Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collections
2.2. Laboratory Preparation of Extracts from Plants
2.3. Green Synthesis of AgNPs from the Extract
2.4. AgNPs Characterization
2.4.1. UV Visible Spectroscopy
2.4.2. X-ray Diffraction (XRD) Analysis
- D = kλβcosθ where k = shape factor (0.94).
- λ = X-ray wavelength (λ = 1.5418 Å);
- β = full width at half maximum (FWHM) in radians.
- and θ = Bragg’s angle.
2.4.3. Fourier Transform InfraRed (FTIR) Spectrum
2.4.4. Scanning Electron Microscope Analysis (SEM)
2.5. In Vitro Biological Application
2.5.1. Antimicrobial Activity
2.5.2. Cell Culture for Cell Line
2.5.3. Assay for Cell Viability
2.6. Physicochemical Parameters
2.7. Mathematical Formulation
3. Results
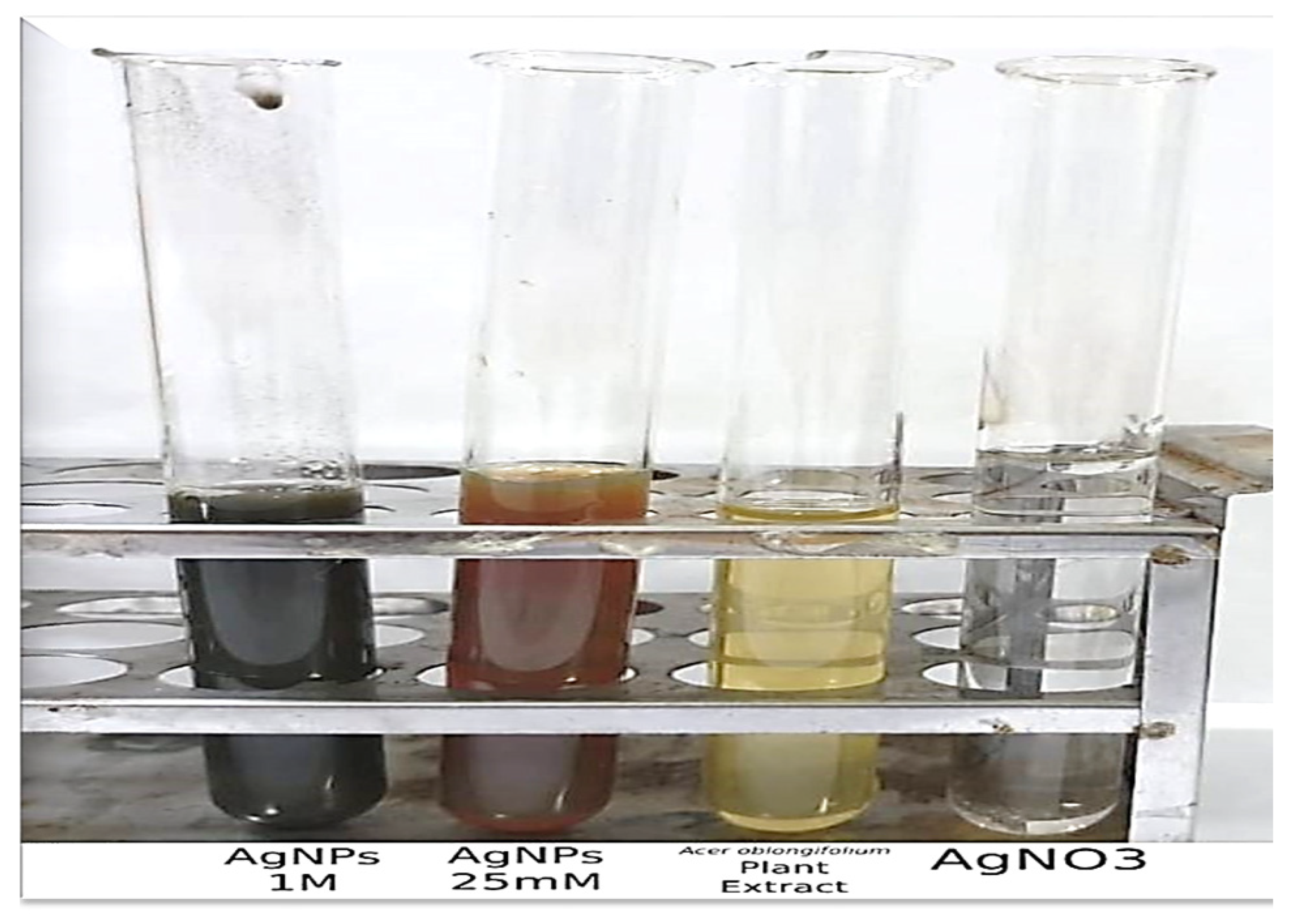
3.1. Color Change of Solution
3.2. UV Visible Spectroscopy
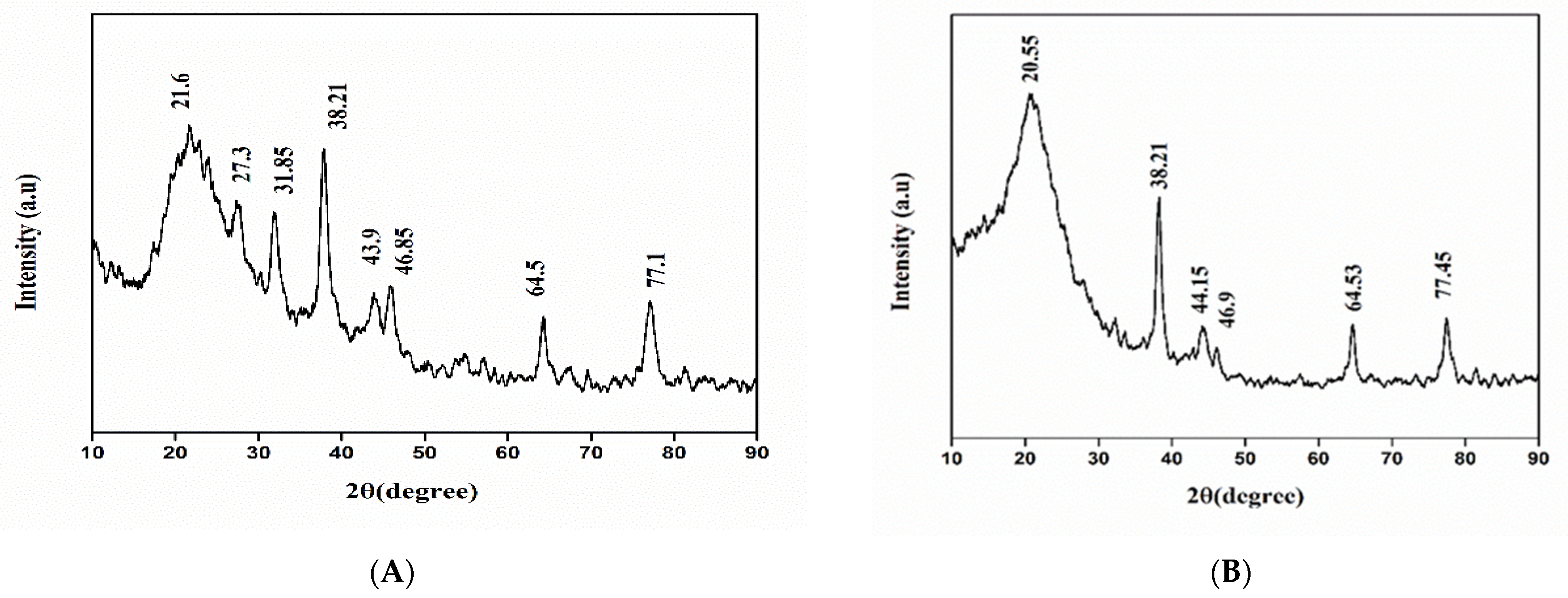
3.3. X-ray Diffraction (XRD) Analysis
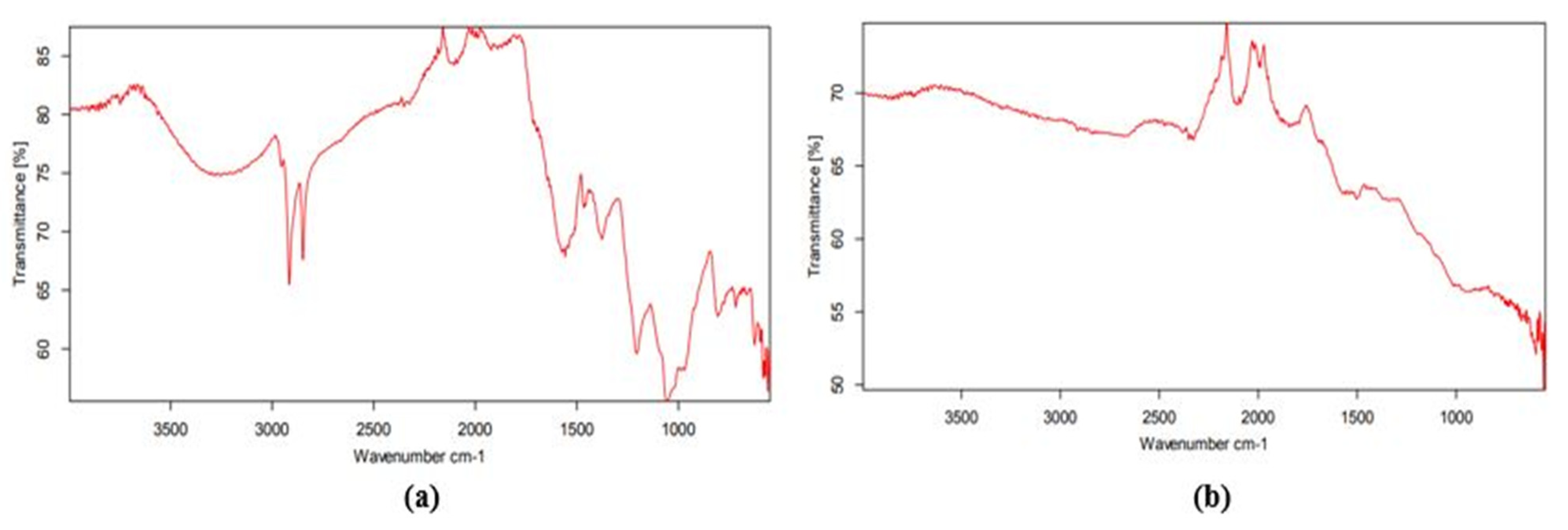
3.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.5. Scanning Electron Microscope (SEM)
3.6. In Vitro Biological Application
3.6.1. Antibacterial Activity
3.6.2. Physicochemical Parameters
3.6.3. Antiproliferative Activity against MCF-7 and HeLa Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Palei, N.N. Green synthesis of silver nanoparticles using leaf extract of Lantana camara and its antimicrobial activity. Int. J. Green Pharm. 2020, 14, 1–7. [Google Scholar]
- Gogoi, B.; Kumar, R.; Upadhyay, J.; Borah, D. Facile biogenic synthesis of silver nanoparticles (AgNPs) by Citrus grandis (L.) Osbeck fruit extract with excellent antimicrobial potential against plant pathogens. SN Appl. Sci. 2020, 2, 1–7. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. 2015, 49, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of silver nanoparticles from oropharyngeal Candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Marinescu, L.; Ficai, D.; Ficai, A.; Oprea, O.; Nicoara, A.I.; Vasile, B.S.; Boanta, L.; Marin, A.; Andronescu, E.; Holban, A.-M. Comparative Antimicrobial Activity of Silver Nanoparticles Obtained by Wet Chemical Reduction and Solvothermal Methods. Int. J. Mol. Sci. 2022, 23, 5982. [Google Scholar] [CrossRef]
- Jain, A.; Malik, A.; Malik, H.K. Mathematical modelling of seed-mediated size-specific growth of spherical silver nanoparticles using Azadirachta indica leaf extract. J. Taibah Univ. Sci. 2020, 14, 873–880. [Google Scholar] [CrossRef]
- Mangindaan, D.; Lin, G.-Y.; Kuo, C.-J.; Chien, H.-W. Biosynthesis of silver nanoparticles as catalyst by spent coffee ground/recycled poly (ethylene terephthalate) composites. Food Bioprod. Process. 2020, 121, 193–201. [Google Scholar] [CrossRef]
- Akinola, P.; Lateef, A.; Asafa, T.; Beukes, L.; Hakeem, A.; Irshad, H. Multifunctional titanium dioxide nanoparticles biofabricated via phytosynthetic route using extracts of Cola nitida: Antimicrobial, dye degradation, antioxidant and anticoagulant activities. Heliyon 2020, 6, e04610. [Google Scholar] [CrossRef]
- Adebayo, E.A.; Ibikunle, J.B.; Oke, A.M.; Lateef, A.; Azeez, M.A.; Oluwatoyin, A.O.; Ayanfe Oluwa, A.V.; Blessing, O.T.; Comfort, O.O.; Adekunle, O.O. Antimicrobial and antioxidant activity of silver, gold and silver-gold alloy nanoparticles phytosynthesized using extract of Opuntia ficus-indica. Rev. Adv. Mater. Sci. 2019, 58, 313–326. [Google Scholar] [CrossRef]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The Role of Calix[n]arenes and Pillar[n]arenes in the Design of Silver Nanoparticles: Self-Assembly and Application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkol, E.K.; Göger, F.; Koşar, M.; Başer, K.H.C. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008, 108, 942–949. [Google Scholar] [CrossRef]
- van den Berg, A.K.; Perkins, T.D. Contribution of anthocyanins to the antioxidant capacity of juvenile and senescing sugar maple (Acer saccharum) leaves. Funct. Plant Biol. 2007, 34, 714–719. [Google Scholar] [CrossRef]
- An, L.; Wang, J.-W.; Liu, J.-D.; Zhao, Z.-M.; Song, Y.-J. Design, preparation, and characterization of novel calix [4] arene bioactive carrier for antitumor drug delivery. Front. Chem. 2019, 7, 732. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vazquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-Gonzalez, M.T.; Castro, E.D.B.; Saucedo-Salazar, E.M.; Morales, R.C.; Soto, D.R.; González, F.T. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Naseh, S. Enhanced photodegradation of dye in wastewater using iron vanadate nanocomposite; ultrasound-assisted preparation and characterization. Ultrason. Sonochem. 2017, 39, 494–503. [Google Scholar] [CrossRef]
- Platania, V.; Kaldeli-Kerou, A.; Karamanidou, T.; Kouki, M.; Tsouknidas, A.; Chatzinikolaidou, M. Antibacterial Effect of Colloidal Suspensions Varying in Silver Nanoparticles and Ions Concentrations. Nanomaterials 2022, 12, 31. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Bogdanchikova, N.; Garibo, D. Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 2021, 26, 2462. [Google Scholar]
- Khan, M.S.; Mei, S.; Shabnam Fernandez-Gamiz, U.; Noeiaghdam, S.; Shah, S.A.; Khan, A. Numerical Analysis of Unsteady Hybrid Nanofluid Flow Comprising CNTs-Ferrous oxide/Water with Variable Magnetic Field. Nanomaterials 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Hemlata, P.R.M.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, M.; Kumar, V.; Pathak, P.; Majee, R.; Ramteke, P.W.; Verma, A. Green Synthesis of Silver Nanoparticles Using Scindapsus officinalis (Gajpipli): In-Vitro Cytotoxic Activity Against HepG-2 & MCF-7 Cancer Cell Lines. Preprints 2019, 2019080118. [Google Scholar]
- Wang, Y.; Chinnathambi, A.; Nasif, O.; Alharbi, S.L. Green synthesis and chemical characterization of a novel anti-human pancreatic cancer supplement by silver nanoparticles containing Zingiber officinale leaf aqueous extract. Arab. J. Chem. 2021, 14, 103081. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria ternatea and Solanum nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J. Nanosci. 2015, 8, 928204. [Google Scholar] [CrossRef] [Green Version]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kivrak, H.; Atbas, D.; Alal, O.; Çögenli, M.S.; Bayrakceken, A.; Mert, S.O.; Sahin, O. A complementary study on novel PdAuCo catalysts: Synthesis, characterization, direct formic acid fuel cell application, and exergy analysis. Int. J. Hydrogen Energy 2018, 43, 21886–21898. [Google Scholar] [CrossRef]
- Bibi, G.; Ullah, N.; Mannan, A.; Mirza, B. Antitumor, cytotoxic and antioxidant potential of Aster thomsonii extracts. Afr. J. Pharm. Pharmacol. 2011, 5, 252–258. [Google Scholar]
- Khan, M.S.; Mei, S.; Fernandez-Gamiz, U.; Noeiaghdam, S.; Khan, A. Numerical Simulation of a Time-Dependent Electroviscous and Hybrid Nanofluid with Darcy-Forchheimer Effect between Squeezing Plates. Nanomaterials 2022, 12, 876. [Google Scholar] [CrossRef]
- Ashour, A.A.; Raafat, D.; El-Gowelli, H.M.; El-Kamel, A.H. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: Characterization and antimicrobial properties. Int. J. Nanomed. 2015, 10, 7207–7221. [Google Scholar]
- Al-Yousef, H.M.; Amina, M.; Alqahtani, A.S.; Alqahtani, M.S.; Malik, A.; Hatshan, M.R.; Siddiqui, M.R.H.; Khan, M.; Shaik, M.R.; Ola, M.S.; et al. Pollen Bee Aqueous Extract-Based Synthesis of Silver Nanoparticles and Evaluation of Their Anti-Cancer and Anti-Bacterial Activities. Processes 2020, 8, 524. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Aromal, S.A.; Philip, D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 83, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 1078–1081. [Google Scholar] [CrossRef]
- Mude, N.; Ingle, A.; Gade, A.; Ra, M. Synthesis of Silver Nanoparticles Using Callus Extract of Carica papaya—A First Report. J. Plant Biochem. Biotechnol. 2009, 18, 83–86. [Google Scholar] [CrossRef]
- Prasad, T.; Elumalai, E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.S.; Pathak, D.; Patel, A.; Dalwadi, P.; Prasad, R.; Patel, P.; Selvaraj, K. Biogenic synthesis of silver nanoparticles using Nicotiana tobaccum leaf extract and study of their antibacterial effect. Afr. J. Biotechnol. 2011, 10, 8122–8130. [Google Scholar]
- Kumar, R.; Nirmalya, K.; Pradipta, K. Mohapatra and Suj Chandrashekhar: India’s Global Powerhouses: How They Are Taking on the World; SAGE Publications: Los Angeles, CA, USA, 2009. [Google Scholar]
- Ponnuchamy, K.; Senthamil Selvi, S.; Prabha, L.; Prem Kumar, K.; Ganeshkumar, R.; Govindaraju, M. Synthesis of silver nanoparticles from Sargassum tenerrimum and screening phytochemicals for its antibacterial activity. Nano. Biomed. Eng. 2012, 4, 12–16. [Google Scholar]
- Iqbal, N.; Iqubal, S.S.; Khan, A.A.; Mohammed, T.; Alshabi, A.M.; Aazam, E.S.; Rafiquee, M. Effect of CTABr (surfactant) on the kinetics of formation of silver nanoparticles by Amla extract. J. Mol. Liq. 2021, 329, 115537. [Google Scholar] [CrossRef]
- Tuama, A.A.; Mohammed, A.A. Phytochemical screening and in vitro antibacterial and anticancer activities of the aqueous extract of Cucumis sativus. Saudi. J. Biol. Sci. 2019, 26, 600–604. [Google Scholar] [CrossRef]
- Chinyerenwa, A.C.; Munna, M.K.H.; Rahman, S.; Mia, M.R.; Yousuf, M.; Hasan, J. Ecofriendly Sweet Scented Osmanthus Leaf Extract Mediated Synthesis of Silver Nanoparticles (SNPs). Int. J. Text. Sci. 2018, 7, 35–42. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver nanoparticles in therapeutics: Development of an antimicrobial gel formulation for topical use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Kap, A.; Kutlu, H.M. Investigation of silver nitrate on cytotoxicity and apoptosis in MCF7 human breast carcinoma cells. Asian Pac. J. Cancer Biol. 2020, 5, 49–56. [Google Scholar]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.; Li, C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf. B Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef]
- Muhammed, R.A.; Hassawi, D.S.; Ibaheem, N.K. Cytotoxic Activity of Taraxacum officinale Ethanolic Plant Extract against Human Breast Cancer (MCF-7) Cells and Human Hepatic (WRL-68) Cells. Iraqi J. Cancer Med. Genet. (IJCMG) 2018, 11, 16–21. [Google Scholar]
- Sigisted, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkinsi, A.R.; Romeroi, A.E.; Pullin, M.J.; Korneinko, A.; Lowrey, T.K.; van Salmbrouck, S.; Steelanti, W.F.A. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef]



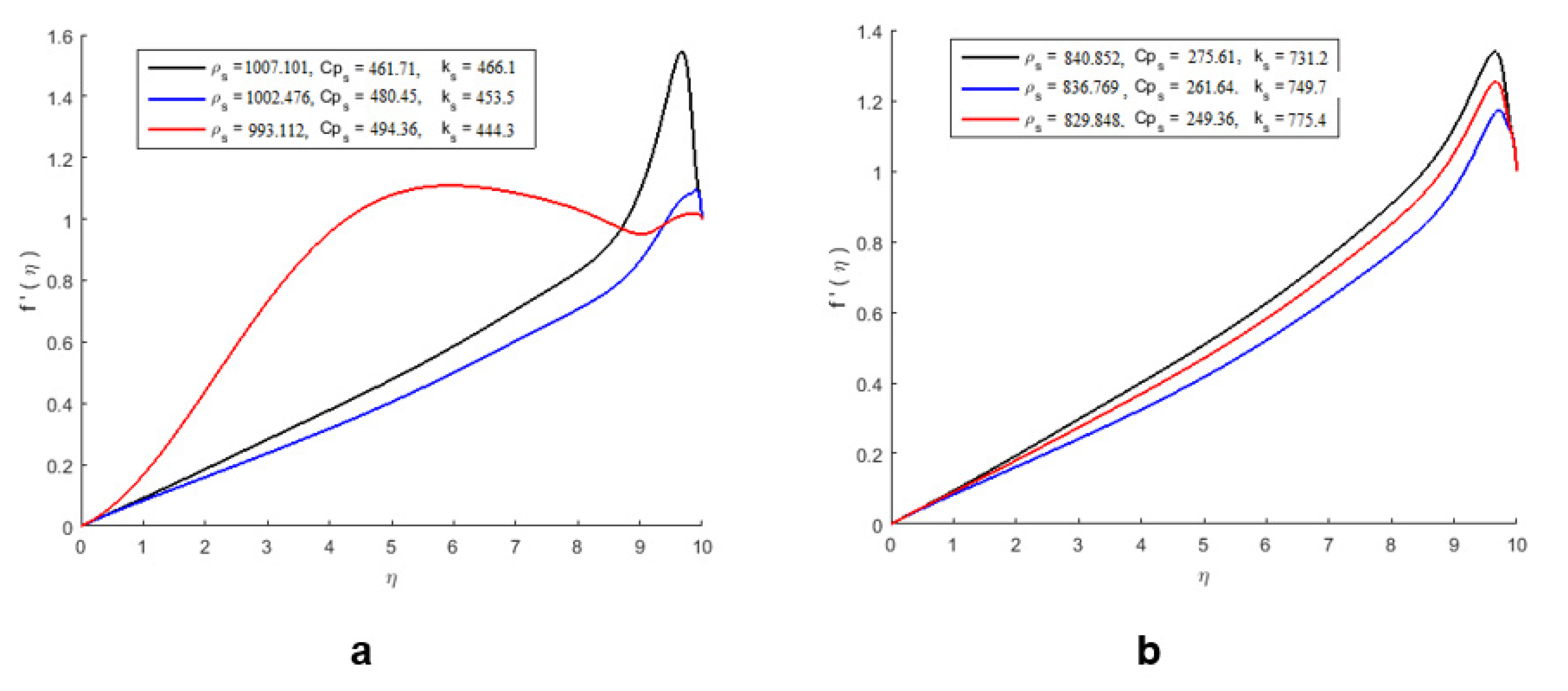
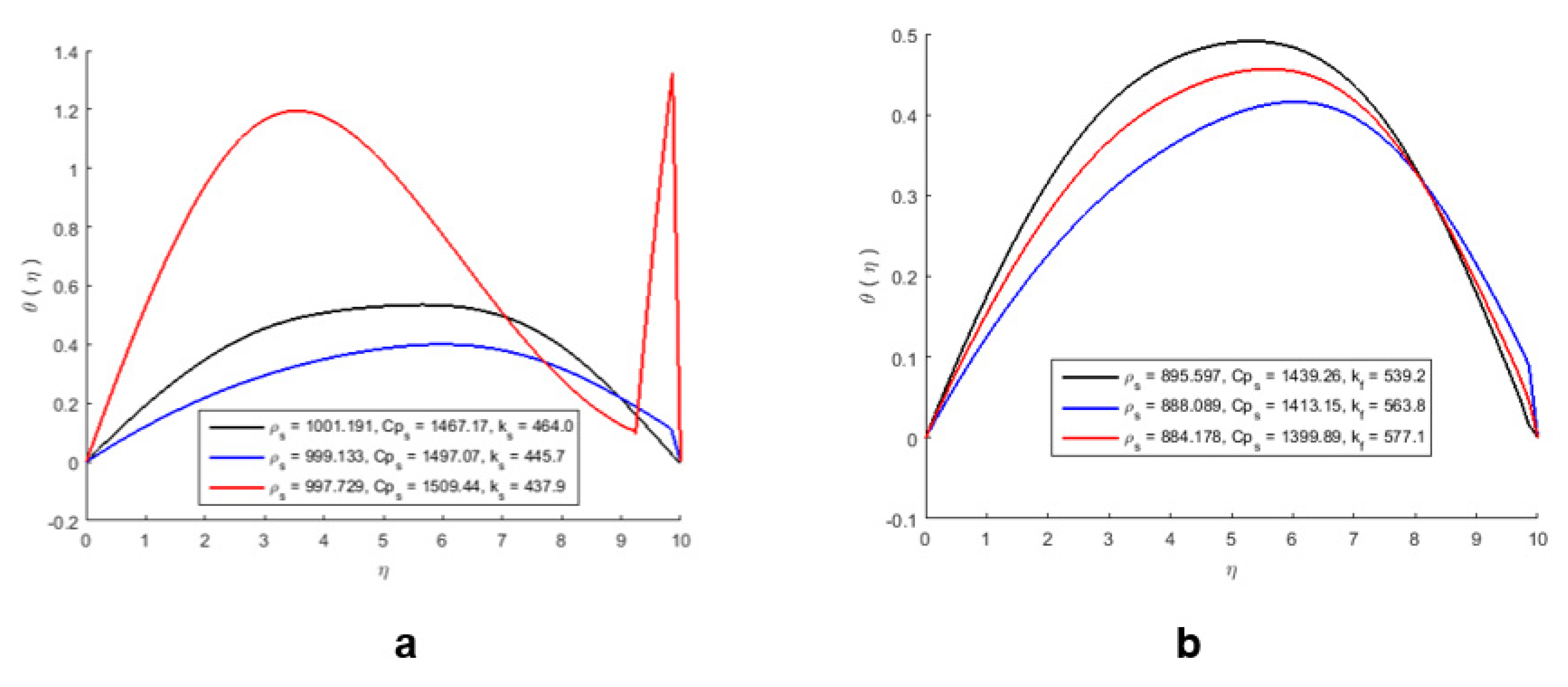
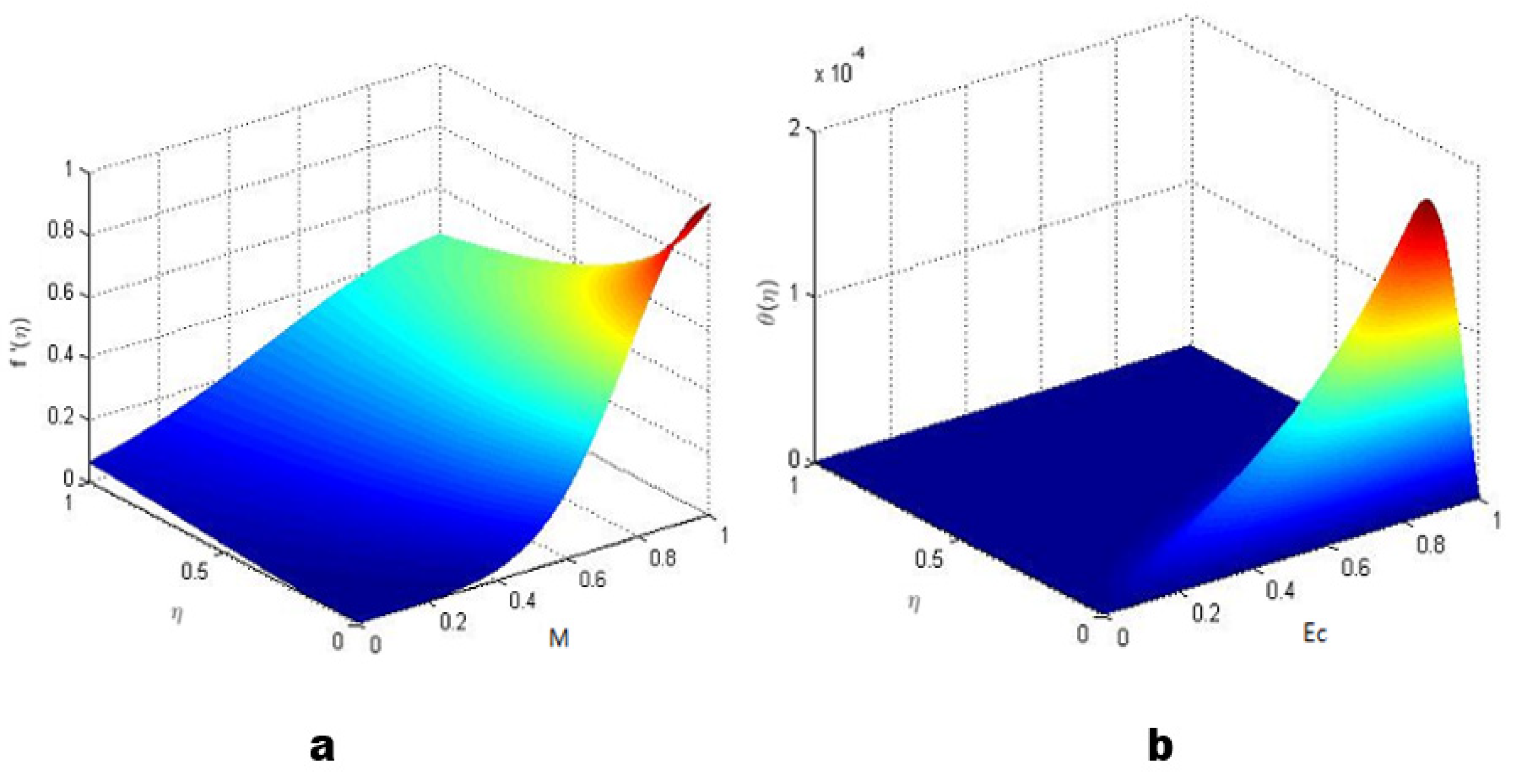
| Temperature (°C) | ρ 1b | ρ 2b | ρ3b | ρ 4b |
|---|---|---|---|---|
| 15 | 1007.101 | 894.880 | 863.323 | 840.852 |
| 20 | 1002.476 | 886.721 | 856.992 | 936.769 |
| 25 | 993.112 | 881.204 | 851.638 | 829.848 |
| 30 | 986.746 | 875.128 | 846.117 | 825.632 |
| 35 | 980.174 | 870.998 | 840.264 | 820.987 |
| 40 | 975.417 | 863.724 | 834.339 | 815.548 |
| 45 | 969.105 | 858.301 | 825.114 | 811.716 |
| Temperature (°C) | ρC p | ρC p2 | ρC P3 | ρC p4 |
|---|---|---|---|---|
| 15 | 461.71 | 435.62 | 333.15 | 275.61 |
| 20 | 480.45 | 424.31 | 321.71 | 261.64 |
| 25 | 494.36 | 411.51 | 304.14 | 249.36 |
| 30 | 507.69 | 396.75 | 292.80 | 231.43 |
| 35 | 519.14 | 388.64 | 277.90 | 217.87 |
| 40 | 527.43 | 371.72 | 264.81 | 199.32 |
| 45 | 534.77 | 356.70 | 249.33 | 181.56 |
| Temperature (°C) | Knf 1 | Knf 2 | Knf 3 | Knf 4 |
|---|---|---|---|---|
| 15 | 466.1 | 537.2 | 652.7 | 731.2 |
| 20 | 453.5 | 550.4 | 669.2 | 749.7 |
| 25 | 444.3 | 562.1 | 688.5 | 775.4 |
| 30 | 436.0 | 571.6 | 707.1 | 802.8 |
| 35 | 430.4 | 592.3 | 727.9 | 822.0 |
| 40 | 424.7 | 607.5 | 748.4 | 848.3 |
| 45 | 419.2 | 624.0 | 769.0 | 874.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naveed, M.; Bukhari, B.; Aziz, T.; Zaib, S.; Mansoor, M.A.; Khan, A.A.; Shahzad, M.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules 2022, 27, 4226. https://doi.org/10.3390/molecules27134226
Naveed M, Bukhari B, Aziz T, Zaib S, Mansoor MA, Khan AA, Shahzad M, Dablool AS, Alruways MW, Almalki AA, et al. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules. 2022; 27(13):4226. https://doi.org/10.3390/molecules27134226
Chicago/Turabian StyleNaveed, Muhammad, Bakhtawar Bukhari, Tariq Aziz, Sumera Zaib, Muhammad Adil Mansoor, Ayaz Ali Khan, Muhammad Shahzad, Anas S. Dablool, Mashael W. Alruways, Abdulraheem Ali Almalki, and et al. 2022. "Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches" Molecules 27, no. 13: 4226. https://doi.org/10.3390/molecules27134226
APA StyleNaveed, M., Bukhari, B., Aziz, T., Zaib, S., Mansoor, M. A., Khan, A. A., Shahzad, M., Dablool, A. S., Alruways, M. W., Almalki, A. A., Alamri, A. S., & Alhomrani, M. (2022). Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules, 27(13), 4226. https://doi.org/10.3390/molecules27134226









