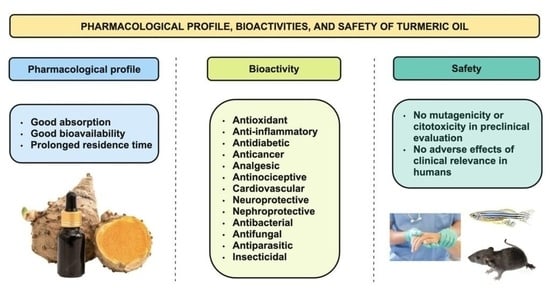
Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil
Abstract
1. Introduction
2. Pharmacological Profile
3. Bioactivity
3.1. Antioxidant
3.2. Anti-Inflammatory
3.3. Antidiabetic
3.4. Anticancer
3.5. Analgesic and Antinociceptive
3.6. Cardiovascular
3.7. Neuroprotective
3.8. Nephroprotective
3.9. Antibacterial
3.10. Antifungal
3.11. Antiparasitic
3.12. Insecticidal
4. Safety
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | adenosine triphosphate-binding cassette |
| ADA | adenosine deaminase |
| AhR | aryl hydrocarbon receptor |
| aPTT | activated partial thromboplastin time |
| Bax | B-cell leukaemia/lymphoma 2-associated X Protein |
| Bcl | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| BPH | benign prostatic hyperplasia |
| CK-MB | creatine kinase MB |
| COX | cyclooxygenase |
| CYP | cytochrome P-450 |
| DHA | docosahexanoic acid |
| eNOS | endothelial NOS |
| EPA | eicosapentaenoic acid |
| GRAS | Generally Recognized As Safe |
| HMGCR | hydroxymethylglutaryl coenzyme A reductase |
| ICAM | intercellular adhesion molecule |
| i.g. | intragastric |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| i.p. | intraperitoneal |
| i.v. | intravenous |
| LXR | liver X receptor |
| LPL | lipoprotein lipase |
| MI/RP | myocardial ischemia/reperfusion |
| NF | nuclear factor |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NPC1L1 | Niemann-Pick C1-Like 1 |
| PGC | peroxisome proliferator-activated receptor gamma coactivator |
| p.o. | per os (oral) |
| PPAR | peroxisome proliferator-activated receptors |
| PT | prothrombin time |
| ROS | reactive oxygen species |
| SCW | streptococcal cell wall |
| SREBP | sterol regulatory element binding protein |
| TACE | transcatheter artery chemoembolization |
| TAG | triacylglycerides |
| TNF | tumor necrosis factor |
| TO | turmeric oil |
References
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; Volume 1, pp. 115–119. [Google Scholar]
- Villegas, I.; Sánchez-Fidalgo, S.; de La Lastra, C.A. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1040–1061. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Durjava, K.; Kouba, M.; López-Alonso, M.; Puente, S.L.; Marcon, F.; Mayo, B.; et al. Safety and efficacy of turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture from Curcuma longa L. rhizome when used as sensory additives in feed for all animal species EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Panel members. EFSA J. 2020, 18, e06146. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.A.; Kitts, D.D. Turmeric and its bioactive constituents trigger cell signaling mechanisms that protect against diabetes and cardiovascular diseases. Mol. Cell. Biochem. 2021, 476, 3785–3814. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Awasthi, P. Chemical Composition of Curcuma Longa Leaves and Rhizome Oil from the Plains of Northern India. J. Young Pharm. 2009, 1, 312. [Google Scholar] [CrossRef]
- Prakash, P.; Misra, A.; Surin, W.R.; Jain, M.; Bhatta, R.S.; Pal, R. Antiplatelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb. Res. 2011, 127, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Paucar, A.M.; Afrikanova, T.; Thomas, J.; Aibuldinov, Y.K.; Dehaen, W.; de Witte, P.A.; Esguerra, C.V. Insights from Zebrafish and Mouse Models on the Activity and Safety of Ar-Turmerone as a Potential Drug Candidate for the Treatment of Epilepsy. PLoS ONE 2013, 8, e81634. [Google Scholar] [CrossRef]
- Peron, G.; Sut, S.; Dal Ben, S.; Voinovich, D.; Dall’Acqua, S. Untargeted UPLC-MS metabolomics reveals multiple changes of urine composition in healthy adult volunteers after consumption of curcuma longa L. extract. Food Res. Int. 2020, 127, 108730. [Google Scholar] [CrossRef]
- Matsumura, S.; Murata, K.; Zaima, N.; Yoshioka, Y.; Morimoto, M.; Kugo, H.; Yamamoto, A.; Moriyama, T.; Matsuda, H. Inhibitory Activities of Essential Oil Obtained from Turmeric and Its Constituents against β-Secretase. Nat. Prod. Commun. 2016, 11, 1785–1788. [Google Scholar] [CrossRef]
- Li, R.; Lindholm, K.; Yang, L.B.; Yue, X.; Citron, M.; Yan, R.; Beach, T.; Sue, L.; Sabbagh, M.; Cai, H. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2004, 101, 3632–3637. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Matsumura, S.; Yoshioka, Y.; Yamamoto, A.; Makino, S.; Moriyama, T.; Zaima, N. Using Turmeric Oil as a Solvent Improves the Distribution of Sesamin-Sesamolin in the Serum and Brain of Mice. Lipids 2019, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Kawamoto, H.; Takeshita, F.; Matsumura, S.; Ayaki, I.; Moriyama, T.; Zaima, N. Mixing Ginkgo biloba Extract with Sesame Extract and Turmeric Oil Increases Bioavailability of Ginkgolide a in Mice Brain. J. Oleo Sci. 2019, 68, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.; Hon, P.M.; Lee, M.Y.H.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells. J. Med. Food 2012, 15, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Xie, T.; Lin, J.; Fan, H.Z.; Huang-Fu, H.J.; Ni, L.F. An investigation of the ability of elemene to pass through the blood-brain barrier and its effect on brain carcinomas. J. Pharm. Pharmacol. 2009, 61, 1653–1656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Marín, R.; Fernandes, S.C.M.; Andrés, M.A.; Labidi, J. Microwave-assisted extraction of curcuma longa l. Oil: Optimization, chemical structure and composition, antioxidant activity and comparison with conventional soxhlet extraction. Molecules 2021, 26, 1516. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Makoter, K.; Razboršek, M.I. Comparative study of chemical composition and antioxidant activity of essential oils and crude extracts of four characteristic zingiberaceae herbs. Plants 2021, 10, 501. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa. L. Indian J. Pharmacol. 2011, 43, 526. [Google Scholar]
- Dohare, P.; Varma, S.; Ray, M. Curcuma oil modulates the nitric oxide system response to cerebral ischemia/reperfusion injury. Nitric Oxide 2008, 19, 1–11. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jena, B.S.; Negi, P.S.; Sakariah, K.K. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: A byproduct from curcumin production. Z. Nat. Sect. C J. Biosci. 2002, 57, 828–835. [Google Scholar] [CrossRef]
- Di Rosa, M.; Giroud, J.P.; Willoughby, D.A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol. 1971, 104, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.N.; Badyal, D.K. Biological studies of turmeric oil, part 3: Anti-inflammatory and analgesic properties of turmeric oil and fish oil in comparison with aspirin. Nat. Prod. Commun. 2014, 9, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Manhas, A.; Khanna, V.; Prakash, P.; Goyal, D.; Malasoni, R.; Naqvi, A. Curcuma oil reduces endothelial cell-mediated inflammation in postmyocardial ischemia/reperfusion in rats. J. Cardiovasc. Pharmacol. 2014, 64, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Zhang, H.; Timmermann, B.N. Anti-Arthritic Effects and Toxicity of the Essential Oils of Turmeric (Curcuma longa L.). J. Agric. Food Chem. 2010, 58, 842. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent advances to improve curcumin oral bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Singh, V.; Jain, M.; Misra, A.; Khanna, V.; Prakash, P.; Malasoni, R. Curcuma oil ameliorates insulin resistance & associated thrombotic complications in hamster & rat. Indian J. Med. Res. 2015, 141, 823. [Google Scholar]
- Lekshmi, P.C.; Arimboor, R.; Indulekha, P.S.; Nirmala Menon, A. Turmeric (Curcuma longa L.) volatile oil inhibits key enzymes linked to type 2 diabetes. Int. J. Food Sci. Nutr. 2012, 63, 832–834. [Google Scholar] [CrossRef]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M. Curcuminoids and Sesquiterpenoids in Turmeric (Curcuma longa L.) Suppress an Increase in Blood Glucose Level in Type 2 Diabetic KK-Ay Mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef]
- Bartoňková, I.; Dvořák, Z. Essential oils of culinary herbs and spices display agonist and antagonist activities at human aryl hydrocarbon receptor AhR. Food Chem. Toxicol. 2018, 111, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Hastak, K.; Lubri, N.; Jakhi, S.D.; More, C.; John, A.; Ghaisas, S.D. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997, 116, 265–269. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Chemopreventive activity of turmeric essential oil and possible mechanisms of action. Asian Pac. J. Cancer Prev. 2014, 15, 6575–6580. [Google Scholar] [CrossRef]
- Zan, X.J.; Rong, D.Y.; Tu, Y.H.; Xue, Y.C.; Ye, Z.Y.; Kang, Y.Q.; Zhou, Y.; Cao, Y. Effect of turmeric volatile oil on proliferation and apoptosis of human skin SCC A431 cells. Zhongguo Zhong Yao Za Zhi 2016, 41, 2883–2887. [Google Scholar] [PubMed]
- Wang, S.; Li, Y.; Li, W.; Zhang, K.; Yuan, Z.; Cai, Y. Curcuma oil ameliorates benign prostatic hyperplasia through suppression of the nuclear factor-kappa B signaling pathway in rats. J. Ethnopharmacol. 2021, 279, 113703. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Baldwin, A.S. NF-κB as a therapeutic target in cancer. Trends Mol. Med. 2002, 8, 385–389. [Google Scholar] [CrossRef]
- Paradkar, P.H.; Juvekar, A.S.; Barkume, M.S.; Amonkar, A.J.; Joshi, J.V.; Soman, G.; Vaidya, A.D.B. In vitro and in vivo evaluation of a standardized Curcuma longa Linn formulation in cervical cancer. J. Ayurveda Integr. Med. 2021, 12, 616–622. [Google Scholar] [CrossRef]
- Santos, P.A.S.R.; Avanço, G.B.; Nerilo, S.B.; Marcelino, R.I.A.; Janeiro, V.; Valadares, M.C. Assessment of Cytotoxic Activity of Rosemary (Rosmarinus officinalis L.), Turmeric (Curcuma longa L.), and Ginger (Zingiber officinale R.) Essential Oils in Cervical Cancer Cells (HeLa). Sci. World J. 2016, 2016, 9273078. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L). Food Chem. Toxicol. 2013, 53, 52–61. [Google Scholar] [CrossRef]
- Clark, S.F. The biochemistry of antioxidants revisited. Nutr. Clin. Pract. 2002, 17, 5–17. [Google Scholar] [CrossRef]
- Yasueda, A.; Urushima, H.; Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr. Cancer Ther. 2016, 15, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Chang, G.; Wu, W.Y. A controlled clinical study between hepatic arterial infusion with embolized curcuma aromatic oil and chemical drugs in treating primary liver cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 2001, 21, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Benny Antony. Composition to Enhance the Bioavailability of Curcumin. U.S. Patent US8895087B2, 25 November 2014.
- Farhana, L.; Sarkar, S.; Nangia-Makker, P.; Yu, Y.; Khosla, P.; Levi, E.; Azmi, A.; Majumdar, A.P.N. Natural agents inhibit colon cancer cell proliferation and alter microbial diversity in mice. PLoS ONE. 2020, 15, e0229823. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Quan, L.; Zhou, H.; Zhao, Y.; Chen, P.; Hu, L. Screening of active fractions from Curcuma Longa Radix isolated by HPLC and GC-MS for promotion of blood circulation and relief of pain. J. Ethnopharmacol. 2019, 234, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Jain, M.; Misra, A.; Khanna, V.; Rana, M.; Prakash, P. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br. J. Nutr. 2013, 110, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.K. Evaluation of anti-atherosclerotic activities of PPAR-α, PPAR-γ, and LXR agonists in hyperlipidemic atherosclerosis-susceptible F(1)B hamsters. Atherosclerosis 2011, 214, 86–93. [Google Scholar] [CrossRef]
- Valasek, M.A.; Repa, J.J.; Quan, G.; Dietschy, J.M.; Turley, S.D. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am. J. Physiol. Gastrointest Liver Physiol. 2008, 295, G813–G822. [Google Scholar] [CrossRef]
- Yu, L.; Li-Hawkins, J.; Hammer, R.E.; Berge, K.E.; Horton, J.D.; Cohen, J.C. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Investg. 2002, 110, 671–680. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Adeniyi, P.A. Effect of Essential Oils from Ginger (Zingiber officinale) and Turmeric (Curcuma longa) Rhizomes on Some Inflammatory Biomarkers in Cadmium Induced Neurotoxicity in Rats. J. Toxicol. 2018, 2018, 4109491. [Google Scholar] [CrossRef]
- Matsumura, S.; Murata, K.; Yoshioka, Y.; Matsuda, H. Search for β-Secretase Inhibitors from Natural Spices. Nat. Prod. Commun. 2016, 11, 507–510. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M.; Serruys, A.S.K.; Afrikanova, T.; Maes, J.; de Borggraeve, W.; Alen, J. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models. Epilepsy Behav. 2012, 24, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Dohare, P.; Garg, P.; Sharma, U.; Jagannathan, N.R.; Ray, M. Neuroprotective efficacy and therapeutic window of curcuma oil: In rat embolic stroke model. BMC Complement. Altern. Med. 2008, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Choo, B.K.M.; Shaikh, M.F. Mechanism of Curcuma longa and Its Neuroactive Components for the Management of Epileptic Seizures: A Systematic Review. Curr. Neuropharmacol. 2021, 19, 1496. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.J.; Faboya, O.L.; Paul, A.A.; Olayide, I.; Faboya, O.A.; Oluwasola, T.A. Nephroprotective Effect of Essential Oils from Ginger (Zingiber officinale) and Turmeric (Curcuma longa) Rhizomes against Cadmium-induced Nephrotoxicity in Rats. J. Oleo Sci. 2018, 67, 1339–1345. [Google Scholar] [CrossRef]
- Hans, V.M.; Grover, H.S.; Deswal, H.; Agarwal, P. Antimicrobial Efficacy of Various Essential Oils at Varying Concentrations against Periopathogen Porphyromonas gingivalis. J. Clin. Diagn. Res. 2016, 10, ZC16. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, B.S.; Keum, K.S.; Yu, H.H.; Kim, Y.H.; Chang, B.S.; Ra, J.-Y.; Moon, H.-D.; Seo, B.-R.; Choi, N.-Y.; et al. Essential Oil of Curcuma longa Inhibits Streptococcus mutans Biofilm Formation. J. Food Sci. 2011, 76, H226–H230. [Google Scholar] [CrossRef]
- Köhler, B.; Birkhed, D.; Olsson, S. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1995, 29, 402–406. [Google Scholar] [CrossRef]
- Nagpal, M.; Sood, S. Role of curcumin in systemic and oral health: An overview. J. Nat. Sci. Biol. Med. 2013, 4, 3–7. [Google Scholar]
- Álvarez, N.M.; Ortíz, A.A.; Martínez, O.C. Actividad antibacteriana in vitro de Curcuma longa (Zingiberaceae) frente a bacterias nosocomiales en Montería, Colombia. Rev. Biol. Trop. 2016, 64, 1201–1208. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999, 47, 4297–4300. [Google Scholar] [CrossRef]
- Teles, A.M.; Rosa, T.D.D.S.; Mouchrek, A.N.; Abreu-Silva, A.L.; da Silva Calabrese, K.; Almeida-Souza, F. Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa Essential Oils: Chemical Composition, Antimicrobial and Antileishmanial Activity. Evid. Based Complement. Altern. Med. 2019, 2019, 2421695. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mahajan, S.; Sharma, R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnol. Rep. 2015, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Ogidi, C.O.; Ojo, A.E.; Ajayi-Moses, O.B.; Aladejana, O.M.; Thonda, O.A.; Akinyele, B.J. Synergistic antifungal evaluation of over-the-counter antifungal creams with turmeric essential oil or Aloe vera gel against pathogenic fungi. BMC Complement. Med. Ther. 2021, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Apisariyakul, A.; Vanittanakom, N.; Buddhasukh, D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). J. Ethnopharmacol. 1995, 49, 163–169. [Google Scholar] [CrossRef]
- Jankasem, M.; Wuthi-udomlert, M.; Gritsanapan, W. Antidermatophytic Properties of Ar-Turmerone, Turmeric Oil, and Curcuma longa Preparations. ISRN Dermatol. 2013, 2013, 250597. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; de Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Romoli, J.C.Z.; Silva, M.V.; Pante, G.C.; Hoeltgebaum, D.; Castro, J.C.; Oliveira da Rocha, G.H. Anti-mycotoxigenic and antifungal activity of ginger, turmeric, thyme and rosemary essential oils in deoxynivalenol (DON) and zearalenone (ZEA) producing Fusarium graminearum. Food Addit. Contam. Part A 2022, 39, 362–372. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Ferreira, F.D.; Mossini, S.A.G.; Ferreira, F.M.D.; Arrotéia, C.C.; da Costa, C.L.; Nakamura, C.V. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Sci. World J. 2013, 2013, 343804. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Negi, P.S.; Anandharamakrishnan, C.; Sakariah, K.K. Chemical composition of turmeric oil -a byproduct from turmeric oleoresin industry and its inhibitory activity against different fungi. Z. Nat. C J. Biosci. 2001, 56, 40–44. [Google Scholar] [CrossRef]
- Achimón, F.; Brito, V.D.; Pizzolitto, R.P.; Ramirez Sanchez, A.; Gómez, E.A.; Zygadlo, J.A. Chemical composition and antifungal properties of commercial essential oils against the maize phytopathogenic fungus Fusarium verticillioides. Rev. Argent. Microbiol. 2021, 53, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Le, T.B.; Beaufay, C.; Nghiem, D.T.; Pham, T.A.; Mingeot-Leclercq, M.P.; Quetin-Leclercq, J. Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components. Molecules 2019, 24, 1158. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.S.; Rao, G.S.; Shankar, J.; Chauhan, L.K.S.; Kapadia, G.J.; Singh, N. Chemoprevention of Leishmaniasis: In-vitro antiparasitic activity of dibenzalacetone, a synthetic curcumin analog leads to apoptotic cell death in Leishmania donovani. Parasitol. Int. 2018, 67, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, H.; Pakravanan, M.; Aflatoonian, M.R.; Khalaf, A.K.; Niazi, M.; Mirbadie, S.R. Efficacy and safety of Curcuma longa essential oil to inactivate hydatid cyst protoscoleces. BMC Complement. Altern. Med. 2019, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, X.; O’Neal, M.; Schultz, G.; Tucker, B.; Coats, J. Mosquito larvicidal activity of botanical-based mosquito repellents. J. Am. Mosq. Control Assoc. 2008, 24, 161–168. [Google Scholar] [CrossRef]
- Groh, I.A.M.; Rudakovski, O.; Gründken, M.; Schroeter, A.; Marko, D.; Esselen, M. Methyleugenol and oxidative metabolites induce DNA damage and interact with human topoisomerases. Arch. Toxicol. 2016, 90, 2809–2823. [Google Scholar] [CrossRef]
- Tascone, O.; Roy, C.; Filippi, J.J.; Meierhenrich, U.J. Use, analysis, and regulation of pesticides in natural extracts, essential oils, concretes, and absolutes. Anal. Bioanal. Chem. 2014, 406, 971–980. [Google Scholar] [CrossRef]
- Donelli, D.; Antonelli, M.; Firenzuoli, F. Considerations about turmeric-associated hepatotoxicity following a series of cases occurred in Italy: Is turmeric really a new hepatotoxic substance? Intern. Emerg. Med. 2020, 15, 725–726. [Google Scholar] [CrossRef]
- Suhail, F.K.; Masood, U.; Sharma, A.; John, S.; Dhamoon, A. Turmeric supplement induced hepatotoxicity: A rare complication of a poorly regulated substance. Clin. Toxicol. 2020, 58, 216–217. [Google Scholar] [CrossRef]
- Lukefahr, A.L.; McEvoy, S.; Alfafara, C.; Funk, J.L. Drug-induced autoimmune hepatitis associated with turmeric dietary supplement use. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Chempakam, B. Toxicity prediction of compounds from turmeric (Curcuma longa L). Food Chem. Toxicol. 2010, 48, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Ghaisas, S.; Vaidya, A.; Vaidya, R.; Kamat, D.V.; Bhagwat, A.N.; Bhide, S. Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. J. Assoc. Physicians India 2003, 51, 1055–1060. [Google Scholar] [PubMed]

| Bioactivity | Main Compounds of Turmeric Oil | Model | Concentration/Dose; Administration Route | Source |
|---|---|---|---|---|
| Antioxidant | ar-turmerone, a-turmerone, β-turmerone | In vitro | 0.025 g/3 mL | [17] |
| ar-turmerone, β-turmerone, ar-curcumene | In vitro | 80% ethanol | [18] | |
| ar-turmerone, curlone, ar-curcumene | In vitro | 200 mg/mL | [19] | |
| In vivo (mouse) | 250 mg/kg; i.p. | [19] | ||
| ar-turmerone, a-turmerone, β-turmerone, curlone | In vivo (rat) | 250 mg/kg; i.p. | [20] | |
| Anti-inflammatory | ar-turmerone, curlone, ar-curcumene | In vivo (mouse) | 500 mg/kg; i.p. | [19] |
| ar-turmerone, a-turmerone, β-turmerone | In vivo (rat) | 100 mg/kg; p.o. | [23] | |
| ar-turmerone, turmerone, and curlone | In vivo (rat) | 250 mg/kg; p.o. | [24] | |
| ar-turmerone, α-turmerone, β-turmerone | In vivo (rat) | >28 mg/kg/day; i.p. | [25] | |
| In vivo (rat) | 560 mg/kg; p.o. | [25] | ||
| Antidiabetic | ar-turmerone, a-turmerone, β-turmerone, curlone | In vivo (hamster) | 300 mg/kg/day; p.o. | [27] |
| In vivo (rat) | 300 mg/kg/day; p.o. | [27] | ||
| ar-turmerone | In vitro | 0.38 mg/mL | [28] | |
| ar-turmerone | In vivo (mouse) | 0.5 g/100 g of diet; p.o. | [29] | |
| Anticancer | p-cymene | In vitro | 100 μg/mL | [32] |
| not reported | In vivo (human) | 600 mg TO + 3 g turmeric extract; p.o. | [33] | |
| ar-turmerone | In vitro | 1 mg/plate | [34] | |
| In vivo (mice) | 50%; topical | [34] | ||
| In vitro | 200 mg/mL | [34] | ||
| not reported | In vitro | 80 mg/L | [35] | |
| not reported | In vitro | 40 mg/mL | [36] | |
| In vivo (rat) | 7.2 mg/kg; i.g. | [36] | ||
| ar-turmerone, a-turmerone, β-turmerone | In vitro | 2100 mg/mL | [39] | |
| ar-turmerone, curlone | In vitro | 3000 mg/plate | [40] | |
| In vivo (rat) | 1 g/kg; p.o. | [40] | ||
| not reported | In vivo (human) | 3 mL (embolized) | [43] | |
| ar-turmerone, a-turmerone, β-turmerone, a-santalene, ar-curcumene | In vivo (mice) | 5 mg (TO + curcumin)/kg; p.o. | [45] | |
| Analgesic and antinociceptive | ar-turmerone, curlone, ar-curcumene | In vivo (mouse) | 100 mg/kg; i.p. | [19] |
| ar-turmerone, a-turmerone, β-turmerone | In vivo (rat) | 100 mg/kg; p.o. | [23] | |
| ar-turmerone, curlone, turmerone | In vivo (mouse) | 9.75 mL/kg; i.p. | [46] | |
| Cardiovascular | ar-turmerone, a-turmerone, β-turmerone, curlone | In vivo (rat) | 1 g/kg; p.o. | [8] |
| In vivo (mouse) | 1 g/kg; p.o. | [8] | ||
| ar-turmerone, a-turmerone, β-turmerone, curlone | In vivo (hamster) | 300 mg/kg/day; p.o. | [27] | |
| In vivo (rat) | 300 mg/kg/day; p.o. | [27] | ||
| ar-turmerone, curlone, turmerone | In vivo (mouse) | 9.75 mL/kg; i.p. | [46] | |
| ar-turmerone, a-turmerone, β-turmerone, curlone | In vivo (hamster) | 300 mg/kg; p.o. | [47] | |
| Neuroprotective | ar-turmerone, a-turmerone, β-turmerone, l-zingiberene, β-sesquiphellandrene | In vitro | 250 mg/mL | [11] |
| eucalyptol | In vivo (rat) | 50 mg/kg; p.o. | [51] | |
| ar-turmerone, a-turmerone, β-turmerone, a-atlantone | In vivo (zebrafish) | 10 mg/mL | [53] | |
| In vivo (mouse) | 100 mg/kg; i.v. | [53] | ||
| ar-turmerone, a-turmerone, β-turmerone, curlone | In vivo (rat) | 50 mg/kg, p.o. | [54] | |
| Nephroprotective | eucalyptol | In vivo (rat) | 50 mg/kg; p.o. | [56] |
| Antibacterial | not reported | In vitro | 100% | [57] |
| a-turmerone, germacrone | In vitro | >0.5 mg/mL | [58] | |
| not reported | In vitro | 1000 ppm | [61] | |
| ar-turmerone, turmerone, curlone | In vitro | 100 ppm | [62] | |
| turmerone, b-turmerone, γ-curcumene | In vitro | 75 mL | [63] | |
| Antifungal | z-citral | In vitro | 10 mg/mL | [65] |
| not reported | In vitro | 114.9 mg/mL | [66] | |
| In vivo (guinea pig) | topical | [66] | ||
| ar-turmerone | In vitro | 6% w/w; topical | [67] | |
| not reported | In vitro | 11,580 mg/mL | [69] | |
| ar-turmerone, turmerone, b-sesquiphellandrene, curcumene | In vitro | 4 mL/mL | [70] | |
| ar-turmerone, a-turmerone, β-turmerone | In vitro | 0.5% v/v | [71] | |
| ar-turmerone, a-zingiberene, b-(Z)-farnesene, ar-curcumene | In vitro | 6 mg/mL | [72] | |
| ar-turmerone, a-turmerone, β-turmerone | In vitro | 1000 ppm | [73] | |
| Antiparasitic | turmerone, β-turmerone, γ-curcumene | In vitro | 500 mg/mL | [63] |
| a-zingiberene, b-sesquiphellandrene, ar-turmerone, curlone | In vitro | 3.17 nL/mL | [74] | |
| a-turmerone, b-turmerone | In vitro | 200 mg/mL | [76] | |
| Insecticidal | turmerone, curcumene | In vivo (mosquito larvae) | 0.2 mg/mL | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana-Paucar, A.M.; Machado-Orellana, M.G. Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil. Molecules 2022, 27, 5055. https://doi.org/10.3390/molecules27165055
Orellana-Paucar AM, Machado-Orellana MG. Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil. Molecules. 2022; 27(16):5055. https://doi.org/10.3390/molecules27165055
Chicago/Turabian StyleOrellana-Paucar, Adriana Monserrath, and María Gabriela Machado-Orellana. 2022. "Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil" Molecules 27, no. 16: 5055. https://doi.org/10.3390/molecules27165055
APA StyleOrellana-Paucar, A. M., & Machado-Orellana, M. G. (2022). Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil. Molecules, 27(16), 5055. https://doi.org/10.3390/molecules27165055