Effective Isolation of Picrocrocin and Crocins from Saffron: From HPTLC to Working Standard Obtaining
Abstract
1. Introduction
2. Results
2.1. HPTLC Fingerprinting Profile
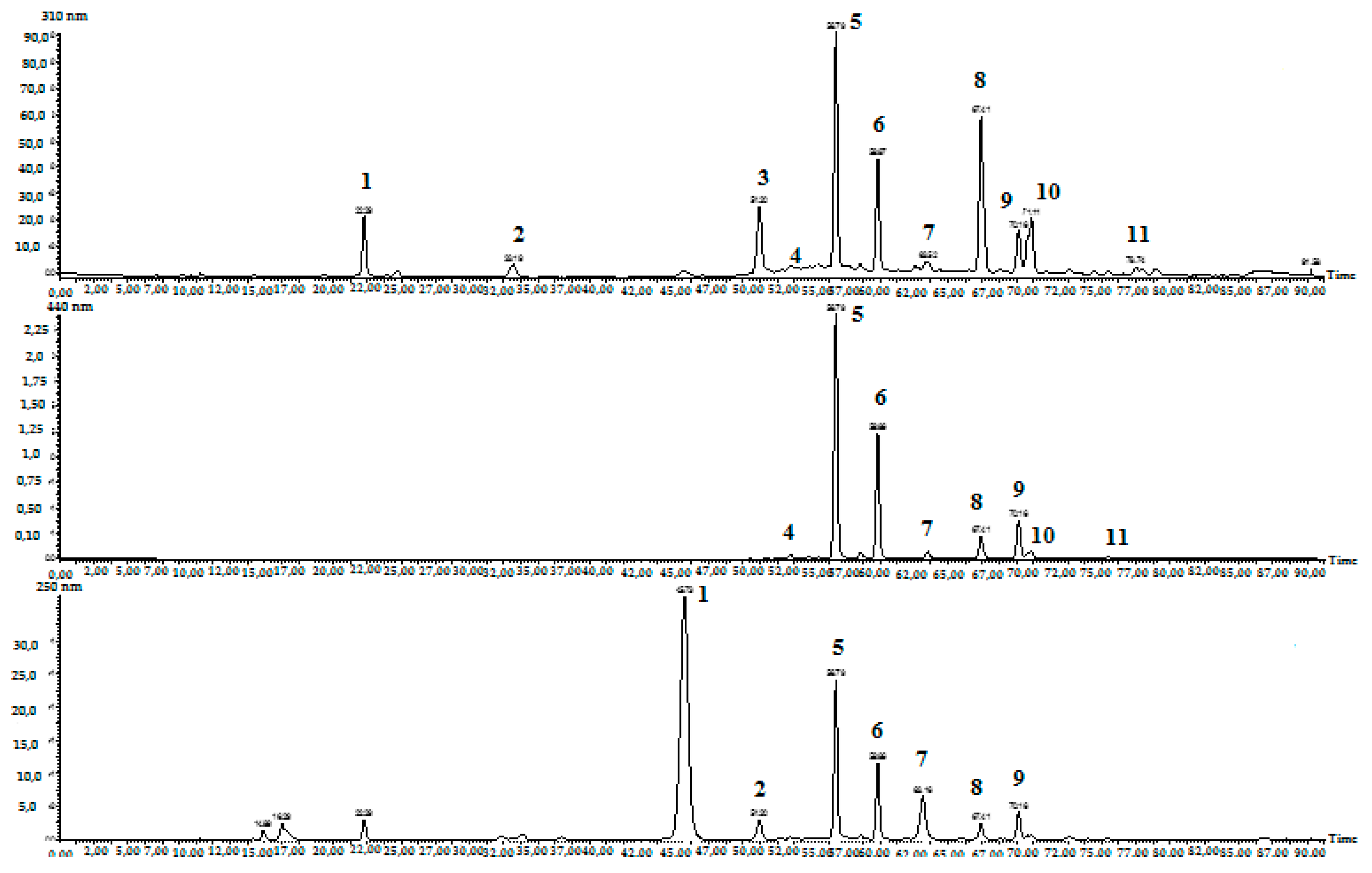
2.2. Preparative Isolation and Purification of Picrocrocin and Crocins
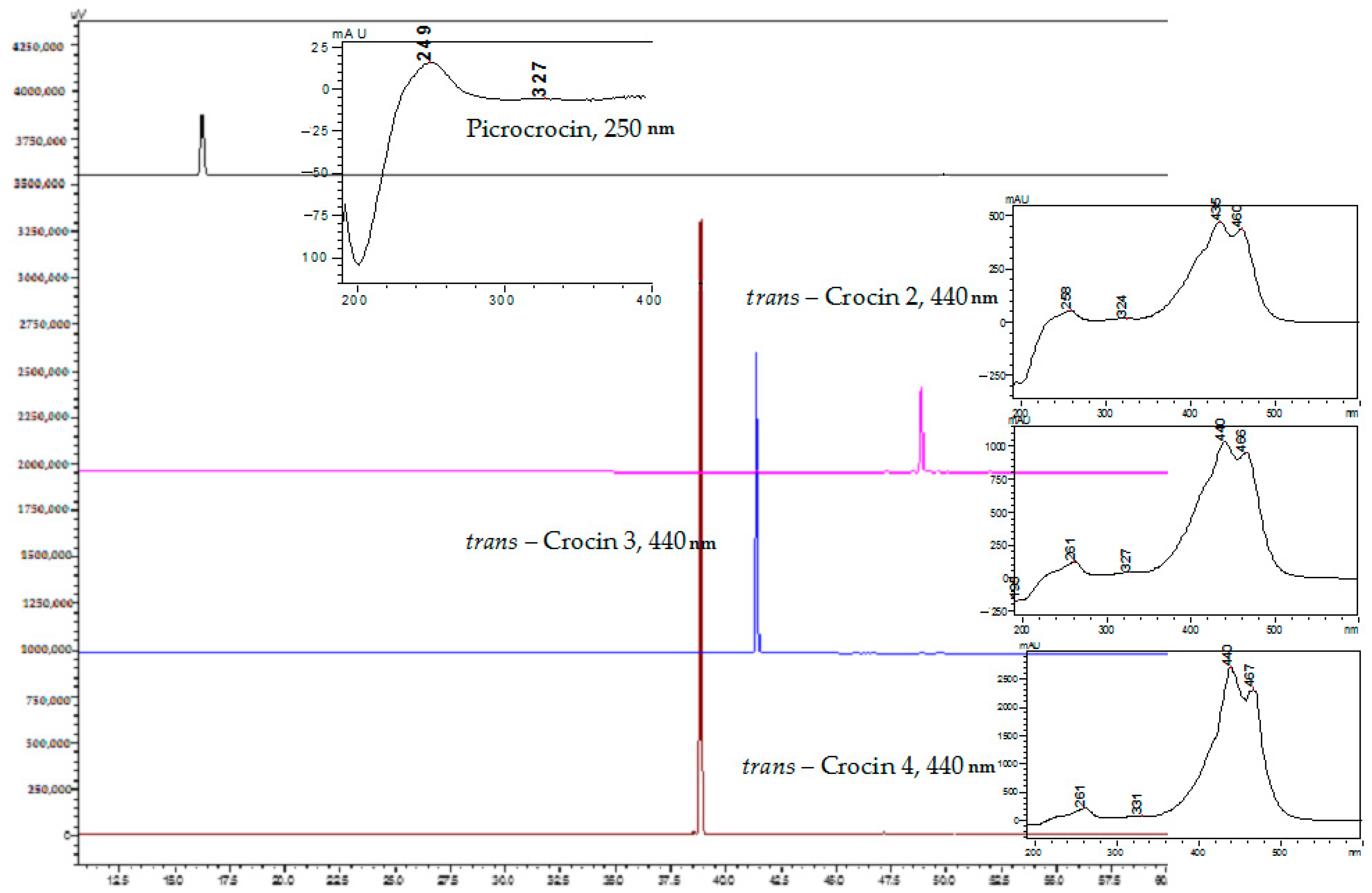
2.3. Structure Confirmation
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Sample Preparation
3.3. HPTLC Conditions
3.4. Compounds Isolation by HPLC
3.5. UPLC-ESI-MS/MS Conditions
3.6. HPTLC Method Validation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO Expert Committee on Specifications for Pharmaceutical Preparations: Fifty-Third Report (WHO Technical Report Series, No. 1019); World Health Organization: Geneva, Switzerland, 2019.
- World Health Organization. Division of drug management and policies. In WHO General Guideline for the Establishment, Maintenance, and Distribution of Chemical Reference Substances; WHO/PHARM/96.590; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Bindra, G.; Jadaun, G.P.S.; Dixit, S.; Saklani, V.; Abbas, Z.; Jain, P.; Dancheck, K.B.; Borer, M.W.; Kumari, M.; Kamal, C.M.; et al. Establishment of the first national reference standard for insulin Lispro: Report of a collaborative study. Biologicals 2019, 58, 1–6. [Google Scholar] [CrossRef]
- Schwarz, M.; Klier, B.; Sievers, H. Herbal reference standards. Planta Med. 2009, 75, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Göber, B.; Kny, L.; Langner, A. Arzneimittel kontrolle—der pharmazeutische Beitrag zur Gewährleistung der Arzneimittelsicherheit. Ein Beitrag zur historischen Entwicklung der Fachgruppe Arzneimittelkontrolle/Pharmazeutische Analytik der Deutschen Pharmazeutischen Gesellschaft–Teil 1 [Quality control of drugs—The contribution of pharmacy to drug safety standards. The historical development of the working group on drug quality control--pharmaceutical analysis of the German Pharmaceutical Society. 1]. Pharmazie in unserer Zeit 2000, 29, 174–182. (In German) [Google Scholar]
- Mohtashami, L.; Sadegh Amiri, M.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. The genus Crocus L.: A review of ethnobotanical uses, phytochemistry and pharmacology. Ind. Crops Prod. 2021, 171, 113923–113948. [Google Scholar] [CrossRef]
- Jafari, S.M.; Tsimidou, M.Z.; Rajabi, H.; Kyriakoudi, A. Bioactive ingredients of saffron: Extraction, analysis, applications. In Saffron; Woodhead Publishing: Sawston, UK, 2020; Volume 16, pp. 261–290. [Google Scholar]
- Mykhailenko, O.; Desenko, V.; Ivanauskas, L.; Georgiyants, V. Standard operating procedure of Ukrainian saffron cultivation according with Good Agricultural and Collection Practices to assure quality and traceability. Ind. Crops Prod. 2020, 151, 112376–112387. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.M.; Tsatsakis, A. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. Phytomed. 2015, 5, 376–391. [Google Scholar] [PubMed]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Koulakiotis, N.S.; Pittenauer, E.; Halabalaki, M.; Tsarbopoulos, A.; Allmaier, G. Comparison of different tandem mass spectrometric techniques (ESI-IT, ESI- and IP-MALDI-QRTOF and vMALDI-TOF/RTOF) for the analysis of crocins and picrocrocin from the stigmas of Crocus sativus L. Rapid Commun. Mass Spectrom. 2012, 26, 670–678. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Bezruk, I.; Ivanauskas, L.; Georgiyants, V. Comparative analysis of apocarotenoids and phenolic constituents of Crocus sativus stigmas from eleven countries: Ecological impact. Arch. Pharm. 2022, 355, e2100468–e2100484. [Google Scholar] [CrossRef]
- Del Campo, C.P.; Garde-Cerdán, T.; Sánchez, A.M.; Maggi, L.; Carmona, M.; Alonso, G.L. Determination of free amino acids and ammonium ion in saffron (Crocus sativus L.) from different geographical origins. Food Chem. 2009, 114, 1542–1548. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Ivanauskas, L.; Bezruk, I.; Lesyk, R.; Georgiyants, V. Comparative investigation of amino acids content in the dry extracts of Juno bucharica, Gladiolus hybrid zefir, Iris hungarica, Iris variegata and Crocus sativus raw materials of Ukrainian flora. Sci. Pharm. 2020, 88, 8. [Google Scholar] [CrossRef]
- Jarukas, L.; Mykhailenko, O.; Baranauskaite, J.; Marksa, M.; Ivanauskas, L. Investigation of organic acids in saffron stigmas (Crocus sativus L.) extract by derivatization method and determination by GC/MS. Molecules 2020, 25, 3427. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Mancini, A.; D’Alessandro, A.M.; Festuccia, C. Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anti-Cancer Agents Med. Chem. 2019, 19, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.L.; Salinas, M.R.; Garijo, J.; Sánchez-Fernández, M.A. Composition of crocins and picrocrocin from Spanish saffron (Crocus sativus L.). J. Food Qual. 2001, 24, 219–233. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z. Latest advances in the extraction and determination of saffron apocarotenoids. Electrophoresis 2018, 39, 1846–1859. [Google Scholar] [CrossRef]
- Wang, W.; He, P.; Zhao, D.; Ye, L.; Dai, L.; Zhang, X.; Sun, Y.; Zheng, J.; Bi, C. Construction of Escherichia coli cell factories for crocin biosynthesis. Microb. Cell Factories 2019, 18, 120–131. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z.; O’Callaghan, Y.C.; Galvin, K.; O’Brien, N.M. Changes in total and individual crocetin esters upon in vitro gastrointestinal digestion of saffron aqueous extracts. J. Agric. Food Chem. 2013, 61, 5318–5327. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 9th ed.; European Department for the Quality of Medicines: Strasbourg, France, 2014; Volume 1, p. 1694.
- British Pharmacopoeia, “Saffron for Homoeopathic Preparations”; Materials for use in the Manufacture of Homoeopathic Preparations; Crown: London, UK, 2009; Volume III, p. 7603.
- German Homoeopathic Pharmacopoeia, “Crocus. Safran”; Deutscher Apotheker Verlag: Gerlingen, Germany; CRC Press: Boca Raton, FL, USA, 2003; Volume 1, p. 547.
- Deutscher Arzneimittel-Codex/Neues Rezeptur-Formularium (DAC/NRF). In Ergänzungsbuch zum Arzneibuch, Bundesvereinigung Deutscher Apothekerverbände (ABDA); Govi-Verlag: Eschborn, Germany, 2016; 6000p.
- Taiwan Herbal Pharmacopeia, 2nd ed; “Croci stigma”; English Version; Ministry of Health and Welfare: Taiwan, China, 2016; 315p.
- Japanese Pharmacopoeia (JP17), 17th ed.; “Crude Drugs Saffron”; English Version; The Ministry of Health, Labour and Welfare: Tokyo, Japan, 2005; p. 173.
- Chinese Pharmacopoeia; “Stigma Croci. Xihonghua”; Chemical Industry Press: Beijing, China, 2005; pp. 310–311.
- Ayurvedic Pharmacopoeia of India; “Kumkuma (Style and stigma)”; Create Space Independent Publishing Platform: Scotts Valley, CA, USA, 2015; Volume IV, p. 59.
- Nassiri-Asl, M.; Hosseinzadeh, H. Chapter 3—Neuropharmacology effects of saffron (Crocus sativus) and its active constituents. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Academic Press: Cambridge, MA, USA, 2015; pp. 29–39. [Google Scholar]
- Fagot, D.; Pham, D.M.; Laboureau, J.; Planel, E.; Guerin, L.; Negre, C.; Donovan, M.; Bernard, B.A. Crocin, a natural molecule with potentially beneficial effects against skin aging. Int. J. Cosmet. Sci. 2018, 40, 388–400. [Google Scholar] [CrossRef]
- Joint FAO/WHO Food Standards Programme Codex Committee on Spices and Culinary Herbs. 3rd Session; CX/SCH 17/3/12. In Proceedings of the CODEX Alimentarius Commission, Chennai, India, 6–10 February 2017.
- Melnyk, N.; Pawłowska, K.A.; Ziaja, M.; Wojnowski, W.; Koshovyi, O.; Granica, S.; Bazylko, A. Characterization of herbal teas containing lime flowers—Tiliae flos by HPTLC method with chemometric analysis. Food Chem. 2021, 346, 128929–128938. [Google Scholar] [CrossRef]
- Dai, H.; Gao, Q.; He, L. Rapid determination of saffron grade and adulteration by thin-layer chromatography coupled with Raman Spectroscopy. Food Anal. Methods 2020, 13, 2128–2137. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2016; 295p. [Google Scholar]
- USP HMC. USP Herbal Medicines Compendium; USP: Rockville, MD, USA, 2015; Available online: http://hmc.usp.org/ (accessed on 10 July 2020).
- Cazes, J. (Ed.) Encyclopedia of Chromatography; CRC Press: Boca Raton, FL, USA, 2005; 22p. [Google Scholar]
- Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Springer: Berlin/Heidelberg, Germany, 1996; 384p. [Google Scholar]
- Mykhailenko, O.; Bezruk, I.; Ivanauskas, L.; Georgiyants, V. Comparative analysis of the major metabolites of Ukrainian saffron samples by HPLC. Plant Foods Hum. Nutr. 2021, 76, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Ataolahi, S.; Aliakbarzadeh, G.; Zarre, S.; Poursorkh, Z. Evaluation of storage time effect on saffron chemical profile using gas chromatography and spectrophotometry techniques coupled with chemometrics. J. Food Sci. Technol. 2018, 55, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Ganai, S.A.; Mansoor, S.; Jan, S.; Mani, P.; Masoodi, K.Z.; Amin, H.; Rehman, M.U.; Ahmad, P. Isolation, purification and characterization of naturally derived crocetin beta-d-glucosyl ester from Crocus sativus L. against breast cancer and its binding chemistry with ER-alpha/HDAC2. Saudi J. Biol. Sci. 2020, 27, 975–984. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Di Donato, F.; Foschi, M.; Maggi, M.A.; Ruggieri, F. UHPLC analysis of saffron (Crocus sativus L.): Optimization of separation using chemometrics and detection of minor crocetin esters. Molecules 2018, 23, 1851. [Google Scholar] [CrossRef]
- Osé Luis Iborra, J.; Rosario Castellar, M.; Anuel Cánovas, M.; Rturo Manjón, A. TLC preparative purification of picrocrocin, HTCC and crocin from saffron. J. Food Sci. 1992, 57, 714–716. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Polissiou, M.; Manfait, M. Separation of picrocrocin, cis-trans-crocins and safranal of saffron using high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. A 1994, 664, 55–61. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Carmona, M.; del Campo, C.P.; Alonso, G.L. Solid-phase extraction for picrocrocin determination in the quality control of saffron spice (Crocus sativus L.). Food Chem. 2009, 116, 792–798. [Google Scholar] [CrossRef]
- Lautenschläger, M.; Lechtenberg, M.; Sendker, J.; Hensel, A. Effective isolation protocol for secondary metabolites from saffron: Semi-preparative scale preparation of crocin-1 and trans-crocetin. Fitoterapia 2014, 92, 290–295. [Google Scholar] [CrossRef]
- Sedaghati, S.; Abbaszadeh, F. Extraction of saffron crocin as a natural pharmaceutical source with crystallization method. Int. J. Pharma Med. Biol. Sci. 2018, 7, 84–87. [Google Scholar] [CrossRef]
- Karkoula, E.; Angelis, A.; Koulakiotis, N.S.; Gikas, E.; Halabalaki, M.; Tsarbopoulos, A.; Skaltsounis, A.L. Rapid isolation and characterization of crocins, picrocrocin, and crocetin from saffron using centrifugal partition chromatography and LC-MS. J. Sep. Sci. 2018, 41, 4105–4114. [Google Scholar] [CrossRef]
- Corti, P.; Mazzei, E.; Ferri, S.; Franchi, G.G.; Dreassi, E. High Performance Thin Layer Chromatographic quantitative analysis of picrocrocin and crocetin, active principles of saffron (Crocus sativus L. Jridaceae): A new method. Phytochem. Anal. 1996, 7, 201–203. [Google Scholar] [CrossRef]
- Kabiri, M.; Rezadoost, H.; Ghassempour, A. A comparative quality study of saffron constituents through HPLC and HPTLC methods followed by isolation of crocins and picrocrocin. LWT Food Sci. Technol. 2017, 84, 441–448. [Google Scholar] [CrossRef]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Cheaib, D.; Darra, N.E.; Rajha, H.N.; El-Ghazzawi, I.; Maroun, R.G.; Louka, N. Effect of the extraction process on the biological activity of lyophilized apricot extracts recovered from apricot pomace. Antioxidants 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Straubinger, M.; Jezussek, M.; Waibel, R.; Winterhalter, P. Two kaempferol sophorosides from Crocus sativus. Nat. Prod. Lett. 1997, 10, 213–216. [Google Scholar] [CrossRef]
- Lech, K.; Witowska-Jarosz, J.; Jarosz, M. Saffron yellow: Characterization of carotenoids by high performance liquid chromatography with electrospraymass spectrometric detection. J. Mass Spectrom. 2009, 44, 1661–1667. [Google Scholar] [CrossRef]
- Bezruk, I.; Marksa, M.; Georgiyants, V.; Ivanauskas, L.; Raudone, L. Phytogeographical profiling of ivy leaf (Hedera helix L.). Ind. Crops Prod. 2020, 154, 112713. [Google Scholar] [CrossRef]
- Zvikas, V.; Pukeleviciene, V.; Ivanauskas, L.; Razukas, A.; Jakstas, V. Variety-based research on the phenolic content in the aerial parts of organically and conventionally grown buckwheat. Food Chem. 2016, 15, 660–667. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH). Validation of Analytical Procedures: Text and Methodology, Q2 (R1); ICH Secretariat: Geneva, Switzerland, 2005. [Google Scholar]
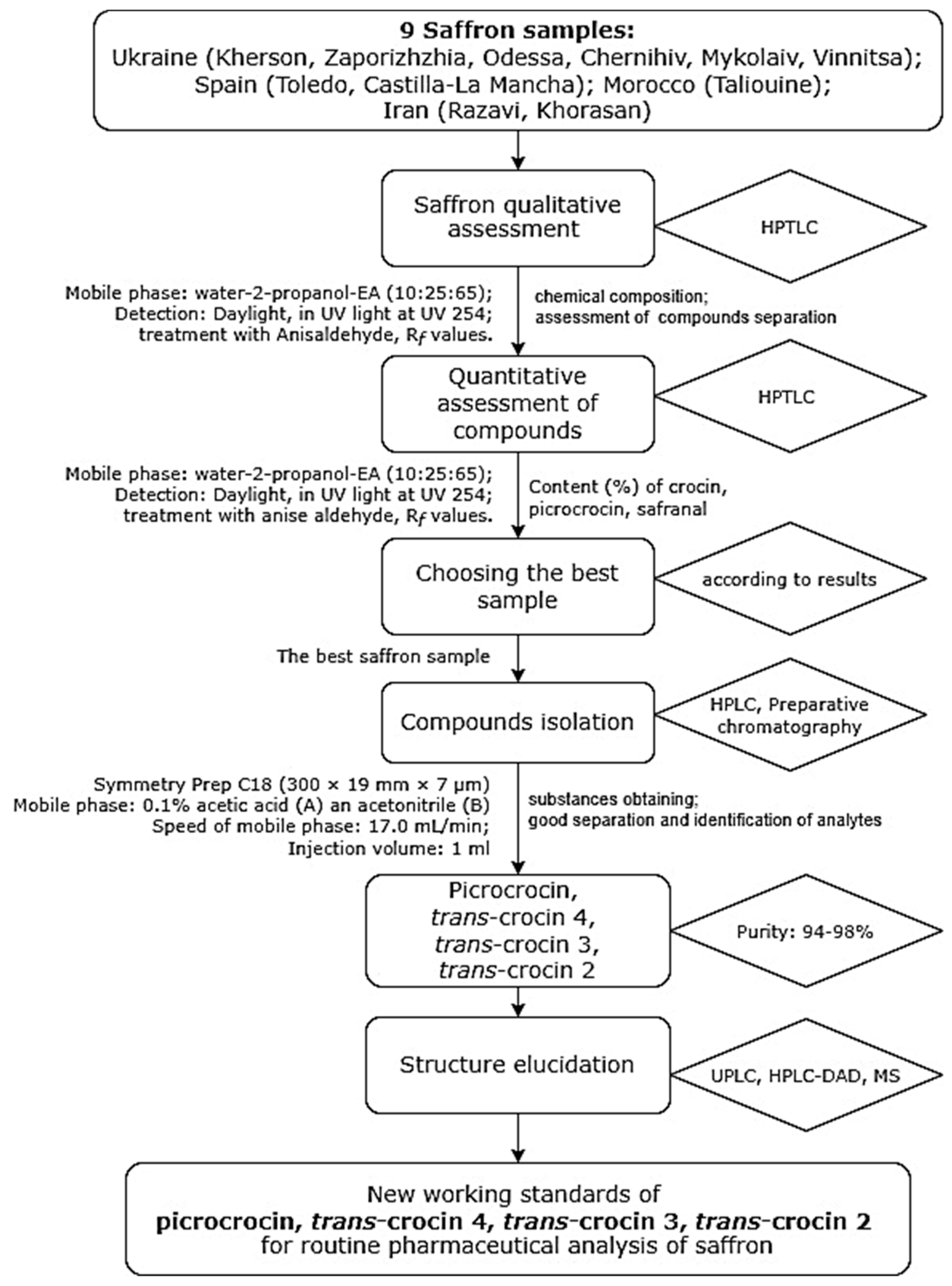


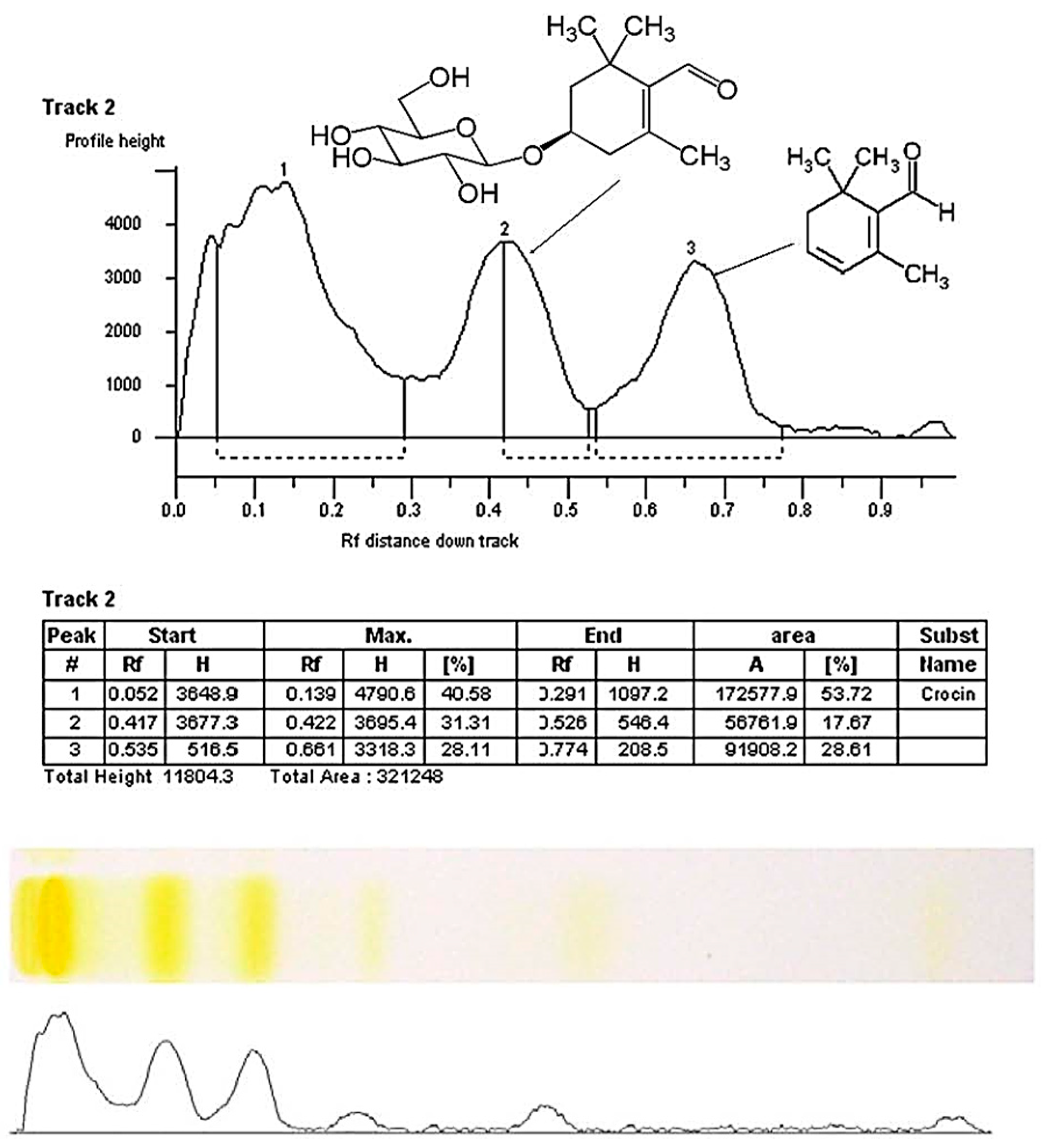
| Track * | Standart/Saffron Origin | Crocin | Picrocrocin | Safranal | |||
|---|---|---|---|---|---|---|---|
| Rf | Content, % | Rf | Content, % | Rf | Content, % | ||
| RSt | Crocin standard | 0.148 | - | - | - | - | - |
| S1 | Kherson, Ukraine | 0.139 | 29.12 ± 3.90 | 0.422 | 9.92 ± 2.50 | 0.661 | 15.51 ± 4.69 |
| S2 | Zaporizhzhia, Ukraine | 0.139 | 33.25 ± 4.39 | 0.413 | 24.20 ± 4.63 | 0.665 | 12.89 ± 2.39 |
| S3 | Odessa, Ukraine | 0.143 | 30.00 ± 3.99 | 0.426 | 21.67 ± 3.21 | 0.674 | 17.14 ± 2.17 |
| S4 | Chernihiv, Ukraine | 0.143 | 17.80 ± 2.42 | 0.426 | 21.02 ± 3.08 | 0.678 | 4.10 ± 0.97 |
| S5 | Mykolaiv, Ukraine | 0.139 | 27.37 ± 2.86 | 0.413 | 20.49 ± 2.56 | 0.674 | 4.13 ± 1.30 |
| S6 | Vinnitsa, Ukraine | 0.148 | 26.08 ± 2.65 | 0.422 | 19.58 ± 2.83 | 0.678 | 4.81 ± 1.49 |
| S7 | Toledo, Spain | 0.126 | 17.26 ± 3.37 | 0.430 | 13.49 ± 2.77 | 0.674 | 10.17 ± 2.08 |
| S8 | Taliouine, Morocco | 0.152 | 24.47 ± 3.54 | 0.430 | 19.92 ± 2.75 | 0.678 | 5.08 ± 1.53 |
| S9 | Razavi, Iran | 0.152 | 32.34 ± 4.61 | 0.430 | 26.55 ± 3.59 | 0.683 | 7.16 ± 1.69 |
| Compound | Calibration Curve a | Correlation Coefficient (r2) | Concentration Range, (μg/mL) | LOD b, (ng/mL) | LOQ c, (ng/mL) |
|---|---|---|---|---|---|
| Crocin | y = 13,662x + 3425.2 | 0.9986 | 5000–39.06 | 0.22 | 0.66 |
| Compound | Abbre-viation * | HPLC, Rtime, min | UV Max, nm | UPLC-MS, Rtime, min | [M − H]−, m/z | Fragment Ions, m/z | Adduct Ions (Formic Acid) [M − H + HAA]− | Molecular Mass, g/moL | Purity, % ** |
|---|---|---|---|---|---|---|---|---|---|
| Picrocrocin | - | 45.70 | 249 | 4.42 | N/O | N/O | 375.1 [M − H + HA]− 365.2 [M − H + HCl]− | 330 | 97.75 |
| Crocin 4 (trans-crocetin di( β-D-gentiobiosyl) ester) | t-4GG | 57.00 | 261, 331, 440, 467 | 5.64 | 975.2 | 651.1 [M – H − Gnt]− | 1021.0 [M − H + HA]− 978.3 [M − H + HCl]− 697.9 [M − H − Gnt + HA]− | 976 | 98.41 |
| Crocin 3 (trans-crocetin (β -D-glucosyl)-( β-D-gentiobiosyl) ester) | t-3Gg | 60.42 | 261, 327, 440, 466 | 6.35 | 813.1 | 651.3 [M − H − Glc]− 489.3 [M − H − Gnt]− | 927.7 [M − H + HA + NaA]− 859.2 [M − H + HA]− 849.5 [M − H + HCl]− | 814 | 97.85 |
| Crocin 2 (trans-crocetin(β -D-gentibiosyl) ester) | t-2G | 70.56 | 258, 324, 435, 460 | 7.29 | 651.4 | N/O | 697.0 [M − H + NaA]− 812.1 [M − H + HA]− | 652 | 94.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarukas, L.; Vitkevicius, K.; Mykhailenko, O.; Bezruk, I.; Georgiyants, V.; Ivanauskas, L. Effective Isolation of Picrocrocin and Crocins from Saffron: From HPTLC to Working Standard Obtaining. Molecules 2022, 27, 4286. https://doi.org/10.3390/molecules27134286
Jarukas L, Vitkevicius K, Mykhailenko O, Bezruk I, Georgiyants V, Ivanauskas L. Effective Isolation of Picrocrocin and Crocins from Saffron: From HPTLC to Working Standard Obtaining. Molecules. 2022; 27(13):4286. https://doi.org/10.3390/molecules27134286
Chicago/Turabian StyleJarukas, Laurynas, Konradas Vitkevicius, Olha Mykhailenko, Ivan Bezruk, Victoriya Georgiyants, and Liudas Ivanauskas. 2022. "Effective Isolation of Picrocrocin and Crocins from Saffron: From HPTLC to Working Standard Obtaining" Molecules 27, no. 13: 4286. https://doi.org/10.3390/molecules27134286
APA StyleJarukas, L., Vitkevicius, K., Mykhailenko, O., Bezruk, I., Georgiyants, V., & Ivanauskas, L. (2022). Effective Isolation of Picrocrocin and Crocins from Saffron: From HPTLC to Working Standard Obtaining. Molecules, 27(13), 4286. https://doi.org/10.3390/molecules27134286








