Effect of Ylang-Ylang (Cananga odorata Hook. F. & Thomson) Essential Oil on Acute Inflammatory Response In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. Analysis of YEO
2.2. YEO Did Not Induce In Vitro Cytotoxicity
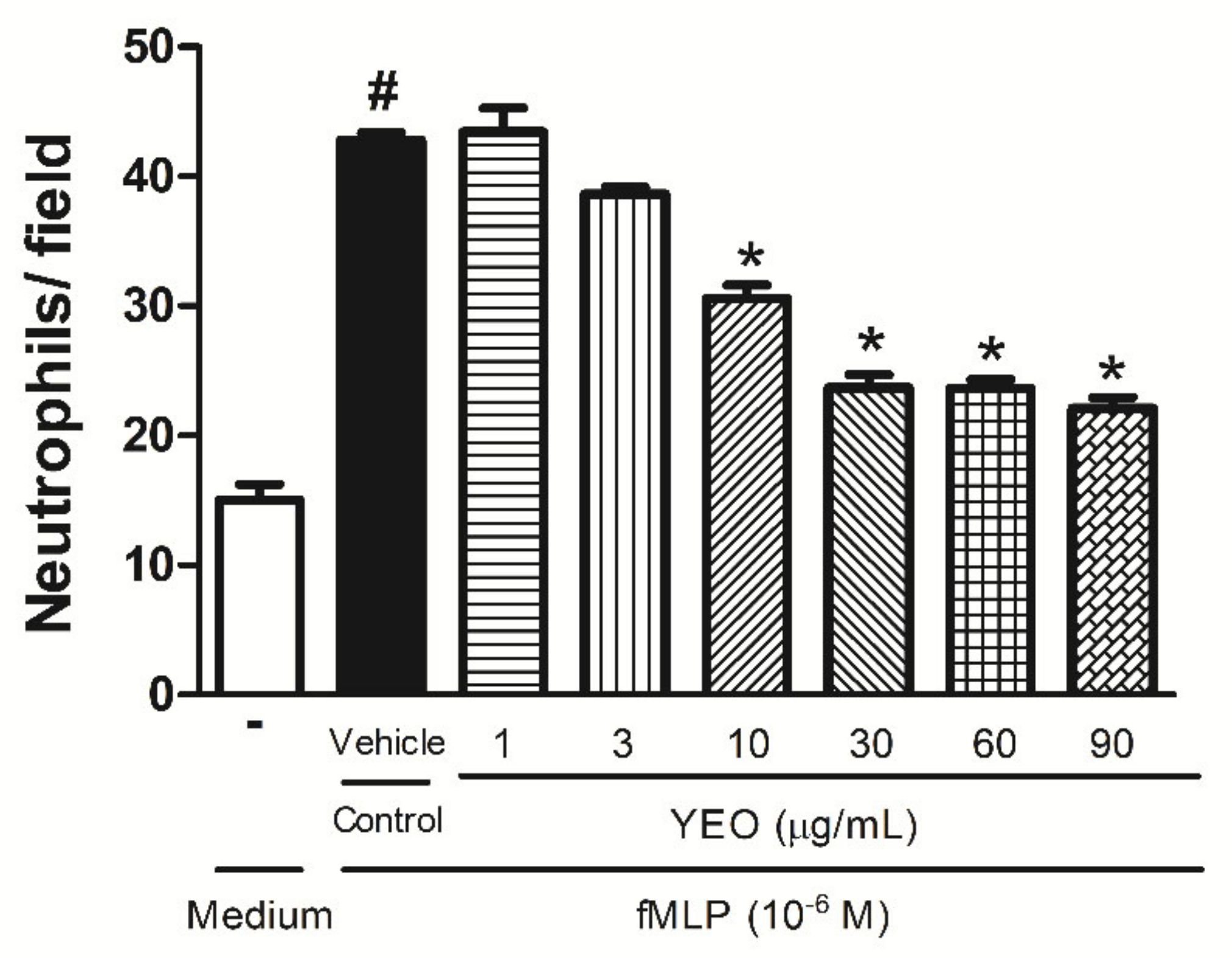
2.3. YEO Reduces Neutrophil Chemotaxis In Vitro
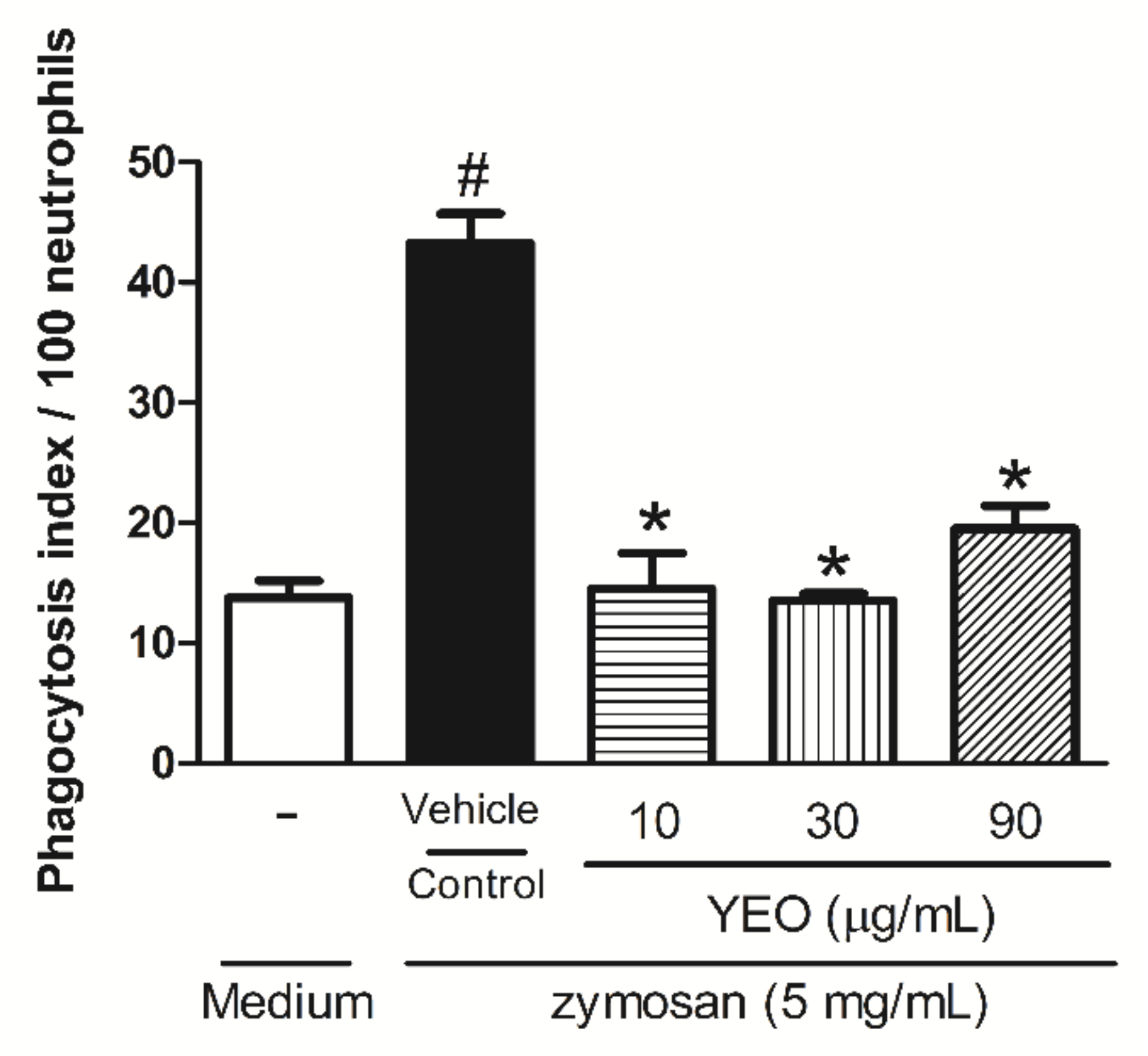
2.4. YEO Reduces Neutrophil Phagocytic Activity
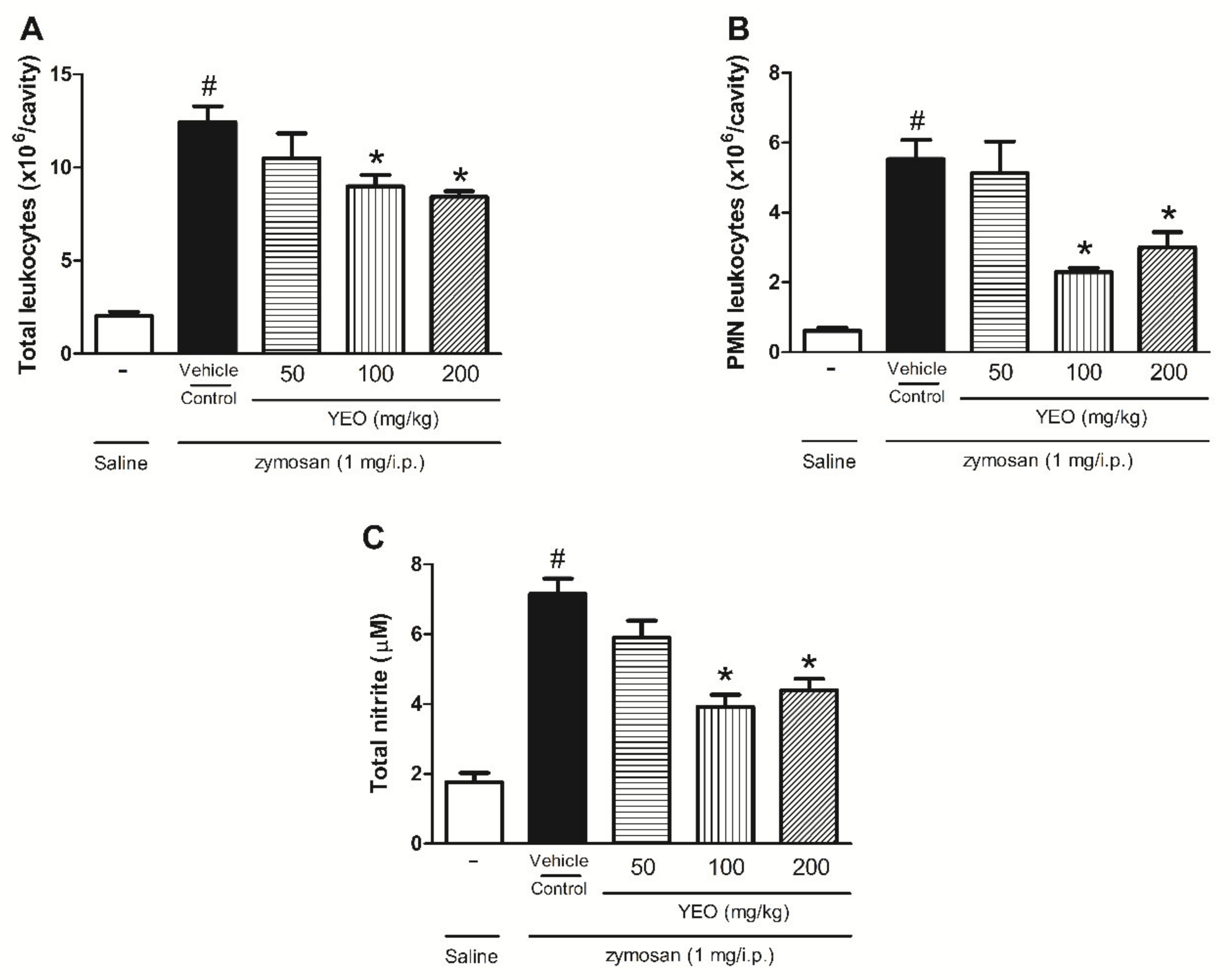
2.5. YEO Reduces Leukocyte Recruitment and Nitric Oxide Production in the Zymosan-Induced Peritonitis Model
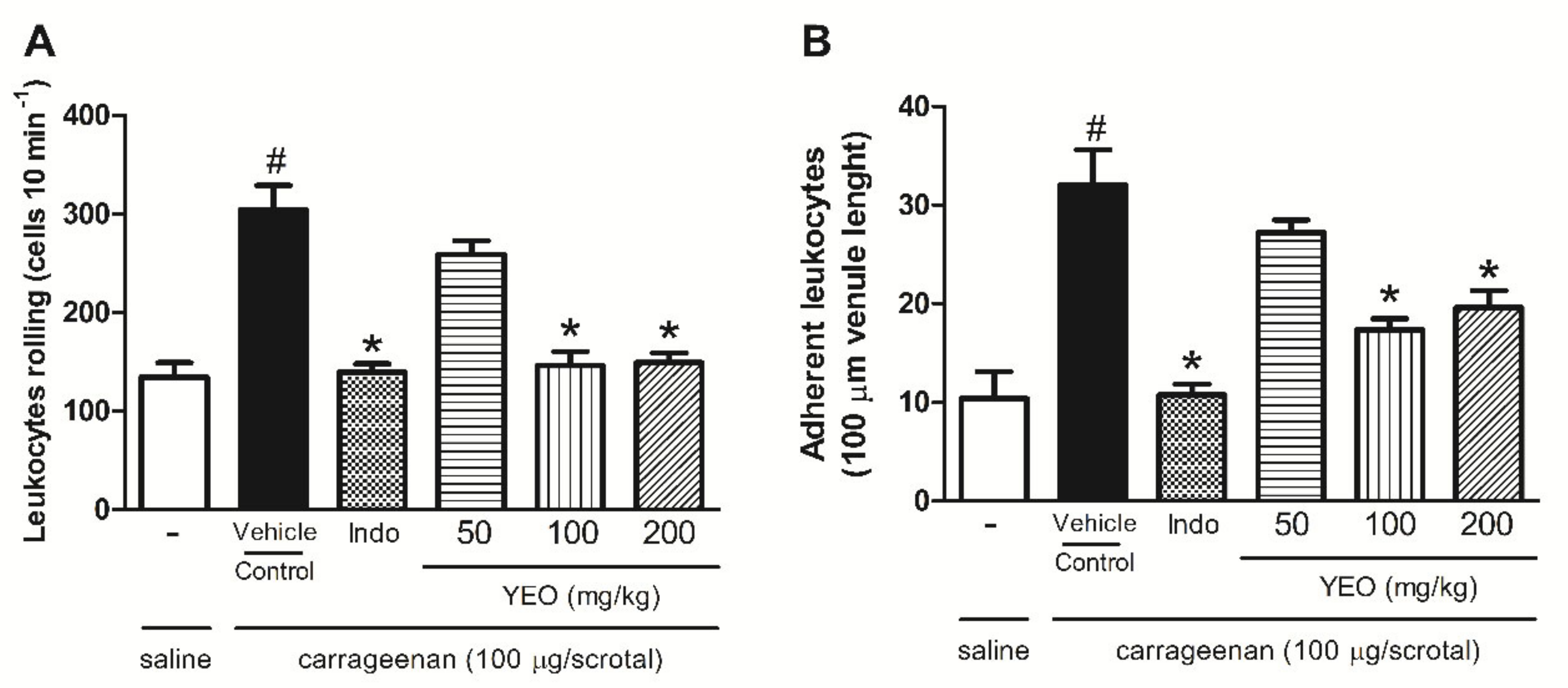
2.6. YEO Treatment Reduces Leukocyte Rolling and Adhesion
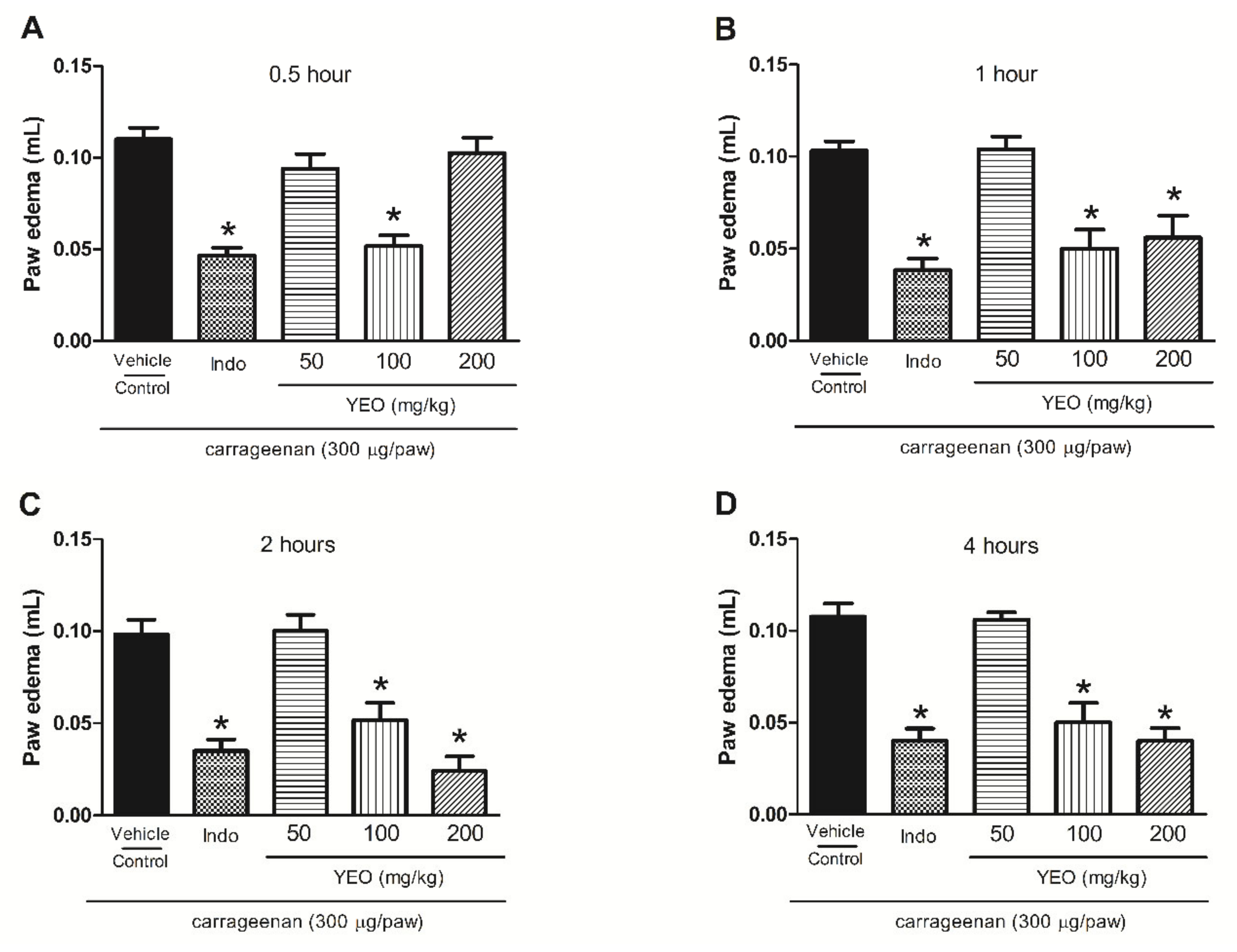
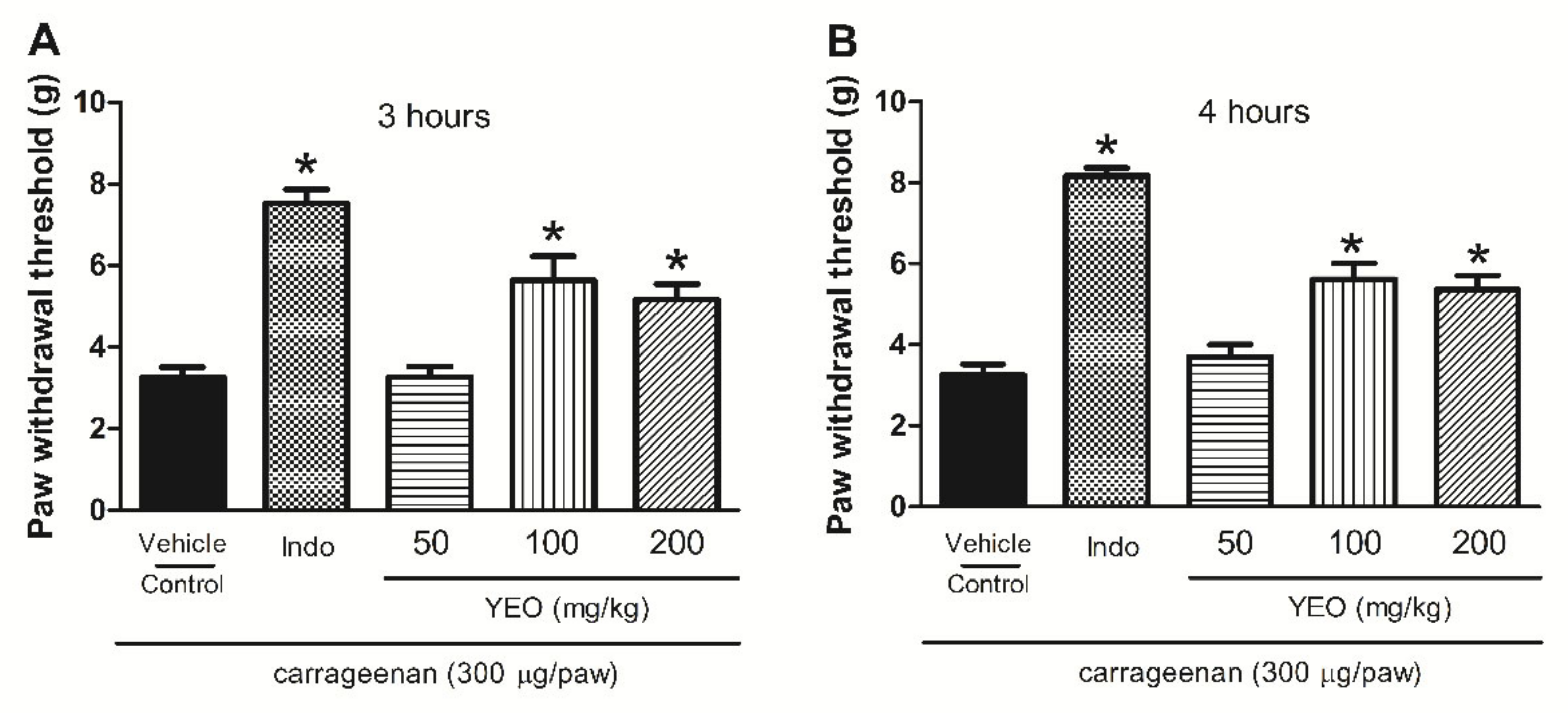
2.7. YEO Treatment Reduces Paw Edema Formation and Mechanical Hyperalgesia Induced by Carrageenan
2.8. YEO Treatment Did Not Induce Acute Oral Toxicity In Vivo
3. Discussion
4. Material and Methods
4.1. Chemicals and Drugs
4.2. Chemical Analysis of YEO
4.3. Animals
4.4. Leukocyte Preparation for In Vitro Assays
4.5. Cell Viability Analysis (MTT Test)
4.6. In Vitro Neutrophil Chemotaxis
4.7. In Vitro Phagocytic Activity of Neutrophils
4.8. Leukocyte Recruitment and Nitric Oxide Levels Determination in Zymosan-Induced Peritonitis Model
4.9. In Situ Intravitral Microscopy Analysis for Rolling and Adhesion Events of Leukocytes in the Microcirculation
4.10. Paw Edema and Mechanical Hyperalgesia Induced by Carrageenan
4.11. Acute Oral Toxicity Study
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The History of Herbs, Medicinal and Aromatic Plants, and Their Extracts. In Feed Additives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Edris, A.E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shrosphire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Ahmed, A.F.; Attia, F.A.K.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant Activity and Total Phenolic Content of Essential Oils and Extracts of Sweet Basil (Ocimum Basilicum L.) Plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga Odorata (Ylang-Ylang). Evid. Based Complement. Altern. Med. 2015, 2015, 896314. [Google Scholar] [CrossRef]
- Indrasetiawan, P.; Aoki-Utsubo, C.; Hanafi, M.; Hartati, S.; Wahyuni, T.S.; Kameoka, M.; Yano, Y.; Hotta, H.; Hayashi, Y. Antiviral Activity of Cananga Odorata Against Hepatitis B Virus. Kobe J. Med. Sci. 2019, 65, E71–E79. [Google Scholar]
- Ahmad, I.; Beg, A.Z. Antimicrobial and Phytochemical Studies on 45 Indian Medicinal Plants against Multi-Drug Resistant Human Pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar] [CrossRef]
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and Cytotoxic Activities of Ethnopharmacologically Selected Medicinal Plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef]
- Kusuma, I.W.; Murdiyanto; Arung, E.T.; Syafrizal; Kim, Y. Antimicrobial and Antioxidant Properties of Medicinal Plants Used by the Bentian Tribe from Indonesia. Food Sci. Hum. Wellness 2014, 3, 191–196. [Google Scholar] [CrossRef]
- Pujiarti, R.; Ohtani, Y.; Widowati, T.B.; Wahyudi, K.; Herath, N.K.; Wang, C.N. Effect of Melaleuca Leucadendron, Cananga Odorata and Pogostemon Cablin Oil Odors on Human Physiological Responses. Wood Res. 2012, 3, 100–105. [Google Scholar] [CrossRef]
- Li, Y.; Zidorn, C. Seasonal variations of natural products in European herbs. Phytochem. Rev. 2022, 21, 1–27. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic Inflammation: Importance of NOD2 and NALP3 in Interleukin-1beta Generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- van Loon, L.M.; Stolk, R.F.; van der Hoeven, J.G.; Veltink, P.H.; Pickkers, P.; Lemson, J.; Kox, M. Effect of Vasopressors on the Macro- and Microcirculation During Systemic Inflammation in Humans In Vivo. Shock 2020, 53, 171–174. [Google Scholar] [CrossRef]
- Robb, C.T.; Regan, K.H.; Dorward, D.A.; Rossi, A.G. Key Mechanisms Governing Resolution of Lung Inflammation. Semin. Immunopathol. 2016, 38, 425–448. [Google Scholar] [CrossRef]
- Silva, M.T. Macrophage Phagocytosis of Neutrophils at Inflammatory/Infectious Foci: A Cooperative Mechanism in the Control of Infection and Infectious Inflammation. J. Leukoc. Biol. 2011, 89, 675–683. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Dey, S.; Pal, C.; Goyal, M.; Alam, A.; Iqbal, M.S.; Sarkar, S.; Azhar Siddiqui, A.; Banerjee, C.; et al. Nonsteroidal Anti-Inflammatory Drug Induces Proinflammatory Damage in Gastric Mucosa through NF-ΚB Activation and Neutrophil Infiltration: Anti-Inflammatory Role of Heme Oxygenase-1 against Nonsteroidal Anti-Inflammatory Drug. Free Radic. Biol. Med. 2013, 65, 456–467. [Google Scholar] [CrossRef]
- Choi, E.-M.; Hwang, J.-K. Screening of Indonesian Medicinal Plants for Inhibitor Activity on Nitric Oxide Production of RAW264.7 Cells and Antioxidant Activity. Fitoterapia 2005, 76, 194–203. [Google Scholar] [CrossRef]
- Estevão-Silva, C.F.; Kummer, R.; Fachini-Queiroz, F.C.; Grespan, R.; Nogueira de Melo, G.A.; Baroni, S.; Cuman, R.K.N.; Bersani-Amado, C.A. Anethole and Eugenol Reduce in Vitro and in Vivo Leukocyte Migration Induced by FMLP, LTB4, and Carrageenan. J. Nat. Med. 2014, 68, 567–575. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-Inflammatory Activity of Linalool and Linalyl Acetate Constituents of Essential Oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef]
- Maniyar, Y.A.; Devi, C.H.J. Evaluation if Anti-inflammatory activity of Ethanolic Extract of Cananga odorata LAM in Experimental Animals. Int. J. Basic Clin. Pharmacol. 2015, 4, 354–357. [Google Scholar] [CrossRef][Green Version]
- Stashenko, E.; Martinez, J.R.; MacKu, C.; Shibamoto, T. HRGC and GC-MS Analysis of Essential Oil from Colombian Ylang-Ylang (Cananga Odorata Hook Fil. et Thomson, Forma Genuina). J. High Resolut. Chromatogr. 1993, 16, 441–444. [Google Scholar] [CrossRef]
- Uchida, N.S.; Silva-Filho, S.E.; Aguiar, R.P.; Wiirzler, L.A.M.; Cardia, G.F.E.; Cavalcante, H.A.O.; Silva-Comar, F.M.D.S.; Becker, T.C.A.; Silva, E.L.; Bersani-Amado, C.A.; et al. Protective Effect of Cymbopogon Citratus Essential Oil in Experimental Model of Acetaminophen-Induced Liver Injury. Am. J. Chin. Med. 2017, 45, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, S.E.; Wiirzler, L.A.M.; Cavalcante, H.A.O.; Uchida, N.S.; de Souza Silva-Comar, F.M.; Cardia, G.F.E.; da Silva, E.L.; Aguiar, R.P.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Patchouli (Pogostemon Cablin) Essential Oil on in Vitro and in Vivo Leukocytes Behavior in Acute Inflammatory Response. Biomed. Pharmacother. 2016, 84, 1697–1704. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula Angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef]
- de Souza Silva-Comar, F.M.; Wiirzler, L.A.M.; Silva-Filho, S.E.; Kummer, R.; Pedroso, R.B.; Spironello, R.A.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Estragole on Leukocyte Behavior and Phagocytic Activity of Macrophages. Evid. Based Complement. Altern. Med. 2014, 2014, 784689. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; de Barros Carvalho, M.D.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus Vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Altern. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Evaluation of Anti-Inflammatory Activity of Citrus Latifolia Tanaka Essential Oil and Limonene in Experimental Mouse Models. Evid. Based Complement. Altern. Med. 2013, 2013, 859083. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/Lipoxygenase Inhibitory Activities and Chemical Compositions of Selected Essential Oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef]
- Moonen, C.G.J.; Hirschfeld, J.; Cheng, L.; Chapple, I.L.C.; Loos, B.G.; Nicu, E.A. Oral Neutrophils Characterized: Chemotactic, Phagocytic, and Neutrophil Extracellular Trap (NET) Formation Properties. Front. Immunol. 2019, 10, 635. [Google Scholar] [CrossRef]
- Mócsai, A.; Jakus, Z.; Vántus, T.; Berton, G.; Lowell, C.A.; Ligeti, E. Kinase Pathways in Chemoattractant-Induced Degranulation of Neutrophils: The Role of P38 Mitogen-Activated Protein Kinase Activated by Src Family Kinases. J. Immunol. 2000, 164, 4321–4331. [Google Scholar] [CrossRef]
- Uthayashanker, R.E.; Rita, M.H. Preliminary Screening of Anti-Inflammatory Effect of Phytochemicals on Chemotaxis of Human Neutrophils. J. Pharmacogn. Phyther. 2015, 7, 183–188. [Google Scholar] [CrossRef]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-Inflammatory Effects of Linalool in RAW 264.7 Macrophages and Lipopolysaccharide-Induced Lung Injury Model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef]
- Dale, D.C.; Boxer, L.; Liles, W.C. The Phagocytes: Neutrophils and Monocytes. Blood 2008, 112, 935–945. [Google Scholar] [CrossRef]
- Aulakh, G.K. Neutrophils in the Lung: “The First Responders”. Cell Tissue Res. 2018, 371, 577–588. [Google Scholar] [CrossRef]
- Saito, E.; Kuo, R.; Pearson, R.M.; Gohel, N.; Cheung, B.; King, N.J.C.; Miller, S.D.; Shea, L.D. Designing Drug-Free Biodegradable Nanoparticles to Modulate Inflammatory Monocytes and Neutrophils for Ameliorating Inflammation. J. Control. Release 2019, 300, 185–196. [Google Scholar] [CrossRef]
- Rao, A.N.; Kazzaz, N.M.; Knight, J.S. Do Neutrophil Extracellular Traps Contribute to the Heightened Risk of Thrombosis in Inflammatory Diseases? World J. Cardiol. 2015, 7, 829–842. [Google Scholar] [CrossRef]
- Safari, H.; Kelley, W.J.; Saito, E.; Kaczorowski, N.; Carethers, L.; Shea, L.D.; Eniola-Adefeso, O. Neutrophils Preferentially Phagocytose Elongated Particles—An Opportunity for Selective Targeting in Acute Inflammatory Diseases. Sci. Adv. 2020, 6, eaba1474. [Google Scholar] [CrossRef]
- Golab, M.; Burdzenia, O.; Majewski, P.; Skwarlo-Sonta, K. Tea Tree Oil Inhalations Modify Immunity in Mice. J. Appl. Biomed. 2005, 3, 101–108. [Google Scholar] [CrossRef]
- Bellavita, R.; Raucci, F.; Merlino, F.; Piccolo, M.; Ferraro, M.G.; Irace, C.; Santamaria, R.; Iqbal, A.J.; Novellino, E.; Grieco, P.; et al. Temporin L-Derived Peptide as a Regulator of the Acute Inflammatory Response in Zymosan-Induced Peritonitis. Biomed. Pharmacother. 2020, 123, 109788. [Google Scholar] [CrossRef]
- Ajuebor, M.N.; Virág, L.; Flower, R.J.; Perretti, M.; Szabó, C. Role of Inducible Nitric Oxide Synthase in the Regulation of Neutrophil Migration in Zymosan-Induced Inflammation. Immunology 1998, 95, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-KappaB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-Inflammatory Effects of Linalool on Ovalbumin-Induced Pulmonary Inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of Nitric Oxide in Inflammatory Diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Alencar, N.M.N.; Oliveira, R.S.B.; Figueiredo, J.G.; Cavalcante, I.J.M.; Matos, M.P.V.; Cunha, F.Q.; Nunes, J.V.S.; Bomfim, L.R.; Ramos, M.V. An Anti-Inflammatory Lectin from Luetzelburgia Auriculata Seeds Inhibits Adhesion and Rolling of Leukocytes and Modulates Histamine and PGE2 Action in Acute Inflammation Models. Inflamm. Res. 2010, 59, 245–254. [Google Scholar] [CrossRef]
- Seely, A.J.E.; Pascual, J.L.; Christou, N.V. Science Review: Cell Membrane Expression (Connectivity) Regulates Neutrophil Delivery, Function and Clearance. Crit. Care 2003, 7, 291–307. [Google Scholar] [CrossRef]
- Díaz-González, F.; González-Alvaro, I.; Campanero, M.R.; Mollinedo, F.; del Pozo, M.A.; Muñoz, C.; Pivel, J.P.; Sánchez-Madrid, F. Prevention of in Vitro Neutrophil-Endothelial Attachment through Shedding of L-Selectin by Nonsteroidal Antiinflammatory Drugs. J. Clin. Investig. 1995, 95, 1756–1765. [Google Scholar] [CrossRef]
- Sakai, A. Diclofenac Inhibits Endothelial Cell Adhesion Molecule Expression Induced with Lipopolysaccharide. Life Sci. 1996, 58, 2377–2387. [Google Scholar] [CrossRef]
- Nogueira de Melo, G.A.; Grespan, R.; Fonseca, J.P.; Farinha, T.O.; da Silva, E.L.; Romero, A.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Inhibitory Effects of Ginger (Zingiber Officinale Roscoe) Essential Oil on Leukocyte Migration in Vivo and in Vitro. J. Nat. Med. 2011, 65, 241–246. [Google Scholar] [CrossRef]
- Ou, Z.; Zhao, J.; Zhu, L.; Huang, L.; Ma, Y.; Ma, C.; Luo, C.; Zhu, Z.; Yuan, Z.; Wu, J.; et al. Anti-Inflammatory Effect and Potential Mechanism of Betulinic Acid on λ-Carrageenan-Induced Paw Edema in Mice. Biomed. Pharmacother. 2019, 118, 109347. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Avoseh, O.N.; Olasunkanmi, K.N.; Lawal, O.A.; Ascrizzi, R.; Flamini, G. Chemical Composition, Anti-Nociceptive and Anti-Inflammatory Activities of Essential Oil of Bougainvillea Glabra. J. Ethnopharmacol. 2019, 232, 188–192. [Google Scholar] [CrossRef]
- dos Santos, E.; Leitão, M.M.; Aguero Ito, C.N.; Silva-Filho, S.E.; Arena, A.C.; de Souza Silva-Comar, F.M.; Nakamura Cuman, R.K.; Oliveira, R.J.; Nazari Formagio, A.S.; Leite Kassuya, C.A. Analgesic and Anti-Inflammatory Articular Effects of Essential Oil and Camphor Isolated from Ocimum Kilimandscharicum Gürke Leaves. J. Ethnopharmacol. 2021, 269, 113697. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. The Anxiolytic Effect of Essential Oil of Cananga Odorata Exposure on Mice and Determination of Its Major Active Constituents. Phytomedicine 2016, 23, 1727–1734. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. Cananga Odorata Essential Oil Reverses the Anxiety Induced by 1-(3-Chlorophenyl) Piperazine through Regulating the MAPK Pathway and Serotonin System in Mice. J. Ethnopharmacol. 2018, 219, 23–30. [Google Scholar] [CrossRef]
- Cavalcante, H.A.O.; Silva-Filho, S.E.; Wiirzler, L.A.M.; Cardia, G.F.E.; Uchida, N.S.; Silva-Comar, F.M.S.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of (-)-α-Bisabolol on the Inflammatory Response in Systemic Infection Experimental Model in C57BL/6 Mice. Inflammation 2020, 43, 193–203. [Google Scholar] [CrossRef]
- Möller, K.A.; Johansson, B.; Berge, O.G. Assessing Mechanical Allodynia in the Rat Paw with a New Electronic Algometer. J. Neurosci. Methods 1998, 84, 41–47. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Guidelines for Testing of Chemical: Paris; Guideline 425: Acute Oral Toxicity—Up-And-Down-Procedure (UDP) 27; Head of Publications Service, OECD: Paris, France, 2008. [Google Scholar]
- Menegati, S.E.L.T.; de Lima, F.F.; Traesel, G.K.; Souza, R.I.C.; dos Santos, A.C.; de Santana Aquino, D.F.; de Oliveira, V.S.; Heredia Vieira, S.C.; Cardoso, C.A.L.; do CarmoVieira, M.; et al. Acute and Subacute Toxicity of the Aqueous Extract of Alibertia Edulis (Rich.) A. Rich. Ex DC. in Rats. J. Ethnopharmacol. 2016, 194, 1096–1102. [Google Scholar] [CrossRef]
- Saleem, U.; Amin, S.; Ahmad, B.; Azeem, H.; Anwar, F.; Mary, S. Acute Oral Toxicity Evaluation of Aqueous Ethanolic Extract of Saccharum Munja Roxb. Roots in Albino Mice as per OECD 425 TG. Toxicol. Rep. 2017, 4, 580–585. [Google Scholar] [CrossRef]
- Malone, M.H.; Robichaud, R.C. A Hippocratic Screen for Pure or Crude Drug Materiais. J. Nat. Prod. Lloydia 1962, 25, 320–331. [Google Scholar]
- Traesel, G.K.; de Souza, J.C.; de Barros, A.L.; Souza, M.A.; Schmitz, W.O.; Muzzi, R.M.; Oesterreich, S.A.; Arena, A.C. Acute and Subacute (28 Days) Oral Toxicity Assessment of the Oil Extracted from Acrocomia Aculeata Pulp in Rats. Food Chem. Toxicol. 2014, 74, 320–325. [Google Scholar] [CrossRef] [PubMed]
| Peak | RT (min) | Compound | RI | % |
|---|---|---|---|---|
| 1 | 4.57 | prenyl acetate | 921 | 0.41 |
| 2 | 7.10 | p-methyl anisole | 1019 | 7.38 |
| 3 | 7.45 | 1,8-cineole | 1030 | 0.05 |
| 4 | 9.62 | methyl benzoate | 1096 | 7.64 |
| 5 | 9.79 | linalool | 1100 | 15.23 |
| 6 | 12.28 | benzyl acetate | 1164 | 18.12 |
| 7 | 15.99 | linalyl acetate | 1255 | 0.21 |
| 8 | 20.91 | α-copaene | 1374 | 0.38 |
| 9 | 21.33 | geranyl acetate | 1384 | 9.46 |
| 10 | 21.60 | β-elemene | 1390 | 0.09 |
| 11 | 22.68 | trans-caryophyllene | 1417 | 5.42 |
| 12 | 23.82 | cinnamyl acetate | 1445 | 6.05 |
| 13 | 24.05 | α-humulene | 1451 | 1.80 |
| 14 | 25.01 | γ-muurolene | 1475 | 0.42 |
| 15 | 25.17 | germacrene D | 1479 | 4.61 |
| 16 | 25.60 | prenyl benzoate | 1489 | 0.17 |
| 17 | 25.79 | bicyclogermacrene | 1494 | 0.13 |
| 18 | 25.98 | α-muurolene | 1499 | 0.21 |
| 19 | 26.12 | γ-bisabolene | 1502 | 0.59 |
| 20 | 26.32 | α-farnesene | 1508 | 2.02 |
| 21 | 26.49 | γ-cadinene | 1512 | 0.22 |
| 22 | 26.87 | δ-cadinene | 1522 | 0.98 |
| 23 | 29.12 | caryophyllene oxide | 1580 | 0.06 |
| 24 | 31.40 | α-cadinol | 1641 | 0.44 |
| 25 | 31.56 | α-muurolol | 1645 | 0.10 |
| 26 | 31.86 | cadin-4-en-10-ol | 1654 | 0.51 |
| 27 | 34.36 | (z,z)-farnesol | 1722 | 0.38 |
| 28 | 35.80 | benzyl benzoate | 1764 | 11.39 |
| 29 | 38.47 | farnesyl acetate | 1841 | 0.83 |
| 30 | 39.34 | benzyl salicylate | 1868 | 4.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas Junior, R.A.; Lossavaro, P.K.d.M.B.; Kassuya, C.A.L.; Paredes-Gamero, E.J.; Farias Júnior, N.C.; Souza, M.I.L.; Silva-Comar, F.M.d.S.; Cuman, R.K.N.; Silva, D.B.; Toffoli-Kadri, M.C.; et al. Effect of Ylang-Ylang (Cananga odorata Hook. F. & Thomson) Essential Oil on Acute Inflammatory Response In Vitro and In Vivo. Molecules 2022, 27, 3666. https://doi.org/10.3390/molecules27123666
de Freitas Junior RA, Lossavaro PKdMB, Kassuya CAL, Paredes-Gamero EJ, Farias Júnior NC, Souza MIL, Silva-Comar FMdS, Cuman RKN, Silva DB, Toffoli-Kadri MC, et al. Effect of Ylang-Ylang (Cananga odorata Hook. F. & Thomson) Essential Oil on Acute Inflammatory Response In Vitro and In Vivo. Molecules. 2022; 27(12):3666. https://doi.org/10.3390/molecules27123666
Chicago/Turabian Stylede Freitas Junior, Robson Araújo, Paloma Kênia de Moraes Berenguel Lossavaro, Cândida Aparecida Leite Kassuya, Edgar Julian Paredes-Gamero, Nelson Carvalho Farias Júnior, Maria Inês Lenz Souza, Francielli Maria de Souza Silva-Comar, Roberto Kenji Nakamura Cuman, Denise Brentan Silva, Mônica Cristina Toffoli-Kadri, and et al. 2022. "Effect of Ylang-Ylang (Cananga odorata Hook. F. & Thomson) Essential Oil on Acute Inflammatory Response In Vitro and In Vivo" Molecules 27, no. 12: 3666. https://doi.org/10.3390/molecules27123666
APA Stylede Freitas Junior, R. A., Lossavaro, P. K. d. M. B., Kassuya, C. A. L., Paredes-Gamero, E. J., Farias Júnior, N. C., Souza, M. I. L., Silva-Comar, F. M. d. S., Cuman, R. K. N., Silva, D. B., Toffoli-Kadri, M. C., & Silva-Filho, S. E. (2022). Effect of Ylang-Ylang (Cananga odorata Hook. F. & Thomson) Essential Oil on Acute Inflammatory Response In Vitro and In Vivo. Molecules, 27(12), 3666. https://doi.org/10.3390/molecules27123666








