Determination of Odor Air Quality Index (OAQII) Using Gas Sensor Matrix
Abstract
:1. Introduction
- Hedonic tone—pleasant or unpleasant sensations during inhalation of a gaseous mixture;
- Odor intensity—relative strength of the odor stimulus induced by a particular odorant;
- Odor threshold—the minimum concentration of a substance at which most test subjects can identify the odor.
- Specific odor emission rate ()where:—base area []—odor concentration []—air flow []
- Odor emission rate ()where:—emitting surface of the considered area []
- Odor emission factor ()where:
- Analytical odor index ()/odor activity value ()where:—concentration of y-th substances detected by GC techniques [ppm]—relative Odor Threshold [ppm]
- Sensorial odor index ()where:—concentration of odor at i-th sampling point []—standard concentration of odor detectable by sensorial analysis []
- Odor index ()where:—odor concentration []
- Odor annoyance index ()where:—total number of observations for the investigated zone or period—number of observations corresponding to the odor rating i (i = 0–6)—corresponding weighting factors
2. Materials and Methods
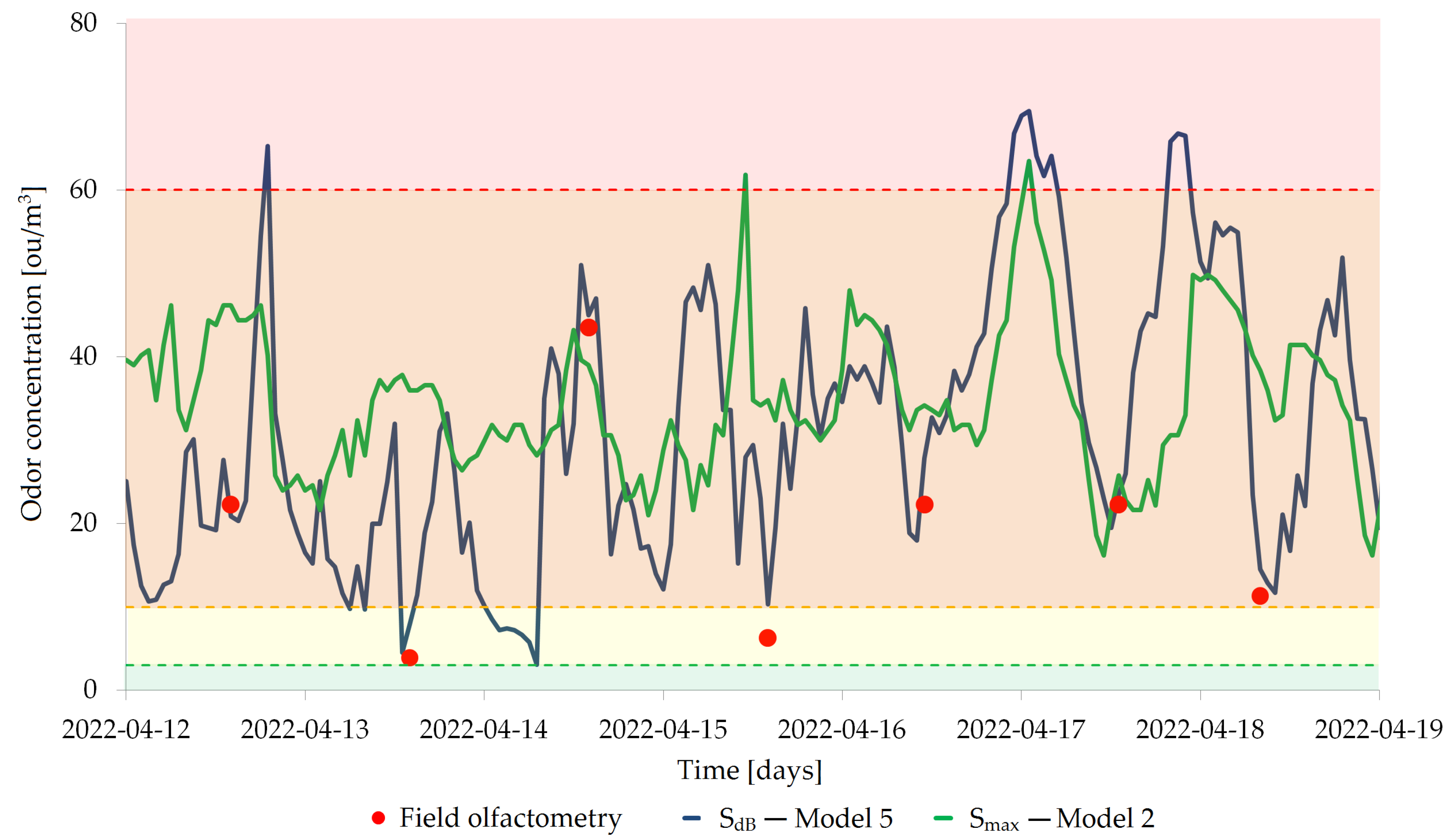
2.1. Field Olfactometry
2.2. Sensory Matrix Development and Measurement
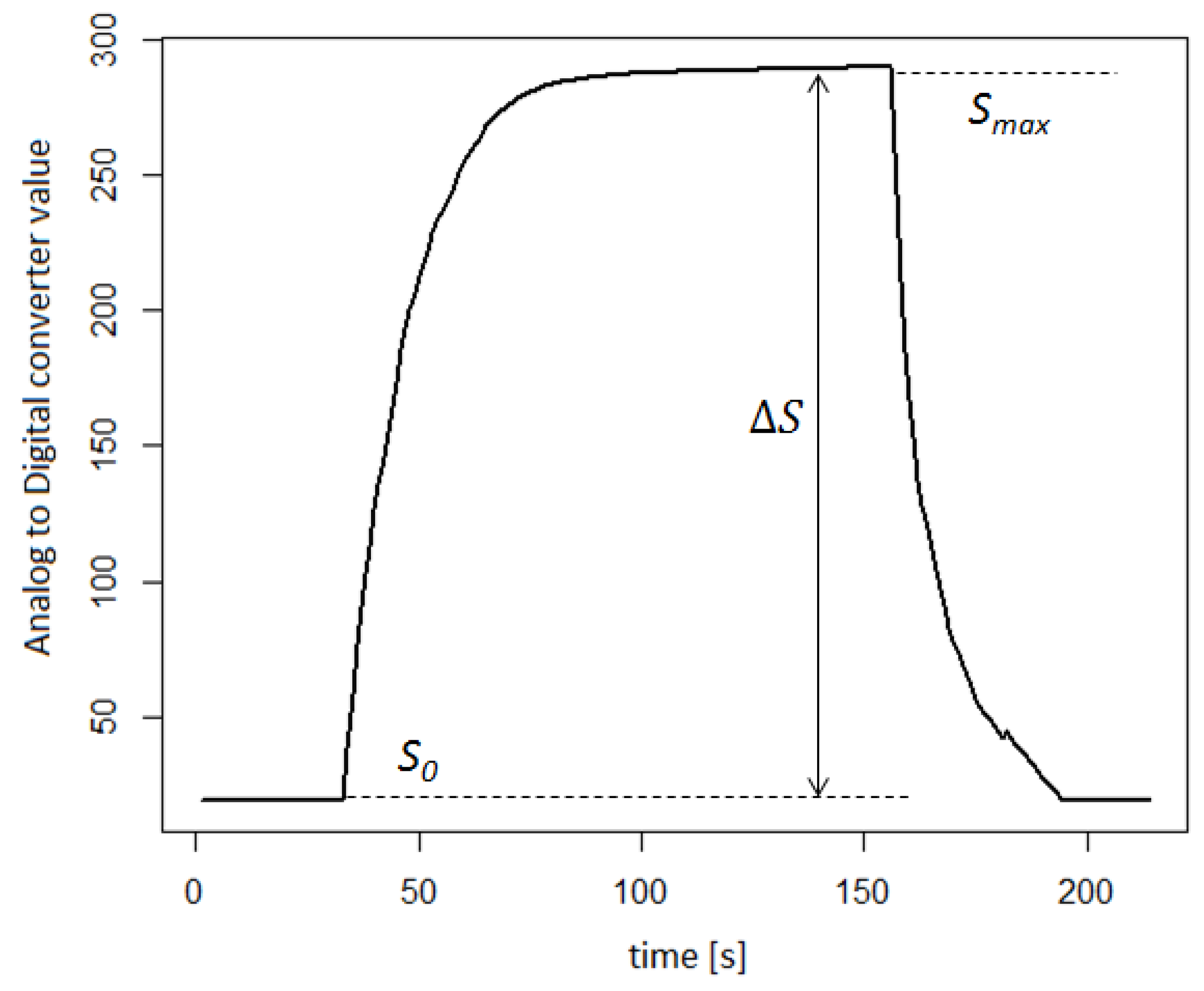
2.3. Signals’ Features Extraction
- The maximum signal value—;
- Logarithm of the ratio of the maximum signal value to the baseline—.
2.4. Mathematical Models for Odor Concentration Determination
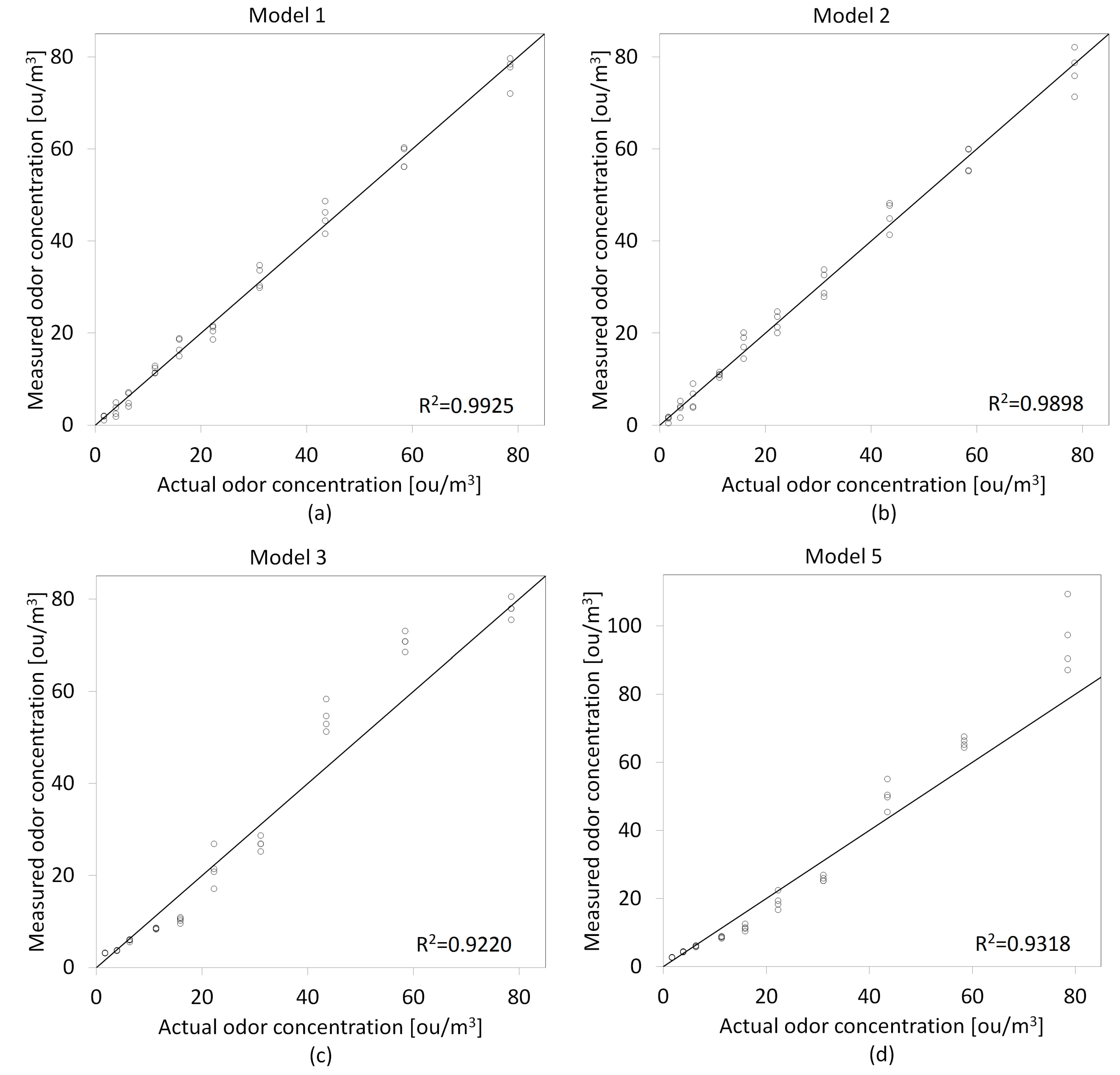
- Model 1: Multiple Linear Regression ()MLR is a statistical technique based on the generation of a linear relationship between the independent variables (sensor signals) and the dependent variable (odor concentration). It is important that the explanatory variables are not correlated with each other and their number is smaller than the number of observations (measurements) made. The general equation is as follows:where:—intercept—regression coefficients—sensor signal (depending on the method of signal’s features extraction)n—number of explanatory variables
- Model 2: Principal Component Regression ()PCR allows for the selection of explanatory variables that have a significant impact on the value of the dependent variable; these are called principal components (PC). PC selection is performed using principal component analysis (PCA) and then the MLR model is created in which are used as explanatory variables. Formally, PCR is defined as follows:where:—intercept—regression coefficients—principal componentsn—number of principal components
- Model 3: Stevens’ power law with a single sensor signal as a power exponentSignals from individual sensors were used as an exponent of the proposed relationship. The model best suited to the training dataset was selected based on the value of the coefficient of determination (). Despite the fact that this coefficient ignores the adjustment of the model to data outside the training set, it is one of the basic measures of the quality of the model matching to this data set. The odor concentration was determined according to the following formula:where:a—first model coefficientb—second model coefficientS—sensor signal (depending on the method of signal’s features extraction)
- Model 4: Stevens’ power law with the geometric mean of the sensor signals as a power exponentThis approach was used only for the second method of extracting signals from the sensor array . The geometric mean of the signals from the individual sensors was used as the exponent of the function in accordance with relationwhere:a—first model coefficientb—second model coefficient—sensor signal value inn—number of sensor in the matrix
- Model 5: Stevens’ power law combined with Principal Component Analysis (PCA)The first principal component of the dataset was used as the exponent of the proposed function. was calculated using PCA and performing mean centering and scaling to unit variance. It shows the direction of the maximum variance in the data and best approximates the data in least squares terms. The odor concentration was computed as follows:where:a—first model coefficientb—second model coefficient—first principal component
2.5. Odor Air Quality Index
3. Results
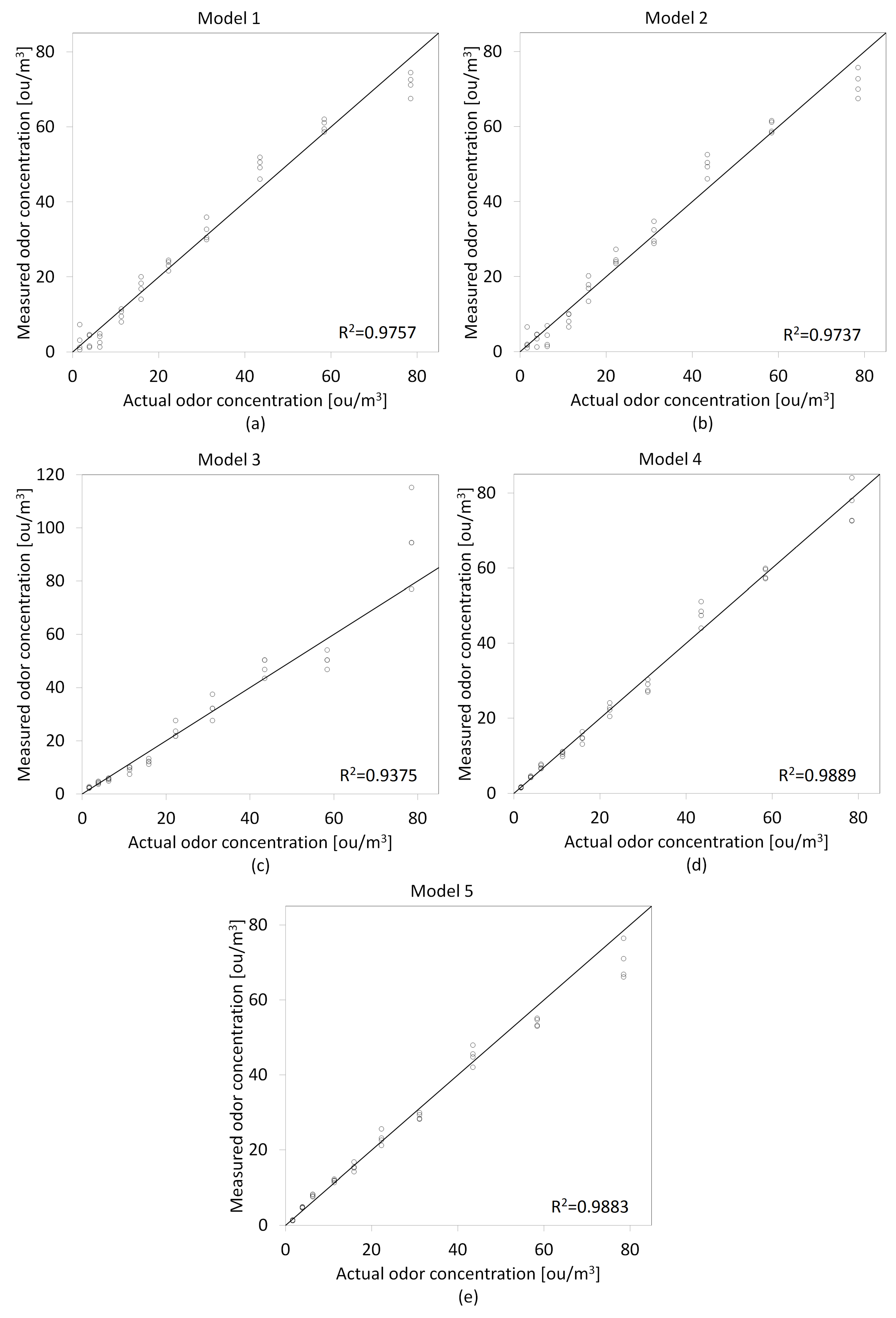
3.1. Models Development
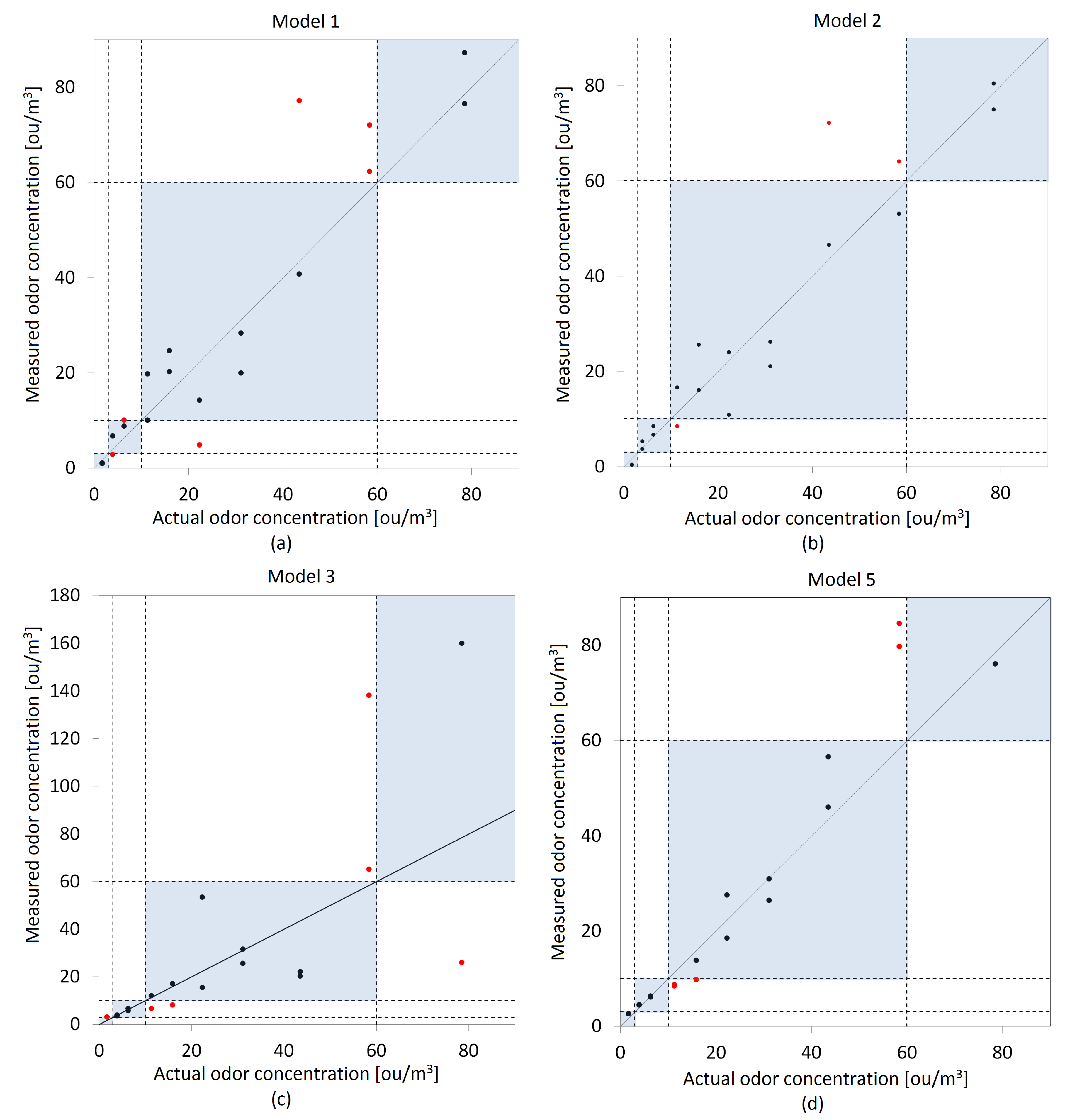
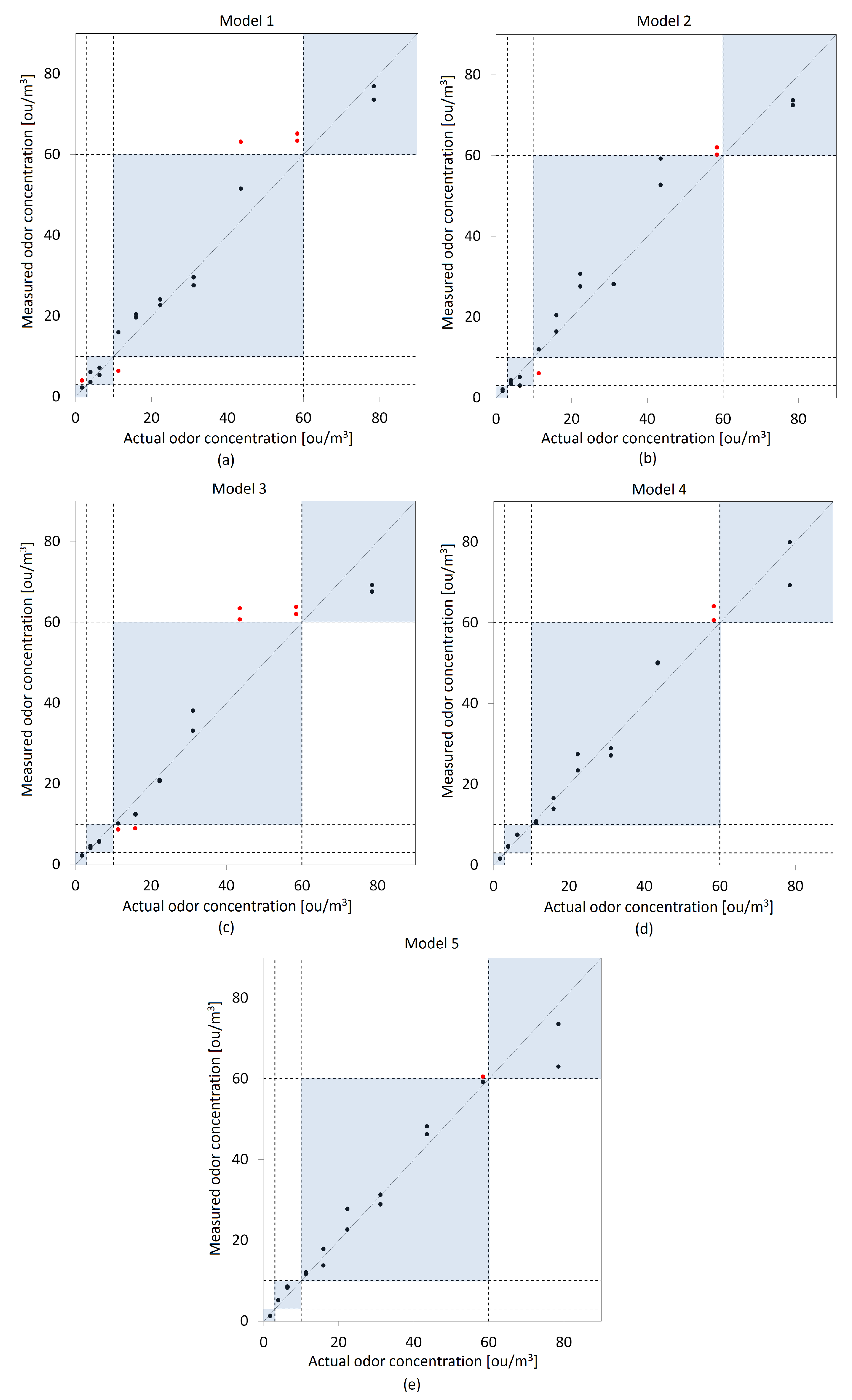
3.2. Models Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vodyanitskii, Y.N. Biochemical processes in soil and groundwater contaminated by leachates from municipal landfills (Mini review). Ann. Agrar. Sci. 2016, 14, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Saarela, J. Pilot investigations of surface parts of three closed landfills and factors affecting them. Environ. Monit. Assess. 2003, 84, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Teta, C.; Hikwa, T. Heavy Metal Contamination of Ground Water from an Unlined Landfill in Bulawayo, Zimbabwe. J. Health Pollut. 2017, 7, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, R.; Ahmed, Z.; Howladar, M.F. Evaluation of heavy metal contamination in water, soil and plant around the open landfill site Mogla Bazar in Sylhet, Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100311. [Google Scholar] [CrossRef]
- Malovanyy, M.; Korbut, M.; Davydova, I.; Tymchuk, I. Monitoring of the Influence of Landfills on the Atmospheric Air Using Bioindication Methods on the Example of the Zhytomyr Landfill, Ukraine. J. Ecol. Eng. 2021, 22, 36–49. [Google Scholar] [CrossRef]
- Mepaiyeda, S.; Madi, K.; Gwavava, O.; Baiyegunhi, C. Geological and geophysical assessment of groundwater contamination at the Roundhill landfill site, Berlin, Eastern Cape, South Africa. Heliyon 2020, 6, e04249. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Nematzadeh, S.; Eskandari, Z.; Aleebrahim Dehkordi, A.; Rezania, S. Gaseous emissions of landfill and modeling of their dispersion in the atmosphere of Shahrekord, Iran. Urban Clim. 2018, 24, 852–862. [Google Scholar] [CrossRef]
- Autelitano, F.; Giuliani, F. Influence of chemical additives and wax modifiers on odor emissions of road asphalt. Constr. Build. Mater. 2018, 183, 485–492. [Google Scholar] [CrossRef]
- Liu, C.; Yang, P.; Wang, H.; Song, H. Identification of odor compounds and odor-active compounds of yogurt using DHS, SPME, SAFE, and SBSE/GC-O-MS. LWT 2022, 154, 112689. [Google Scholar] [CrossRef]
- Nordin, S.; Lidén, E. Environmental odor annoyance from air pollution from steel industry and bio-fuel processing. J. Environ. Psychol. 2006, 26, 141–145. [Google Scholar] [CrossRef]
- Sá, M.F.; Castro, V.; Gomes, A.I.; Morais, D.F.; Silva Braga, R.V.; Saraiva, I.; Souza-Chaves, B.M.; Park, M.; Fernández-Fernández, V.; Rodil, R.; et al. Tracking pollutants in a municipal sewage network impairing the operation of a wastewater treatment plant. Sci. Total. Environ. 2022, 817, 152518. [Google Scholar] [CrossRef] [PubMed]
- Agus, E.; Zhang, L.; Sedlak, D.L. A framework for identifying characteristic odor compounds in municipal wastewater effluent. Water Res. 2012, 46, 5970–5980. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.; Kaiser, R.; Natsch, A.; Gautschi, M. Investigation of odors in the fragrance industry. Chemoecology 2010, 20, 135–147. [Google Scholar] [CrossRef]
- Weihua, Y.; Xiande, X.; Gen, W.; Jie, M.; Zengxiu, Z.; Jiayin, L. Emission characteristics of volatile odorous organic compounds in fragrance and flavor industry. Environ. Chem. 2021, 1071–1077. [Google Scholar]
- Han, Z.; Qi, F.; Li, R.; Wang, H.; Sun, D. Health impact of odor from on-situ sewage sludge aerobic composting throughout different seasons and during anaerobic digestion with hydrolysis pretreatment. Chemosphere 2020, 249, 126077. [Google Scholar] [CrossRef]
- Schlegelmilch, M.; Streese, J.; Biedermann, W.; Herold, T.; Stegmann, R. Odour control at biowaste composting facilities. Waste Manag. 2005, 25, 917–927. [Google Scholar] [CrossRef]
- Han, Z.; Qi, F.; Wang, H.; Li, R.; Sun, D. Odor assessment of NH3 and volatile sulfide compounds in a full-scale municipal sludge aerobic composting plant. Bioresour. Technol. 2019, 282, 447–455. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, Z.; Zhu, S.; Lou, Z.; Zhu, N.; Feng, L. The identification and health risk assessment of odor emissions from waste landfilling and composting. Sci. Total. Environ. 2019, 649, 1038–1044. [Google Scholar] [CrossRef]
- Zhang, Y.; Ning, X.; Li, Y.; Wang, J.; Cui, H.; Meng, J.; Teng, C.; Wang, G.; Shang, X. Impact assessment of odor nuisance, health risk and variation originating from the landfill surface. Waste Manag. 2021, 126, 771–780. [Google Scholar] [CrossRef]
- Kim, J.R.; Dec, J.; Bruns, M.A.; Logan, B.E. Removal of odors from swine wastewater by using microbial fuel cells. Appl. Environ. Microbiol. 2008, 74, 2540–2543. [Google Scholar] [CrossRef] [Green Version]
- Guffanti, P.; Pifferi, V.; Falciola, L.; Ferrante, V. Analyses of odours from concentrated animal feeding operations: A review. Atmos. Environ. 2018, 175, 100–108. [Google Scholar] [CrossRef]
- Wang, Y.C.; Han, M.F.; Jia, T.P.; Hu, X.R.; Zhu, H.Q.; Tong, Z.; Lin, Y.T.; Wang, C.; Liu, D.Z.; Peng, Y.Z.; et al. Emissions, measurement, and control of odor in livestock farms: A review. Sci. Total. Environ. 2021, 776, 145735. [Google Scholar] [CrossRef] [PubMed]
- Shareefdeen, Z.; Herner, B.; Wilson, S. Biofiltration of nuisance sulfur gaseous odors from a meat rendering plant. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2002, 77, 1296–1299. [Google Scholar] [CrossRef]
- Amon, M.; Dobeic, M.; Sneath, R.W.; Phillips, V.; Misselbrook, T.H.; Pain, B.F. A farm-scale study on the use of clinoptilolite zeolite and De-Odorase® for reducing odour and ammonia emissions from broiler houses. Bioresour. Technol. 1997, 61, 229–237. [Google Scholar] [CrossRef]
- Kusic, H.; Koprivanac, N.; Bozic, A.L. Minimization of organic pollutant content in aqueous solution by means of AOPs: UV- and ozone-based technologies. Chem. Eng. J. 2006, 123, 127–137. [Google Scholar] [CrossRef]
- Yao, H.; Feilberg, A. Characterisation of photocatalytic degradation of odorous compounds associated with livestock facilities by means of PTR-MS. Chem. Eng. J. 2015, 277, 341–351. [Google Scholar] [CrossRef]
- Andersen, K.B.; Beukes, J.A.; Feilberg, A. Non-thermal plasma for odour reduction from pig houses—A pilot scale investigation. Chem. Eng. J. 2013, 223, 638–646. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Odor reduction rate in the confinement pig building by spraying various additives. Bioresour. Technol. 2008, 99, 8464–8469. [Google Scholar] [CrossRef]
- Lewkowska, P.; Cieślik, B.; Dymerski, T.; Konieczka, P.; Namieśnik, J. Characteristics of odors emitted from municipal wastewater treatment plant and methods for their identification and deodorization techniques. Environ. Res. 2016, 151, 573–586. [Google Scholar] [CrossRef]
- Zarra, T.; Naddeo, V.; Belgiorno, V.; Reiser, M.; Kranert, M. Odour monitoring of small wastewater treatment plant located in sensitive environment. Water Sci. Technol. 2008, 58, 89–94. [Google Scholar] [CrossRef]
- Franco-Luesma, E.; Ferreira, V. Reductive off-odors in wines: Formation and release of H2S and methanethiol during the accelerated anoxic storage of wines. Food Chem. 2016, 199, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, R.; Wrona, M.; Nerín, C.; Bertochi Veroneze, I.; Gavril, G.L.; Andrea Cruz, S. Importance of profile of volatile and off-odors compounds from different recycled polypropylene used for food applications. Food Chem. 2021, 350, 129250. [Google Scholar] [CrossRef] [PubMed]
- Vera, P.; Canellas, E.; Nerín, C. Compounds responsible for off-odors in several samples composed by polypropylene, polyethylene, paper and cardboard used as food packaging materials. Food Chem. 2020, 309, 125792. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B.; Inanloo, B. Odor impact zones around landfills: Delineation based on atmospheric conditions and land use characteristics. Waste Manag. 2019, 88, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, H.; Lu, W. VOCs released from municipal solid waste at the initial decomposition stage: Emission characteristics and an odor impact assessment. J. Environ. Sci. 2020, 98, 143–150. [Google Scholar] [CrossRef]
- Anet, B.; Lemasle, M.; Couriol, C.; Lendormi, T.; Amrane, A.; Le Cloirec, P.; Cogny, G.; Fillières, R. Characterization of gaseous odorous emissions from a rendering plant by GC/MS and treatment by biofiltration. J. Environ. Manag. 2013, 128, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Amoore, J.E.; Hautala, E. Odor as an ald to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 1983, 3, 272–290. [Google Scholar] [CrossRef]
- Wysocka, I.; Gębicki, J.; Namieśnik, J. Technologies for deodorization of malodorous gases. Environ. Sci. Pollut. Res. 2019, 26, 9409–9434. [Google Scholar] [CrossRef] [Green Version]
- Nagata, Y.; Takeuchi, N. Measurement of odor threshold by triangle odor bag method. Odor Meas. Rev. 2003, 118, 118–127. [Google Scholar]
- Szulczyński, B.; Gębicki, J.; Namieśnik, J. Monitoring and efficiency assessment of biofilter air deodorization using electronic nose prototype. Chem. Pap. 2018, 72, 527–532. [Google Scholar] [CrossRef]
- López, R.; Cabeza, I.; Giráldez, I.; Díaz, M. Biofiltration of composting gases using different municipal solid waste-pruning residue composts: Monitoring by using an electronic nose. Bioresour. Technol. 2011, 102, 7984–7993. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Dunlop, M.; Hudson, N.; Kim, T.I.; Yoo, Y.H. Non-specific conducting polymer-based array capable of monitoring odour emissions from a biofiltration system in a piggery building. Sens. Actuators B Chem. 2009, 135, 455–464. [Google Scholar] [CrossRef]
- Cabeza, I.; López, R.; Giraldez, I.; Stuetz, R.; Díaz, M. Biofiltration of α-pinene vapours using municipal solid waste (MSW)—Pruning residues (P) composts as packing materials. Chem. Eng. J. 2013, 233, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. Volatile organic compounds, ammonia and hydrogen sulphide removal using a two-stage membrane biofiltration process. Chem. Eng. Res. Des. 2021, 165, 69–80. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, J.; Zhang, Y.; Han, C.; Ma, S.; Chen, J.; Li, G.; An, T. Removal of volatile organic compounds (VOCs) emitted from a textile dyeing wastewater treatment plant and the attenuation of respiratory health risks using a pilot-scale biofilter. J. Clean. Prod. 2020, 253, 120019. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Szulczyński, B.; Gębicki, J. Simultaneous Removal of Hexane and Ethanol from Air in a Biotrickling Filter—Process Performance and Monitoring Using Electronic Nose. Sustainability 2020, 12, 387. [Google Scholar] [CrossRef] [Green Version]
- Dobrzyniewski, D.; Szulczyński, B.; Dymerski, T.; Gębicki, J. Development of gas sensor array for methane reforming process monitoring. Sensors 2021, 21, 4983. [Google Scholar] [CrossRef]
- Zhou, J.; Welling, C.M.; Vasquez, M.M.; Grego, S.; Chakrabarty, K. Sensor-Array optimization based on time-series data analytics for sanitation-related malodor detection. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 705–714. [Google Scholar] [CrossRef]
- Romero-Flores, A.; McConnell, L.L.; Hapeman, C.J.; Ramirez, M.; Torrents, A. Evaluation of an electronic nose for odorant and process monitoring of alkaline-stabilized biosolids production. Chemosphere 2017, 186, 151–159. [Google Scholar] [CrossRef]
- Guz, Ł.; Łagód, G.; Jaromin-Gleń, K.; Suchorab, Z.; Sobczuk, H.; Bieganowski, A. Application of gas sensor arrays in assessment of wastewater purification effects. Sensors 2014, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Gębicki, J.; Dymerski, T.; Namieśnik, J. Monitoring of odour nuisance from landfill using electronic nose. Chem. Eng. Trans. 2014, 40, 85–90. [Google Scholar]
- Matindoust, S.; Baghaei-Nejad, M.; Abadi, M.H.S.; Zou, Z.; Zheng, L.R. Food quality and safety monitoring using gas sensor array in intelligent packaging. Sens. Rev. 2016, 36, 169–183. [Google Scholar] [CrossRef]
- Xiao-Wei, H.; Xiao-Bo, Z.; Ji-Yong, S.; Zhi-Hua, L.; Jie-Wen, Z. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Carrasco, A.; Saby, C.; Bernadet, P. Discrimination of Yves Saint Laurent perfumes by an electronic nose. Flavour Fragr. J. 1998, 13, 335–348. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Dymerski, T.; Gębicki, J.; Wardencki, W.; Namieśnik, J. Quality evaluation of agricultural distillates using an electronic nose. Sensors 2013, 13, 15954–15967. [Google Scholar] [CrossRef]
- Haddi, Z.; Amari, A.; Alami, H.; El Bari, N.; Llobet, E.; Bouchikhi, B. A portable electronic nose system for the identification of cannabis-based drugs. Sens. Actuators B Chem. 2011, 155, 456–463. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Pawlowski, W. Metal oxide sensor arrays for detection of explosives at sub-parts-per million concentration levels by the differential electronic nose. Sens. Actuators B Chem. 2012, 161, 528–533. [Google Scholar] [CrossRef]
- Patil, S.J.; Duragkar, N.; Rao, V.R. An ultra-sensitive piezoresistive polymer nano-composite microcantilever sensor electronic nose platform for explosive vapor detection. Sens. Actuators B Chem. 2014, 192, 444–451. [Google Scholar] [CrossRef]
- de Oliveira, L.F.; Mallafré-Muro, C.; Giner, J.; Perea, L.; Sibila, O.; Pardo, A.; Marco, S. Breath analysis using electronic nose and gas chromatography-mass spectrometry: A pilot study on bronchial infections in bronchiectasis. Clin. Chim. Acta 2022, 526, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Smulko, J.; Chludziński, T.; Majchrzak, T.; Kwiatkowski, A.; Borys, S.; Lisset Jaimes-Mogollón, A.; Manuel Durán-Acevedo, C.; Geovanny Perez-Ortiz, O.; Ionescu, R. Analysis of exhaled breath for dengue disease detection by low-cost electronic nose system. Measurement 2022, 190, 110733. [Google Scholar] [CrossRef]
- Saidi, T.; Moufid, M.; de Jesus Beleño-Saenz, K.; Welearegay, T.G.; El Bari, N.; Lisset Jaimes-Mogollon, A.; Ionescu, R.; Bourkadi, J.E.; Benamor, J.; El Ftouh, M.; et al. Non-invasive prediction of lung cancer histological types through exhaled breath analysis by UV-irradiated electronic nose and GC/QTOF/MS. Sens. Actuators B Chem. 2020, 311, 127932. [Google Scholar] [CrossRef]
- Bax, C.; Prudenza, S.; Gaspari, G.; Capelli, L.; Grizzi, F.; Taverna, G. Drift compensation on electronic nose data for non-invasive diagnosis of prostate cancer by urine analysis. iScience 2022, 25, 103622. [Google Scholar] [CrossRef]
- Zaim, O.; Diouf, A.; El Bari, N.; Lagdali, N.; Benelbarhdadi, I.; Ajana, F.Z.; Llobet, E.; Bouchikhi, B. Comparative analysis of volatile organic compounds of breath and urine for distinguishing patients with liver cirrhosis from healthy controls by using electronic nose and voltammetric electronic tongue. Anal. Chim. Acta 2021, 1184, 339028. [Google Scholar] [CrossRef]
- Ma, H.; Wang, T.; Li, B.; Cao, W.; Zeng, M.; Yang, J.; Su, Y.; Hu, N.; Zhou, Z.; Yang, Z. A low-cost and efficient electronic nose system for quantification of multiple indoor air contaminants utilizing HC and PLSR. Sens. Actuators B Chem. 2022, 350, 130768. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, F.; Liu, S.; Guo, J.; Hu, B.; Ye, Q.; Dang, L.; Peng, X.; Kadri, C.; Feng, J. Chaos based neural network optimization for concentration estimation of indoor air contaminants by an electronic nose. Sens. Actuators B Chem. 2013, 189, 161–167. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, F.; Nie, H.; Dang, L.; Li, G.; Ye, Q.; Kadri, C. Classification of multiple indoor air contaminants by an electronic nose and a hybrid support vector machine. Sens. Actuators B Chem. 2012, 174, 114–125. [Google Scholar] [CrossRef]
- Gebicki, J.; Szulczynski, B.; Kaminski, M. Determination of authenticity of brand perfume using electronic nose prototypes. Meas. Sci. Technol. 2015, 26, 125103. [Google Scholar] [CrossRef]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of key odorant compounds in freshly distilled cognac using GC-O, GC-MS, and sensory evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, L.; Koziel, J.A.; Hoff, S.J.; Schmidt, D.R.; Clanton, C.J.; Jacobson, L.D.; Parker, D.B.; Heber, A.J. Field air sampling and simultaneous chemical and sensory analysis of livestock odorants with sorbent tubes and GC–MS/olfactometry. Sens. Actuators B Chem. 2010, 146, 427–432. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography–olfactometry (GC–O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ruth, S.M. Methods for gas chromatography-olfactometry: A review. Biomol. Eng. 2001, 17, 121–128. [Google Scholar] [CrossRef]
- Romain, A.C.; Godefroid, D.; Nicolas, J. Monitoring the exhaust air of a compost pile with an e-nose and comparison with GC–MS data. Sens. Actuators B Chem. 2005, 106, 317–324. [Google Scholar] [CrossRef]
- Byliński, H.; Kolasińska, P.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Determination of odour concentration by TD-GC× GC–TOF-MS and field olfactometry techniques. Monatshefte Chem.-Chem. Mon. 2017, 148, 1651–1659. [Google Scholar] [CrossRef] [Green Version]
- Zarra, T.; Naddeo, V.; Oliva, G.; Belgiorno, V. Odour emissions characterization for impact prediction in anaerobic-aerobic integrated treatment plants of municipal solid waste. Chem. Eng. Trans. 2016, 54, 91–96. [Google Scholar]
- Sironi, S.; Capelli, L.; Céntola, P.; Del Rosso, R.; Il Grande, M. Odour emission factors for the prediction of odour emissions from plants for the mechanical and biological treatment of MSW. Atmos. Environ. 2006, 40, 7632–7643. [Google Scholar] [CrossRef]
- Lucernoni, F.; Tapparo, F.; Capelli, L.; Sironi, S. Evaluation of an Odour Emission Factor (OEF) to estimate odour emissions from landfill surfaces. Atmos. Environ. 2016, 144, 87–99. [Google Scholar] [CrossRef]
- Sironi, S.; Capelli, L.; Céntola, P.; Del Rosso, R.; Grande, M.I. Odour emission factors for assessment and prediction of Italian MSW landfills odour impact. Atmos. Environ. 2005, 39, 5387–5394. [Google Scholar] [CrossRef]
- Sironi, S.; Capelli, L.; Céntola, P.; Del Rosso, R.; Grande, M.I. Odour emission factors for assessment and prediction of Italian rendering plants odour impact. Chem. Eng. J. 2007, 131, 225–231. [Google Scholar] [CrossRef]
- Zarra, T.; Naddeo, V.; Belgiorno, V.; Reiser, M.; Kranert, M. Instrumental characterization of odour: A combination of olfactory and analytical methods. Water Sci. Technol. 2009, 59, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Cors, M.; Romain, A.C.; Delva, J. Identification of odour sources in an industrial park from resident diaries statistics. Atmos. Environ. 2010, 44, 1623–1631. [Google Scholar] [CrossRef]
- Nicolas, J.; Romain, A.C.; Ledent, C. The electronic nose as a warning device of the odour emergence in a compost hall. Sens. Actuators B Chem. 2006, 116, 95–99. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Del Rosso, R.; Céntola, P. Predicting odour emissions from wastewater treatment plants by means of odour emission factors. Water Res. 2009, 43, 1977–1985. [Google Scholar] [CrossRef]
- Naddeo, V.; Zarra, T.; Kubo, A.; Uchida, N.; Higuchi, T.; Belgiorno, V. Odour measurement in wastewater treatment plant using both European and Japanese standardized methods: Correlation and comparison study. Glob. Nest J. 2016, 18, 728–733. [Google Scholar]
- Zarra, T.; Reiser, M.; Naddeo, V.; Belgiorno, V.; Kranert, M. Odour emissions characterization from wastewater treatment plants by different measurement methods. Chem. Eng. Trans. 2014, 40, 37–42. [Google Scholar]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Wolbert, D.; Le Roux, S.; Soutrel, I.; Nunes, G. Odor concentration (OC) prediction based on odor activity values (OAVs) during composting of solid wastes and digestates. Atmos. Environ. 2019, 201, 1–12. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Yan, L.; Chen, H.; Shao, H.; Meng, T. Assessment of odor activity value coefficient and odor contribution based on binary interaction effects in waste disposal plant. Atmos. Environ. 2015, 103, 231–237. [Google Scholar] [CrossRef]
- Sohn, J.; Smith, R.; Yoong, E.; Leis, J.; Galvin, G. Quantification of Odours from Piggery Effluent Ponds using an Electronic Nose and an Artificial Neural Network. Biosyst. Eng. 2003, 86, 399–410. [Google Scholar] [CrossRef]
- Misselbrook, T.; Hobbs, P.; Persaud, K. Use of an Electronic Nose to Measure Odour Concentration Following Application of Cattle Slurry to Grassland. J. Agric. Eng. Res. 1997, 66, 213–220. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Céntola, P.; Del Rosso, R.; Il Grande, M. Electronic noses for the continuous monitoring of odours from a wastewater treatment plant at specific receptors: Focus on training methods. Sens. Actuators B Chem. 2008, 131, 53–62. [Google Scholar] [CrossRef]
- Sironi, S.; Capelli, L.; Céntola, P.; Del Rosso, R. Development of a system for the continuous monitoring of odours from a composting plant: Focus on training, data processing and results validation methods. Sens. Actuators B Chem. 2007, 124, 336–346. [Google Scholar] [CrossRef]
- Stuetz, R.; Fenner, R.; Engin, G. Assessment of odours from sewage treatment works by an electronic nose, H2S analysis and olfactometry. Water Res. 1999, 33, 453–461. [Google Scholar] [CrossRef]
- Sohn, J.H.; Hudson, N.; Gallagher, E.; Dunlop, M.; Zeller, L.; Atzeni, M. Implementation of an electronic nose for continuous odour monitoring in a poultry shed. Sens. Actuators B Chem. 2008, 133, 60–69. [Google Scholar] [CrossRef]
- Brattoli, M.; De Gennaro, G.; De Pinto, V.; Demarinis Loiotile, A.; Lovascio, S.; Penza, M. Odour detection methods: Olfactometry and chemical sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.figaro.co.jp/en/product/entry/tgs2602.html (accessed on 21 June 2022).
- Available online: https://www.figaro.co.jp/en/product/entry/tgs2603.html. (accessed on 21 June 2022).
- Available online: https://www.figaro.co.jp/en/product/entry/tgs2612-D00.html (accessed on 21 June 2022).
- Available online: https://www.alphasense.com/wp-content/uploads/2019/10/H2S-A4.pdf (accessed on 21 June 2022).
- Available online: https://www.alphasense.com/wp-content/uploads/2021/05/NH3-B1.pdf (accessed on 21 June 2022).

| Chemical Compound | Olfactory Threshold | Unit | Odor Character |
|---|---|---|---|
| Acetaldehyde | 0.015–0.066 | ppm | Fruity, apple |
| Formaldehyde | 0.50–0.80 | ppm | Pungent, suffocating |
| Acrolein | 0.0036–0.16 | ppm | Pungent, suffocating |
| Phenol | 0.0056–0.040 | ppm | Pungent |
| Hydrogen sulfide | 0.41–0.81 | ppb | Rotten eggs |
| Carbon disulfide | 0.11–0.21 | ppm | Rotten vegetables |
| Dimethyl sulfide | 2.70–3.00 | ppb | Rotten vegetables, garlic |
| Ammonia | 1.50–5.20 | ppm | Sharp, pungent |
| Methylamine | 0.035–4.70 | ppm | Fish, piscine |
| Dimethylamine | 0.033–0.34 | ppm | Fish, piscine |
| Acetone | 13.00–42.00 | ppm | Fruity, sweet |
| Acetic acid | 0.0060–0.48 | ppm | Vinegar |
| Acetonitrile | 13–170 | ppm | Etheric |
| Propionic acid | 0.0057–0.16 | ppm | Pungent |
| Acrylonitrile | 1.60–17.0 | ppm | Etheric |
| Sulfur dioxide | 0.87–1.10 | ppm | Pungent, suffocating |
| Ethyl mercaptan | 0.0087–0.76 | ppb | Rotten eggs, rotten cabbage |
| Nitrogen dioxide | 0.12–0.36 | ppm | Harsh |
| Pyridine | 0.063–0.17 | ppm | Strong sickening |
| Hexane | 1.5–130 | ppm | slightly disagreeable |
| Cyclohexane | 2.5–25 | ppm | Sweet |
| Toluene | 0.33–2.50 | ppm | Paint thinners |
| Benzene | 2.70–12.00 | ppm | Sweet, aromatic, gasoline |
| Facility | Index | Scope of Research | References |
|---|---|---|---|
| WWTP | AOI SOI | Investigation of correlation between odors concentration measured by means of dynamic olfactometry (DO) and chromatographic GC-MS-FID analysis | [83] |
| MSWTP | SOEF OER OEF | A study of large anaerobic–aerobic treatment plant, identifying its odor sources, characterizing them in terms of odor concentration and emissions using dynamic olfactometry | [78] |
| Industrial park | OAI | 13 potential odor emitting facilities; assessment of the odor annoyance using the residents as measuring tools—resident diary method | [84] |
| MSWTP | OER OEF | Mechanical and biological MSWTPs; calculation of OEFs, based on the results of olfactometric measurements, as a function of plants capacity which differ in constructional features, in type of treated waste and geographical locations in Italy | [79] |
| Compost facility | OAI | Assessment of odor annoyance generated by the composting facility and demonstration of the feasibility of gas sensor array to monitor the emission of the odorous substances | [85] |
| MSW landfills | SOER OER OEF | Estimation of odor emissions from landfills, focusing on the odor related to the emissions of landfill gas (LFG) from plant surface | [80] |
| MSW landfills | SOER OEF | Seven dimensionally different landfills, the odor concentration was calculated as the geometric mean of the odor threshold values of each panelist, using dynamic olfactometry | [81] |
| MRP | SOER OER OEF | Determination of odor nuisance from the rendering industry based on experimental data obtained by means of dynamic olfactometry, mass of processed material was used as “activity index” for OEF calculation | [82] |
| WWTP | OEF | Calculation of OEFs based on the results of olfactometric measurements that were carried out on a significant number of WWTPs, which differ in constructional features, in type of treated wastewater and in geographical locations in Italy; yearly treatment plant capacity was used as “activity index” | [86] |
| WWTP | OI | Investigation of the relationship odor index assessed by Japanese standard methods (triangle odor bag method) and odor concentrations measured with dynamic olfactometry | [87] |
| WWTP | OI | Relationship between odor concentrations emitted by WWTP assessed by Japanese standard methods and odor concentrations measured with dynamic olfactometry and compared to the measurement carried out by novel prototype of e-nose | [87] |
| WWTP | AOI SOI | Comparison and evaluation of the principal odor measurement methods (GC-MS, dynamic olfactometry, electronic nose) used to identify and characterize the odor emission from a WWTP with the aim of analyzing the weaknesses and strengths of the different techniques | [88] |
| Compost facility | OAV | The ability of OAV to predict odor concentrations during composting of six solid wastes and three digestates was evaluated, dynamic olfactometry and GC-MS were used as measurement methods | [89] |
| MSW landfills | OAV | Evaluation of odorant interaction effect to accurately estimate the contribution of odors, samples from a food waste treatment plant were analyzed by instrumental and olfactory methods, an odorant coefficient was proposed to assess the type and level of binary interaction effects based on OAV variation | [90] |
| Sensor Type | Manufacturer | Model | Detected Gases | Signal Processing |
|---|---|---|---|---|
| MOS | Figaro Engineering Inc. (Osaka, Japan) | TGS2602 [98] | hydrogen (1–30 ppm), toluene (1–30 ppm), ethanol (1–30 ppm), ammonia (1–30 ppm), hydrogen sulfide (0.1–3 ppm) | Voltage divider |
| MOS | Figaro Engineering Inc. (Osaka, Japan) | TGS2603 [99] | hydrogen (1-30 ppm), hydrogen sulfide (0.3–3.0 ppm), ethanol (1–30 ppm), methyl mercaptan (0.3–3.0 ppm), trimethyl amine (0.1–3.0 ppm) | Voltage divider |
| MOS | Figaro Engineering Inc. (Osaka, Japan) | TGS2612 [100] | methane (300–10,000 ppm), propane (300–10,000 ppm), ethanol (300–10,000 ppm), iso-butane (300–10,000 ppm) | Voltage divider |
| EC | Alphasense (Braintree, United Kingdom) | H2S-A4 [101] | hydrogen sulfide (limit of performance warranty 0–50 ppm) | I-U converter |
| EC | Alphasense (Braintree, United Kingdom) | NH3-B1 [102] | ammonia (limit of performance warranty 0–100 ppm) | I-U converter |
| Scale | Odor Intensity | Hedonic Tone | Proposed Range |
|---|---|---|---|
| 0—very good | non-perceptible and very weak | neutral and slightly unpleasant | |
| 1—moderate | weak | moderately unpleasant | |
| 2—bad | distinct and strong | very unpleasant | |
| 3—very bad | very and extremely strong | extremely unpleasant |
| Signals Features | Model | RMSE | Accordance |
|---|---|---|---|
| Model 1 | 10.302 | 0.70 | |
| Model 2 | 8.092 | 0.85 | |
| Model 3 | 67.973 | 0.65 | |
| Model 5 | 7.399 | 0.75 | |
| Model 1 | 5.754 | 0.75 | |
| Model 2 | 5.420 | 0.85 | |
| Model 3 | 7.120 | 0.70 | |
| Model 4 | 3.667 | 0.90 | |
| Model 5 | 4.220 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyniewski, D.; Szulczyński, B.; Gębicki, J. Determination of Odor Air Quality Index (OAQII) Using Gas Sensor Matrix. Molecules 2022, 27, 4180. https://doi.org/10.3390/molecules27134180
Dobrzyniewski D, Szulczyński B, Gębicki J. Determination of Odor Air Quality Index (OAQII) Using Gas Sensor Matrix. Molecules. 2022; 27(13):4180. https://doi.org/10.3390/molecules27134180
Chicago/Turabian StyleDobrzyniewski, Dominik, Bartosz Szulczyński, and Jacek Gębicki. 2022. "Determination of Odor Air Quality Index (OAQII) Using Gas Sensor Matrix" Molecules 27, no. 13: 4180. https://doi.org/10.3390/molecules27134180
APA StyleDobrzyniewski, D., Szulczyński, B., & Gębicki, J. (2022). Determination of Odor Air Quality Index (OAQII) Using Gas Sensor Matrix. Molecules, 27(13), 4180. https://doi.org/10.3390/molecules27134180







