Synthesis of Spin-Labeled Ibuprofen and Its Interaction with Lipid Membranes
Abstract
1. Introduction
2. Results and Discussion
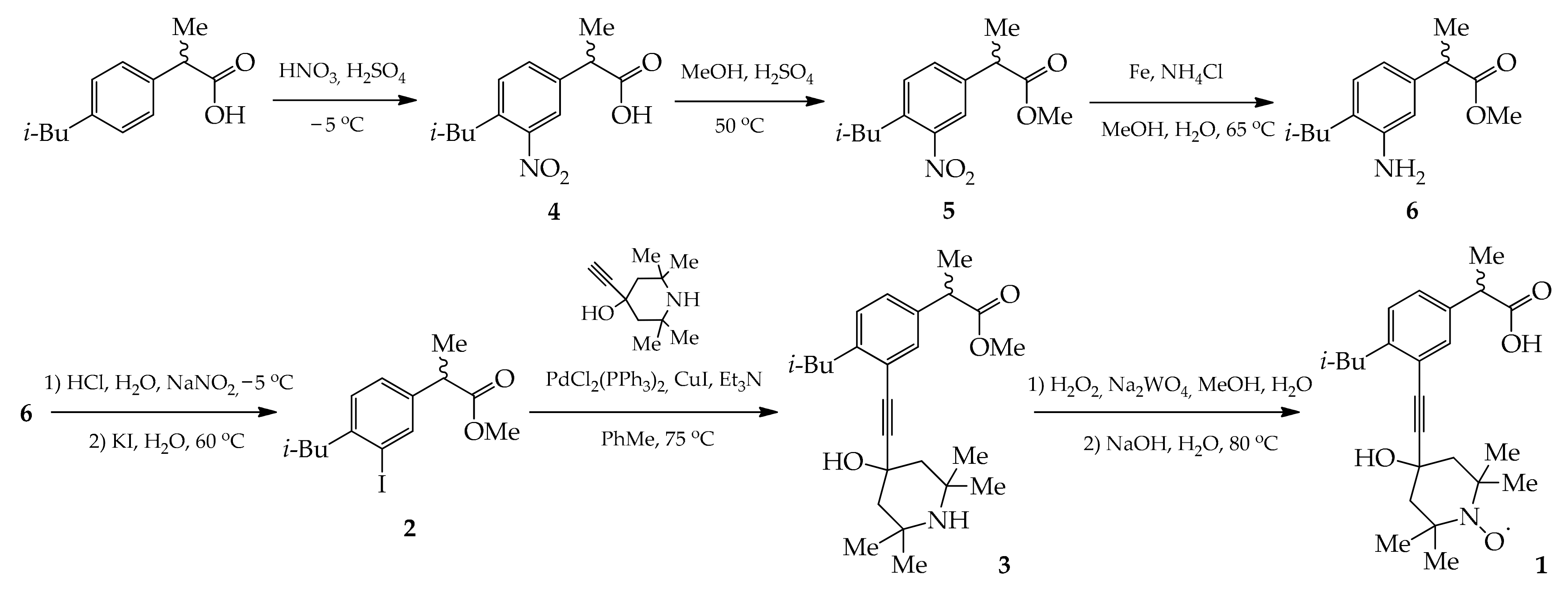
2.1. Synthesis
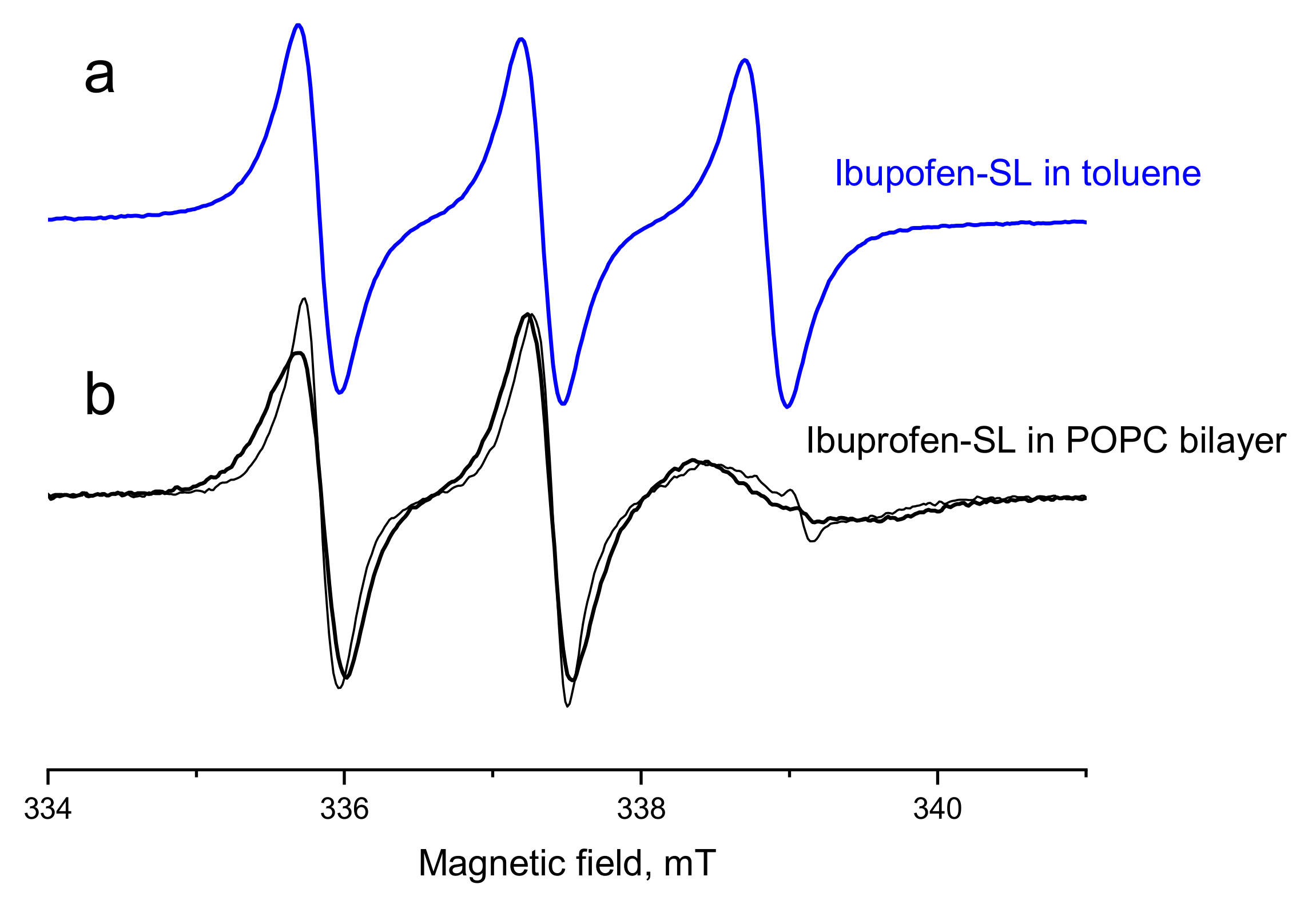
2.2. Conventional EPR Spectra: Interaction with the POPC Membrane
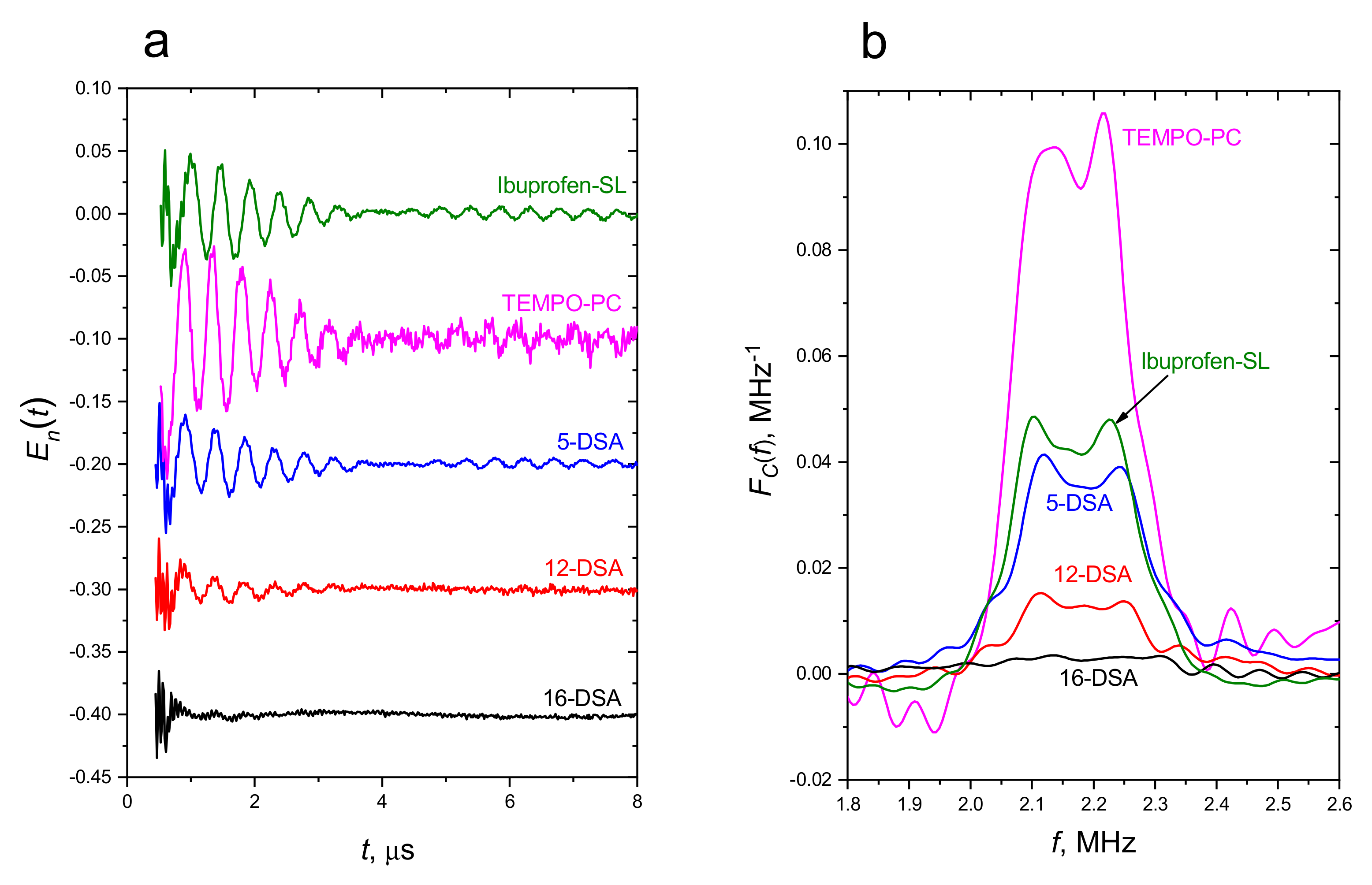
2.3. Pulsed EPR: Location in the POPC Membrane
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.3. Sample Preparations for EPR Investigation
3.4. EPR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rainsford, K.D. Ibuprofen: Discovery, Development and Therapeutics; John Wiley & Sons: West Sussex, UK, 2015. [Google Scholar] [CrossRef]
- Radman, M.; Babic, A.; Runjic, E.; Jelicic Kadic, A.; Jeric, M.; Moja, L.; Puljak, L. Revisiting established medicines: An overview of systematic reviews about ibuprofen and paracetamol for treating pain in children. Eur. J. Pain 2019, 23, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, G.; Pergolizzi, J.V.; Dowling, P.; Paladini, A. Ibuprofen Safety at the Golden Anniversary: Are all NSAIDs the Same? A Narrative Review. Adv. Ther. 2020, 37, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Ibuprofen: Pharmacology, Therapeutics and Side Effects; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Ibuprofen, inflammation and Alzheimer disease. Nat. Med. 2000, 6, 973–974. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Schwarzschild, M.A.; Ascherio, A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011, 76, 863–869. [Google Scholar] [CrossRef]
- Van Dam, D.; Coen, K.; De Deyn, P.P. Ibuprofen modifies cognitive disease progression in an Alzheimer’s mouse model. J. Psychopharmacol. 2010, 24, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, F.; Allen, J.; Lynch, J.; Andrews, P.; Djakiew, D. Ibuprofen inhibits survival of bladder cancer cells by induced expression of the p75NTR tumor suppressor protein. Cancer Res. 2004, 64, 6207–6213. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Endo, H.; Yano, M.; Okumura, Y.; Kido, H. Ibuprofen enhances the anticancer activity of cisplatin in lung cancer cells by inhibiting the heat shock protein 70. Cell Death Dis. 2014, 5, e1027. [Google Scholar] [CrossRef]
- Greenspan, E.J.; Madigan, J.P.; Boardman, L.A.; Rosenberg, D.W. Ibuprofen inhibits activation of nuclear β-catenin in human colon adenomas and induces the phosphorylation of GSK-3β. Cancer Prev. Res. 2011, 4, 161–171. [Google Scholar] [CrossRef]
- Cajaraville, J.P. Ibuprofen arginate for rapid-onset pain relief in daily practice: A review of its use in different pain conditions. J. Pain Res. 2021, 14, 117–126. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Ferris, D.P.; Holcroft, J.M.; Kandela, I.; Stern, C.L.; Nassar, M.S.; Botros, Y.Y.; Stoddart, J.F. Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies. Mol. Pharm. 2017, 14, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Leyva, R.; Kellstein, D.; Arthur, E.; Cruz-Rivera, M. Efficacy of a Fixed-Dose Combination of Ibuprofen and Acetaminophen Compared with Individual Monocomponents in Adult Male Subjects With Endotoxin-Induced Fever: A Randomized Controlled Trial. Clin. Ther. 2021, 43, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Brazier, D.; Perry, R.; Keane, J.; Barrett, K.; Elmaleh, D.R. Pharmacokinetics of Cromolyn and Ibuprofen in Healthy Elderly Volunteers. Clin. Drug Investig. 2017, 37, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Kivitz, A.J.; Bello, A.E.; Grahn, A.Y.; Schiff, M.H.; Taha, A.S. Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers. Am. J. Gastroenterol. 2012, 107, 379–386. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Villena, F.; Sotomayor, C.P.; Edwards, A.M.; Muñoz, M.A.; Garidel, P.; Suwalsky, M. Human cells and cell membrane molecular models are affected in vitro by the nonsteroidal anti-inflammatory drug ibuprofen. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2656–2664. [Google Scholar] [CrossRef][Green Version]
- Alsop, R.J.; Armstrong, C.L.; Maqbool, A.; Toppozini, L.; Dies, H.; Rheinstädter, M.C. Cholesterol expels ibuprofen from the hydrophobic membrane core and stabilizes lamellar phases in lipid membranes containing ibuprofen. Soft Matter 2015, 11, 4756–4767. [Google Scholar] [CrossRef]
- Sreij, R.; Prévost, S.; Dargel, C.; Dattani, R.; Hertle, Y.; Wrede, O.; Hellweg, T. Interaction of the Saponin Aescin with Ibuprofen in DMPC Model Membranes. Mol. Pharm. 2018, 15, 4446–4461. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mamontov, E.; Tyagi, M. Effects of NSAIDs on the nanoscopic dynamics of lipid membrane. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183100. [Google Scholar] [CrossRef]
- Yefimova, S.L.; Tkacheva, T.N.; Kasian, N.A. Study of the Combined Effect of Ibuprofen and Cholesterol on the Microviscosity and Ordering of Model Lipid Membranes by Timeresolved Measurement of Fluorescence Anisotropy Decay. J. Appl. Spectrosc. 2017, 84, 284–290. [Google Scholar] [CrossRef]
- Sharma, V.K.; Nagao, M.; Rai, D.K.; Mamontov, E. Membrane softening by nonsteroidal anti-inflammatory drugs investigated by neutron spin echo. Phys. Chem. Chem. Phys. 2019, 21, 20211–20218. [Google Scholar] [CrossRef]
- Ramadurai, S.; Sarangi, N.K.; Maher, S.; Macconnell, N.; Bond, A.M.; McDaid, D.; Flynn, D.; Keyes, T.E. Microcavity-Supported Lipid Bilayers; Evaluation of Drug-Lipid Membrane Interactions by Electrochemical Impedance and Fluorescence Correlation Spectroscopy. Langmuir 2019, 35, 8095–8109. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.; Morales, M.; Miller, E.; Braziel, S.; Giancaspro, J.; Scollan, P.; Rosario, J.; Gayapa, A.; Krmic, M.; Lee, S. Ibuprofen and the Phosphatidylcholine Bilayer: Membrane Water Permeability in the Presence and Absence of Cholesterol. Langmuir 2021, 37, 4468–4480. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sendecki, A.M.; Pullanchery, S.; Huang, D.; Yang, T.; Cremer, P.S. Multistep Interactions between Ibuprofen and Lipid Membranes. Langmuir 2018, 34, 10782–10792. [Google Scholar] [CrossRef] [PubMed]
- Kremkow, J.; Luck, M.; Huster, D.; Müller, P.; Scheidt, H.A. Membrane interaction of ibuprofen with cholesterol-containing lipid membranes. Biomolecules 2020, 10, 1384. [Google Scholar] [CrossRef]
- De Souza Teixeira, L.; Chagas, T.V.; Alonso, A.; Gonzalez-Alvarez, I.; Bermejo, M.; Polli, J.; Rezende, K.R. Biomimetic artificial membrane permeability assay over franz cell apparatus using bcs model drugs. Pharmaceutics 2020, 12, 988. [Google Scholar] [CrossRef]
- Aloi, E.; Rizzuti, B.; Guzzi, R.; Bartucci, R. Association of ibuprofen at the polar/apolar interface of lipid membranes. Arch. Biochem. Biophys. 2018, 654, 77–84. [Google Scholar] [CrossRef]
- Marsh, D. Spin-Label Electron Paramagnetic Resonance Spectroscopy; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Erilov, D.A.; Bartucci, R.; Guzzi, R.; Shubin, A.A.; Maryasov, A.G.; Marsh, D.; Dzuba, S.A.; Sportelli, L. Water concentration profiles in membranes measured by ESEEM of spin-labeled lipids. J. Phys. Chem. B 2005, 109, 12003–12013. [Google Scholar] [CrossRef]
- Salnikov, E.S.; Erilov, D.A.; Milov, A.D.; Tsvetkov, Y.D.; Peggion, C.; Formaggio, F.; Toniolo, C.; Raap, J.; Dzuba, S.A. Location and aggregation of the spin-labeled peptide trichogin GA IV in a phospholipid membrane as revealed by pulsed EPR. Biophys. J. 2006, 91, 1532–1540. [Google Scholar] [CrossRef]
- Milov, A.D.; Samoilova, R.I.; Shubin, A.A.; Grishin, Y.A.; Dzuba, S.A. ESEEM measurements of local water concentration in D2O-containing spin-labeled systems. Appl. Magn. Reson. 2008, 35, 73–94. [Google Scholar] [CrossRef]
- Dzuba, S.A.; Marsh, D. ESEEM of Spin Labels to Study Intermolecular Interactions, Molecular Assembly and Conformation. In A Specialist Periodic Report, Electron Paramagnetic Resonance; Gilbert, C., Chechik, V., Murphy, D.M., Eds.; RSC Publishing: Cambridge, UK, 2015; Volume 24, pp. 102–121. [Google Scholar] [CrossRef]
- Milov, A.D.; Maryasov, A.G.; Tsvetkov, Y.D. Pulsed Electron Double Resonance (PELDOR) and its Applications in Free-Radicals Research. Appl. Magn. Reson. 1998, 15, 107–143. [Google Scholar] [CrossRef]
- Schiemann, O.; Prisner, T.F. Long-Range Distance Determinations in Biomacromolecules by EPR Spectroscopy. Quart. Rev. Biophys. 2007, 40, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. DEER Distance Measurements on Proteins. Ann. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Fábregas-Ibáñez, L.; Tessmer, M.H.; Jeschke, G.; Stoll, S. Dipolar pathways in dipolar EPR spectroscopy. Phys. Chem. Chem. Phys. 2022, 24, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Unguryan, V.V.; Golysheva, E.A.; Dzuba, S.A. Double Electron-Electron Resonance of Spin-Labeled Cholestane in Model Membranes: Evidence for Substructures inside the Lipid Rafts. J. Phys. Chem. B 2021, 125, 9557–9563. [Google Scholar] [CrossRef]
- Sasaki, K.; Ito, T.; Fujii, H.G.; Sato, S. Synthesis and reduction kinetics of five ibuprofen-nitroxides for ascorbic acid and methyl radicals. Chem. Pharm. Bull. 2016, 64, 1509–1513. [Google Scholar] [CrossRef][Green Version]
- Akdogan, Y.; Emrullahoglu, M.; Tatlidil, D.; Ucuncu, M.; Cakan-Akdogan, G. EPR studies of intermolecular interactions and competitive binding of drugs in a drug-BSA binding model. Phys. Chem. Chem. Phys. 2016, 18, 22531–22539. [Google Scholar] [CrossRef]
- Selinsky, B.S.; Gupta, K.; Sharkey, C.T.; Loll, P.J. Structural analysis of NSAID binding by prostaglandin H2 synthase: Time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry 2001, 40, 5172–5180. [Google Scholar] [CrossRef]
- Gupta, K.; Kaub, C.J.; Carey, K.N.; Casillas, E.G.; Selinsky, B.S.; Loll, P.J. Manipulation of kinetic profiles in 2-aryl propionic acid cyclooxygenase inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 667–671. [Google Scholar] [CrossRef]
- Boggara, M.B.; Faraone, A.; Krishnamoorti, R. Effect of pH and ibuprofen on the phospholipid bilayer bending modulus. J. Phys. Chem. B 2010, 114, 8061–8066. [Google Scholar] [CrossRef]
- Boggara, M.B.; Krishnamoorti, R. Partitioning of nonsteroidal antiinflammatory drugs in lipid membranes: A molecular dynamics simulation study. Biophys. J. 2010, 98, 586–595. [Google Scholar] [CrossRef]
- Bode, B.E.; Margraf, D.; Prisner, T.F.; Schiemann, O. Counting the Monomers in Nanometer-Sized Oligomers by Pulsed Electron-Electron Double Resonance. J. Am. Chem. Soc. 2007, 129, 6736–6745. [Google Scholar] [CrossRef] [PubMed]
- Kálai, T.; Schindler, J.; Balog, M.; Fogassy, E.; Hideg, K. Synthesis and resolution of new paramagnetic α-amino acids. Tetrahedron 2008, 64, 1094–1100. [Google Scholar] [CrossRef]
- Saha, S.; Hetzke, T.; Prisner, T.F.; Sigurdsson, S.T. Noncovalent spin-labeling of RNA: The aptamer approach. Chem. Commun. 2018, 54, 11749–11752. [Google Scholar] [CrossRef]
- Smorygina, A.S.; Golysheva, E.S.; Dzuba, S.A. Clustering of Stearic Acids in Model Phospholipid Membranes Revealed by Double Electron-Electron Resonance. Langmuir 2021, 37, 13909–13916. [Google Scholar] [CrossRef]
- Konov, K.B.; Isaev, N.P.; Dzuba, S.A. Glycerol penetration profile in phospholipid bilayers measured by ESEEM of spin-labelled lipids. Mol. Phys. 2013, 111, 2882–28863. [Google Scholar] [CrossRef]
- Stimson, L.; Dong, L.; Karttunen, M.; Wisniewska, A.; Dutka, M.; Róg, T. Stearic Acid Spin Labels in Lipid Bilayers: Insight through Atomistic Simulations. J. Phys. Chem. B 2007, 111, 12447–12453. [Google Scholar] [CrossRef]
- Khajeh, A.; Modarress, H. The influence of cholesterol on interactions and dynamics of ibuprofen in a lipid bilayer. Biochim. Biophys. Acta 2014, 1838, 2431–2438. [Google Scholar] [CrossRef]
- Jämbeck, J.P.M.; Lyubartsev, A.P. Exploring the Free Energy Landscape of Solutes Embedded in Lipid Bilayers. J. Phys. Chem. Lett. 2013, 4, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Litvin, E.F.; Kozlova, L.M.; Shapiro, A.B.; Freidlin, L.K.; Rozantsev, E.G.; Skripnichenko, L.N. Hydrogenation of stable nitroxyl radicals with acetylenic bonds on Ni, Pd, and Pt catalysts. Bull. Acad. Sci. USSR Div. Chem. Sci. 1979, 28, 97–102. [Google Scholar] [CrossRef]
- Pabst, G.; Danner, S.; Podgornik, R.; Katsaras, J. Entropy-Driven Softening of Fluid Lipid Bilayers by Alamethicin. Langmuir 2007, 23, 11705–11711. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranov, D.S.; Smorygina, A.S.; Dzuba, S.A. Synthesis of Spin-Labeled Ibuprofen and Its Interaction with Lipid Membranes. Molecules 2022, 27, 4127. https://doi.org/10.3390/molecules27134127
Baranov DS, Smorygina AS, Dzuba SA. Synthesis of Spin-Labeled Ibuprofen and Its Interaction with Lipid Membranes. Molecules. 2022; 27(13):4127. https://doi.org/10.3390/molecules27134127
Chicago/Turabian StyleBaranov, Denis S., Anna S. Smorygina, and Sergei A. Dzuba. 2022. "Synthesis of Spin-Labeled Ibuprofen and Its Interaction with Lipid Membranes" Molecules 27, no. 13: 4127. https://doi.org/10.3390/molecules27134127
APA StyleBaranov, D. S., Smorygina, A. S., & Dzuba, S. A. (2022). Synthesis of Spin-Labeled Ibuprofen and Its Interaction with Lipid Membranes. Molecules, 27(13), 4127. https://doi.org/10.3390/molecules27134127






