The Anti-Inflammatory Properties of Chaga Extracts Obtained by Different Extraction Methods against LPS-Induced RAW 264.7
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition
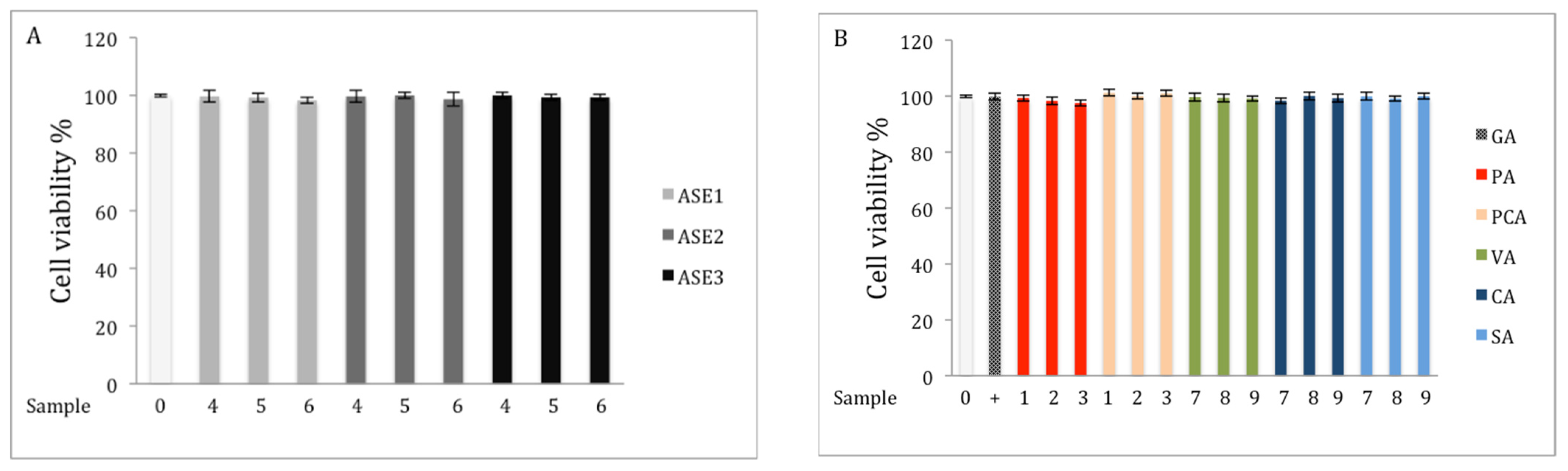
2.2. Cell Viability
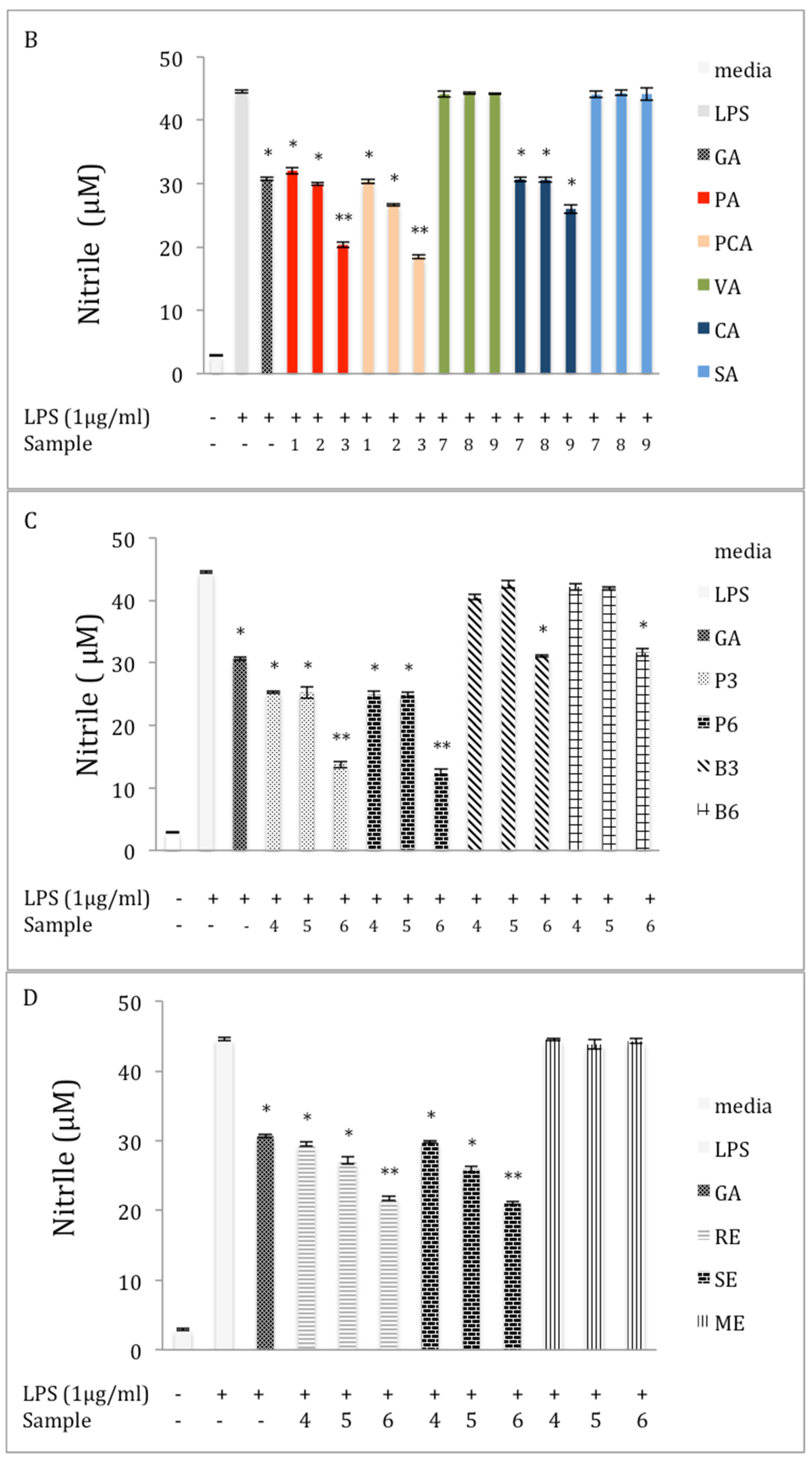
2.3. Inhibition of NO Production in LPS-Stimulated RAW 264.7 Macrophages
2.4. Inhibition of TNF-α Production in LPS-Stimulated RAW 264.7 Macrophages
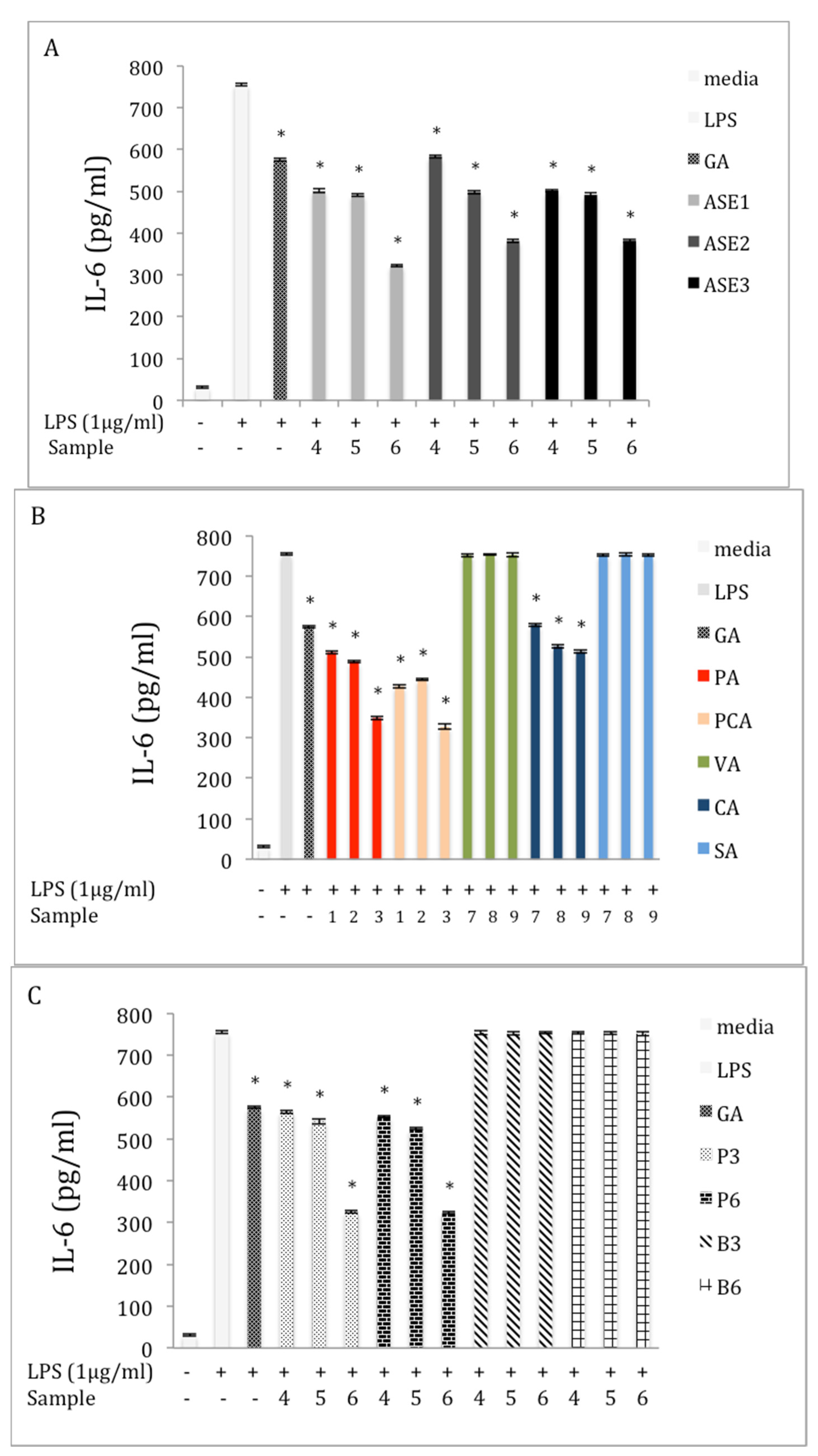
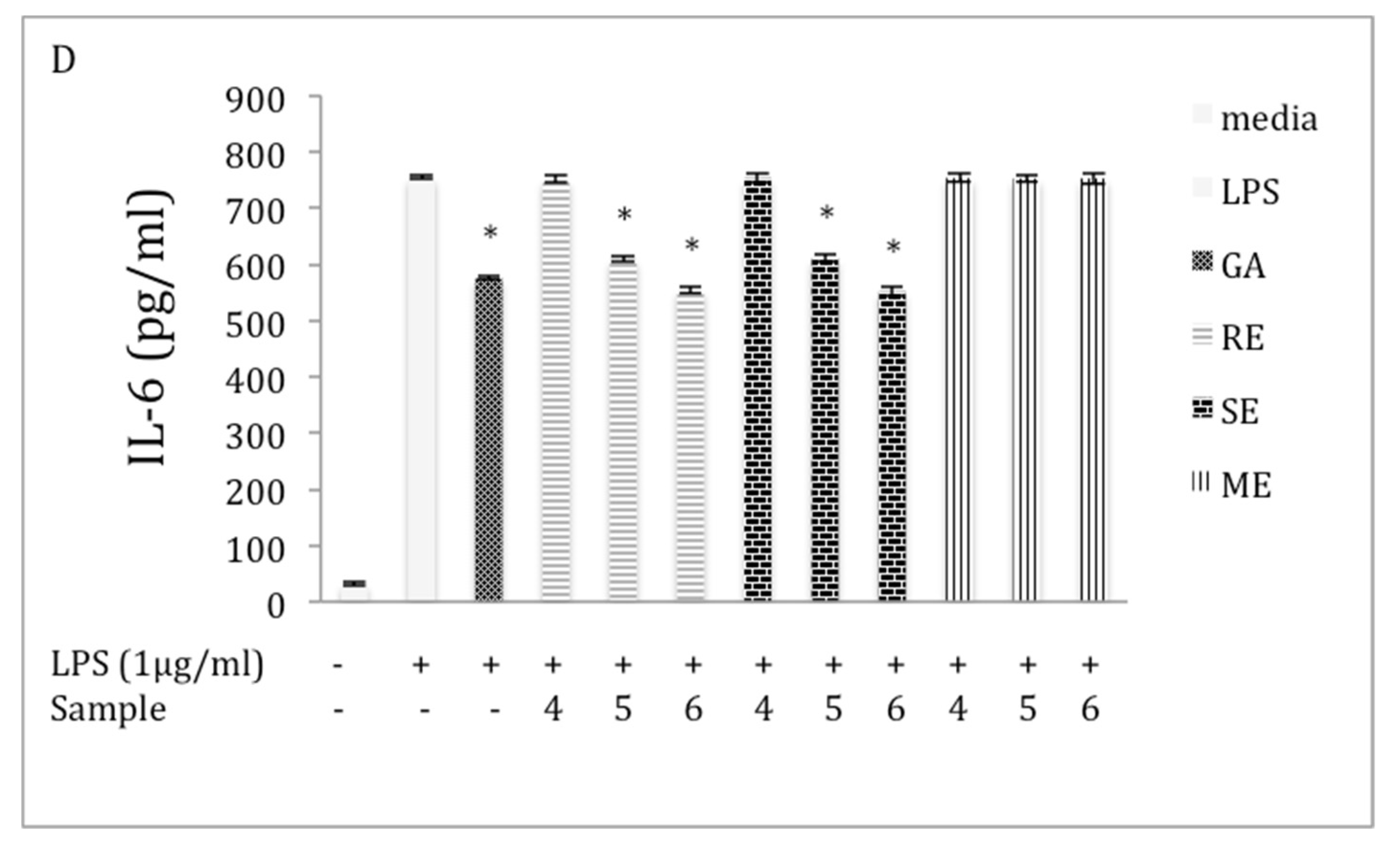
2.5. Inhibition of IL-6 Production in LPS-Stimulated RAW 264.7 Macrophages
2.6. Inhibition of IL-β Production in LPS-Stimulated RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Fungal Material
4.2. Reagents
4.3. Preparation of Polysaccharide Extracts
4.4. Preparation of Phenolic Extracts
4.4.1. Green Extraction, Accelerated Solvent Extraction (ASE)
4.4.2. Conventional Solvent Extraction (CSE)
Maceration Extraction (ME)
Reflux Extraction (RE)
Soxhlet Extraction (SE)
4.5. Determination of Total Phenolic Content (TPC)
4.6. Determination of Total Neutral Carbohydrate Contents
4.6.1. Determination of Uronic Acid Content
4.6.2. Determination of Protein Content
4.7. Cell Culture
4.7.1. Measurement of Cell Viability
4.7.2. Measurement of NO Production
4.7.3. Cytokine Measurement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C. Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Garlanda, C.; Di Liberto, D.; Vecchi, A.; La Manna, M.P.; Buracchi, C.; Caccamo, N.; Salerno, A.; Dieli, F.; Mantovani, A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 2007, 179, 3119–3125. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.B.; Jeong, H.J. Rheosmin, a naturally occurring phenolic compound inhibits LPS-induced iNOS and COX-2 expression in RAW264.7 cells by blocking NF-κB activation pathway. Food Chem. Toxicol. 2010, 48, 2148–2153. [Google Scholar] [CrossRef]
- Dai, B.; Wei, D.; Zheng, N.-N.; Chi, Z.-H.; Xin, N.; Ma, T.-X.; Zheng, L.-Y.; Sumi, R.; Sun, L. Coccomyxa Gloeobotrydiformis polysaccharide inhibits lipopolysaccharide-induced inflammation in RAW 264.7 Macrophages. Cell. Physiol. Biochem. 2018, 51, 2523–2535. [Google Scholar] [CrossRef] [Green Version]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytotherapy Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Burmasova, M.A.; Sysoeva, M.A. Chemical composition and biological activity of the BuOH Fraction from chaga melanin. Pharm. Chem. J. 2017, 51, 292–294. [Google Scholar] [CrossRef]
- Shashkina, M.Y.; Shashkin, P.N.; Sergeev, A.V. Chemical and medicobiological properties of chaga (review). Pharm. Chem. J. 2006, 40, 560–568. [Google Scholar] [CrossRef]
- Mazurkiewicz, W. Analysis of aqueous extract of Inonotus obliquus. Acta Pol. Pharm. Drug Res. 2007, 63, 497–501. [Google Scholar]
- Zheng, W.; Zhang, M.; Zhao, Y.; Miao, K.; Pan, S.; Cao, F.; Dai, Y. Analysis of antioxidant metabolites by solvent extraction from sclerotia of Inonotus obliquus (Chaga). Phytochem. Anal. 2011, 22, 95–102. [Google Scholar] [CrossRef]
- Géry, A.; Dubreule, C.; André, V.; Rioult, J.-P.; Bouchart, V.; Heutte, N.; De Pécoulas, P.E.; Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a future potential medicinal fungus in oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and human bronchial epithelial cells (BEAS-2B). Integr. Cancer Ther. 2018, 17, 832–843. [Google Scholar] [CrossRef] [Green Version]
- Diao, B.-Z.; Jin, W.-R.; Yu, X.-J. Protective Effect of polysaccharides from Inonotus obliquus on Streptozotocin-induced diabetic symptoms and their potential mechanisms in rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 841496. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Kang, J.-H.; Kim, D.-K.; Oh, S.H.; Kim, M.K. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J. Ethnopharmacol. 2012, 143, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.I.; Al-Tuwariijiri, M.M.; Prasad, C. Chaga mushroom (Inonotus obliquus) inhibits growth of both lung adeno-carcinoma (A549) cells and Aspergillus fumigtatus. Curr. Top. Nutraceutical Res. 2018, 16, 289–296. [Google Scholar] [CrossRef]
- Glamočlija, J.; Ciric, A.; Nikolic, M.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.; Sokovic, M.; van Griensven, L.J. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Van, Q.; Nayak, B.; Reimer, M.; Jones, P.; Fulcher, R.; Rempel, C. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. J. Ethnopharmacol. 2009, 125, 487–493. [Google Scholar] [CrossRef]
- Nakajima, Y.; Sato, Y.; Konishi, T. Antioxidant Small Phenolic Ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem. Pharm. Bull. 2007, 55, 1222–1226. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, Y.; Nishida, H.; Matsugo, S.; Konishi, T. Cancer cell cytotoxicity of extracts and small phenolic compounds from chaga [Inonotus obliquus (persoon) Pilat]. J. Med. Food 2009, 12, 501–507. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, H.B.; Jeon, E.-J.; Jung, H.S.; Kang, M.-H. Chaga mushroom extract inhibits oxidative DNA damage in human lymphocytes as assessed by comet assay. BioFactors 2004, 21, 109–112. [Google Scholar] [CrossRef]
- Park, Y.-M.; Won, J.-H.; Kim, Y.-H.; Choi, J.-W.; Park, H.-J.; Lee, K.-T. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J. Ethnopharmacol. 2005, 101, 120–128. [Google Scholar] [CrossRef]
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T. Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus obliquus) compared with hot water extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.-K.; Lee, S.-C. Antioxidant activity of subcritical water extracts from chaga mushroom (Inonotus obliquus). Sep. Sci. Technol. 2010, 45, 198–203. [Google Scholar] [CrossRef]
- Alhallaf, W.; Bishop, K.; Perkins, L.B. Optimization of accelerated solvent extraction of phenolic compounds from chaga using response surface methodology. Food Anal. Methods 2022, in press. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [Google Scholar] [CrossRef]
- Han, S.; Sung, K.-H.; Yim, D.; Lee, S.; Cho, K.; Lee, C.-K.; Ha, N.-J.; Kim, K. Activation of murine macrophage cell line RAW 264.7 by Korean propolis. Arch. Pharmacal Res. 2002, 25, 895–902. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, I.-T.; Won, J.-H.; Jeong, S.-H.; Park, E.-Y.; Nam, J.-H.; Choi, J.; Lee, K.-T. Anti-inflammatory activities of ent-16αH,17-hydroxy-kauran-19-oic acid isolated from the roots of Siegesbeckia pubescens are due to the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB inactivation. Eur. J. Pharmacol. 2007, 558, 185–193. [Google Scholar] [CrossRef]
- Paige, J.S.; Jaffrey, S. Pharmacologic manipulation of nitric oxide signaling: Targeting NOS dimerization and protein-protein interactions. Curr. Top. Med. Chem. 2007, 7, 97–114. [Google Scholar] [CrossRef] [Green Version]
- Alderton, W.; Cooper, C.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Gilmore, T.D. The Rel/NF-κB signal transduction pathway: Introduction. Oncogene 1999, 18, 6842–6844. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Ye, S.-K.; Cho, I.H.; Jung, J.E.; Kim, D.-H.; Choi, S.; Kim, Y.S.; Park, C.-G.; Kim, T.-Y.; Lee, J.W.; et al. 8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic. Biol. Med. 2006, 41, 1392–1403. [Google Scholar] [CrossRef]
- Adams, D.O.; Hamilton, T.A. The cell biology of macrophage activation. Annu. Rev. Immunol. 1984, 2, 283–318. [Google Scholar] [CrossRef]
- Beutler, B.; Cerami, A. The Biology of Cachectin/TNF—A Primary Mediator of the Host Response. Annu. Rev. Immunol. 1989, 7, 625–655. [Google Scholar] [CrossRef]
- Dinarello, C.A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002, 20, S1–S13. [Google Scholar]
- Sánchez-Miranda, E.; Lemus-Bautista, J.; Pérez, S.; Pérez-Ramos, J. Effect of Kramecyne on the Inflammatory Response in Lipopolysaccharide-Stimulated Peritoneal Macrophages. Evid.-Based Complement. Altern. Med. 2013, 2013, 762020. [Google Scholar] [CrossRef] [Green Version]
- Juman, S.; Yasui, N.; Ikeda, K.; Ueda, A.; Sakanaka, M.; Negishi, H.; Miki, T. Caffeic Acid phenethyl Ester suppresses the production of pro-inflammatory cytokines in hypertrophic adipocytes through lipopolysaccharide-stimulated macrophages. Biol. Pharm. Bull. 2012, 35, 1941–1946. [Google Scholar] [CrossRef] [Green Version]
- Miles, E.A.; Zoubouli, P.; Calder, P. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef]
- Jaramillo-Flores, M.E.; González-Cruz, L.; Cornejo-Mazón, M.; Alvarez, L.D.; Gutierrez, G.; Hernández-Sánchez, H. Effect of thermal treatment on the antioxidant activity and content of carotenoids and phenolic compounds of cactus pear cladodes (Opuntia ficus-indica). Food Sci. Technol. Int. 2003, 9, 271–278. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Allen, J.; Brock, S.A. Tailoring the message. Minn. Med. 2000, 83, 45–48. [Google Scholar]
- Emami Bistgani, Z.; Siadat, S.A.; Bakhshandeh, A.; Ghasemi Pirbalouti, A.; Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef] [Green Version]






| Sample | Yield% | Carbohydrate% | Protein% | Uronic Acid% | TPC% |
|---|---|---|---|---|---|
| P3 | 30.66 ± 0.05 a | 17.02 ± 0.01 a | 10.53 ±1.66 a | 4.11 ± 0.47 a | 16.77 ± 0.13 ab |
| P6 | 30.21 ± 0.01 a | 17.56 ± 0.01 a | 10.23 ±1.02 a | 4.12 ± 0.44 a | 17.61 ± 0.05 a |
| P10 | 31.33 ± 0.04 a | 18.31 ± 0.05 a | 11.02 ±1.28 a | 4.11± 0.26 a | 17.04 ± 0.03 a |
| B3 | 15.53 ± 0.01 c | 10.26 ± 0.08 b | 7.14 ± 1.83 b | 3.01 ± 0.53 b | 6.49 ± 0.04 d |
| B6 | 17.33 ± 0.03 b | 11.56 ± 0.01 b | 7.11 ± 0.5 b | 2.57 ± 0.71 b | 8.33 ± 0.05 c |
| B10 | 17.33 ± 0.02 b | 11.81 ± 0.04 b | 7.23 ± 2.01 b | 2.84 ± 1.22 b | 8.33 ± 0.06 c |
| Extraction Conditions | Extract | |
|---|---|---|
| Temperature °C | ETOH% | |
| 170 | 66 | ASE1 |
| 150 | 70 | ASE2 |
| 130 | 70 | ASE3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhallaf, W.; Perkins, L.B. The Anti-Inflammatory Properties of Chaga Extracts Obtained by Different Extraction Methods against LPS-Induced RAW 264.7. Molecules 2022, 27, 4207. https://doi.org/10.3390/molecules27134207
Alhallaf W, Perkins LB. The Anti-Inflammatory Properties of Chaga Extracts Obtained by Different Extraction Methods against LPS-Induced RAW 264.7. Molecules. 2022; 27(13):4207. https://doi.org/10.3390/molecules27134207
Chicago/Turabian StyleAlhallaf, Weaam, and Lewis B. Perkins. 2022. "The Anti-Inflammatory Properties of Chaga Extracts Obtained by Different Extraction Methods against LPS-Induced RAW 264.7" Molecules 27, no. 13: 4207. https://doi.org/10.3390/molecules27134207
APA StyleAlhallaf, W., & Perkins, L. B. (2022). The Anti-Inflammatory Properties of Chaga Extracts Obtained by Different Extraction Methods against LPS-Induced RAW 264.7. Molecules, 27(13), 4207. https://doi.org/10.3390/molecules27134207





