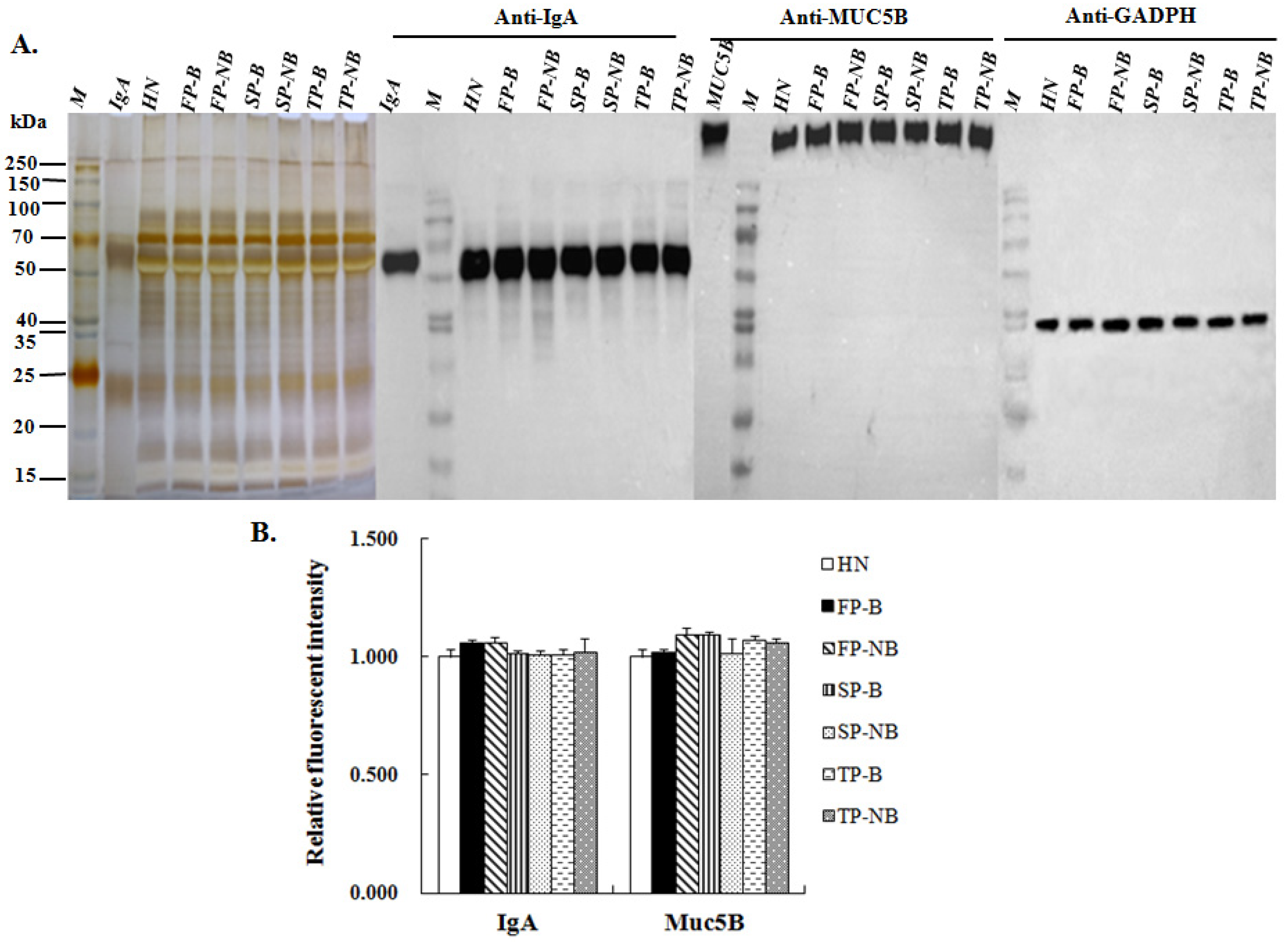
2.6. Western Blot Analysis
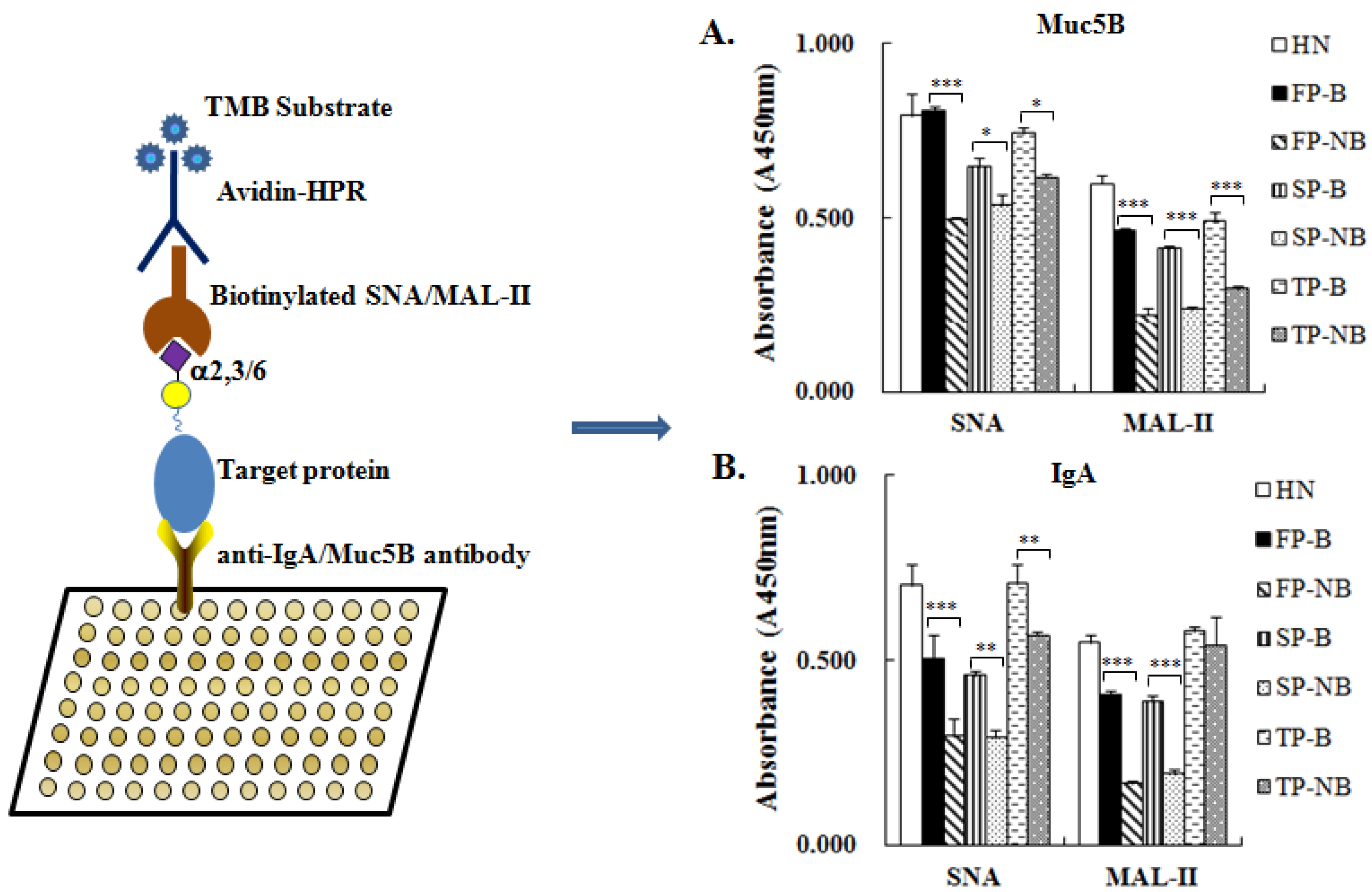
2.7. ELISA Based Lectin Binding Assay
2.8. Statistical Analysis
3. Results
3.1. Relative Expression Levels of Terminal α2-3/6-Linked Siain Saliva Groups of Postpartum Women with and without Breastfeeding
3.2. Analysis for the Salivary Proteins and the Variation in Terminal α2-3/6-Linked Sia Expression Level on Glycoproteins of Different Groups
3.3. Comparison of Salivary Proteins Binding Ability to AIVs between Postpartum Women with and without Breastfeeding
3.4. Expression Level of IgA and MUC5B in the Saliva of Postpartum Women with and without Breastfeeding
3.5. Expression Level of Terminal α2-3/6-Linked Sia Expressed on IgA and MUC5B in Different Saliva Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Li, Y.T.; Linster, M.; Mendenhall, I.H.; Su, Y.C.F.; Smith, G.J.D. Avian influenza viruses in humans: Lessons from past outbreaks. Br. Med. Bull. 2019, 132, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Huang, Q.S.; Wood, T.; Aminisani, N.; McArthur, C.; Baker, M.G.; Seeds, R.; Thompson, M.G.; Widdowson, M.A.; Newbern, E.C. Influenza-associated outcomes among pregnant, postpartum, and nonpregnant women of reproductive Age. J. Infect. Dis. 2019, 219, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S. Glycobiology of human milk. Biochemistry 2013, 78, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Lis-Kuberka, J.; Królak-Olejnik, B.; Berghausen-Mazur, M.; Orczyk-Pawiłowicz, M. Lectin-based method for deciphering human milk IgG sialylation. Molecules 2019, 24, 3797. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Fouani, M.; Basset, C.A.; Jurjus, A.R.; Leone, L.G.; Tomasello, G.; Leone, A. Salivary gland proteins alterations in the diabetic milieu. J. Mol. Histol. 2021, 52, 893–904. [Google Scholar] [CrossRef]
- Fang, L.; Liu, Q.; He, P.; Wang, X.; Wang, Y.; Wei, M.; Chen, L. Alteration of salivary glycopatterns in oral lichen planus. Biomarkers 2018, 23, 188–195. [Google Scholar] [CrossRef]
- Guruaribam, V.D.; Sarumathi, T. Relevance of serum and salivary sialic acid in oral cancer diagnostics. J. Cancer Res. Ther. 2020, 16, 401–404. [Google Scholar]
- Tada, A.; Senpuku, H. The impact of oral health on respiratory viral infection. Dent. J. 2021, 9, 43. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Veerman, E.C.I.; van’t Hof, W. Research on salivary proteins: From properties to functions. Ned. Tijdschr. Voor Tandheelkd. 2020, 127, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.; Olausson, P.; Hedenberg-Magnusson, B.; Ernberg, M.; Ghafouri, B. The proteomic profile of whole and glandular saliva in healthy pain-free subjects. Sci. Rep. 2016, 6, 39073. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Kamerling, J.P. Exploration of the sialic acid world. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–213. [Google Scholar] [PubMed]
- Pearce, O.M.; Läubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2016, 26, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Adams, O.J.; Stanczak, M.A.; von Gunten, S.; Läubli, H. Targeting sialic acid-siglec interactions to reverse immune suppression in cancer. Glycobiology 2018, 28, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Arena, A.; Fierabracci, A. Sialic acid-siglec axis in human immune regulation, involvement in autoimmunity and cancer and potential therapeutic treatments. Int. J. Mol. Sci. 2021, 22, 5774. [Google Scholar] [CrossRef]
- Sieben, C.; Sezgin, E.; Eggeling, C.; Manley, S. Influenza A viruses use multivalent sialic acid clusters for cell binding and receptor activation. PLoS Pathog. 2020, 16, e1008656. [Google Scholar] [CrossRef]
- Wen, F.; Wan, X.F. Influenza neuraminidase: Underrated role in receptor binding. Trends Microbiol. 2019, 27, 477–479. [Google Scholar] [CrossRef]
- Byrd-Leotis, L.; Cummings, R.D.; Steinhauer, D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int. J. Mol. Sci. 2017, 18, 1541. [Google Scholar] [CrossRef]
- Heida, R.; Bhide, Y.C.; Gasbarri, M.; Kocabiyik, Ö.; Stellacci, F.; Huckriede, A.L.W.; Hinrichs, W.L.J.; Frijlink, H.W. Advances in the development of entry inhibitors for sialic-acid-targeting viruses. Drug Discov. Today 2021, 26, 122–137. [Google Scholar] [CrossRef]
- Han, J.; Perez, J.; Schafer, A.; Cheng, H.; Peet, N.; Rong, L.; Manicassamy, B. Influenza virus: Small molecule therapeutics and mechanisms of antiviral resistance. Curr. Med. Chem. 2018, 25, 5115–5127. [Google Scholar] [CrossRef] [PubMed]
- Limsuwat, N.; Suptawiwat, O.; Boonarkart, C.; Puthavathana, P.; Wiriyarat, W.; Auewarakul, P. Sialic acid content in human saliva and anti-influenza activity against human and avian influenza viruses. Arch. Virol. 2016, 161, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Barnard, K.N.; Alford-Lawrence, B.K.; Buchholz, D.W.; Wasik, B.R.; LaClair, J.R.; Yu, H.; Honce, R.; Ruhl, S.; Pajic, P.; Daugherity, E.K.; et al. Modified sialic acids on mucus and erythrocytes inhibit influenza A virus hemagglutinin and neuraminidase functions. J. Virol. 2020, 94, e01567-19. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, B.; Edenborough, K.; McVernon, J.; Brown, L.E. Inhibition of influenza A virus by human infant saliva. Viruses 2019, 11, 766. [Google Scholar] [CrossRef]
- Tan, M.; Cui, L.; Huo, X.; Xia, M.; Shi, F.; Zeng, X.; Huang, P.; Zhong, W.; Li, W.; Xu, K.; et al. Saliva as a source of reagent to study human susceptibility to avian influenza H7N9 virus infection. Emerg. Microbes Infect. 2018, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhong, Y.; Zhu, M.; Dang, L.; Yu, H.; Chen, Z.; Chen, W.; Wang, X.; Zhang, H.; Li, Z. Age- and sex-associated differences in the glycopatterns of human salivary glycoproteins and their roles against influenza A virus. J. Proteome Res. 2013, 12, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Qin, Y.; Yu, H.; Yu, J.; Wu, H.; Chen, L.; Zhang, P.; Wang, X.; Jia, Z.; Guo, Y.; et al. Avian influenza virus infection risk in humans with chronic diseases. Sci. Rep. 2015, 5, 8971. [Google Scholar] [CrossRef]
- Ding, L.; Fu, X.; Guo, W.; Cheng, Y.; Chen, X.; Zhang, K.; Zhu, G.; Yang, F.; Yu, H.; Chen, Z.; et al. Pregnancy-associated decrease of Siaα2-3Gal-linked glycans on salivary glycoproteins affects their binding ability to avian influenza virus. Int. J. Biol. Macromol. 2021, 184, 339–348. [Google Scholar] [CrossRef]
- Maurer, M.A.; Meyer, L.; Bianchi, M.; Turner, H.L.; Le, N.P.L.; Steck, M.; Wyrzucki, A.; Orlowski, V.; Ward, A.B.; Crispin, M.; et al. Glycosylation of Human IgA Directly Inhibits Influenza A and Other Sialic-Acid-Binding Viruses. Cell Rep. 2018, 23, 90–99. [Google Scholar] [CrossRef]
- Littauer, E.Q.; Skountzou, I. Hormonal Regulation of Physiology, Innate immunity and antibody response to H1N1 influenza virus infection during pregnancy. Front. Immunol. 2018, 9, 2455. [Google Scholar] [CrossRef]
- Finch, C.L.; Zhang, A.; Kosikova, M.; Kawano, T.; Pasetti, M.F.; Ye, Z.; Ascher, J.R.; Xie, H. Pregnancy level of estradiol attenuated virus-specific humoral immune response in H5N1-infected female mice despite inducing anti-inflammatory protection. Emerg. Microbes Infect. 2019, 8, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Vieira Borba, V.; Shoenfeld, Y. Prolactin, autoimmunity, and motherhood: When should women avoid breastfeeding? Clin. Rheumatol. 2019, 38, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Peeva, E.; Zouali, M. Spotlight on the role of hormonal factors in the emergence of autoreactive B-lymphocytes. Immunol. Lett. 2005, 101, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikova, A.; Garcia, S.C.; Hovey, R.C. A Comparative review of the extrinsic and intrinsic factors regulating lactose synthesis. J. Mammary Gland. Biol. Neoplasia 2021, 26, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Kurabuchi, S.; Matsuoka, T.; Hosoi, K. Hormone-induced granular convoluted tubule-like cells in mouse parotid gland. J. Med. Investig. 2009, 56, 290–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramos-Roman, M.A.; Syed-Abdul, M.M.; Adams-Huet, B.; Casey, B.M.; Parks, E.J. Lactation versus formula feeding: Insulin, glucose, and fatty acid metabolism during the postpartum period. Diabetes 2020, 69, 1624–1635. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.; Low, W.Y. The Long-term public health benefits of breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef]
- Sattari, M.; Serwint, J.R.; Levine, D.M. Maternal implications of breastfeeding: A review for the internist. Am. J. Med. 2019, 132, 912–920. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Cheng, Y.; Guo, W.; Sun, S.; Chen, X.; Zhang, T.; Cheng, H.; Hao, J.; Lu, Y.; Wang, X.; et al. High Expression Level of α2-3-Linked Sialic Acids on Salivary Glycoproteins of Breastfeeding Women May Help to Protect Them from Avian Influenza Virus Infection. Molecules 2022, 27, 4285. https://doi.org/10.3390/molecules27134285
Ding L, Cheng Y, Guo W, Sun S, Chen X, Zhang T, Cheng H, Hao J, Lu Y, Wang X, et al. High Expression Level of α2-3-Linked Sialic Acids on Salivary Glycoproteins of Breastfeeding Women May Help to Protect Them from Avian Influenza Virus Infection. Molecules. 2022; 27(13):4285. https://doi.org/10.3390/molecules27134285
Chicago/Turabian StyleDing, Li, Yimin Cheng, Wei Guo, Siyue Sun, Xiangqin Chen, Tiantian Zhang, Hongwei Cheng, Jiayue Hao, Yunhua Lu, Xiurong Wang, and et al. 2022. "High Expression Level of α2-3-Linked Sialic Acids on Salivary Glycoproteins of Breastfeeding Women May Help to Protect Them from Avian Influenza Virus Infection" Molecules 27, no. 13: 4285. https://doi.org/10.3390/molecules27134285
APA StyleDing, L., Cheng, Y., Guo, W., Sun, S., Chen, X., Zhang, T., Cheng, H., Hao, J., Lu, Y., Wang, X., & Li, Z. (2022). High Expression Level of α2-3-Linked Sialic Acids on Salivary Glycoproteins of Breastfeeding Women May Help to Protect Them from Avian Influenza Virus Infection. Molecules, 27(13), 4285. https://doi.org/10.3390/molecules27134285





