Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview
Abstract
:1. Introduction
2. ANs Chemistry, Biosynthesis and Stability
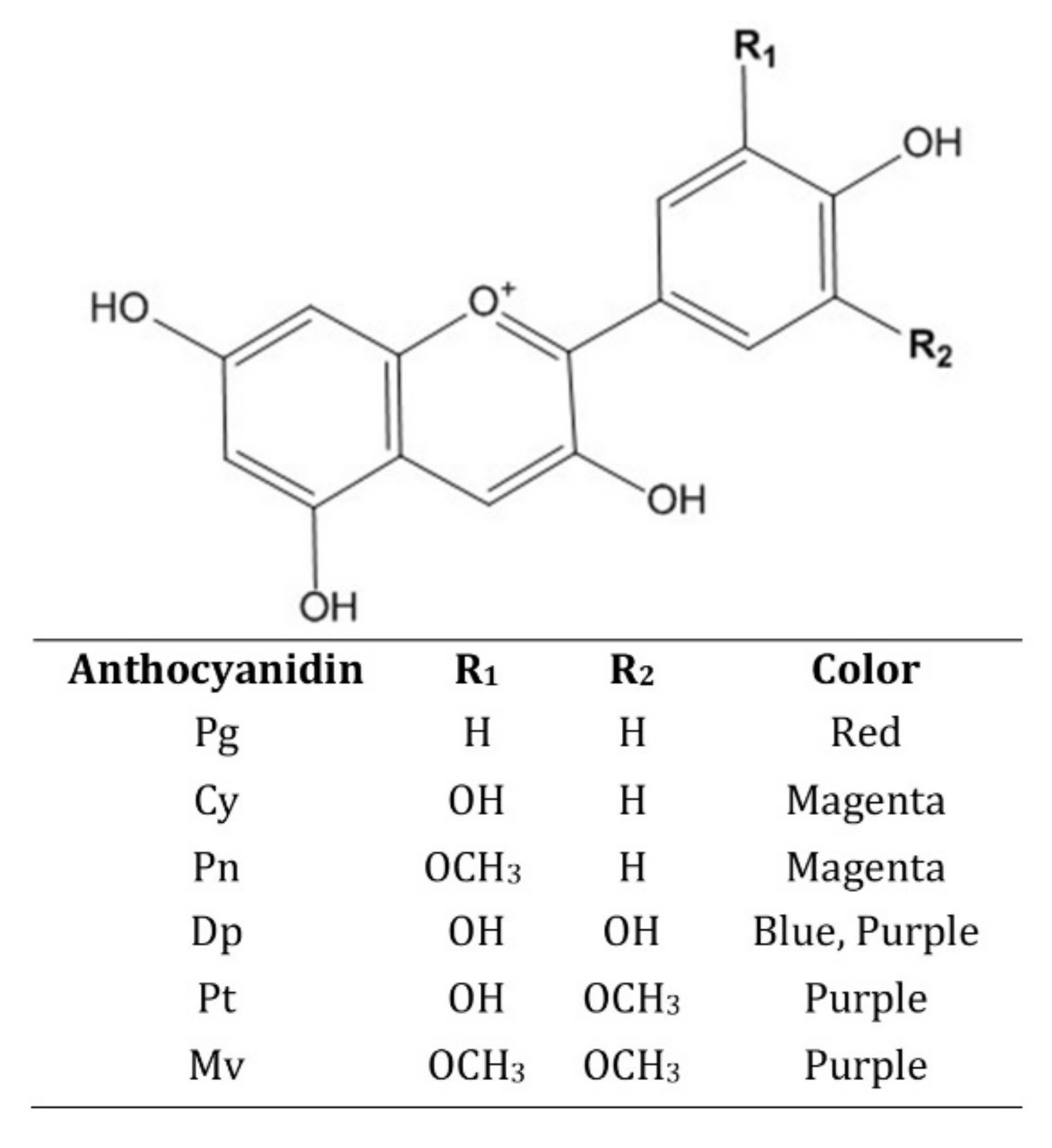
2.1. Chemistry
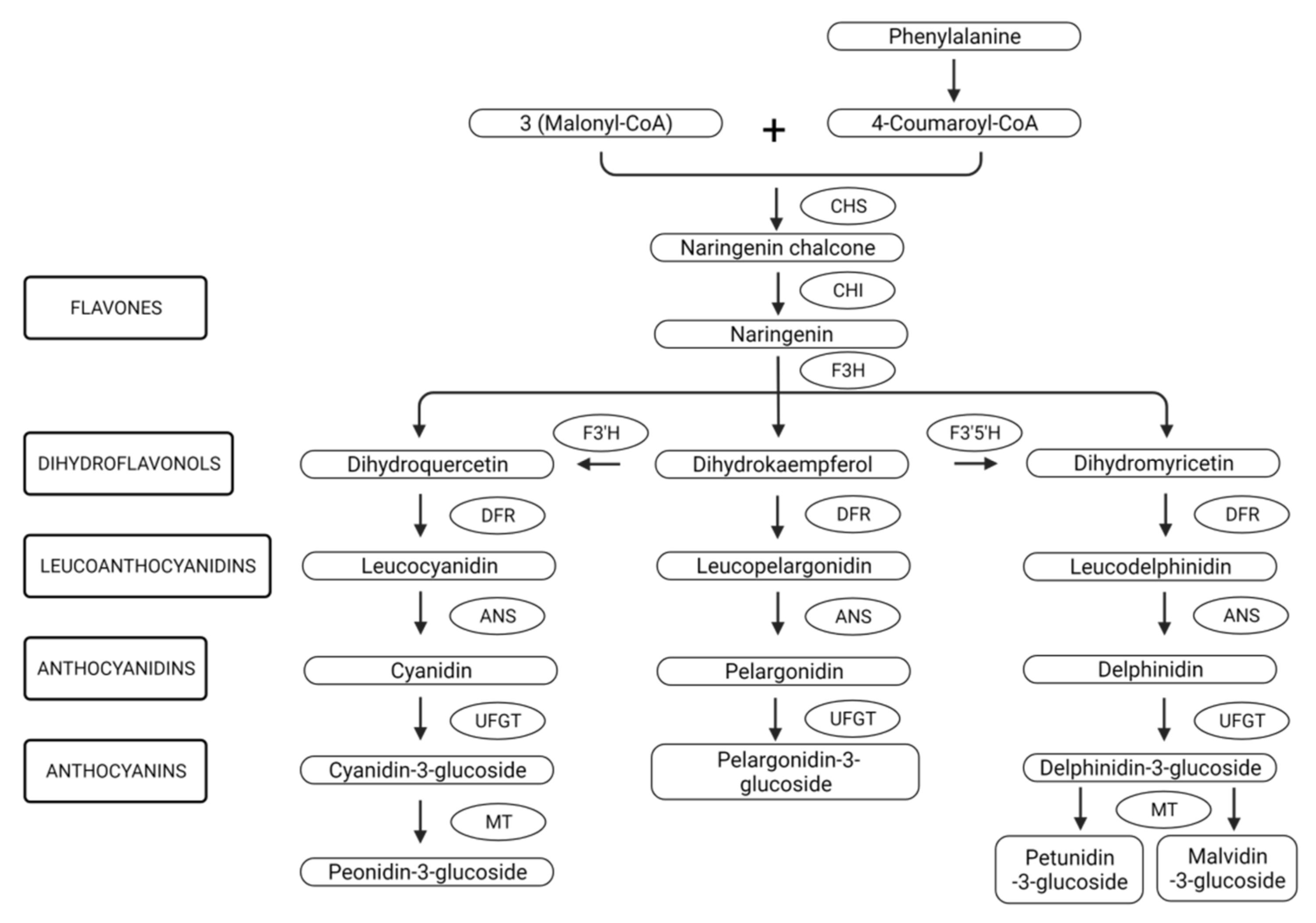
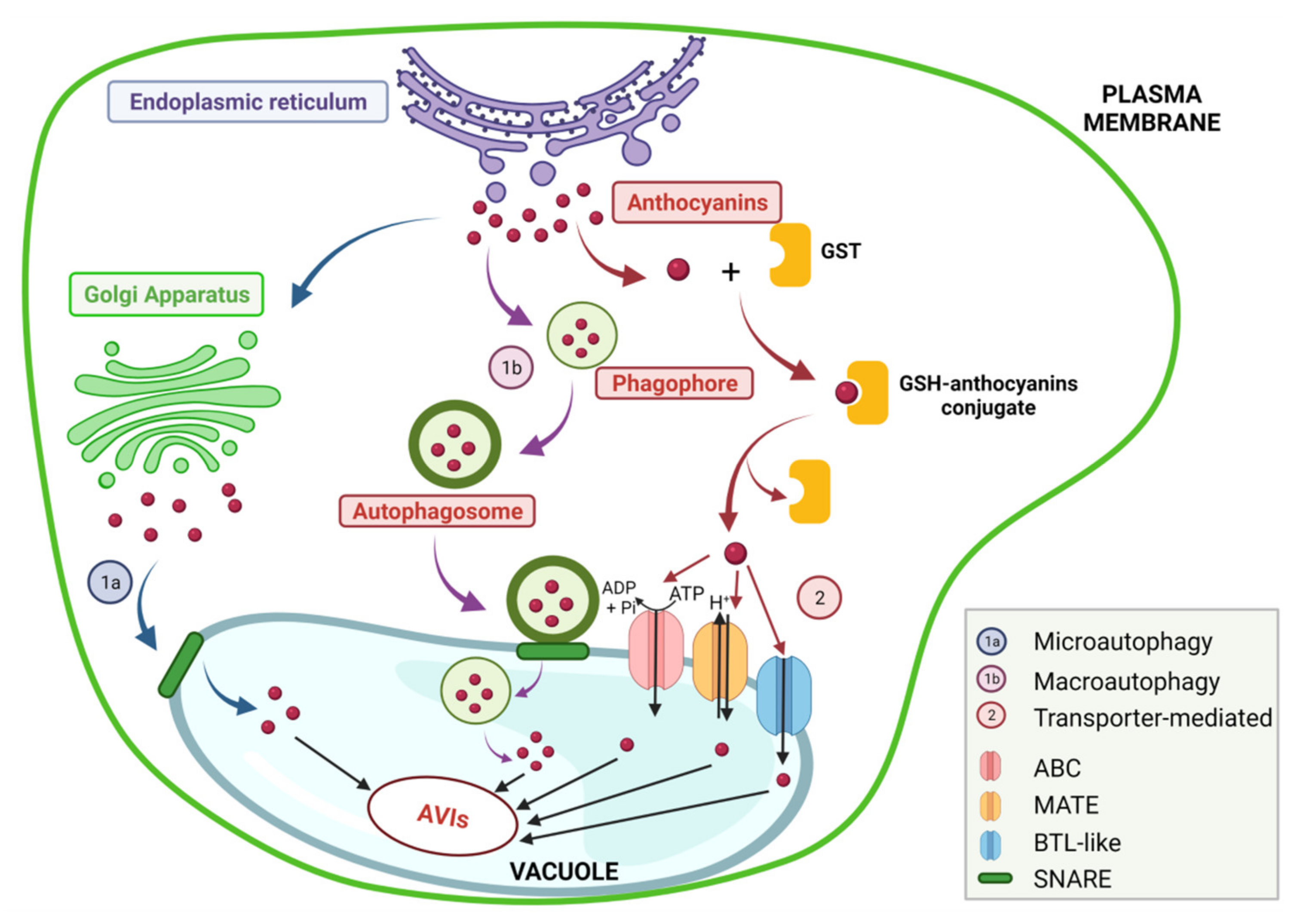
2.2. Biosynthesis, Genetic Regulation and Stability
2.2.1. Influence of pH
2.2.2. Temperature and Oxygen Influence
2.2.3. Light
2.2.4. Copigmentation/Glycosylation and Acylation
2.3. Effects of Extraction Methods on ANs
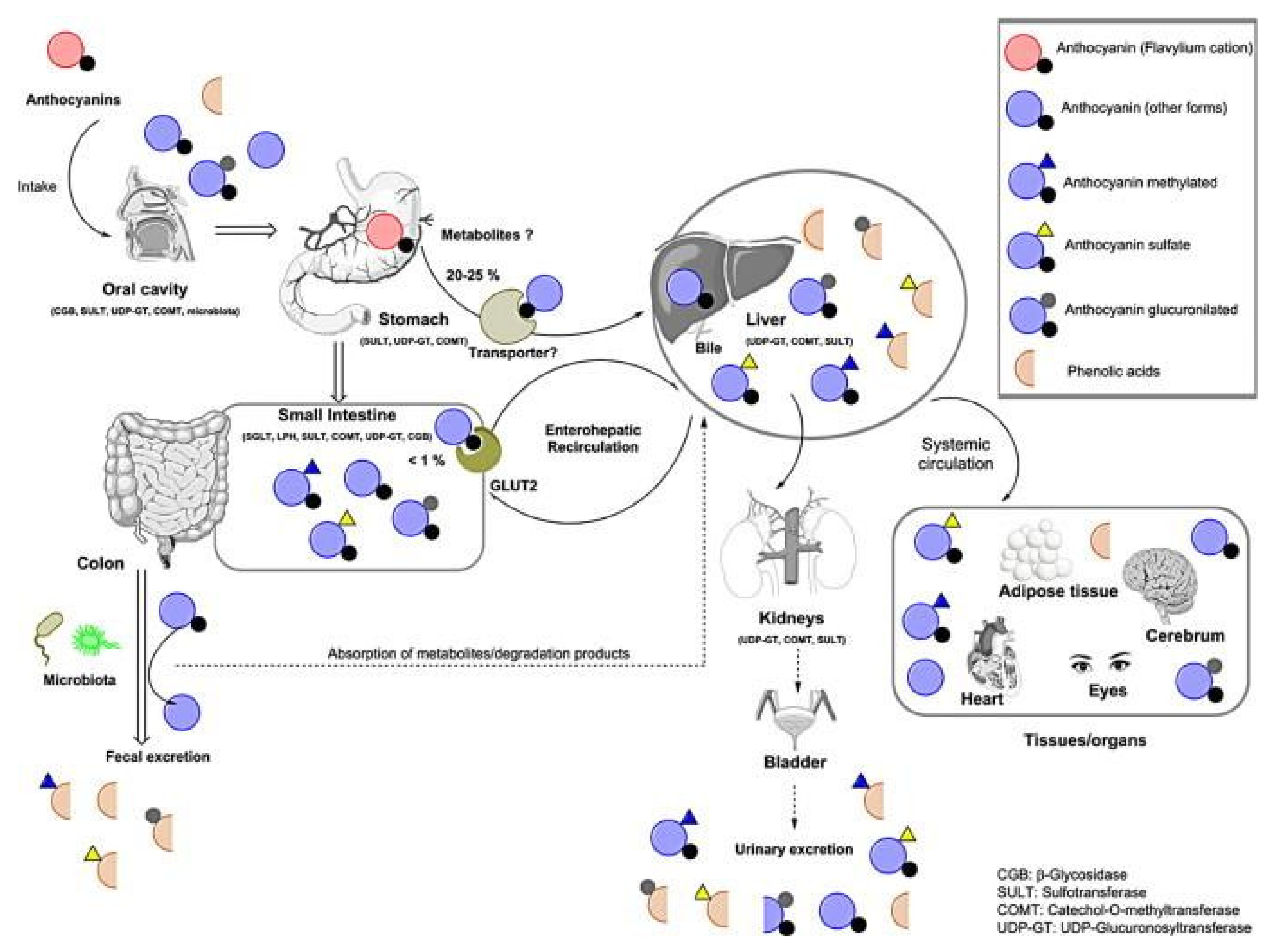
3. ANs Bioavailability
3.1. Oral Cavity Absorption
3.2. Gastric and Intestinal Absorption
3.2.1. Stomach
3.2.2. Intestine
4. ANs and Preventive Action on Diseases
4.1. In Vitro Studies
4.2. In Vivo/Clinical Studies
Neuroprotection Sustained by ANs
4.3. Medicinal Products Developed with ANs
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-OhdG | 8-hydroxydeoxyguanosine |
| Aβ | amyloid-β |
| ABC | ATP-binding cassette |
| AMPK | adenosine monophosphate-activated protein kinase |
| ANs | anthocyanins |
| ANS | anthocyanidin synthase |
| AOX1 | aldehyde oxidase 1 |
| ASE | accelerated solvent extraction |
| AVIs | anthocyanins vacuolar inclusions |
| BACE-1 | beta site amyloid precursor protein cleaving enzyme 1 |
| BBB | Blood–brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| BTL-like | bilitranslocase-like transporters |
| BW | body weight |
| CAT | catalase |
| CCL-2 | C-C motif chemokine ligand 2 |
| CCR2 | C-C motif receptor 2 |
| CDH4 | cadherin 4 |
| CHI | chalcone isomerase |
| CHS | chalcone synthase |
| CLDN14 | claudin 14 |
| COMT | catechol-O-methyltransferase |
| COX-2 | cyclooxygenase-2 |
| CPR | C-reactive protein |
| CRB3 | Crumbs cell polarity complex component 3 |
| Cy | cyanidin |
| CYP2E1 | cytochrome P450 2E1 |
| DFR | dihydroflavonol 4-reductase |
| D-HAEC | diabetic human aortic endothelial cells |
| Dp | delphinidin |
| EAE | enzyme-assisted extraction |
| eIF2α | eukaryotic initiation factor 2 α |
| ER | endoplasmic reticulum |
| F3H | flavanone 3-hydroxylase |
| F3′H | flavonoid 3′-hydroxylase |
| F3′5′H | flavonoid 3′,5′-hydroxylase |
| FAK | focal adhesion kinase |
| FMD | flow-mediated dilation |
| GCLM | glutamate-cysteine ligase modifier subunit |
| GLP-1 | glucagon-like peptide-1 |
| GLUT2 | glucose transporter 2 |
| GSH | glutathione |
| GSH-PX/GPX | glutathione peroxidase |
| GSTs | glutathione S-transferases |
| H2O2 | hydrogen peroxide |
| HDLc | high-density lipoprotein cholesterol |
| HHPE | high hydrostatic pressure extraction |
| HIF-1a | hypoxia-inducible factor 1-alpha |
| HO• | hydroxyl radical |
| HO-1 | heme oxygenase 1 |
| hsCRP | high-sensitivity C-reactive protein |
| HUVECs | human umbilical vein endothelial cells |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFN-γ | Interferon-gamma |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| LDH | lactate dehydrogenase |
| LED | light-emitting diodes |
| MAE | Microwave-assisted extraction |
| MAP | mitogen-activated protein |
| MATE | multidrug and toxic compound extrusion |
| MDA | melanoma differentiation-associated protein |
| MEF | moderate electric field |
| MMD | monocyte to macrophage differentiation-associated |
| MMP-1, MMP-2, MMP-9 | matrix metallopeptidase 1, 2, 9 |
| Mv | malvidin |
| MT | O-methyl transferase |
| NF-ƙB | nuclear factor kappa-B |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| O2− | superoxide anion |
| oxLDL | oxidized low-density lipoprotein |
| PD-1, PD-L1 | programmed death-1, -ligand 1 |
| PEF | pulsed electric field-assisted extraction |
| Pg | pelargonidin |
| PGE2 | prostaglandin E2 |
| PI3K | phosphoinositide 3-kinase |
| PLE | pressurized liquid extraction |
| Pn | peonidin |
| Pt | petunidin |
| p-tau | hyperphosphorylated tau |
| RAGE | receptor for advanced glycation end products |
| RBP4 | retinol binding protein 4 |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelium |
| SFE | supercritical fluid extraction |
| SGLT1 | sodium dependent glucose co-transporter 1 |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment protein receptors |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid reactive substances |
| TG | triglycerides |
| TNF | tumor necrosis factor |
| TXNIP | thioredoxin-interacting protein |
| UAE | Ultrasound-assisted extraction |
| UFGT | flavonoid 3-O-glucosyltransferase |
| UMAE | ultrasound/microwave-assisted extraction |
| uPA | urokinase plasminogen activator |
| sVCAM-1 | soluble vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| XBP-1 | X-box binding protein 1 |
References
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T. Chapter 8—Plant Polyphenols: Recent Advances in Epidemiological Research and Other Studies on Cancer Prevention. In Studies in Natural Products Chemistry; Rahman, A.-u., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. 269–295. [Google Scholar]
- Dixon, R.A.; Liu, C.; Jun, J.H. Metabolic engineering of anthocyanins and condensed tannins in plants. Curr. Opin. Biotechnol. 2013, 24, 329–335. [Google Scholar] [CrossRef]
- Mackon, E.; Ma, Y.; Jeazet Dongho Epse Mackon, G.C.; Li, Q.; Zhou, Q.; Liu, P. Subcellular Localization and Vesicular Structures of Anthocyanin Pigmentation by Fluorescence Imaging of Black Rice (Oryza sativa L.) Stigma Protoplast. Plants 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Hatier, J.-H.; Gould, K. Anthocyanin Function in Vegetative Organs. In Anthocyanins; Springer: Berlin, Germany, 2008; pp. 1–19. [Google Scholar]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateus, N.; de Freitas, V. Anthocyanins as Food Colorants. In Anthocyanins: Biosynthesis, Functions, and Applications; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2009; pp. 284–304. [Google Scholar]
- He, J.A.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K. Chapter 20—Phyto-Pharmaceuticals, Nutraceuticals and Their Evaluation. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 707–722. [Google Scholar]
- Wang, E.; Yin, Y.; Xu, C.; Liu, J. Isolation of high-purity anthocyanin mixtures and monomers from blueberries using combined chromatographic techniques. J. Chromatogr. A 2014, 1327, 39–48. [Google Scholar] [CrossRef]
- Pan, F.; Liu, Y.; Liu, J.; Wang, E. Stability of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin pigments and their inhibitory effects and mechanisms in human cervical cancer HeLa cells. RSC Adv. 2019, 9, 10842–10853. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, I.; de Freitas, V.; Mateus, N. Anthocyanins and human health: How gastric absorption may influence acute human physiology. Nutr. Aging 2014, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free. Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure-activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef]
- Nurtiana, W. Anthocyanin as natural colorant: A review. Food ScienTech J. 2019, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, A.; Cerretani, L.; Cichelli, A. Colors: Properties and Determination of Natural Pigments. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 273–283. [Google Scholar]
- Husain, A.; Chanana, H.; Khan, S.A.; Dhanalekshmi, U.M.; Ali, M.; Alghamdi, A.A.; Ahmad, A. Chemistry and Pharmacological Actions of Delphinidin, a Dietary Purple Pigment in Anthocyanidin and Anthocyanin Forms. Front. Nutr. 2022, 9, 746881. [Google Scholar] [PubMed]
- Ku, S.-K.; Yoon, E.-K.; Lee, W.; Kwon, S.; Lee, T.; Bae, J.-S. Antithrombotic and antiplatelet activities of pelargonidin in vivo and in vitro. Arch. Pharmacal Res. 2016, 39, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Rajan, V.K.; Hasna, C.K.; Muraleedharan, K. The natural food colorant Peonidin from cranberries as a potential radical scavenger—A DFT based mechanistic analysis. Food Chem. 2018, 262, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dormán, G.; Flachner, B.; Hajdú, I.; András, C. Chapter 23—Target identification and polypharmacology of nutraceuticals. In Nutraceuticals, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 315–343. [Google Scholar]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.J.; Cooperstone, J.L.; Cichon, M.J.; Joachim, H.; Giusti, M.M. Colorants. In Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2017; pp. 681–752. [Google Scholar]
- Jimenez-Garcia, S.N.; Guevara-Gonzalez, R.G.; Miranda-Lopez, R.; Feregrino-Perez, A.A.; Torres-Pacheco, I.; Vazquez-Cruz, M.A. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Res. Int. 2013, 54, 1195–1207. [Google Scholar] [CrossRef]
- Carbone, F.; Preuss, A.; De Vos, R.C.H.; D’amico, E.; Perrotta, G.; Bovy, A.G.; Martens, S.; Rosati, C. Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant Cell Environ. 2009, 32, 1117–1131. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids—Biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Kapoor, P.; Chunduri, V.; Pandey, A.K.; Garg, M. Spotlight on the overlapping routes and partners for anthocyanin transport in plants. Physiol. Plant 2021, 171, 868–881. [Google Scholar] [CrossRef]
- Zhang, T.K.; Yuan, Z.H. Quick Convergent Evolution of MBW Complex for Pomegranate Fruit Coloration. In Proceedings of the 4th International Symposium on Pomegranate and Minor Mediterranean Fruits, Elche, Spain, 18–22 September 2017; pp. 135–142. [Google Scholar]
- Xu, P.B.; Wu, L.; Cao, M.H.; Ma, C.; Xiao, K.; Li, Y.B.; Lian, H.L. Identification of MBW Complex Components Implicated in the Biosynthesis of Flavonoids in Woodland Strawberry. Front. Plant Sci. 2021, 12, 774943. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, Z.; Sebastian, A.; Xu, W.; Grain, D.; Salsac, F.; Avon, A.; Berger, N.; Tran, J.; Dubreucq, B.; Lurin, C.; et al. Analysis of the DNA-Binding Activities of the Arabidopsis R2R3-MYB Transcription Factor Family by One-Hybrid Experiments in Yeast. PLoS ONE 2015, 10, e0141044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Heppel, S.C.; Pillet, J.; Léon, C.; Czemmel, S.; Delrot, S.; Lauvergeat, V.; Bogs, J. The Basic Helix-Loop-Helix Transcription Factor MYC1 Is Involved in the Regulation of the Flavonoid Biosynthesis Pathway in Grapevine. Mol. Plant 2010, 3, 509–523. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; John, P.; Lovegrove, J.A.; Vysini, E.; Wagstaffe, A.; Hadley, P. Development of colour and firmness in strawberry crops is UV light sensitive, but colour is not a good predictor of several quality parameters. J. Sci. Food Agric. 2012, 92, 1597–1604. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Mao, K.; Zhao, C.; Zhao, X.-Y.; Zhang, H.-L.; Shu, H.-R.; Hao, Y.-J. MdCOP1 Ubiquitin E3 Ligases Interact with MdMYB1 to Regulate Light-Induced Anthocyanin Biosynthesis and Red Fruit Coloration in Apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Biosynthesis and Stability of Anthocyanins; Springer: Berlin, Germany, 2016; pp. 71–86. [Google Scholar]
- Enaru, B.; Dretcanu, G.; Pop, T.D.; Stanila, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Liu, Y. Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, I.B.; Freitas, A.; Maçanita, A.L.; Lima, J.C. Effect of water content on the acid-base equilibrium of cyanidin-3-glucoside. Food Chem. 2015, 172, 476–480. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Iland, P.G.; Tate, M.E.; Jones, G.P. Charge equilibria and pK(a) of malvidin-3-glucoside by electrophoresis. Anal. Biochem. 2003, 318, 291–299. [Google Scholar] [CrossRef]
- Khoo, H.E.; Chew, L.; Ismail, A.; Azlan, A. Anthocyanins in purple colored fruits. In Polyphenols: Chemistry, Dietary Sources and Health Benefits; Nova Science Publishers: New York, NY, USA, 2012; pp. 133–152. ISBN 9871620818688. [Google Scholar]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Z.; Jiang, S.; Xu, H.; Wang, Y.; Feng, S.; Chen, X. Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2016, 127, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Sikorski, Z.E. Fennema’s Food Chemistry, 5th ed.; Damodaran, S., Parkin, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2017; p. 1107. ISBN 9781482208122. [Google Scholar]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- West, M.E.; Mauer, L.J. Color and chemical stability of a variety of anthocyanins and ascorbic acid in solution and powder forms. J. Agric. Food Chem. 2013, 61, 4169–4179. [Google Scholar] [CrossRef]
- Marquez, A.; Perez-Serratosa, M.; Varo, M.A.; Merida, J. Effect of Temperature on the Anthocyanin Extraction and Color Evolution during Controlled Dehydration of Tempranillo Grapes. J. Agric. Food Chem. 2014, 62, 7897–7902. [Google Scholar] [CrossRef]
- Muche, B.M.; Speers, R.A.; Rupasinghe, H.P.V. Storage Temperature Impacts on Anthocyanins Degradation, Color Changes and Haze Development in Juice of “Merlot” and “Ruby” Grapes (Vitis vinifera). Front. Nutr. 2018, 5, 100. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes in bioactive composition of fresh-cut strawberries stored under superatmospheric oxygen, low-oxygen or passive atmospheres. J. Food Compos. Anal. 2010, 23, 37–43. [Google Scholar] [CrossRef]
- Rehman, R.N.U.; You, Y.; Zhang, L.; Goudia, B.D.; Khan, A.R.; Li, P.; Ma, F. High Temperature Induced Anthocyanin Inhibition and Active Degradation in Malus profusion. Front. Plant Sci. 2017, 8, 1401. [Google Scholar] [CrossRef]
- Thewes, F.R.; Brackmann, A.; de Oliveira Anese, R.; Bronzatto, E.S.; Schultz, E.E.; Wagner, R. Dynamic controlled atmosphere storage suppresses metabolism and enhances volatile concentrations of ‘Galaxy’ apple harvested at three maturity stages. Postharvest Biol. Technol. 2017, 127, 1–13. [Google Scholar] [CrossRef]
- Attoe, E.; Elbe, J.H. Photochemial Degradation of Betanine and Selected Anthocyanins. J. Food Sci. 2006, 46, 1934–1937. [Google Scholar] [CrossRef]
- Hernández, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedling physiological responses under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Y.; Wang, J.; Li, P.; Zhao, C.; Chen, Y.; Bi, Y. Phytochrome-interacting factors PIF4 and PIF5 negatively regulate anthocyanin biosynthesis under red light in Arabidopsis seedlings. Plant Sci. 2015, 238, 64–72. [Google Scholar] [CrossRef]
- Mazzucato, A.; Willems, D.; Bernini, R.; Picarella, M.E.; Santangelo, E.; Ruiu, F.; Tilesi, F.; Soressi, G.P. Novel phenotypes related to the breeding of purple-fruited tomatoes and effect of peel extracts on human cancer cell proliferation. Plant Physiol. Biochem. 2013, 72, 125–133. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Zhang, D.; Zhou, F.; Wang, D.; Wei, Y.; Gao, F.; Xie, L.; Jia, G.; Wu, W.; et al. Blueberry anthocyanins: Protection against ageing and light-induced damage in retinal pigment epithelial cells. Br. J. Nutr. 2012, 108, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Lopez, E.; Castañeda, A.; González-Olivares, L.; Añorve Morga, J.; Jaimez, J. Effect of Light on Stability of Anthocyanins in Ethanolic Extracts of Rubus fruticosus. Food Nutr. Sci. 2014, 5, 488–494. [Google Scholar]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammerer, D.R. 3—Anthocyanins. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Shaston, UK, 2016; pp. 61–80. [Google Scholar]
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. Chapter 6—Sweet Potato Anthocyanins. In Sweet Potato Processing Technology; Mu, T., Sun, H., Zhang, M., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 279–355. [Google Scholar]
- Morata, A.; López, C.; Tesfaye, W.; González, C.; Escott, C. 12—Anthocyanins as Natural Pigments in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 383–428. [Google Scholar]
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, T.; Szymusiak, H.; Gliszczyńska-Swigło, A.; Tyrakowska, B. The effect of 3-O-β-glucosylation on structural transformations of anthocyanidins. Food Res. Int. 2005, 38, 1031–1037. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Gençdağ, E.; Özdemir, E.E.; Demirci, K.; Görgüç, A.; Yılmaz, F.M. Copigmentation and stabilization of anthocyanins using organic molecules and encapsulation techniques. Curr. Plant Biol. 2022, 29, 100238. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [Green Version]
- Bimpilas, A.; Tsimogiannis, D.; Balta-Brouma, K.; Lymperopoulou, T.; Oreopoulou, V. Evolution of phenolic compounds and metal content of wine during alcoholic fermentation and storage. Food Chem. 2015, 178, 164–171. [Google Scholar] [CrossRef]
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Regenstein, J.M.; Laokuldilok, T. Copigmentation of cyanidin 3-O-glucoside with phenolics: Thermodynamic data and thermal stability. Food Biosci. 2019, 30, 100419. [Google Scholar] [CrossRef]
- Nistor, M.; Diaconeasa, Z.; Frond, A.D.; Stirbu, I.; Socaciu, C.; Pintea, A.; Rugina, D. Comparative efficiency of different solvents for the anthocyanins extraction from chokeberries and black carrots, to preserve their antioxidant activity. Chem. Pap. 2020, 75, 813–822. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trac-Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.H.; Sastry, S. Extraction from Food and Natural Products by Moderate Electric Field: Mechanisms, Benefits, and Potential Industrial Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1040–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socaciu, C. Food Colorants: Chemical and Functional Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Bochi, V.C.; Barcia, M.T.; Rodrigues, D.; Speroni, C.S.; Giusti, M.M.; Godoy, H.T. Polyphenol extraction optimisation from Ceylon gooseberry (Dovyalis hebecarpa) pulp. Food Chem. 2014, 164, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.; Collins, T.; Walsh, K.; Naiker, M. Solvent extractions and spectrophotometric protocols for measuring the total anthocyanin, phenols and antioxidant content in plums. Chem. Pap. 2020, 74, 4481–4492. [Google Scholar] [CrossRef]
- Azwanida, N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.M.E. Production of a food grade blueberry extract rich in anthocyanins: Selection of solvents, extraction conditions and purification method. J. Food Meas. Charact. 2017, 11, 1248–1253. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Atnip, A.; Bomser, J.; Giusti, M.M. Aglycone structures and glycosylations affect anthocyanin transport and uptake in human gastric epithelial (NCI-N87) cells. J. Food Compos. Anal. 2018, 65, 33–39. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-L.; Shah, V.; Patnaik, R.; Adams, W.; Hussain, A.; Conner, D.; Mehta, M.; Malinowski, H.; Lazor, J.; Huang, S.-M.; et al. Bioavailability and Bioequivalence: An FDA Regulatory Overview. Pharm. Res. 2001, 18, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Nave, F.; Petrov, V.; Pina, F.; Teixeira, N.; Mateus, N.; de Freitas, V. Thermodynamic and Kinetic Properties of a Red Wine Pigment: Catechin-(4,8)-malvidin-3-O-glucoside. J. Phys. Chem. B 2010, 114, 13487–13496. [Google Scholar] [CrossRef] [PubMed]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Remesy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Texier, O.; Garcin, P.; Besson, C.; Lamaison, J.L.; Scalbert, A. Tissue distribution of anthocyanins in rats fed a blackberry anthocyanin-enriched diet. Mol. Nutr. Food Res. 2009, 53, 1098–1103. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Sakakibara, H.; Ichikawa, Y.; Tajima, S.; Makino, Y.; Wakasugi, Y.; Shimoi, K.; Kobayashi, S.; Kumazawa, S.; Goda, T. Practical application of flavonoid-poor menu meals to the study of the bioavailability of bilberry anthocyanins in human subjects. Biosci. Biotechnol. Biochem. 2014, 78, 1748–1752. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Liu, C.; Gao, W.; Li, Z.; Qin, G.; Qi, S.; Jiang, H.; Li, X.; Liu, M.; Yan, F.; et al. Anthocyanins Are Converted into Anthocyanidins and Phenolic Acids and Effectively Absorbed in the Jejunum and Ileum. J. Agric. Food Chem. 2021, 69, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of Cyanidin Glycosides from Natural Chokeberry (Aronia melanocarpa) Juice with Dietary-Relevant Dose of Anthocyanins in Humans. J. Agric. Food Chem. 2010, 58, 12130–12136. [Google Scholar] [CrossRef]
- Kalt, W. Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals. Molecules 2019, 24, 4024. [Google Scholar] [CrossRef] [Green Version]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Jia, Y.; Du, X.; Wang, Y.; Yang, Z.; Li, K. Study of physicochemical stability of anthocyanin extracts from black peanut skin and their digestion enzyme and adipogenesis inhibitory activities. LWT 2019, 107, 107–116. [Google Scholar] [CrossRef]
- Lang, Y.; Meng, X.; Gao, H.; Tian, J.; Shu, C.; Sun, R.; Li, B. Protective effects of α-casein or β-casein on the stability and antioxidant capacity of blueberry anthocyanins and their interaction mechanism. LWT 2019, 115, 108434. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003, 544, 210–213. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wallace, T.C.; Keatley, K.E.; Failla, M.L.; Giusti, M.M. Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J. Agric. Food Chem. 2009, 57, 3141–3148. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Failla, M.L.; Kumar, P.S.; Giusti, M.M. Anthocyanin Structure Determines Susceptibility to Microbial Degradation and Bioavailability to the Buccal Mucosa. J. Agric. Food Chem. 2014, 62, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodukula, K.; Faller, D.V.; Harpp, D.N.; Kanara, I.; Pernokas, J.; Pernokas, M.; Powers, W.R.; Soukos, N.S.; Steliou, K.; Moos, W.H. Gut Microbiota and Salivary Diagnostics: The Mouth Is Salivating to Tell Us Something. Biores Open Access 2017, 6, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, Y.; Guan, Y.; Moon, K.; Williams, L. Anthocyanins: Natural Sources and Traditional Therapeutic Uses. In Flavonoids—A Coloring Model for Cheering up Life; Intechopen: London, UK, 2019. [Google Scholar]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Remesy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef]
- Borges, G.; Roowi, S.; Rouanet, J.M.; Duthie, G.G.; Lean, M.E.; Crozier, A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007, 51, 714–725. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef]
- Walton, M.C.; McGhie, T.K.; Reynolds, G.W.; Hendriks, W.H. The flavonol quercetin-3-glucoside inhibits cyanidin-3-glucoside absorption in vitro. J. Agric. Food Chem. 2006, 54, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; de Pascual-Teresa, S. Metabolism of Anthocyanins by Human Gut Microflora and Their Influence on Gut Bacterial Growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Marques, F.; de Freitas, V.; Mateus, N. Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food Chem. 2013, 141, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Bonsignore, G.; Patrone, M.; Ranzato, E. Mediterranean Diet Polyphenols: Anthocyanins and Their Implications for Health. Mini-Rev. Med. Chem. 2021, 21, 1692–1700. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mazeikiene, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins—Nature’s Bold, Beautiful, and Health-Promoting Colors. Foods 2019, 8, 550. [Google Scholar] [CrossRef] [Green Version]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.Z.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eker, M.; Aaby, K.; Budić-Leto, I.; Rimac, S.; El, S.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Buve, C.; Panozzo, A.; Grauwet, T.; Hendrickx, M. Role of structural barriers in the in vitro bioaccessibility of anthocyanins in comparison with carotenoids. Food Chem. 2017, 227, 271–279. [Google Scholar] [CrossRef]
- Garcia-Diaz, D.F.; Johnson, M.H.; de Mejia, E.G. Anthocyanins from fermented berry beverages inhibit inflammation-related adiposity response in vitro. J. Med. Food 2015, 18, 489–496. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Aboonabi, A.; Singh, I.; Rose’ Meyer, R. Cytoprotective effects of berry anthocyanins against induced oxidative stress and inflammation in primary human diabetic aortic endothelial cells. Chem.-Biol. Interact. 2020, 317, 108940. [Google Scholar] [CrossRef]
- Krga, I.; Monfoulet, L.-E.; Konic-Ristic, A.; Mercier, S.; Glibetic, M.; Morand, C.; Milenkovic, D. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to TNFα-activated endothelial cells at physiologically relevant concentrations. Arch. Biochem. Biophys. 2016, 599, 51–59. [Google Scholar] [CrossRef]
- Joo, H.K.; Choi, S.; Lee, Y.R.; Lee, E.O.; Park, M.S.; Park, K.B.; Kim, C.-S.; Lim, Y.P.; Park, J.-T.; Jeon, B.H. Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and Apo E(-/-) Mice. Int. J. Mol. Sci. 2018, 19, 816. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.S.; Vissers, M.C.M.; Anderson, R.F.; Sreebhavan, S.; Bozonet, S.M.; Scheepens, A.; Melton, L.D. Bioavailable Blueberry-Derived Phenolic Acids at Physiological Concentrations Enhance Nrf2-Regulated Antioxidant Responses in Human Vascular Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1700647. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Ling, W.; Zhu, H.; Ma, J.; Wang, Q.; Hou, M.; Tang, Z.; Guo, H.; Liu, C.; Ye, Q. Anthocyanin attenuates CD40-mediated endothelial cell activation and apoptosis by inhibiting CD40-induced MAPK activation. Atherosclerosis 2009, 202, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.-S.; Tseng, T.-H.; Lee, H.-J.; Chan, K.-C.; Wang, C.-J. Anthocyanin extracted from Hibiscus attenuate oxidized LDL-mediated foam cell formation involving regulation of CD36 gene. Chem.-Biol. Interact. 2009, 179, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-tumor properties of anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Heeba, G.H.; Elwy, H.M.; Murali, C.; El-Awady, R.; Amin, A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: In vivo and in vitro analyses. Sci. Rep. 2018, 8, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-C.; Hung, C.-H.; Hung, T.-W.; Lin, Y.-C.; Wang, C.-J.; Kao, S.-H. Dietary delphinidin inhibits human colorectal cancer metastasis associating with upregulation of miR-204-3p and suppression of the integrin/FAK axis. Sci. Rep. 2019, 9, 18954. [Google Scholar] [CrossRef] [Green Version]
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef] [Green Version]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K.P. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef]
- Forni, C.; Braglia, R.; Mulinacci, N.; Urbani, A.; Ronci, M.; Gismondi, A.; Tabolacci, C.; Provenzano, B.; Lentini, A.; Beninati, S. Antineoplastic activity of strawberry (Fragariaxananassa Duch.) crude extracts on B16-F10 melanoma cells. Mol. Biosyst. 2014, 10, 1255–1263. [Google Scholar] [CrossRef]
- Huang, H.P.; Shih, Y.W.; Chang, Y.C.; Hung, C.N.; Wang, C.J. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. J. Agric. Food Chem. 2008, 56, 9286–9293. [Google Scholar] [CrossRef]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.S.; Kumar, M.S.; Das, S.M. Evaluation of antiproliferative activity of red sorghum bran anthocyanin on a human breast cancer cell line (mcf-7). Int. J. Breast Cancer 2011, 2011, 891481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugină, D.; Sconţa, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, A.; Pestana, D.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytother. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, L.; Giampieri, F.; Alvarez Suarez, J.M.; Gasparrini, M.; Mezzetti, B.; Forbes Hernandez, T.Y.; Battino, M.A. Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines. Food Funct. 2019, 10, 7103–7120. [Google Scholar] [CrossRef] [PubMed]
- Rugină, D.; Hanganu, D.; Diaconeasa, Z.; Tăbăran, F.; Coman, C.; Leopold, L.; Bunea, A.; Pintea, A. Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, K.L.; Stuart, C.M.; McCarthy, J.J.; Kaur, B.; Cantu, E.J.; Forester, S.C. Enhancing the Cancer Cell Growth Inhibitory Effects of Table Grape Anthocyanins. J. Food Sci. 2018, 83, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.P.; Han, B.; Yu, X.P.; Chen, X.Y.; Zhou, J.; Chen, W.; Zhu, Y.F.; Peng, X.L.; Zou, Q.; Li, S.Y. Anti-metastasis activity of black rice anthocyanins against breast cancer: Analyses using an ErbB2 positive breast cancer cell line and tumoral xenograft model. Asian Pac. J. Cancer Prev. 2014, 15, 6219–6225. [Google Scholar] [CrossRef] [Green Version]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Bunea, A.; Rugina, D.; Sconta, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tabaran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Neuroprotective effects of cyanidin against Aβ-induced oxidative and ER stress in SK-N-SH cells. Neurochem. Int. 2016, 101, 15–21. [Google Scholar] [CrossRef]
- Cásedas, G.; González-Burgos, E.; Smith, C.; López, V.; Gómez-Serranillos, M.P. Regulation of redox status in neuronal SH-SY5Y cells by blueberry (Vaccinium myrtillus L.) juice, cranberry (Vaccinium macrocarpon A.) juice and cyanidin. Food Chem. Toxicol. 2018, 118, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Molina-Ayala, M.A.; Rodriguez-Amador, V.; Suarez-Sanchez, R.; Leon-Solis, L.; Gomez-Zamudio, J.; Mendoza-Zubieta, V.; Cruz, M.; Suarez-Sanchez, F. Expression of obesity- and type-2 diabetes-associated genes in omental adipose tissue of individuals with obesity. Gene 2022, 815, 146181. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [PubMed]
- Liu, X.; Li, S.; Wang, Z.; Wang, X.; He, Y.; Wen, L. Ultrahigh Pressure Facilitates the Acylation of Malvidin and Chlorogenic Acid to Increase the Stability and Protective Effect of Malvidin Derivatives on H2O2-Induced ARPE-19 Cells. J. Agric. Food Chem. 2021, 69, 13990–14003. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Kim, E.K.; Lee, S.J.; Kim, Y.S.; Moon, S.H.; Jeon, B.T.; Sung, S.H.; Kim, E.T.; Park, P.J. Antioxidant activity and protective effect of anthocyanin oligomers on H(2)O(2)-triggered G2/M arrest in retinal cells. J. Agric. Food Chem. 2012, 60, 4282–4288. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, F.; Li, S.; Wang, Z.; Wang, X.; Wen, L.; He, Y. Malvidin and its derivatives exhibit antioxidant properties by inhibiting MAPK signaling pathways to reduce endoplasmic reticulum stress in ARPE-19 cells. Food Funct. 2021, 12, 7198–7213. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.X.; Sun, H.; Qi, W.; Li, A. Bilberry anthocyanin-rich extract protects against retinal photooxidative damage via activation of HO-1 and inhibition of NF-κB. Food Agric. Immunol. 2019, 30, 829–840. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization, Regional Office for the Eastern Mediterranean. Healthy Diet; World Health Organization, Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2019. [Google Scholar]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E.; Kobayashi, K.; Tabuchi, M.; Lopez, L.R. Oxidative modification of low-density lipoprotein and immune regulation of atherosclerosis. Prog. Lipid Res. 2006, 45, 466–486. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2022, 118, 115–129. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Z.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.K. Anthocyanin Rich-Black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in Overweight/Obese Korean Adults: A Randomized Controlled Trial. J. Med. Food 2016, 19, 995–1003. [Google Scholar] [CrossRef]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef] [Green Version]
- Azzini, E.; Venneria, E.; Ciarapica, D.; Foddai, M.S.; Intorre, F.; Zaccaria, M.; Maiani, F.; Palomba, L.; Barnaba, L.; Tubili, C.; et al. Effect of Red Orange Juice Consumption on Body Composition and Nutritional Status in Overweight/Obese Female: A Pilot Study. Oxid. Med. Cell. Longev. 2017, 2017, 1672567. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Guo, X.; Zhang, M.; Liu, R.; Sui, W. Raspberry anthocyanin consumption prevents diet-induced obesity by alleviating oxidative stress and modulating hepatic lipid metabolism. Food Funct. 2018, 9, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Guo, X.; Zhang, M.; Yang, L.; Liu, R.; Yin, J. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mice. Food Funct. 2017, 8, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef]
- Sasaki, R.; Nishimura, N.; Hoshino, H.; Isa, Y.; Kadowaki, M.; Ichi, T.; Tanaka, A.; Nishiumi, S.; Fukuda, I.; Ashida, H.; et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007, 74, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. An anthocyanin-rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition 2013, 29, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-K.; Lim, S.S.; Lee, J.-Y.; Yeo, K.M.; Kang, Y.-H. Anthocyanin-rich purple corn extract inhibit diabetes-associated glomerular angiogenesis. PLoS ONE 2013, 8, e79823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tani, T.; Nishikawa, S.; Kato, M.; Tsuda, T. Delphinidin 3-rutinoside-rich blackcurrant extract ameliorates glucose tolerance by increasing the release of glucagon-like peptide-1 secretion. Food Sci. Nutr. 2017, 5, 929–933. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Alhosin, M.; León-González, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.M.; Mauvieux, L.; Herbrecht, R.; et al. Bilberry extract (Antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad pathway. Sci. Rep. 2015, 5, 8996. [Google Scholar] [CrossRef] [Green Version]
- Keravis, T.; Favot, L.; Abusnina, A.A.; Anton, A.; Justiniano, H.; Soleti, R.; Alabed Alibrahim, E.; Simard, G.; Andriantsitohaina, R.; Lugnier, C. Delphinidin Inhibits Tumor Growth by Acting on VEGF Signalling in Endothelial Cells. PLoS ONE 2015, 10, e0145291. [Google Scholar] [CrossRef] [Green Version]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an Anthocyanidin in Pigmented Fruits and Vegetables, Protects Human HaCaT Keratinocytes and Mouse Skin Against UVB-Mediated Oxidative Stress and Apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Pratheeshkumar, P.; Son, Y.O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-kappaB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2017, 57, 1169–1180. [Google Scholar] [CrossRef]
- Whyte, A.R.; Schafer, G.; Williams, C.M. Cognitive effects following acute wild blueberry supplementation in 7- to 10-year-old children. Eur. J. Nutr. 2016, 55, 2151–2162. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins Reversed D-Galactose-Induced Oxidative Stress and Neuroinflammation Mediated Cognitive Impairment in Adult Rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3β Signaling in LPS-Treated Adult Mice. Mol. Neurobiol. 2019, 56, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J. Nutr. Biochem. 2018, 56, 193–204. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J.; Zhou, Z. Anthocyanins from Black Chokeberry (Aroniamelanocarpa Elliot) Delayed Aging-Related Degenerative Changes of Brain. J. Agric. Food Chem. 2017, 65, 5973–5984. [Google Scholar] [CrossRef]
- Goetz, M.E.; Judd, S.E.; Safford, M.M.; Hartman, T.J.; McClellan, W.M.; Vaccarino, V. Dietary flavonoid intake and incident coronary heart disease: The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am. J. Clin. Nutr. 2016, 104, 1236–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, O.R.; Netzel, G.A.; Sakzewski, A.R. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: The QUENCH trial. Can. J. Physiol. Pharmacol. 2013, 91, 480–488. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Limsitthichaikoon, S.; Khampaenjiraroch, B.; Damrongrungruang, T.; Limphirat, W.; Thapphasaraphong, S.; Priprem, A. Topical oral wound healing potential of anthocyanin complex: Animal and clinical studies. Ther. Deliv. 2018, 9, 359–374. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alawadh, S.H.; Bouazzaoui, A.; Alhowail, A.H.; Mohammed, H.A. Anthocyanins rich pomegranate cream as a topical formulation with anti-aging activity. J. Dermatol. Treat. 2020, 32, 983–990. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Source of ANs | Major ANs/ Metabolites | Effects | References | |
|---|---|---|---|---|---|
| Diabetes and obesity | RAW264.7 (murine macrophages); 3T3-L1 (human preadipocyte) | Blackberry/ Blueberry beverages | Cy-3-glucoside | ↓NF-ƙB ↓nitric oxide ↓TNF-α ↓isoproterenol-induced lipolysis ↓fat accumulation | [143] |
| HepG2 (human hepatocellular carcinoma) | Blueberry extract Mv Mv-3-glucoside Mv-3-galactoside | Mv Mv-3-glucoside Mv-3-galactoside | ↓ROS ↓lipogenesis/glycogenolysis enzymes ↑lipolysis via AMPK pathway | [144] | |
| D-HAEC (diabetic human aortic endothelial cells) | Bilberry/blueberry ANs capsules | Cy, Dp, Pt, Pn, Mv | ↓NF-ƙB pathway ↓inflammatory and oxidative process | [145] | |
| Cardiovascular diseases | HUVECs (human umbilical vein endothelial cells) | Standard solutions | Cy, Pn, Dp, 4-hydroxy- benzaldehyde | ↓inflammatory and oxidative process ↓monocytes adhesion to HUVECs | [146] |
| Red Chinese cabbage | Cy | ↓TNF-α-induced NF-κB activity | [147] | ||
| Blueberry juice | Protocatechuic acid Vanillic acid trans-ferulic acid p-coumaric acid | ↑antioxidant response of Nrf2-regulated heme oxygenase 1 (HO-1) and glutamate-cysteine ligase modifier subunit (GCLM) | [148] | ||
| Standard solutions | Cy-3-glucoside Pn-3-glucoside | ↓levels of VCAM-1, ICAM-1, MMP-1, MMP-9 ↓activity of caspase-3, JNK, p38 | [149] | ||
| J774A.1 (murine monocyte macrophage) | Hibiscus sabdariffa L. | Dp, Cy | ↓oxLDL ↓CD36 expression | [150] | |
| Cancer | SMMC-7721 (human hepatoma cells) | Lonicera caerulea ‘Beilei’ fruit | Cy, Pn | ↓cell proliferation ↑apoptosis | [151] |
| HepG2 (human hepatocyte carcinoma) | Grape seeds | Pro-ANs | ✓activation of caspase-3 ✓G2/M, G1/M cell cycle arrest | [152] | |
| DLD-1 SW480 SW620 Human colon cancer | Standard solution | Dp | ↓tumor cells adhesion, migration, invasion, epithelial-to-mesenchymal transition ↓integrin and FAK signaling pathways ↑miR-204-3p upregulation | [153] | |
| HCT-29 HCT-116 Human colorectal cancer | Standard solutions | Dp-3-glucoside Cy-3-glucoside | ↓PD-1, PD-L1 | [154] | |
| Colon cancer stem cells | Purple fleshed potato extract | Pt, Mv, Cy, Pn | ↓cell proliferation ↑cell apoptosis | [155] | |
| B16-F10 (murine melanoma cells) | Strawberry fruits | Cy, Pg, p-cumaroyl monohexose | ↑cell differentiation ↓cell proliferation | [156] | |
| B16-F1 (murine melanoma cells) | Mulberry fruits | Cy, Pg | ↓PI3K expression ↓Ras ↓NF-kβ | [157] | |
| MCF-7 HER2 MDA-MB-231 MDA-MB-453 Breast cancer cells | Black rice | Cy-3-glucoside Pn-3-glucoside | ↓cell viability ↑caspase-3 activation ↑cytochrome C release | [158] | |
| MCF-7 (human breast cancer cells) | Red sorghum bran | - | ↑apoptosis ↓tumor proliferation | [159] | |
| HeLa (human cervical tumor Cells) | Chokeberry | Cy-3-galactoside | ✓antioxidant activity ↓cell proliferation | [160] | |
| MDA-MB-231 and MCF7 (human breast cancer cells) | Blueberry | Dp-3-glucoside Cy-3-glucoside Mv-3-glucoside | ↓cell invasion capacity ✓activation of caspase-3 in MCF7 cells | [161] | |
| N202/1A, N202/1E (murine melanoma) | Strawberry | Pg-3-glucose | ↓cell viability ✓ROS induction ✓mitochondrial damage | [162] | |
| B16-F10 (murine melanoma cells) | Elderberries | Cy-3-sambubioside-5-glucoside | ↓cell proliferation ↑LDH activity | [163] | |
| Caco-2, HT-29 (colon cancer); MDA-MB-231 (breast cancer) | Table grapes with entacapone | Cy-3-glucoside Dp-3-glucose | ↓cell proliferation ↑extracellular ROS levels | [164] | |
| MDA-MB-453 (breast cancer) | Black rice | Cy-3-glucoside Pn-3-glucoside | ↓cell migration, adhesion, motility, invasion ↓urokinase-type plasminogen activator activity ↓transfer promoting factor activity | [165] | |
| MCF-7 (breast); SF-268 (CNS); NCI-H460 (lung); HCT-116 (colon); AGS (gastric) human tumor cells | Black/Red raspberry, Blackberry | Cy-3-glucoside Pg-3-glucoside Cy-3-glucosylrutinoside | ↓cell proliferation ↓lipid peroxidation | [166] | |
| B16-F10 (murine melanoma) | Blueberry | Mv-3-galactoside Pt-3-galactoside Dp-3-galactoside | ✓antioxidant activity ↓cells proliferation ✓apoptosis ↑LDH activity | [167] | |
| Neurological disorders | SK-N-SH (human neuroblastoma) treated with Aβ25-35 (neurotoxic) | Standard solution | Cy-3-glucoside | ↓ROS accumulation ↓ER stress response proteins ↓eIF2α, XBP-1, caspase-12 | [168] |
| SH-SY5Y (human neuroblastoma) treated with hydrogen peroxide (neurotoxic) | Blueberry/cranberry juices | Cy, Dp, Pn, Pt | ↑SOD and CAT activity ↓ROS and TBARS accumulation | [169] |
| Medical Condition | Source of ANs | Target Group | Treatment Details | Dose of Treatment | Effect | Reference | |
|---|---|---|---|---|---|---|---|
| Diabetes and Obesity | Metabolic syndrome | Fresh bilberries | 15 volunteers | 8 weeks | 400 g | ↓IL-6, IL-12 ↓C-reactive protein ↓MMD and CCR2 expression | [186] |
| Overweight/obesity | Black soybean extract | 63 obese volunteers | 8 weeks | 2.5 g/day (ANs conc: 12.58 mg/g) | ↓LDLc ↓TG ↓Non-HDLc | [187] | |
| Weight control over time | Blueberries Strawberries Apples Pears | 124,086 volunteers | 24 years | - | ✓0.07–0.10 kg less weight gained every 4 years ✓weight control | [188] | |
| Overweight/obesity | Commercial red orange juice | 11 women | 12 weeks | 500 mL/day | ↓LDL | [189] | |
| Obesity | Raspberry extract | Male C57BL/6 mice | 4 weeks | 200 mg/kg food | ↓TNFα, IL-6, NF-κB gene expression ↓63.7% less body weight ↑SOD, GSH-PX activity | [190] | |
| Obesity | Black rice Clack soybean Purple corn | C57BL/6 mice | 12 weeks | 200 mg/kg food | ↓TNFα, IL-6, iNOS, NF-κB gene expression ↓lipid peroxidation ↑peroxide dismutase | [191] | |
| Obesity | Cherry Mulberry | C57BL/6 mice | 8 weeks | 200 mg/kg food | ↓29.6 and 32.7% less body weight ↓TNFα, IL-6, iNOS, NF-κB gene ↑SOD, GPX activity | [192] | |
| Metabolic syndrome | Wild blueberries | Obese Zucker rats | 8 weeks | 8% of diet | ↓IL-6, TNF-α, Nf-κB ↓C-reactive protein | [193] | |
| Diabetes | Cy-3-glucoside | KK-A(y) mice | 5 weeks | 0.2% of diet | ↓RBP4 expression ↓blood glucose ↑Glut4 | [194] | |
| Pre-diabetes | Kamchatka honeysuckle extract | 24 Wistar rats | 4 weeks | 327 mg ANs/g | ↑gut α and β glucosidase activity ✓ameliorates abnormal lipid/glucose metabolism | [195] | |
| Diabetic nephropathy | Purple corn extract | C57BLKS/J-Leprdb mice | 8 weeks | 10 mg/kg BW | ↓VEGF, HIF-1a ↓angiogenesis | [196] | |
| Pre-diabetes | Black currant extract | Sprague- Dawley rats | - | 5 mg/kg BW | ↑GLP-1 | [197] | |
| Cardiovascular diseases/Obesity | Myocardial infarction (MI) | ANs-rich fruits and vegetables | 93,600 women, ages 25–42 | 18 years | - | ↓MI risk | [198] |
| Vascular impairments | Blueberry fruits | 21 healthy men | 1, 2, 4, 6 h after ingestion | 319, 637, 766, 1278, and 1791 mg total | ↑vascular function | [199] | |
| Cardiovascular risk | Strawberries | Healthy volunteers | 1 month | 500 g fruits/day | ↓cholesterol ↓triglycerides ↓activated platelets ↑plasma antioxidant capacity | [200] | |
| Hypercholesterolemia | ANs mixture | 150 volunteers | 24 weeks | 320 mg/day | ↓hsCRP ↓sVCAM-1 ↓IL-1β | [201] | |
| Cancer | Chronic B cell lymphocytic leukemia | Bilberry extract | 30 patients | 24 h | - | ✓activation of caspase-3 ✓apoptosis of B CLL cells ↓Bcl-2/Bad pathway | [202] |
| Induced melanoma | Dp pure solution | C57BL/6N mice | 30 days | 10 mg delphinidin/kg | ↓melanoma-induced tumor growth | [203] | |
| UVB-mediated apoptosis | Dp | Female SKH-1 mice | 1 and 8 h | 1 mg/0.1 DMSO/mouse | ↓apoptosis ↓cyclobutane pyrimidine dimers ↓8-OhdG ↓DNA damage | [204] | |
| UVB-induced inflammation | Cy-3-glucoside | Female SKH-1 mice | 24 h | 250 and 500 µM | ↓COX-2, iNOS, PGE2, NF-κB ↓proinflammatory cytokines ↓p38 MAP kinase signaling | [205] | |
| Neuroprotection | Dementia | Cherry juice | 49 older adults (+70 years) | 12 weeks | 200 mL/day | ↑cognition ↑speech fluency ↑short/long memory | [206] |
| Cognitive degradation | Freeze-dried blueberries | 37 older adults (60–75 years) | 90 days | 24 g/day | ↓verbal errors ↓switch cost on task-switching test | [207] | |
| Cognition improvement | Freeze-dried wild blueberries | 21 children (7–10 years) | 1, 3, 6 h | 15 or 30 g/day | ↑cognitive performance | [208] | |
| Neuroinflammation mediated cognitive impairment | Korean black soybean | Male Sprague-Dawley rats | 7 weeks | 100 mg/kg ANs | ✓memory improved ✓astrocytes and microglia activation ↓RAGE, BACE-1, Aβ expression | [209] | |
| Neuroinflammation | Korean black soybean | Male C57BL/6N mice | 14 days | 24 mg/kg/day | ↓p-NF-κB, TNF-α, and IL-1β ↓cell apoptosis | [210] | |
| Alzheimer dementia | ANs | Male Wistar rats | - | 200 mg/kg/day | ↑SOD, CAT, GPX ↓ROS | [211] | |
| Age-related brain deficiency | Chokeberry extract | Male Kunming mice | 8 weeks | 15 or 30 mg/kg | ↓COX2, TGF-β1 and IL-1 ↓DNA degradation | [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, M.; Pop, R.; Daescu, A.; Pintea, A.; Socaciu, C.; Rugina, D. Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview. Molecules 2022, 27, 4254. https://doi.org/10.3390/molecules27134254
Nistor M, Pop R, Daescu A, Pintea A, Socaciu C, Rugina D. Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview. Molecules. 2022; 27(13):4254. https://doi.org/10.3390/molecules27134254
Chicago/Turabian StyleNistor, Madalina, Roxana Pop, Adela Daescu, Adela Pintea, Carmen Socaciu, and Dumitrita Rugina. 2022. "Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview" Molecules 27, no. 13: 4254. https://doi.org/10.3390/molecules27134254
APA StyleNistor, M., Pop, R., Daescu, A., Pintea, A., Socaciu, C., & Rugina, D. (2022). Anthocyanins as Key Phytochemicals Acting for the Prevention of Metabolic Diseases: An Overview. Molecules, 27(13), 4254. https://doi.org/10.3390/molecules27134254









