Comparison of Different Extraction Processes on the Physicochemical Properties, Nutritional Components and Antioxidant Ability of Xanthoceras sorbifolia Bunge Kernel Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Oil Extraction Methods
2.3. Determination of Basic Indicators
2.4. Determination of Physicochemical Properties
2.5. Analysis of Fatty Acid Composition
2.6. Determination of Nutritional Components
2.6.1. Tocopherol Content
2.6.2. Sterol Content
2.7. Determination of DPPH Free Radical Scavenging Rate
- As: absorbance of test solution and DPPH measured at 517 nm;
- Ar: absorbance of test solution and absolute ethanol measured at 517 nm;
- A0: absorbance of ethanol and DPPH measured at 517 nm.
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Physicochemical Properties
3.3. Fatty Acid Composition
3.4. Nutrient Content
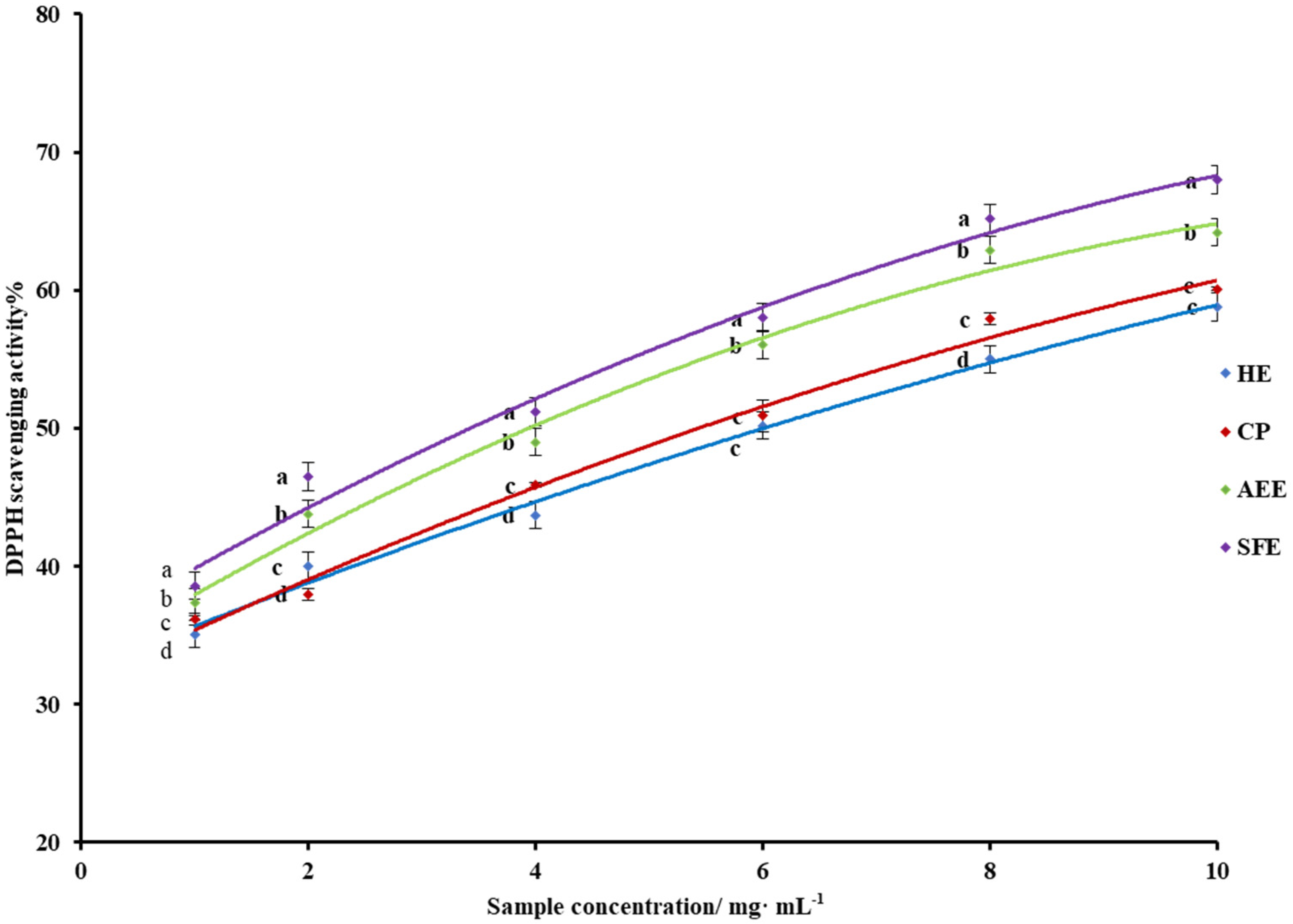
3.5. DPPH Scavenging Activity
3.6. Multiple Linear Regression Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yao, Z.-Y.; Qi, J.-H.; Yin, L.-M. Biodiesel production from Xanthoceras sorbifolia in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2013, 24, 57–65. [Google Scholar] [CrossRef]
- Ruan, C.-J.; Yan, R.; Wang, B.-X.; Mopper, S.; Guan, W.-K.; Zhang, J. The importance of yellow horn (Xanthoceras sorbifolia) for restoration of arid habitats and production of bioactive seed oils. Ecol. Eng. 2016, 99, 504–512. [Google Scholar] [CrossRef]
- Ma, Y.; Bi, Q.; Li, G.; Liu, X.; Fu, G.; Zhao, Y.; Wang, L. Provenance variations in kernel oil content, fatty acid profile and biodiesel properties of Xanthoceras sorbifolium Bunge in northern China. Ind. Crop. Prod. 2020, 151, 112487. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, K.; Ao, Y.; Ma, L.; Duan, J. Evaluation of biodiesel from Xanthoceras sorbifolia Bunge seed kernel oil from 13 areas in China. J. For. Res. 2018, 30, 869–877. [Google Scholar] [CrossRef]
- Thandapilly, S.J.; Raj, P.D.; Louis, X.L.; Perera, D.; Yamanagedara, P.; Zahradka, P.; Taylor, C.G.; Netticadan, T. Canola oil rich in oleic acid improves diastolic heart function in diet-induced obese rats. J. Physiol. Sci. 2016, 67, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Qipshidze-Kelm, N.; Piell, K.M.; Solinger, J.C.; Cole, M.P. Co-treatment with conjugated linoleic acid and nitrite protects against myocardial infarction. Redox Biol. 2013, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Umemoto, H.; Yasugi, S.; Tsuda, S.; Yoda, M.; Ishiguro, T.; Kaba, N.; Itoh, T. Protective effect of nervonic acid against 6-hydroxydopamine-induced axidative stress in PC-12 cells. J. Oleo Sci. 2021, 70, 95–102. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, W.; Yuan, F.; Liu, X.; Li, D.; Yang, K.Q. Characterization of yuanbaofeng (Acer truncatum Bunge) samaras: Oil, fatty acid, and phytosterol content. Ind. Crop. Prod. 2019, 135, 344–351. [Google Scholar] [CrossRef]
- Guneser, B.A.; Yilmaz, E. Bioactives, Aromatics and Sensory Properties of Cold-Pressed and Hexane-Extracted Lemon (Citrus Limon L.) Seed Oils. J. Am. Oil Chem. Soc. 2017, 94, 723–731. [Google Scholar] [CrossRef]
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Jahirul, M.; Brown, J.; Senadeera, W.; Ashwath, N.; Laing, C.; Leski-Taylor, J.; Rasul, M. Optimisation of Bio-Oil Extraction Process from Beauty Leaf (Calophyllum Inophyllum) Oil Seed as a Second Generation Biodiesel Source. Procedia Eng. 2013, 56, 619–624. [Google Scholar] [CrossRef]
- Balvardi, M.; Rezaei, K.; Mendiola, J.A.; Ibáñez, E. Optimization of the Aqueous Enzymatic Extraction of Oil from Iranian Wild Almond. J. Am. Oil Chem. Soc. 2015, 92, 985–992. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Gordon, M.H.; Ezeh, O.; Niranjan, K. Aqueous enzymatic extraction of Moringa oleifera oil. Food Chem. 2016, 211, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Papatheodorou, A.; Karasavva, S.; Kaderides, K. Ultrasound-Assisted Aqueous Enzymatic Extraction of Oil from Pomegranate Seeds. Waste Biomass-Valorization 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Karrar, E.; Sheth, S.; Wei, W.; Wang, X. Supercritical CO2 extraction of gurum (Citrulluslanatus var. Colocynthoide) seed oil and its properties comparison with conventional methods. J. Food Process. Eng. 2019, 42, e13129. [Google Scholar] [CrossRef]
- Aiello, A.; Pizzolongo, F.; Scognamiglio, G.; Romano, A.; Masi, P.; Romano, R. Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil. Int. J. Food Sci. Technol. 2019, 55, 2472–2480. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Effects of processing methods on the chemical composition and antioxidant capacity of walnut (Juglans regia L.) oil. LWT-Food Sci. Technol. 2020, 135, 109958. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, S.; Li, M.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Evaluation on the formation of lipid free radicals in the oxidation process of peanut oil. LWT-Food Sci. Technol. 2019, 104, 24–29. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, L.; Jin, Q.; Wang, X. Effects of Adsorption on Polycyclic Aromatic Hydrocarbon, Lipid Characteristic, Oxidative Stability, and Free Radical Scavenging Capacity of Sesame Oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1700150. [Google Scholar] [CrossRef]
- Jing, Z.; Cheng, J.; Guo, C.; Wang, X. Seed traits, nutrient elements and assessment of genetic diversity for almond (Amygdalus spp.) endangered to China as revealed using SRAP markers. Biochem. Syst. Ecol. 2013, 49, 51–57. [Google Scholar] [CrossRef]
- Ma, J.; Ye, H.; Rui, Y.; Chen, G.; Zhang, N. Fatty acid composition of Camellia oleifera oil. J. Für Verbrauch. Und Lebensm. 2011, 6, 9–12. [Google Scholar] [CrossRef]
- Soo, P.; Ali, Y.; Lai, O.; Kuan, C.; Tang, T.; Lee, Y.; Phuah, E. Enzymatic and Mechanical Extraction of Virgin Coconut Oil. Eur. J. Lipid Sci. Technol. 2020, 122, 1900220. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Crowe, T.D.; White, P.J. Oxidative stability of walnut oils extracted with supercritical carbon dioxide. J. Am. Oil Chem. Soc. 2003, 80, 575–578. [Google Scholar] [CrossRef]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? Phospholipids in foods. J. Sci. Food Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.-B.; Zhang, G.-J.; Du, L.; Du, J.; Qi, K.; Zhu, X.-L.; Zhang, X.-Y.; Jiang, Z.-H. Comparative study on the extraction of Xanthoceras sorbifolia Bunge (yellow horn) seed oil using subcritical n-butane, supercritical CO2, and the Soxhlet method. LWT-Food Sci. Technol. 2019, 111, 548–554. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.-G.; Luo, M.; Gu, C.-B.; Zhao, C.-J.; Efferth, T.; Fu, Y.-J. Aqueous enzymatic process assisted by microwave extraction of oil from yellow horn (Xanthoceras sorbifolia Bunge.) seed kernels and its quality evaluation. Food Chem. 2013, 138, 2152–2158. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, T.; Li, S.; Wen, J.; Zhu, L.; Yin, T.; Yan, K.; Xu, X.; Li, S.; Mao, J.; et al. The Acer truncatum genome provides insights into nervonic acid biosynthesis. Plant J. 2020, 104, 662–678. [Google Scholar] [CrossRef]
- Tu, X.; Wan, J.; Xie, Y.; Wei, F.; Quek, S.; Lv, X.; Du, L.; Chen, H. Lipid analysis of three special nervonic acid resources in China. Oil Crop. Sci. 2020, 5, 180–186. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. Oil Content, Fatty Acid Composition and Distributions of Vitamin-E-Active Compounds of Some Fruit Seed Oils. Antioxidants 2015, 4, 124–133. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.E.; Ahmed, I.A.M. Influence of Sumac Extract on the Physico-chemical Properties and Oxidative Stability of Some Cold Pressed Citrus Seed Oils. J. Oleo Sci. 2020, 69, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Weeghel, V.M.; Brinke, H.T.; Lenthe, V.H.; Kulik, W.; Minkler, P.E.; Stoll, M.S.K.; Sass, J.O.; Janssen, U.; Stoffel, W.; Schwab, K.O.; et al. Functional redundancy of mitochondrial enoyl-CoA isomerases in the oxidation of unsaturated fatty acids. FASEB J. 2012, 26, 4316–4326. [Google Scholar] [CrossRef] [PubMed]
| Composition | Crude Fat | Crude Protein | Moisture | Ash | Crude Fiber |
|---|---|---|---|---|---|
| content (%) | 58.16 ± 0.18 | 29.53 ± 0.25 | 4.43 ± 0.13 | 2.32 ± 0.09 | 1.61 ± 0.12 |
| HE | CP | AEE | SFE | |
|---|---|---|---|---|
| oil yield (%) | 98.04 ± 0.29 a | 87.81 ± 0.29 c | 68.74 ± 0.18 d | 89.63 ± 0.17 b |
| moisture and volatile matter (%) | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 1.81 ± 0.05 a | 0.01 ± 0.00 b |
| AV (KOH)/(mg/g) | 0.19 ± 0.02 c | 0.12 ± 0.01 d | 0.25 ± 0.02 b | 0.58 ± 0.02 a |
| PV (mmol/kg) | 0.58 ± 0.02 b | 0.65 ± 0.03 a | 0.47 ± 0.02 d | 0.51 ± 0.01 c |
| OSI (h) | 9.20 ± 0.05 a | 7.85 ± 0.04 c | 8.53 ± 0.18 b | 7.86 ± 0.06 c |
| HE | CP | AEE | SFE | |
|---|---|---|---|---|
| C16:0 | 5.40 ± 0.061 a | 5.23 ± 0.087 b | 4.60 ± 0.061 c | 5.31 ± 0.064 a,b |
| C18:0 | 2.22 ± 0.031 c | 2.26 ± 0.070 b | 2.12 ± 0.031 d | 2.32 ± 0.061 a |
| C18:1(n−9) | 31.48 ± 0.055 a,b | 31.36 ± 0.065 b,c | 31.28 ± 0.090 c | 31.53 ± 0.046 a |
| C18:2(n−6) | 40.55 ± 0.036 b | 40.58 ± 0.060 b | 40.86 ± 0.101 a | 40.59 ± 0.089 b |
| C18:3(n−3) | 0. 50 ± 0.017 a | 0.46 ± 0.058 b | 0.40 ± 0.026 b | 0.40 ± 0.026 b |
| C20:0 | 0.44 ± 0.010 a | 0.38 ± 0.025 b | 0.28 ± 0.029 d | 0.32 ± 0.029 c |
| C20:1 | 6.88 ± 0.061 b | 6.89 ± 0.040 b | 7.13 ± 0.078 a | 6.84 ± 0.067 c |
| C20:2 | 0.38 ± 0.021 b | 0.43 ± 0.030 a | 0.43 ± 0.030 a | 0.37 ± 0.023 c |
| C22:0 | 0.54 ± 0.015 c | 0.58 ± 0.021 a,b | 0.59 ± 0.010 a | 0.57 ± 0.023 b |
| C22:1 | 8.22 ± 0.026 c | 8.47 ± 0.032 b | 8.88 ± 0.081 a | 8.44 ± 0.078 b |
| C24:0 | 0.31 ± 0.006 b | 0.34 ± 0.052 a | 0.34 ± 0.052 a | 0.31 ± 0.006 b |
| C24:1 | 2.73 ± 0.025 c | 3.02 ± 0.075 b | 3.09 ± 0.047 a | 3.03 ± 0.085 b |
| SFA | 8.91 ± 0.07 a | 8.77 ± 0.07 b | 7.93 ± 0.07 c | 8.83 ± 0.05 a,b |
| MUFA | 49.31 ± 0.05 c | 49.75 ± 0.11 b | 50.38 ± 0.17 a | 49.81 ± 0.04 b |
| PUFA | 41.78 ± 0.03 a | 41.48 ± 0.04 b | 41.69 ± 0.11 a | 41.37 ± 0.08 b |
| HE | CP | AEE | SFE | |
|---|---|---|---|---|
| α-Tocopherol | 94.51 ± 0.70 a | 73.53 ± 2.18 b | 74.28 ± 2.72 b | 75.83 ± 4.40 b |
| γ-Tocopherol | 361.37 ± 3.85 a | 329.18 ± 7.31 b | 333.84 ± 16.22 b | 343.69 ± 16.37 a,b |
| δ-Tocopherol | 74.27 ± 0.60 a | 49.09 ± 5.09 b | 53.78 ± 2.12 b | 53.33 ± 2.07 b |
| Total tocopherol | 530.15 ± 5.14 a | 451.80 ± 0.74 b | 461.90 ± 15.61 b | 472.85 ± 18.70 b |
| Sterol | 2104.07 ± 14.44 a | 1340.20 ± 31.00 c | 1274.71 ± 6.65 d | 2004.51 ± 8.93 b |
| Dependent Variable | Variable | R | VIF | Equation |
|---|---|---|---|---|
| DPPH | (Constant) | −6.548 × 10−15 | Y = −6.548 × 10−15 − 0.957 (C20:0) − 0.336 (PUFA) + 0.432 (sterol) | |
| C20:0 | −0.957 | 1.576 | ||
| PUFA | −0.336 | 1.164 | ||
| sterol | 0.432 | 1.405 | ||
| OSI | (Constant) | 1.931 × 10−16 | Y = 1.931 × 10−16 − 0.513 (C18:0) + 0.784 (sterol) + 0.074 (C18:2(n−6)) | |
| C18:0 | −0.513 | 1.162 | ||
| C18:2(n−6) | 0.074 | 1.409 | ||
| Sterol | 0.784 | 1.232 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Gao, P.; Wang, S.; Ruan, Y.; Zhong, W.; Hu, C.; He, D. Comparison of Different Extraction Processes on the Physicochemical Properties, Nutritional Components and Antioxidant Ability of Xanthoceras sorbifolia Bunge Kernel Oil. Molecules 2022, 27, 4185. https://doi.org/10.3390/molecules27134185
Zheng Y, Gao P, Wang S, Ruan Y, Zhong W, Hu C, He D. Comparison of Different Extraction Processes on the Physicochemical Properties, Nutritional Components and Antioxidant Ability of Xanthoceras sorbifolia Bunge Kernel Oil. Molecules. 2022; 27(13):4185. https://doi.org/10.3390/molecules27134185
Chicago/Turabian StyleZheng, Yuling, Pan Gao, Shu Wang, Yuling Ruan, Wu Zhong, Chuanrong Hu, and Dongping He. 2022. "Comparison of Different Extraction Processes on the Physicochemical Properties, Nutritional Components and Antioxidant Ability of Xanthoceras sorbifolia Bunge Kernel Oil" Molecules 27, no. 13: 4185. https://doi.org/10.3390/molecules27134185
APA StyleZheng, Y., Gao, P., Wang, S., Ruan, Y., Zhong, W., Hu, C., & He, D. (2022). Comparison of Different Extraction Processes on the Physicochemical Properties, Nutritional Components and Antioxidant Ability of Xanthoceras sorbifolia Bunge Kernel Oil. Molecules, 27(13), 4185. https://doi.org/10.3390/molecules27134185







