The Chemical Composition and Biological Activities of Essential Oil from Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.)
Abstract
1. Introduction
2. Botanical Characteristics
3. Agronomic Characteristics
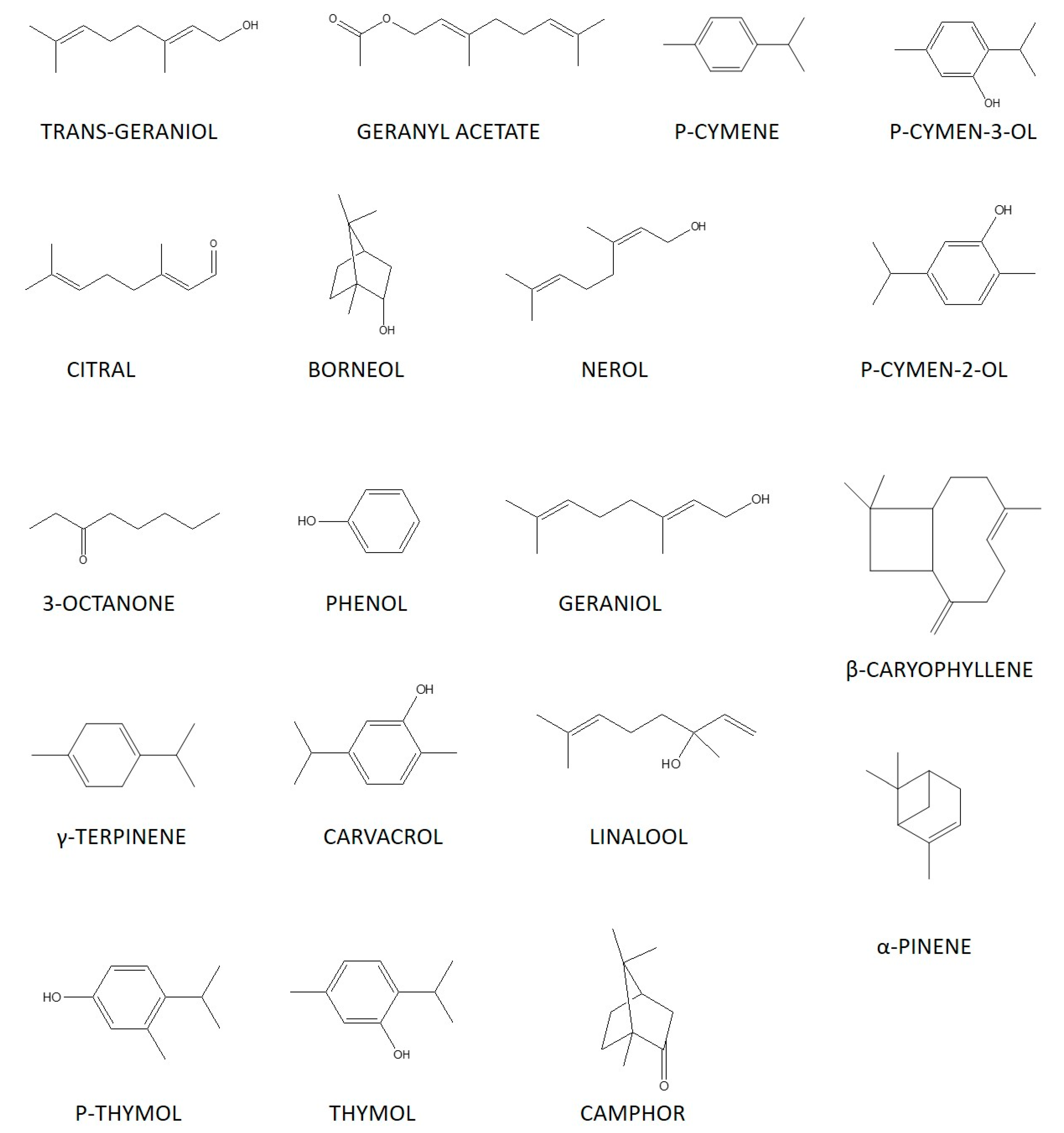
4. Chemical Composition of Essential Oils
5. Biological Properties
5.1. Antioxidant Activity
5.2. Antimicrobial Activity
5.3. Antiviral Activity
5.4. Anti-Inflammatory Activity
5.5. Anticancer Activity
5.6. Analgesic Activity
5.7. Sleep Prolongation Activity
5.8. Calming Activity
5.9. Whitening Activity
5.10. Anti-Obesity Activity
5.11. Skin Protection Activity
5.12. Skin Aging Inhibitory Activity
5.13. Anti-Acne Activity
5.14. Plant Growth Inhibitory Activity
5.15. Aromatherapy Activity
5.16. Ground Cover Plant
| S. No. | Place of Collection | Extraction Method | Major Components | References |
|---|---|---|---|---|
| 1. | Wolchul, Jiri, and Odae mountains, South Korea | Steam distillation | Odae cultivar—thymol (30.54%), γ-terpinene (23.92%), and p-cymene (11.13%) Wolchul cultivar—geraniol (42.94%), geranyl acetate (26.49%), and borneol (5.91%) Jiri cultivar—linalool (47.89%), thymol (15.98%), and caryophyllene (7.02%) | [31] |
| 2. | Yantai city, Shandong Province, China | Hydro-distillation | Linalool (52.003%), borneol (10.911%), and anethole (5.325%) | [117] |
| 3. | China | Hydro-distillation | o-Cymene, carvacrol, caryophyllene, 2-isopropyl-1-methoxy-4-methylbenzene, and gamma-terpene | [118] |
| 4. | Jeju Island, South Korea | Hydro-distillation | p-Cymen-3-ol (50.41%), p-cymen-2-ol (24.06%), and cymene (19.04%) | [35] |
| 5. | Jeju high mountain, Jeju middle mountain, Kyeonggi Province, Ulleung Island, and Gangwon Province, South Korea | Thermal desorption gas chromatograph and mass spectrometer | Jeju high mountain—γ-terpinene (18.51%), thymol (13.89%), bicyclo [2.2.1] heptan-2-one (10.61%), and limonene (5.80%) Jeju middle mountain—thymol (35.91%), γ-terpinene (12.13%), and benzene (5.82%) Kyeonggi Province—carvacrol (18.25%), γ-terpinene (8.73%), and thymol (6.69%) Ulleung Island—phenol (13.48%), δ-terpinene (4.21%), and caryophyllene (3.46%) Gangwon Province—carvacrol (19.20%), γ-terpinene (8.83%), and sabinene hydrate (5.55%) | [41] |
| 6. | Gangwon Province, South Korea | Supercritical fluid extraction and water and steam distillation | Supercritical fluid extraction—thymol (77.63%), carvacrol (5.65%), and β-bisabolene (20.65%) Water and steam distillation—thymol (30.44%), β-bisabolene (20.65%), and caryophyllene (6.46%) | [37] |
| 7. | South Korea | Simultaneous and steam distillation extraction | Thymol (39.8%), γ-terpinene (10%), p-cymene (9.2%), camphor (5.9%) | [28] |
| 8. | Chungbuk, South Korea | Solid-phase microextraction and simultaneous distillation and extraction | Citral (24.90% and 33.67%), trans-geraniol (36.85% and 39.75%), and geranyl acetate (3.43% and 6.00%) | [33] |
| 9. | Cultivated in Seoul, South Korea | Steam distillation | Thymol (41.7%), γ-terpinene (16%), and p-cymene (13%) | [50] |
| 10. | Four regions in China: YL—Shaanxi Province, JB—Shaanxi Province, QY—Gansu Province, and LD—Ningxia Hui Autonomous Region | Hydro-distillation | YL—Shaanxi Province—carvacrol ethyl ether (31.80%), 1,8-cineole (7.23%%), borneol (6.50%), and terpinen-4-ol (4.96%) JB—Shaanxi Province—carvacrol ethyl ether (23.32%), p-cymene (19.20%), terpinen-4-ol (10.56%), borneol (5.61%), and 1,8-cineole (5.22%) QY—Gansu Province—p-vinyl guaiacol (23.55%), thymol (16.32%), o-cymene (12.10%), γ-terpinene (11.11%), and 1,8-cineole (10.16%) LD—Ningxia Hui Autonomous Region—linalool (12.80%) and γ-terpineol (3.04%) | [119] |
| 11. | Laoshan Mountains, Qingdao, China | Steam distillation | Growth period, flowering period, and nearly withered period: linalool—40.31, 39.10, and 45.44%, respectively | [120] |
| S. No. | Sample | Biological Activity | Model | References |
|---|---|---|---|---|
| 1. | Essential oil | Antibacterial | Streptococcus pneumoniae, Staphylococcus aureus, Salmonella enteritidis, and Salmonella typhimurium | [67] |
| 2. | Essential oil | Insecticidal and repellent | Tribolium castaneum, Lasioderma serricorne, and Liposcelis bostrychophila | [117] |
| 3. | Essential oil | Antimicrobial | Propionibacterium | [35] |
| 4. | Essential oil | Antibacterial | E. coli 1-deoxy-d-xylulose-5-phosphate reductoisomerase | [73] |
| 5. | Essential oil | Antifungal | Experimental vaginal candidiasis in mice by Candida albicans | [61] |
| 6. | Essential oil | Antifungal | Aspergillus niger, Aspergillus flavus, Candida albicans, Candida utilis, Cryptococcus neoformans, Trichosporon mucoides, and Blastoschyzomyces capitatus | [50] |
| 7. | Essential oil | Antioxidant | DPPH, ABTS, FRAP thiobarbituric acid reactive substances (TBARS) and oxidative stress in zebrafish | [119] |
| 8. | Thymol | Hepatoprotective | Tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in Chang liver cells. | [121] |
| 9. | Thymol (2-isopropyl-5-methylphenol) | Anti-melanogenic | B16F10 cells, inhibitory effect of thymol to tyrosinase, expression level of tyrosinase in B16F10 cells | [90] |
| 10. | Galuteolin | Skin whitening | B16/F10 melanoma cells | [122] |
| 11. | Water extract | Antioxidant | (LPS) To induce inflammation and oxidative stress in RAW 264.7 macrophages; nitric oxide and H2O2 assay and mitochondrial ATP assay | [1] |
| 12. | Polysaccharides and its fractions | Antioxidant and inhibition of digestive enzymes | DPPH, ABTSagainst 2, 2’-azo-bis-(2-methylpropylimid)-dihydrochloride (AAPH)-induced oxidative stress in a zebrafish model; α-amylase and α-glucosidase | [123] |
| 13. | Water and 70% ethanolic extracts | Antioxidant, cytoprotective, and anti-apoptotic | FRAP, ferric thiocyanate (FTC) and thiobarbituric acid (TBA) methods; t-BHP-induced toxicity | [124] |
| 14. | Extracts obtained by supercritical fluid extraction, simultaneous distillation and extraction, and microwave-assisted extraction | Antioxidant and antimicrobial | Staphylococcus aureus, Bacillus cereus, Salmonella typhimurium, Bacillus subtilis, Escherichia coli, and Saccharomyces cerevisiae; nitrite scavenging, and DPPH | [36] |
| 15. | 50% methanol extract | Alpha-amyalse/-glucosidase inhibition and antioxidant | Alpha-amyalse/-glucosidase ORAC system; maltase and sucrose inhibition | [56] |
| 16. | Extract | Hepatoprotective | Chronic alcohol-induced liver injury in C57 mice | [125] |
| 17. | Ethanol extracts—ethyl acetate fraction | Anti-tumor | Human leukemia cell lines K562 and HL-60 | [126] |
| 18. | Methanol extract—the ethyl acetate fraction | Antioxidant, antimicrobial, and antidiabetic | DPPH scavenging and reducing power assays; Kocuria rhizophila and Staphylococcus epidermidis; α-glucosidase and α-amylase inhibition | [55] |
| 19. | 70% Ethanol | Anti-aging effect | Human keratinocytes | [100] |
| 20. | Ethyl acetate Extract 2(S)-5,7,3’,5’-tetrahydroxyflavanone, (+)-taxifolin, (+)-aromadendrin, rosmarinic acid, caffeic acid, protocatechuic acid, and protocatechuic aldehyde | Pancreatic lipase inhibition | Enzyme-based method | [92] |
| 21. | 70% Ethanol extract | Antioxidant and antimicrobial | DPPH scavenging activity; Enterococcus faecalis, Listeria monocytogenes, Citrobacter Freundii, and Escherichia coli | [58] |
| 22. | Supercritical fluid extraction | Antimicrobial | Fungus—Ascosphaera apis | [64] |
| 23. | Ethanol extract: (1) danshensu, (2) vanillic acid, (3) chlorogenic acid, (4) galuteolin, (5) scutellarin, (6) apigenin | Antioxidant | Response surface methodology based on its DPPH radical scavenging activity | [127] |
| 24. | Polyphenol-rich fraction | Cardioprotective | Myocardial ischemia injury in mice | [128] |
| 25. | High-polar extract (ethanol) and polyphenol-rich fraction (PRF) | Anticerebral ischemia-reperfusion injury effect | Free radicals and zebrafish embryos; transient middle cerebral artery occlusion (tMCAO) model in rats | [129] |
| 26. | Extract | Antifungal | Cladosporium cucumerinum | [62] |
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, M.J.; Kim, J.H.; Kim, H.Y.; Kim, M.J.; Kim, S.M. Chemical composition and biological activity of essential oil of Agastache rugosa (Fisch. & C. A. Mey.) O. Kuntze. Korean J. Crop Sci. 2020, 28, 95–110. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential oils in medicine: Principles of therapy. Parassitologia 2008, 50, 89–91. [Google Scholar] [PubMed]
- Hartmans, K.J.; Diepenhorst, P.; Bakker, W.; Gorris, L.G.M. The use of carvone in agriculture: Sprout suppression of potatoes and antifungal activity against potato tuber and other plant diseases. Ind. Crops Prod. 1995, 4, 3–13. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 1995, 78, 264–269. [Google Scholar]
- Hong, S.G.; Kim, J.J.; Im, H.T. Studies on the shade tolerance of the woody and herbaceous plants for urban forest aromatic bath. J. Korean For. Soc. 2000, 89, 585–590. [Google Scholar]
- Yun, M.S.; Cho, H.M.; Yeon, B.R.; Choi, J.S.; Kim, S. Herbicidal activities of essential oils from Pine, Nut Pine, Larch and Khingan Fir in Korea. Weed Turf. Sci. 2013, 2, 30–37. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Agostini, G.; Atti-Serfini, L.; Paroul, N.; Pauletti, G.F.; dos Santos, A.C. Correlation between the chemical and genetic relationships among commercial thyme cultivars. J. Agric. Food Chem. 2001, 49, 4220–4223. [Google Scholar] [CrossRef]
- Maissa, B.J.; Walid, H. Antifungal activity of chemically different essential oils from wild Tunisian Thymus spp. Nat. Prod. Res. 2015, 29, 869–873. [Google Scholar] [CrossRef]
- Li, X.; He, T.; Wang, X.; Shen, M.; Yan, X.; Fan, S.; Wang, L.; Wang, X.; Xu, X.; Sui, H.; et al. Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem. Biodivers. 2019, 16, e1900254. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. A review on applications and uses of Thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Lee, K.J.; Lee., W.H.; Ryu, K.S. Effect of feeding Thymus vulgaris powder on the productivity, egg quality and egg yolk fatty acid composition in laying hens. Korean J. Poult. Sci. 2012, 39, 157–161. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Leem, H.; Han, S.; Ji, S.J.; So, S. Conservation measures and distribution of vulnerable species for climate change in Gayasan National Park. Korean J. Plant Res. 2017, 30, 167–175. [Google Scholar] [CrossRef][Green Version]
- Lim, D.O.; Kim, Y.S.; Hwang, I.C. Floristic characteristics and rare and endangered plant species in Woraksan National Park. Korean J. Environ. Ecol. 2005, 19, 112–118. [Google Scholar]
- Ahn, Y. Ecological study of the flora at Tumen river area, border of North Korea and China. J. Environ. Sci. 2003, 12, 125–132. [Google Scholar]
- Mizuno, T.; Nakahara, Y.; Fujimori, T.; Yoshida, H. Natural revegetation potential of Japanese wild thyme (Thymus quinquecostatus Celak.) on serpentine quarries. Ecol. Res. 2018, 33, 777–788. [Google Scholar] [CrossRef]
- Kubitzki, K. Flowering Plants Dicotyledons; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; pp. 167–275. [Google Scholar]
- Morales, R. The history, botany and taxonomy of the genus Thymus. In Thyme: The Genus Thymus; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–28. [Google Scholar]
- Bartolucci, F. Verso una revisione biosistematica del genere Thymus L. (Lamiaceae martinov) in Italia: Considerazioni nomenclaturali, sistematiche e criticit tassonomica. Thymus 2010, 50, 1. [Google Scholar]
- Federici, S.; Galimberti, A.; Bartolucci, F.; Bruni, I.; De Mattia, F.; Cortis, P.; Labra, M. DNA barcoding to analyse taxonomically complex groups in plants: The case of Thymus (Lamiaceae). Bot. J. Linn. Soc. 2013, 171, 687–699. [Google Scholar] [CrossRef]
- Shin, H.; Yu, S. Ultrastructure of capitate glandular trichome in leaf of Thymus quinquecostatus. Appl. Microsc. 1998, 28, 159–170. [Google Scholar]
- Jing, H.; Liu, J.; Liu, H.; Xin, H. Histochemical investigation and kinds of alkaloids in leaves of different developmental stages in Thymus quinquecostatus. Sci. World J. 2014, 2014, 839548. [Google Scholar] [CrossRef] [PubMed]
- Korea National Arboretum. Plant Encyclopedia. Korea Forest Service. 2016. Available online: http://www.nature.go.kr/kbi/plant/pilbk/selectPlantPilbkDtl.do?plantPilbkNo=33913 (accessed on 7 March 2021).
- Nakada, M.; Sugawara, T. Floral dimorphism and gynodioecy in Thymus quinquecostatus (Lamiaceae). Acta Phytotaxon. Geobot. 2011, 62, 25–34. [Google Scholar]
- Lim, Y.S.; Kim, Y.D.; Shin, H.C. Lectotypification and identity of Thymus quinquecostatus var. magnus (Nakai) Kitam. (Labiatae). Korean J. Pl. Taxon. 2006, 36, 129–136. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.C.; Choi, Y.H. Essential oil of Thymus quinquecostatus Celakov. and Thymus magnus Nakai. Korean J. Med. Crop Sci. 1994, 2, 234–240. [Google Scholar]
- Chen, G.; Tang, Y.; Qu, C.T.; Zhang, Q.Z.; Mu, S.Z. Study on the chemical components of essential oil of Thymus quinquecostatus Celak. from Shandong Yimeng. Jingxi Huagong Zhongjianti 2009, 39, 70–72. [Google Scholar]
- Minli, Y.; Fengxia, H.; Jun, H. A study on the chemical components of essential oil of Thymus quinquecostatus in Ningxia Guyuan by GC-MS. J. Ningxia Univ. 2004, 25, 353–355. [Google Scholar]
- Kim, M.; Moon, J.C.; Kim, S.; Sowndhararajan, K. Morphological, chemical, and genetic characteristics of Korean native thyme Bak-ri-hyang (Thymus quinquecostatus Celak.). Antibiotics 2020, 9, 289. [Google Scholar] [CrossRef]
- Choi, I.Y.; Cho, C.H.; Moon, J.S.; Song, Y.J.; Choi, D.C. Determination of optimum cultivation technique of Thymus quinquecostatus Celakov for high quality herb production. J. Agric. Life Sci. 2010, 41, 19–24. [Google Scholar]
- Song, Y.E.; Ku, C.S.; Mun, S.P.; Ryu, J.S.; Kim, D.H.; Choi, J.S.; Choi, Y.G. Volatile aroma compounds and their characteristics of Labiatae by solid-phase microextraction (SPME). Korean J. Med. Crop Sci. 2002, 10, 120–125. [Google Scholar]
- Choi, I.Y.; Song, Y.J.; Choi, D.C.; Lee, W.H. A comparative study for obtaining maximum essential oil from six herbs on the basis of harvesting time, cultivation regions & type, and drying methods. Korean J. Hortic. Sci. Technol. 2010, 28, 492–496. [Google Scholar]
- Oh, T.H.; Kim, S.S.; Yoon, W.J.; Kim, J.Y.; Yang, E.J.; Lee, N.H.; Hyun, C.G. Chemical composition and biological activities of Jeju Thymus quinquecostatus essential oils against Propionibacterium species inducing acne. J. Gen. Appl. Microbiol. 2009, 55, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.K.; Kim, S.W.; Kim, Y.W.; Choi, Y.H.; Kwon, J.H. Evaluation of functional properties of the traditional herbs in Korea. Food Eng. Prog. 2005, 9, 249–261. [Google Scholar]
- Lee, S.E.; Kim, S.; Lim, W.C.; Kang, K.C.; Pyo, H.B. Comparison of volatile compounds from Thymus magnus Nakai by three different extraction methods. J. Soc. Cosmet. Sci. Korea 2014, 40, 171–178. [Google Scholar]
- Cardoso-Ugarte, G.A.; Juarez-Becerra, G.P.; SosaMorales, M.E.; Lopez-Malo, A. Microwave-assisted extraction of essential oils from herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef]
- Kokolakis, A.K.; Golfinopoulos, S.K. Microwave-assisted techniques (MATs); a quick way to extract a fragrance: A review. Nat. Prod. Commun. 2013, 8, 1493–1504. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.B.; Kim, K.S.; Kim, M.S. Changes of growth characteristics, rosmarinic acid and essential oil contents according to harvest time in Agastache rugosa O. Kuntze. Korean J. Med. Crop Sci. 1999, 7, 83–88. [Google Scholar]
- Chiang, M.H.; Lee, K.W.; Baik, J. Volatile aroma compounds of several domestic Thymus quinquecostatus by thermal desorption gas chromatograph mass spectrometer. J. Bio-Environ. Con. 2011, 20, 14–20. [Google Scholar]
- Ceylan, R.; Zengin, G.; Uysal, S.; Ilhan, V.; Aktumsek, A.; Kandemir, A.; Anwar, F. GC-MS analysis and in vitro antioxidant and enzyme inhibitory activities of essential oil from aerial parts of endemic Thymus spathulifolius Hausskn. et Velen. J. Enzyme Inhib. Med. Chem. 2016, 31, 983–990. [Google Scholar] [CrossRef]
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Costa, M.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Latif, S.; Sherazi, S.T.H.; Ahmad, A.; Worthington, J.; Sarker, S.D. Chemical composition and bioactivity studies of the essential oils from two Thymus species from the Pakistani flora. Food Sci. Technol. 2013, 50, 185–192. [Google Scholar] [CrossRef]
- Zani, F.; Massimo, G.; Benvenuti, S.; Bianchi, A.; Albasini, A.; Melegari, M.; Vampa, G.; Bellotti, A.; Mazza, P. Studies on the genotoxic properties of essential oils with Bacillus subtilis rec-assay and salmonella/microsome reversion assay. Planta Med. 1991, 57, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Cioni, P.L.; Tomei, P.E.; Catalano, S.; Morelli, I. Study of variation in individual essential oils in a micropopulation of Thymus vulgaris L. plants. Riv. Ital. 1990, 1, 3–6. [Google Scholar]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Zeljkovic, S.C.; Maksimovic, M. Chemical composition and bioactivity of essential oil from Thymus species in Balkan Peninsula. Phytochem. Rev. 2015, 14, 335–352. [Google Scholar] [CrossRef]
- Morales, R. Synopsis of the genus Thymus L. in the Mediterranean area. Lagascalia. 1997, 19, 249–262. [Google Scholar]
- Shin, S.; Kim, J.H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Med. 2004, 70, 1090–1092. [Google Scholar] [CrossRef]
- Shin, H.C.; Choi, H.K. A taxonomic study on Thymus in Korea-numerical analyses of morphological characters. Korean J. Pl. Taxon. 1997, 27, 117–135. [Google Scholar] [CrossRef]
- Baik, J.; Baek, Y.; Chiang, M. Phenol contents of solvent extraction in several domestic Thymus quinquecostatus Celak. J. Bio-Environ. Con. 2009, 18, 468–474. [Google Scholar]
- Sin, D.H. Research and prospects of natural antioxidants. Bull. Food Technol. 1995, 8, 28–36. [Google Scholar]
- Farag, R.S.; Badei, A.Z.M.A.; Hewedi, F.M.; El-Baroty, G.S.A. Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J. Am. Oil Chem. Soc. 1989, 66, 792–799. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.; Kim, J. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 2014, 52, 611–616. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, H.J.; Jang, H.; Kwon, Y. In vitro α-glucosidase inhibitory potential and antioxidant activity of selected Lamiaceae species inhabited in Korean penninsula. Food Sci. Biotechnol. 2009, 18, 239–244. [Google Scholar]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Choi, I.Y.; Song, Y.J.; Lee, W.H. DPPH radical scavenging effect and antimicrobial activities of some herbal extracts. Korean J. Hortic. Sci. Technol. 2010, 28, 871–876. [Google Scholar]
- Khadir, A.; Sobeh, M.; Gad, H.A.; Benbelaid, F.; Bendahou, M.; Peixoto, H.; Sporer, F.; Ashour, M.L.; Wink, M. Chemical composition and biological activity of the essential oil from Thymus lanceolatus. Z. Naturforsch. C J. Biosci. 2016, 71, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, S.W. In vivo anti-fungal activity of the essential oil fraction from Thymus species and in vitro synergism with clotrimazole. Nat. Prod. Sci. 2007, 13, 258–262. [Google Scholar]
- Park, S.H.; Hong, S.J.; Shim, C.K.; Kim, M.J.; Park, J.H.; Han, E.J.; Kim, Y.K. Screening for effective organic farming materials for the control of cucumber scab caused by Cladosporium cucumerinum. Res. Plant Dis. 2017, 23, 159–167. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Wurster, M.; Schroder, G.; Lindequist, U. In vitro antimicrobial, cytotoxic and radical scavenging activities and chemical constituents of the endemic Thymus laevigatus (Vahl). Rec. Nat. Prod. 2010, 4, 49–63. [Google Scholar]
- Choi, S.W.; Lee, M.Y.; Hong, I.P.; Choi, Y.S.; Kim, H.K.; Kim, N.S.; Lee, K.G.; Kim, J.M.; Hwang, C.Y. Antimicrobial activity of herbal plants extracts on Ascosphaera apis. Korean J. Apic. 2013, 28, 211–216. [Google Scholar]
- Oliveira, A.P.; Santos, A.A.; Santana, A.S.; Lima, A.P.S.; Melo, C.R.; Santana, E.D.; Sampaio, T.S.; Blank, A.F.; Araujo, A.P.A.; Cristaldo, P.F. Essential oil of Lippia sidoides and its major compound thymol: Toxicity and walking response of populations of Sitophilus zeamais (Coleoptera: Curculionidae). Crop Prot. 2018, 112, 33–38. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, K.Y.; Jang, D.Y.; Um, D.Y.; Kim, T.J.; Jung, B.J. Phytopathogenic activities of essential oils and their main compounds. Korean. J. Pestic. Sci. 2006, 10, 201–209. [Google Scholar]
- Shin, S.; Kim, J.H. In vitro inhibitory activities of essential oils from two Korean Thymus species against antibiotic-resistant pathogens. Arch. Pharm. Res. 2005, 28, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Benabdelkader, T.; Chelghoum, C. Studies on the essential oil composition and antimicrobial activity of Thymus algeriensis Boiss. et Reut. Int. J. Aromather. 2006, 16, 95–100. [Google Scholar] [CrossRef]
- Karaman, S.; Digrak, M.; Ravid, U.; Ilcim, A. Antibacterial and antifungal activity of the essential oils of Thymus revolutus Celak from Turkey. J. Ethnopharmacol. 2001, 76, 183–186. [Google Scholar] [CrossRef]
- Rasooli, I.; Mirmostafa, S.A. Antibacterial properties of Thymus pubescens and Thymus serpyllum essential oils. Fitoterapia 2002, 73, 244–250. [Google Scholar] [CrossRef]
- Tantaoui-Elaraki, A.; Lattaoui, N.; Errifi, A.; Benjilali, B. Composition and antimicrobial activity of the essential oils of Thymus broussonettii, T. zygis and T. satureioides. J. Essent. Oil Res. 1993, 5, 45–53. [Google Scholar] [CrossRef]
- Yan, G.; Zhu, B.R.; Tian, F.L.; Hui, X.; Li, H.; Li, Y.M.; Gao, W.Y. Inhibitory activity of plant essential oils against E. coli 1-deoxy-d-xylulose-5-phosphate reductoisomerase. Molecules 2019, 24, 2518. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbi. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Huang, S.T.; Sun, F.M.; Chiang, Y.L.; Chiang, C.J.; Tsai, C.M.; Weng, C.J. Transformation of cinnamic acid from trans-to cis-form raises a notable bactericidal and synergistic activity against multiple-drug resistant Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2011, 43, 188–194. [Google Scholar] [CrossRef]
- Bolla, J.; Alibert-Franco, S.; Handzlik, J.; Chevalier, J.; Mahamoud, A.; Boyer, G.; Kiec-Kononowicz, K.; Pages, J. Strategies for bypassing the membrane barrier in multidrug resistant gram-negative bacteria. FEBS Lett. 2011, 585, 1682–1690. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–92. [Google Scholar] [CrossRef]
- Kim, S.M.; Suk, K.D. Anti-nociceptive, anti-inflammatory, mental effects of essential oil from Thymus magnus. Yakhak Hoeji. 2007, 51, 508–516. [Google Scholar]
- Islam, M.T.; Khalipha, A.B.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer activity of thymol: A literature-based review and docking study with emphasis on its anticancer mechanisms. IUBMB Life 2019, 71, 9–19. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef]
- Zeng, Q.; Che, Y.; Zhang, Y.; Chen, M.; Guo, Q.; Zhang, W. Thymol Isolated from Thymus vulgaris L. Inhibits Colorectal Cancer Cell Growth and Metastasis by Suppressing the Wnt/β-Catenin Pathway. Drug Des. Devel. Ther. 2020, 14, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Rep. 2021, 11, 13178. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.; Lukanov, J.; Sagorchev, P. Effect of thymol on the spontaneous contractile activity of the smooth muscles. Phytomedicine 2007, 14, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Suh, H.S.; Song, I.J.; Shon, J.W. The Factors that were associated with prescription of hypnotics in inpatients of a large mental hospital. J. Korean Soc. Biol. Ther. Psychiatry 1997, 3, 100–111. [Google Scholar]
- Enforcement Decree of the Cosmetics Act. Functional Cosmetics Standards and Test Methods. Ministry of Food and Drug Safety; 2020. Available online: https://www.law.go.kr/%EB%B2%95%EB%A0%B9/%ED%99%94%EC%9E%A5%ED%92%88%EB%B2%95%20%EC%8B%9C%ED%96%89%EB%A0%B9 (accessed on 7 March 2021).
- Park, J.A.; Jeong, S.H. Melanin inhibitory activity and changes in the melanin and erythema values of Rumex crispus L. extracts. Asian J. Beauty Cosmetol. 2012, 10, 291–297. [Google Scholar]
- Choi, D.; Park, C.I.; Lee, S.; Baek, J. Anti-melanogenic effect of thymol, a major odorant in essential oils of family Lamiaceae. Korean J. Herbol. 2019, 34, 19–25. [Google Scholar]
- Lee, J.H.; Lee, E.S.; Bae, I.H.; Hwang, J.A.; Kim, S.H.; Kim, D.Y.; Park, N.H.; Rho, H.S.; Kim, Y.J.; Oh, S.G. Antimelanogenic efficacy of melasolv (3, 4, 5-trimethoxycinnamate thymol ester) in melanocytes and three-dimensional human skin equivalent. Skin Pharmacol. Physiol. 2017, 30, 190–196. [Google Scholar] [CrossRef]
- Lee, I.; Bae, J.S.; Kim, T.; Kwon, O.J.; Kim, T.H. Polyphenolic constituents from the aerial parts of Thymus quinquecostatus var. japonica collected on Ulleung island. Appl. Biol. Chem. 2011, 54, 811–816. [Google Scholar]
- Sedighi, R.; Zhao, Y.; Yerke, A.; Sang, S. Preventive and protective properties of rosemary (Rosmarinus officinalis L.) in obesity and diabetes mellitus of metabolic disorders: A brief review. Curr. Opin. Food Sci. 2015, 2, 58–70. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D‘Orazio, J. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Guo, W.; An, Y.; Jiang, L.; Geng, C.; Zhong, L. The protective effects of hydroxytyrosol against UVB-induced DNA damage in HaCaT cells. Phytother. Res. 2010, 24, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Urbach, F. Potential effects of altered solar ultraviolet radiation on human skin cancer. Photochem. Photobiol. 1989, 50, 507–513. [Google Scholar] [CrossRef]
- Yam, J.C.; Kwok, A.K. Ultraviolet light and ocular diseases. Int. Ophthalmol. 2014, 34, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Cornaghi, L.; Arnaboldi, F.; Calo, R.; Landoni, F.; Preis, W.F.B.; Marabini, L.; Donetti, E. Effects of UV rays and thymol/Thymus vulgaris L. extract in an ex vivo human skin model: Morphological and genotoxicological assessment. Cells Tissues Organs 2016, 201, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Choi, J.; Choi, Y.; Shin, S.; Kang, S.; Han, S.J.; Kang, Y. (−) Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food Chem. Toxicol. 2008, 46, 1298–1307. [Google Scholar] [CrossRef]
- Jung, H.; Jeong, H.J.; Shin, K.; Kim, Y.S.; Moon, J.H.; Lee, T.H. Protective effect of Thymus quinquecostatus extracts UVB-induced matrix metalloproteinase-1 via suppressing MAPKs phosphorylation in human keratinocyte. J. Appl. Biol. Chem. 2018, 61, 417–421. [Google Scholar] [CrossRef]
- Calo, R.; Visone, C.M.; Marabini, L. Thymol and Thymus vulgaris L. activity against UVA- and UVB-induced damage in NCTC 2544 cell line. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 791, 30–37. [Google Scholar] [CrossRef]
- Ku, J.E.; Park, C.H. Research trends in effective medicinal plants for acne. Korean Soc. Cosmet. Cosmetol. 2018, 8, 431–445. [Google Scholar]
- Bassett, I.B.; Barnetson, R.S.C.; Pannowitz, D.L. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [CrossRef]
- Athikomkulchai, S.; Watthanachaiyingcharoen, R.; Tunvichien, S.; Vayumhasuwan, P.; Karnsomkiet, P.; Saejong, P.; Ruangrungsi, N. The development of anti-acne products from Eucalyptus globulus and Psidium guajava oil. J. Health Res. 2008, 22, 109–113. [Google Scholar]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Abies koreana essential oil inhibits drug-resistant skin pathogen growth and LPS-induced inflammatory effects of murine macrophage. Lipids 2009, 44, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Baik, J.S.; Oh, T.; Yoon, W.; Lee, N.H.; Hyun, C. Biological activities of Korean Citrus obovoides and Citrus natsudaidai essential oils against acne-inducing bacteria. Biosci. Biotechnol. Biochem. 2008, 72, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production. Pol. J. Microbiol. 2009, 58, 61–68. [Google Scholar] [PubMed]
- Lertsatitthanakorn, P.; Taweechaisupapong, S.; Aromdee, C.; Khunkitti, W. In vitro bioactivities of essential oils used for acne control. Int. J. Aromather. 2006, 16, 43–49. [Google Scholar] [CrossRef]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Viyoch, J.; Pisutthanan, N.; Faikreua, A.; Nupangta, K.; Wangtorpol, K.; Ngokkuen, J. Evaluation of in vitro antimicrobial activity of Thai basil oils and their micro-emulsion formulas against Propionibacterium acnes. Int. J. Cosmet. Sci. 2006, 28, 125–133. [Google Scholar] [CrossRef]
- Sinha, P.; Srivastava, S.; Mishra, N.; Yadav, N.P. New perspectives on antiacne plant drugs: Contribution to modern therapeutics. Biomed. Res. Int. 2014, 2014, 301304. [Google Scholar] [CrossRef]
- Kim, K.W.; Hong, K.S. Herbicidal activities of methanol extracts from Korean native plants against barnyardgrass and duckweed. Korean J. Weed Sci. 2005, 25, 209–220. [Google Scholar]
- Manion, C.R.; Widder, R.M. Essentials of essential oils. Am. J. Health Syst. Pharm. 2017, 74, e153–e162. [Google Scholar] [CrossRef]
- Cristina, E.D. Understanding true aromatherapy: Understanding essential oils. Home Health Care Manag. Pract. 2004, 16, 474–479. [Google Scholar] [CrossRef]
- Bang, K.; Ju, J.; Kim, S. Effects of soil depth and irrigation period on some of the native plants in and artificial substrate of roof garden. J. Korean Soc. Environ. Restor. Technol. 2004, 7, 75–83. [Google Scholar]
- Youn, H.J.; Jang, S.W.; Lee, E.H. Temperature monitoring of vegetation models for the extensive green roof. KIEAE J. 2013, 13, 89–96. [Google Scholar]
- Lu, X.X.; Feng, Y.X.; Du, Y.S.; Zheng, Y.; Borjigidai, A.; Zhang, X.; Du, S.S. Insecticidal and repellent activity of Thymus quinquecostatus Celak. essential oil and major compositions against three stored-product insects. Chem. Biodivers. 2021, 18, e2100374. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Yuan, Y.; Ai, K.H. Study on the essential oil components in Thymus quinquecostatus Celak. Yao Xue Xue Bao Acta Pharm. Sin. 2001, 36, 233–234. [Google Scholar]
- He, T.; Li, X.; Wang, X.; Xu, X.; Yan, X.; Li, X.; Sun, S.; Dong, Y.; Ren, X.; Liu, X.; et al. Chemical composition and anti-oxidant potential on essential oils of Thymus quinquecostatus Celak. from Loess Plateau in China, regulating Nrf2/Keap1 signaling pathway in zebrafish. Sci. Rep. 2020, 10, 11280. [Google Scholar] [CrossRef]
- Jia, P.; Liu, H.; Gao, T.; Xin, H. Glandular trichomes and essential oil of Thymus quinquecostatus. Sci. World J. 2013, 2013, 387952. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, J.W.; Kang, S.H.; Kim, E.H.; Jeon, Y.J.; Jeong, J.H.; Kim, H.R.; Moon, S.H.; Jeon, B.T.; Park, P.J. Thymol from Thymus quinquecostatus Celak. protects against tert-butyl hydroperoxide-induced oxidative stress in Chang cells. J. Nat. Med. 2014, 68, 154–162. [Google Scholar] [CrossRef]
- Kim, M.H.; Kwon, B.; Kim, K.S.; Kim, M.S.; Kim, M.J.; Kim, H.J.; Choi, D.I.; Park, M.; Kim, M.; Shin, M.K.; et al. Galuteolin, identified in the extract of Thymus quinquecostatus flowers, is involved in inhibiting melanin biosynthesis in B16/F10 melanoma cells. Nat. Prod. Res. 2021, 35, 5389–5391. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Dong, Y.; Song, R.; Wei, J.; Yu, A.; Fan, Q.; Yao, J.; Shan, D.; Lv, F.; et al. Preparation, structural analysis, antioxidant and digestive enzymes inhibitory activities of polysaccharides from Thymus quinquecostatus Celak. leaves. Ind. Crops Prod. 2022, 175, 114288. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, S.J.; Hwang, J.W.; Kim, E.K.; Kim, S.E.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. In vitro protective effects of Thymus quinquecostatus Celak extracts on t-BHP-induced cell damage through antioxidant activity. Food Chem. Toxicol. 2012, 50, 4191–4198. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Ren, X.Y.; Liu, X.Y.; Ma, J.M.; Song, R.L.; Wang, X.H.; Dong, Y.; Yu, A.X.; Fan, Q.Q.; et al. Gut dysbiosis correction contributes to the hepatoprotective effects of Thymus quinquecostatus Celak extract against alcohol through the gut-liver axis. Food Funct. 2021, 12, 10281–10290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.X.; Zhang, Y.H.; Cheng, S.; Ma, Q.W.; Guo, S.L.; Zhang, J.B. Anti-tumor effect of ethanol extracts from Thymus quinquecostatus Celak on human leukemia cell line. Chin. J. Integr. Med. 2005, 3, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Shen, M.; Ren, X.Y.; He, T.; Wang, L.; Fan, S.S.; Wang, X.H.; Li, X.; Wang, X.P.; Chen, X.Y.; et al. Multi-Response extraction optimization based on anti-oxidative activity and quality evaluation by main indicator ingredients coupled with chemometric analysis on Thymus quinquecostatus Celak. Molecules 2018, 23, 957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, S.; Niu, F.; Liu, Y.; Liu, X.; Ren, X.; Yang, Y.; Fan, G.; Dong, H.; Shen, M.; et al. Polyphenol-rich fraction from Thymus quinquelostatus Celak attenuates the myocardial ischemia injury in mice induced by isoproterenol through inhibiting apoptosis, antioxidation and activating PI3K/AKT pathway. J. Funct. Foods. 2021, 87, 104805. [Google Scholar] [CrossRef]
- Fan, S.; Liu, X.; Wang, Y.; Ren, X.; Liu, Y.; Dong, Y.; Fan, Q.; Wei, J.; Ma, J.; Yu, A.; et al. Thymus quinquecostatus Celak. ameliorates cerebral ischemia-reperfusion injury via dual antioxidant actions: Activating Keap1/Nrf2/HO-1 signaling pathway and directly scavenging ROS. Phytomedicine 2021, 91, 153673. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Sowndhararajan, K.; Kim, S. The Chemical Composition and Biological Activities of Essential Oil from Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules 2022, 27, 4251. https://doi.org/10.3390/molecules27134251
Kim M, Sowndhararajan K, Kim S. The Chemical Composition and Biological Activities of Essential Oil from Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules. 2022; 27(13):4251. https://doi.org/10.3390/molecules27134251
Chicago/Turabian StyleKim, Minju, Kandhasamy Sowndhararajan, and Songmun Kim. 2022. "The Chemical Composition and Biological Activities of Essential Oil from Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.)" Molecules 27, no. 13: 4251. https://doi.org/10.3390/molecules27134251
APA StyleKim, M., Sowndhararajan, K., & Kim, S. (2022). The Chemical Composition and Biological Activities of Essential Oil from Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules, 27(13), 4251. https://doi.org/10.3390/molecules27134251






