Novel D-A-π-A1 Type Organic Sensitizers from 4,7-Dibromobenzo[d][1,2,3]thiadiazole and Indoline Donors for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of D-A-π-A1 Dyes
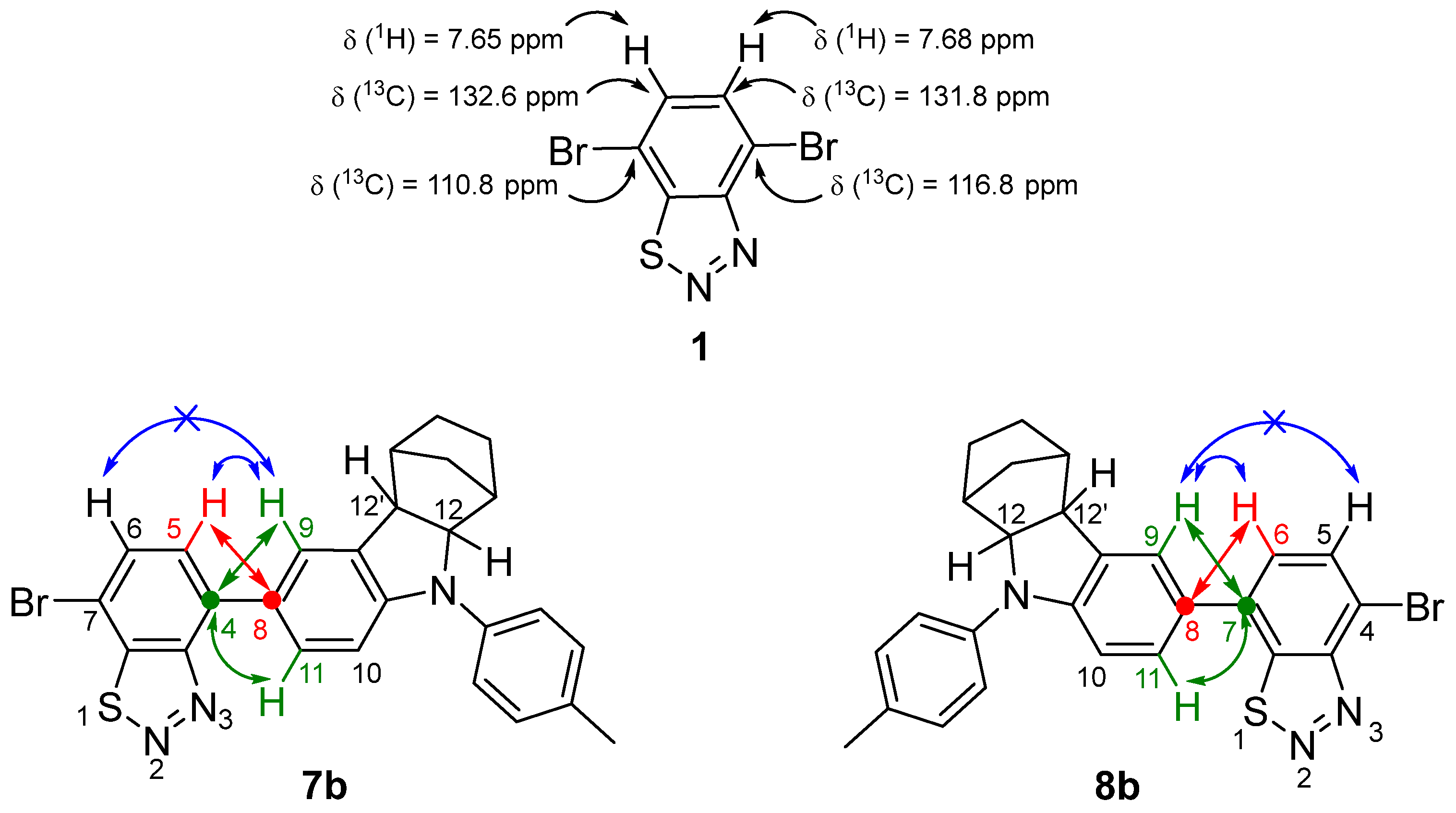
2.2. Proof of the Structure of Monobromo Derivatives 7 and 8 by NMR Spectroscopy and X-ray Diffraction
2.3. Synthesis and Characterization of the Dyes
2.4. Optical Properties
2.5. Electrochemical Properties
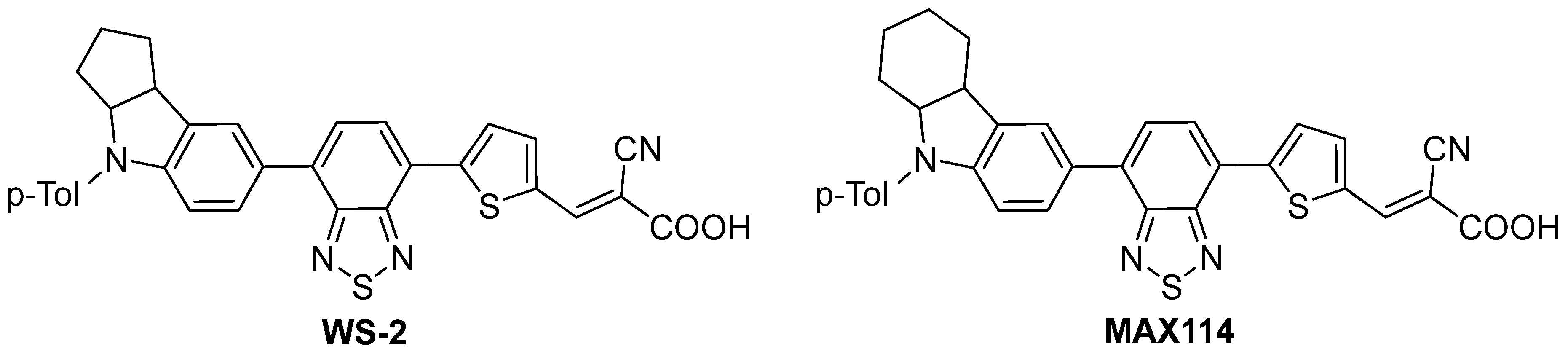
2.6. Photovoltaic Performance
3. Materials and Methods
3.1. Materials and Reagents
3.2. Analytical Instruments
3.3. X-ray Analysis
3.4. General Procedure for Suzuki-Miyamura Coupling Reactions
3.5. Synthesis of Dyes KEA321 and KEA337
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grätzel, M. Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 10720–10728. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Masuda, H.; Yamanaka, N.; Minami, M.; Nakamura, T.; Nishikitani, Y. Efficient Electron Transfer Ruthenium Sensitizers for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2009, 113, 2618–2623. [Google Scholar] [CrossRef]
- Elmorsy, M.R.; Su, R.; Fadda, A.A.; Etman, H.A.; Tawfik, E.H.; El-Shafei, A. Effect of terthiophene spacer position in Ru(II) bipyridyl complexes on the photocurrent and photovoltage for high efficiency dye-sensitized solar cells. Dye. Pigment. 2018, 156, 348–356. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K.; Lu, Y.; Tang, W.; Zhao, S.; Liu, Q.; Zhu, W.; Tian, H.; Xie, Y. Efficient solar cells sensitized by a promising new type of porphyrin: Dye-aggregation suppressed by double strapping. Chem. Sci. 2019, 10, 2186–2192. [Google Scholar] [CrossRef] [Green Version]
- Kurumisawa, Y.; Higashino, T.; Nimura, S.; Tsuji, Y.; Iiyama, H.; Imahori, H. Renaissance of Fused Porphyrins: Substituted Methylene-Bridged Thiophene-Fused Strategy for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2019, 141, 9910–9919. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, M.; Wu, H.; Yang, L.; Li, R.; Wang, P. Donor/Acceptor Indenoperylene Dye for Highly Efficient Organic Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 3799–3802. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-T.; Kuo, Y.-L.; Kumar, C.P.; Huang, P.-T.; Lin, J.T. Tetraphenylethylene tethered phenothiazine-based double-anchored sensitizers for high performance dye-sensitized solar cells. J. Mater. Chem. A 2019, 7, 23225–23233. [Google Scholar] [CrossRef]
- Wu, H.; Xie, X.; Mei, Y.; Ren, Y.; Shen, Z.; Li, S.; Wang, P. Phenalenothiophene-Based Organic Dye for Stable and Efficient Solar Cells with a Cobalt Redox Electrolyte. ACS Photonics 2019, 6, 1216–1225. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, K.; Huang, Z.-S.; Wang, L.; Tang, H.; Meier, H.; Cao, D. Effect of structural engineering of π-spacers on anti-aggregation of D–A–π–A dyes. J. Mater. Chem. C 2019, 7, 10379–10388. [Google Scholar] [CrossRef]
- Lee, C.-P.; Lin, R.Y.-Y.; Lin, L.-Y.; Li, C.-T.; Chu, T.-C.; Sun, S.-S.; Lin, J.T.; Ho, K.-C. Recent progress in organic sensitizers for dye-sensitized solar cells. RSC Adv. 2015, 5, 23810–23825. [Google Scholar] [CrossRef]
- Ji, J.-M.; Zhou, H.; Kim, H.K. Rational design criteria for D–π–A structured organic and porphyrin sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 2018, 6, 14518–14545. [Google Scholar] [CrossRef]
- Prachumrak, N.; Sudyoadsuk, T.; Thangthong, A.; Nalaoh, P.; Jungsuttiwong, S.; Daengngern, R.; Namuangruk, S.; Pattanasattayavong, P.; Promarak, V. Improvement of D–π–A organic dye-based dye-sensitized solar cell performance by simple triphenylamine donor substitutions on the π-linker of the dye. Mater. Chem. Front. 2017, 1, 1059–1072. [Google Scholar] [CrossRef]
- Parker, T.C.; Patel, D.G.; Moudgil, K.; Barlow, S.; Risko, C.; Brédas, J.-L.; Reynolds, J.R.; Marder, S.R. Heteroannulated acceptors based on benzothiadiazole. Mater. Horizons 2015, 2, 22–36. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Zhang, Q.; Tian, H.; Zhu, W. D-A-π-A Featured Sensitizers Bearing Phthalimide and Benzotriazole as Auxiliary Acceptor: Effect on Absorption and Charge Recombination Dynamics in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2012, 4, 1822–1830. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W.-H.; Zakeeruddin, S.M.; Grätzel, M. Insight into D–A−π–A Structured Sensitizers: A Promising Route to Highly Efficient and Stable Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 9307–9318. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230–152241. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. 4,7-Dibromo-substituted 2,1,3-benzothia(selena,oxa)diazoles and [1,2,5]thia(selena)diazolo[3,4-c]pyridines as building blocks in solar cells components (microreview). Chem. Heterocycl. Compd. 2017, 53, 855–857. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Knyazeva, E.A.; Rakitin, O.A. Recent Developments in the Synthesis and Applications of 1,2,5-Thia- and Selenadiazoles. A Review. Org. Prep. Proc. Int. 2014, 46, 475–544. [Google Scholar] [CrossRef]
- Chen, Z.; Brown, J.; Drees, M.; Seger, M.; Hu, Y.; Xia, Y.; Boudinet, D.; McCray, M.; Delferro, M.; Marks, T.J.; et al. Benzo[d][1,2,3]thiadiazole (isoBT): Synthesis, Structural Analysis, and Implementation in Semiconducting Polymers. Chem. Mater. 2016, 28, 6390–6400. [Google Scholar] [CrossRef]
- Gudim, N.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Golovanov, I.S.; Popov, V.V.; Obruchnikova, N.V.; Rakitin, O.A. Benzothiadiazole vs. iso-Benzothiadiazole: Synthesis, Electrochemical and Optical Properties of D–A–D Conjugated Molecules Based on Them. Molecules 2021, 26, 4931. [Google Scholar] [CrossRef]
- Gudim, N.S.; Knyazeva, E.A.; Obruchnikova, N.V.; Rakitin, O.A.; Popov, V.V. 4-(7-Bromobenzo[d][1,2,3]thiadiazol-4-yl)morpholine. Molbank 2021, 2021, M1202. [Google Scholar] [CrossRef]
- Moro, A.V.; Ferreira, P.C.; Migowski, P.; Rodembusch, F.S.; Dupont, J.; Lüdtke, D.S. Synthesis and photophysical properties of fluorescent 2,1,3-benzothiadiazole-triazole-linked glycoconjugates: Selective chemosensors for Ni(II). Tetrahedron 2013, 69, 201–206. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Chmovzh, T.N.; Ustimenko, O.O.; Chkhetiani, G.R.; Paleva, I.S.; Konstantinova, L.S.; Mikhal’chenko, L.V.; Rakitin, O.A. Suzuki cross-coupling reactions of 4,7-dibromo[1,2,5]selenadiazolo[3,4-c]pyridine—A path to new solar cell components. Chem. Heterocycl. Comp. 2017, 53, 608–614. [Google Scholar] [CrossRef]
- Henson, Z.B.; Welch, G.C.; van der Poll, T.; Bazan, G.C. Pyridalthiadiazole-Based Narrow Band Gap Chromophores. J. Am. Chem. Soc. 2012, 134, 3766–3779. [Google Scholar] [CrossRef]
- Braga, A.A.C.; Morgon, N.H.; Ujaque, G.; Lledós, A.; Maseras, F. Computational study of the transmetalation process in the Suzuki–Miyaura cross-coupling of aryls. J. Organomet. Chem. 2006, 691, 4459–4466. [Google Scholar] [CrossRef]
- Sicre, C.; Braga, A.A.C.; Maseras, F.; Cid, M.M. Mechanistic insights into the transmetalation step of a Suzuki–Miyaura reaction of 2(4)-bromopyridines: Characterization of an intermediate. Tetrahedron 2008, 64, 7437–7443. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Li, W.; Wang, Z.-S.; Tian, H.; Zhu, W. Hexylthiophene-Featured D-A-π-A Structural Indoline Chromophores for Coadsorbent-Free and Panchromatic Dye-Sensitized Solar Cells. Adv. Energy Mater. 2012, 2, 149–156. [Google Scholar] [CrossRef]
- Mikhailov, M.S.; Gudim, N.S.; Knyazeva, E.A.; Tanaka, E.; Zhang, L.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole—A new donor building-block in the design of sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2020, 391, 112333. [Google Scholar] [CrossRef]
- Oskam, G.; Bergeron, B.V.; Meyer, G.J.; Searson, P.C. Pseudohalogens for Dye-Sensitized TiO2 Photoelectrochemical Cells. J. Phys. Chem. B 2001, 105, 6867–6873. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Swetha, T.; Kapil, G.; Pandey, S.S.; Hayase, S.; Singh, S.P. Simple Metal-Free Dyes Derived from Triphenylamine for DSSC: A Comparative Study of Two Different Anchoring Group. Electrochim. Acta 2015, 169, 256–263. [Google Scholar] [CrossRef]
- Gudim, N.; Mikhailov, M.; Knyazeva, E.A.; Almenningen, D.M.; Mikhalchenko, L.V.; Economopoulos, S.; Rakitin, O.A. Monitoring the dependence of photovoltaic properties of dye-sensitized solar cells from the structure of D-A-π-A type sensitizers with 9-(p-tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazole donor building block. Mol. Syst. Des. Eng. 2022, 7. [Google Scholar] [CrossRef]
- Akhtaruzzaman, M.; Seya, Y.; Asao, N.; Islam, A.; Kwon, E.; El-Shafei, A.; Han, L.; Yamamoto, Y. Donor–acceptor dyes incorporating a stable dibenzosilole π-conjugated spacer for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 10771. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamabuki, K.; Oishi, T.; Onimura, K. Synthesis and fluorescent properties of conjugated co-oligomers containing maleimide and carbazole units at the main chain. Polym. J. 2014, 46, 94–103. [Google Scholar] [CrossRef]
- Moleele, S.S.; Michael, J.P.; de Koning, C.B. Methodology for the synthesis of 1,2-disubstituted arylnaphthalenes from α-tetralones. Tetrahedron 2006, 62, 2831–2844. [Google Scholar] [CrossRef]
- Kim, B.; Yeom, H.R.; Yun, M.H.; Kim, J.Y.; Yang, C. A selenophene analogue of PCDTBT: Selective fine-tuning of lumo to lower of the bandgap for efficient polymer solar cells. Macromolecules 2012, 45, 8658–8664. [Google Scholar] [CrossRef]
- CrysAlisPro. Version 1.171.41.106a. Rigaku Oxford Diffraction. 2021. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 4 April 2022).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]

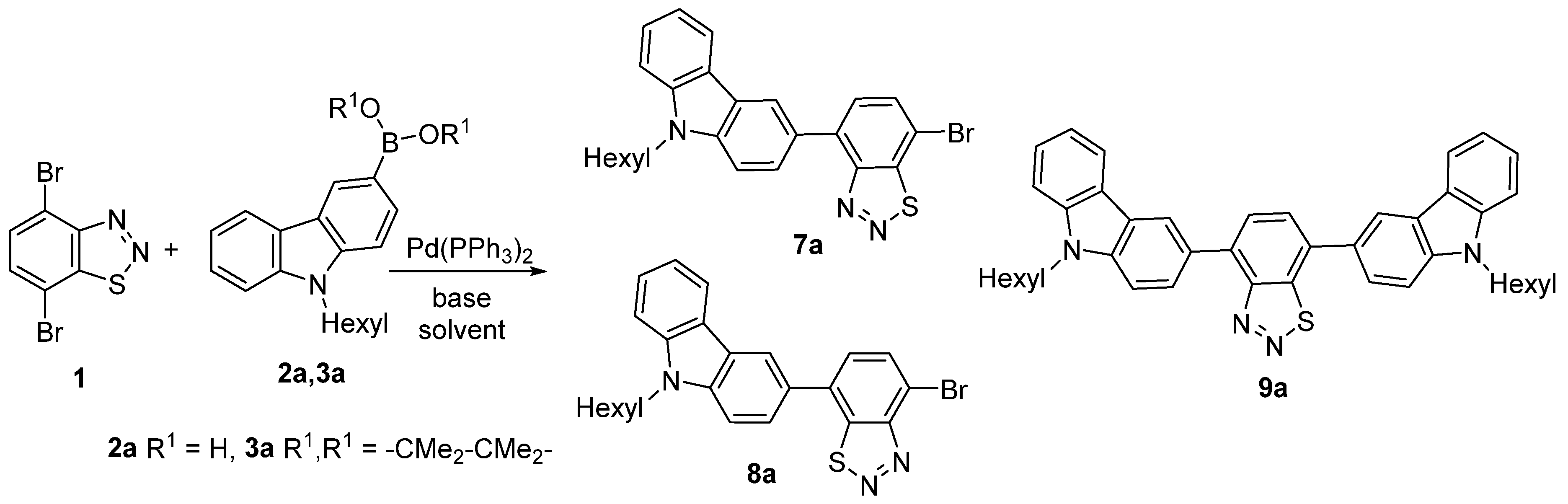






| Entry | B(OR)2 | Solvent | Base | Conditions | Yields (%) | |||
|---|---|---|---|---|---|---|---|---|
| 7a | 8a | 9a | 1 | |||||
| 1 | BPin | THF/H2O | K2CO3 | 70 °C, 8 h | 23 | 24 | 23 | 15 |
| 2 | BPin | THF | Cs2CO3 | 70 °C, 8 h | 20 | 22 | 18 | 17 |
| 3 | BPin | dioxane/H2O | K2CO3 | 110 °C, 6 h | 19 | 22 | 21 | 20 |
| 4 | BPin | toluene/H2O | K2CO3 | 120 °C, 5 h | 19 | 21 | 22 | 17 |
| 5 | BPin | DMF/H2O | K2CO3 | 80 °C, 12 h | traces | 0 | 0 | 90 |
| 6 | BPin | Toluene/H2O | K2CO3 | rt, 24 h | 12 | 14 | 15 | 38 |
| 7 | BPin | dioxane/H2O | K2CO3 | rt, 1.5 h | 4 | 4 | traces | 87 |
| 8 | B(OH)2 | THF/H2O | K2CO3 | 80 °C, 8 h | 19 | 22 | 20 | 18 |
| Entry | B(OR)2 | Yields, % | ||
|---|---|---|---|---|
| 7 | 8 | 9 | ||
| 1 | 2a | 23 | 24 | 23 |
| 2 | 3b | 25 | 16 | 8 |
| 3 | 3c | 25 | 27 | 24 |
| 4 | 3d | 21 | 22 | 13 |
| 5 | 2e | 47 | 0 | 26 |
| 6 | 2f | 44 | 0 | 23 |
| Compound | Electrode | Eoxonset a, V | Eoxonset (vs. Fc/Fc+) a, V | EHOMO b, eV |
|---|---|---|---|---|
 10a | Pt | 1.24 | 0.74 | −5.84 |
| glassy carbon | 1.25 | 0.75 | −5.85 | |
 10c | Pt | 0.72 | 0.22 | −5.32 |
| glassy carbon | 0.71 | 0.21 | −5.31 | |
 10d | Pt | 0.63 | 0.12 | −5.22 |
| glassy carbon | 0.63 | 0.12 | −5.22 | |
 10e | Pt | 2.33 | 1.92 | −7.02 |
| glassy carbon | 2.24 | 1.82 | −6.92 | |
 10f | Pt | 1.91 | 1.5 | −6.6 |
| glassy carbon | 1.85 | 1.44 | −6.54 |
| Compound | C4 | C5 | C5-H | C6 | C6-H | C7 |
|---|---|---|---|---|---|---|
| 1 | 116.83 | 131.82 | 7.68 | 132.65 | 7.65 | 110.83 |
| 7a | 138.17 | 128.05 | 7.57 | 132.00 | 7.87 | 109.03 |
| 7b | 137.61 | 126.90 | 7.55 | 132.10 | 7.77 | 108.31 |
| 7c | 137.86 | 129.36 | 7.57 | 132.61 | 7.78 | 108.90 |
| 7d | 137.72 | 129.60 | 7.56 | 132.15 | 7.79 | 108.31 |
| 7e | 136.95 | 128.07 | 7.58 | 131.89 | 7.88 | 110.29 |
| 7f | 138.26 | 125.90 | 7.55 | 132.03 | 7.78 | 109.51 |
| 8a | 115.43 | 131.42 | 7.93 | 126.57 | 7.53 | 134.82 |
| 8b | 114.66 | 131.44 | 7.84 | 127.41 | 7.53 | 134.39 |
| Dye | λmax1, [nm] a | εmax1 × 103, [M−1cm−1] a | λmax2, [nm] a | εmax2 × 103, [M−1cm−1] a |
|---|---|---|---|---|
| KEA321 | 404 | 29.5 | 504 | 22.9 |
| KEA337 | 398 | 14.5 | 454 | 16.2 |
| WS-2 b | 394 | 14.1 | 533 | 16.7 |
| MAX114 c | 392 | 16.3 | 507 | 20.4 |
| Dye | Eredonset (vs. Fc/Fc+) a, V | Eoxonset (vs. Fc/Fc+) a, V | ELUMO b, eV | EHOMO b, eV | Eg c, eV |
|---|---|---|---|---|---|
| KEA321 | −1.31 | 0.21 | −3.79 | −5.31 | −1.52 |
| KEA337 | −1.49 | 0.15 | −3.61 | −5.25 | −1.64 |
| Dye | Voc (mV) | Jsc (mA·cm−2) | FF | η (%) |
|---|---|---|---|---|
| KEA321 | 0.65 | 12.94 | 0.61 | 5.15 |
| KEA337 | 0.66 | 10.99 | 0.67 | 4.83 |
| WS-2 a | 0.59 | 11.80 | 0.63 | 5.07 |
| MAX114 b | 0.57 | 11.50 | 0.75 | 4.90 |
| Compound 7b | Compound 8b | |
|---|---|---|
| Empirical formula | C26H22BrN3S | C26H22BrN3S |
| Formula weight | 488.43 | 488.43 |
| Temperature | 100(2) K | 100(2) K |
| Wavelength | 0.71073 Å | 0.71073 Å |
| Crystal system | monoclinic | Triclinic |
| Space group | P21/c | P-1 |
| Unit cell dimensions | a = 11.4845(11) Å | a = 9.3264(2) Å |
| b = 10.3419(10) Å | b = 9.7012(2) Å | |
| c = 18.4029(18) Å | c = 12.0096(3) Å | |
| α = 90° | α = 78.2162(7)° | |
| β = 101.867(3)° | β = 89.0002(7)° | |
| γ = 90° | γ = 88.9216(8)° | |
| Volume | 2139.0(4) Å3 | 1063.41(4) Å3 |
| Z | 4 | 2 |
| Density (calculated) | 1.517 | 1.525 |
| Absorption coefficient | 2.039 | 2.051 |
| F(000) | 1000 | 500 |
| Crystal size | 0.370 × 0.310 × 0.260 mm | 0.570 × 0.300 × 0.120 mm |
| Theta range for data collection | 2.591 to 34.000° | 2.467 to 26.998° |
| Index ranges | −18 ≤ h ≤ 18, −16 ≤ k ≤ 16, −28 ≤ l ≤ 28 | −11 ≤ h ≤ 11, −12 ≤ k ≤ 12, −15 ≤ l ≤ 15 |
| Reflections collected | 81,377 | 30,186 |
| Independent reflections | 8716 | 4636 |
| [R(Int) = 0.0541] | [R(int) = 0.0311] | |
| Completeness to theta | 99.7 | 99.9 |
| Absorption correction | Multi-scan | Semi-empirical from equivalents |
| Max. and min. transmission | (not specified) | 0.1004 and 0.0595 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 8716/0/281 | 4636/1/281 |
| Goodness-of-fit on F2 | 1.014 | 1.073 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0333, wR2 = 0.0909 | R1 = 0.0719, wR2 = 0.1870 |
| R indices (all data) | R1 = 0.0418, wR2 = 0.0980 | R1 = 0.0781, wR2 = 0.1928 |
| Largest diff. peak and hole | 0.823 and −0.622 Å−3 | 2.446 and −1.031 Å−3 |
| Compound 7e | Compound 7f | |
|---|---|---|
| Empirical formula | C12H7BrN2S | C10H5BrN2S2 |
| Formula weight | 291.17 | 297.19 |
| Temperature | 100.00(10) K | 100.00(10) K |
| Wavelength | 0.71073 Å | 0.71073 Å |
| Crystal system | monoclinic | monoclinic |
| Space group | P121/c1 | P121/c1 |
| Unit cell dimensions | a = 11.4348(3) | a = 3.85910(10) |
| b = 14.7803(5) | b = 20.3833(6) | |
| c = 6.7783(3) | c = 12.7488(5) | |
| α = 90° | α = 90° | |
| β = 106.295(4)° | β = 90.357(3)° | |
| γ = 90° | γ = 90° | |
| Volume | 1099.58(7) Å3 | 1002.82(6) Å3 |
| Z | 4 | 4 |
| Density (calculated) | 1.759 | 1.968 |
| Absorption coefficient | 3.897 | 4.476 |
| F(000) | 576 | 584 |
| Crystal size | 0.103 × 0.11 × 0.59 mm | 0.04 mm3 |
| Theta range for data collection | 2.311 to 33.370° | 2.559 to 34.479 |
| Index ranges | −16 ≤ h ≤ 16; −22 ≤ k ≤ 19; −10 ≤ l ≤ 9 | −6 ≤ h ≤ 5; −31 ≤ k ≤ 32; −19 ≤ l ≤ 19 |
| Reflections collected | 3686 | 3832 |
| Independent reflections | 3014 | 3069 |
| [R(Int) = 0.0522] | [R(Int) = 0.0588] | |
| Completeness to theta | 99.94 | 99.9 |
| Absorption correction | spherical harmonics | spherical harmonics |
| Max. and min. transmission | (not specified) | (not specified) |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3686/0/145 | 3832/2/146 |
| Goodness-of-fit on F2 | 1.091 | 1.029 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0367, wR2 = 0.0925 | R1 = 0.0389, wR2 = 0.0779 |
| R indices (all data) | R1 = 0.0478, wR2 = 0.0962 | R1 = 0.0565, wR2 = 0.0823 |
| Largest diff. peak and hole | 1.044 and −0.706 Å−3 | 1.026 and −0.538 Å−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudim, N.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Mikhailov, M.S.; Zhang, L.; Robertson, N.; Rakitin, O.A. Novel D-A-π-A1 Type Organic Sensitizers from 4,7-Dibromobenzo[d][1,2,3]thiadiazole and Indoline Donors for Dye-Sensitized Solar Cells. Molecules 2022, 27, 4197. https://doi.org/10.3390/molecules27134197
Gudim NS, Knyazeva EA, Mikhalchenko LV, Mikhailov MS, Zhang L, Robertson N, Rakitin OA. Novel D-A-π-A1 Type Organic Sensitizers from 4,7-Dibromobenzo[d][1,2,3]thiadiazole and Indoline Donors for Dye-Sensitized Solar Cells. Molecules. 2022; 27(13):4197. https://doi.org/10.3390/molecules27134197
Chicago/Turabian StyleGudim, Nikita S., Ekaterina A. Knyazeva, Ludmila V. Mikhalchenko, Maksim S. Mikhailov, Lu Zhang, Neil Robertson, and Oleg A. Rakitin. 2022. "Novel D-A-π-A1 Type Organic Sensitizers from 4,7-Dibromobenzo[d][1,2,3]thiadiazole and Indoline Donors for Dye-Sensitized Solar Cells" Molecules 27, no. 13: 4197. https://doi.org/10.3390/molecules27134197
APA StyleGudim, N. S., Knyazeva, E. A., Mikhalchenko, L. V., Mikhailov, M. S., Zhang, L., Robertson, N., & Rakitin, O. A. (2022). Novel D-A-π-A1 Type Organic Sensitizers from 4,7-Dibromobenzo[d][1,2,3]thiadiazole and Indoline Donors for Dye-Sensitized Solar Cells. Molecules, 27(13), 4197. https://doi.org/10.3390/molecules27134197







