Domino Nitro Reduction-Friedländer Heterocyclization for the Preparation of Quinolines
Abstract
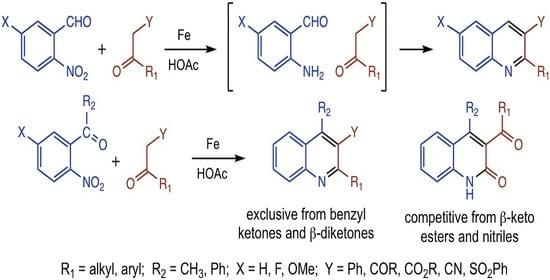
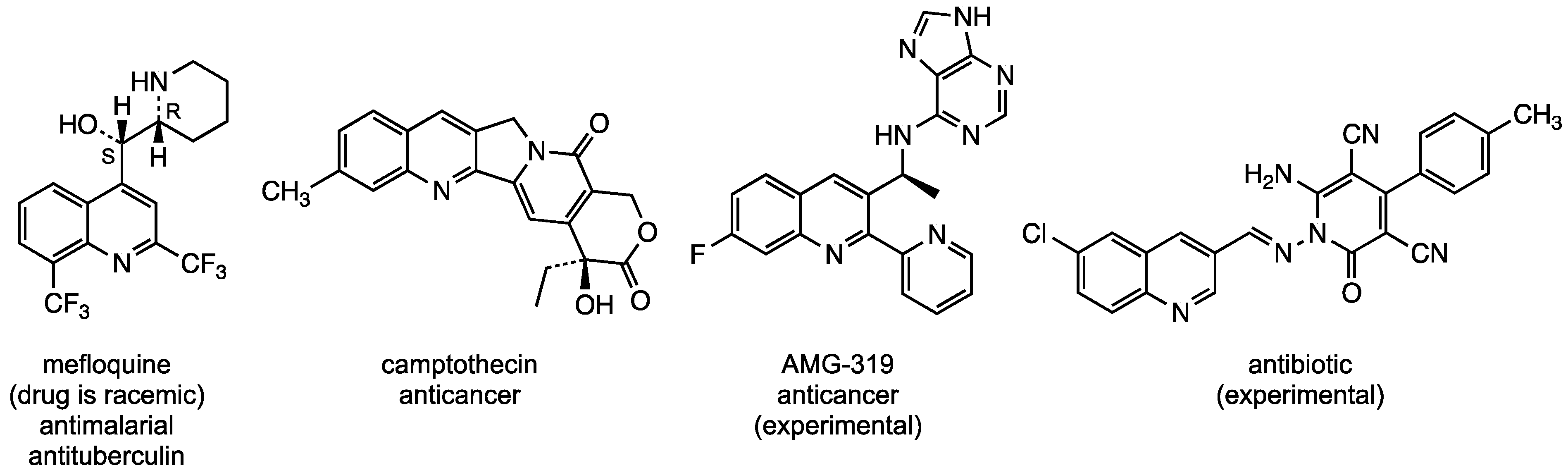
1. Introduction
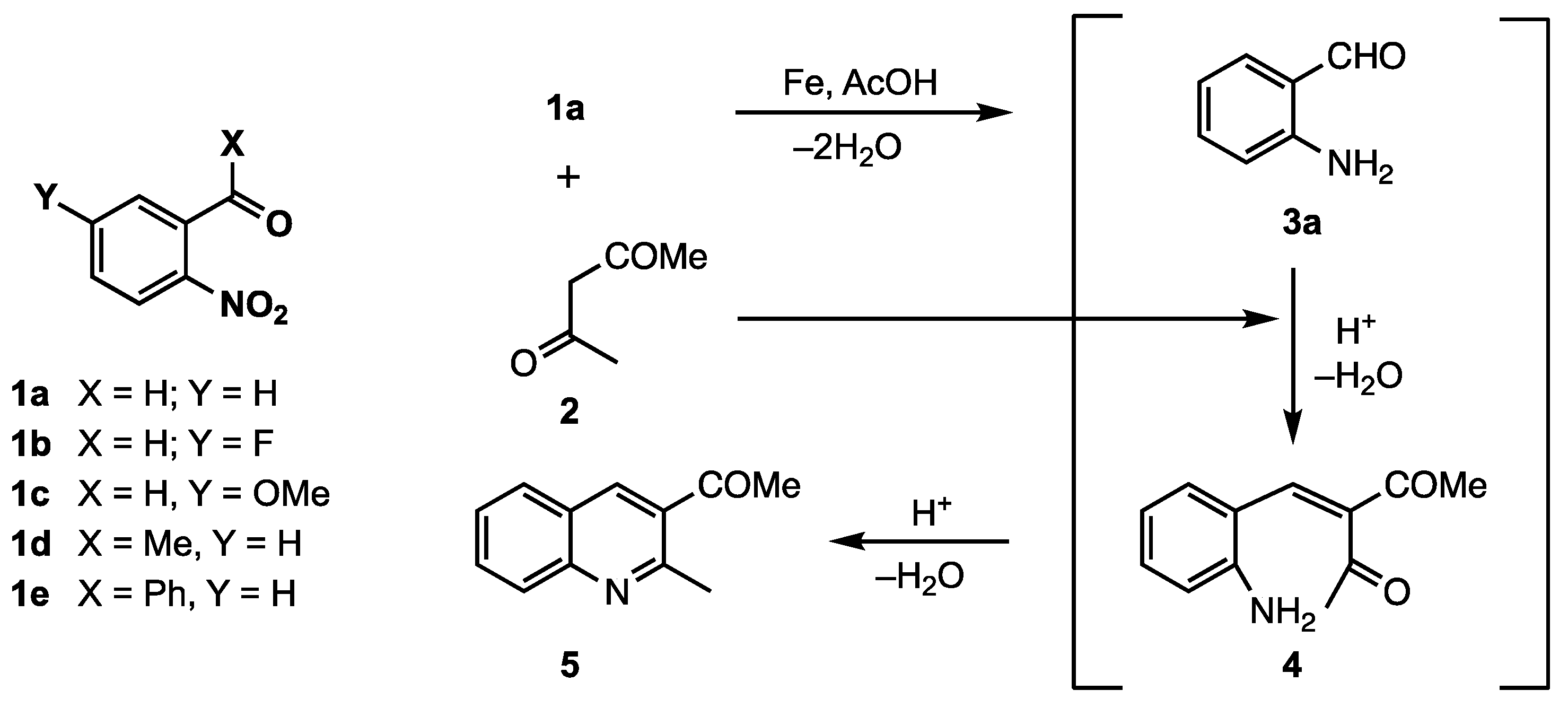
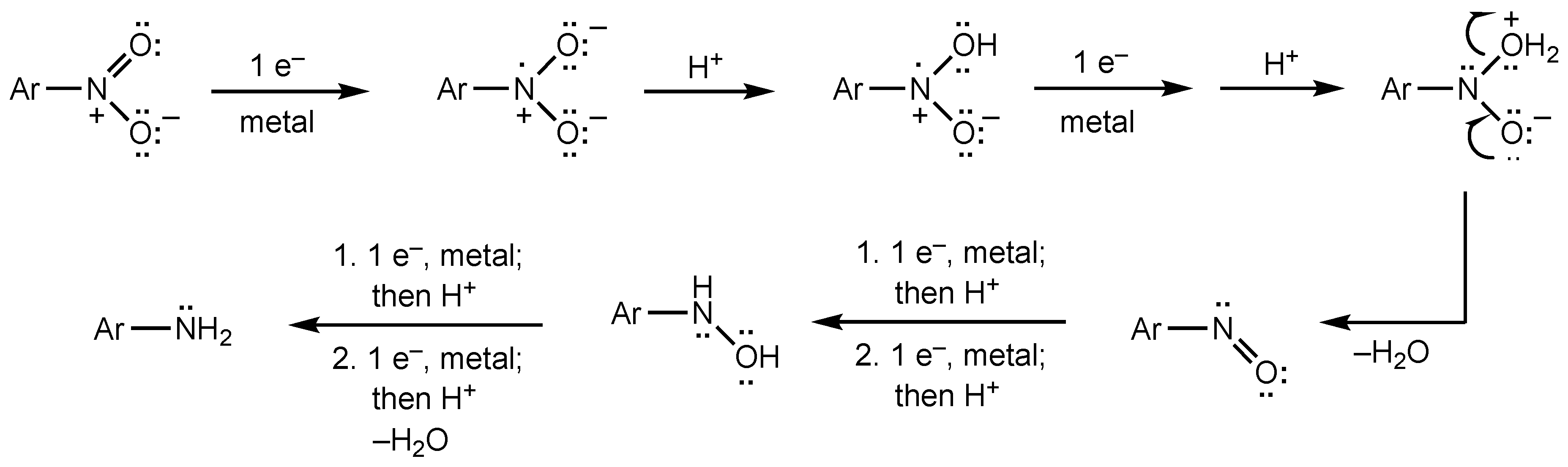
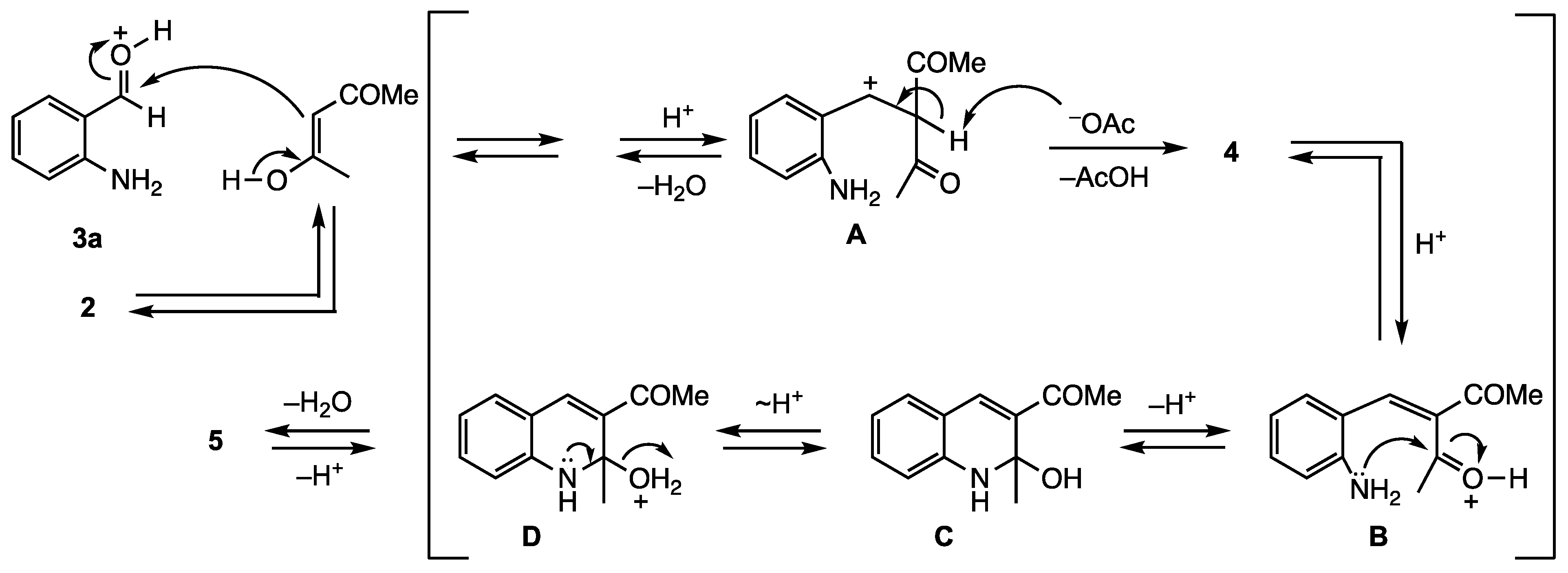
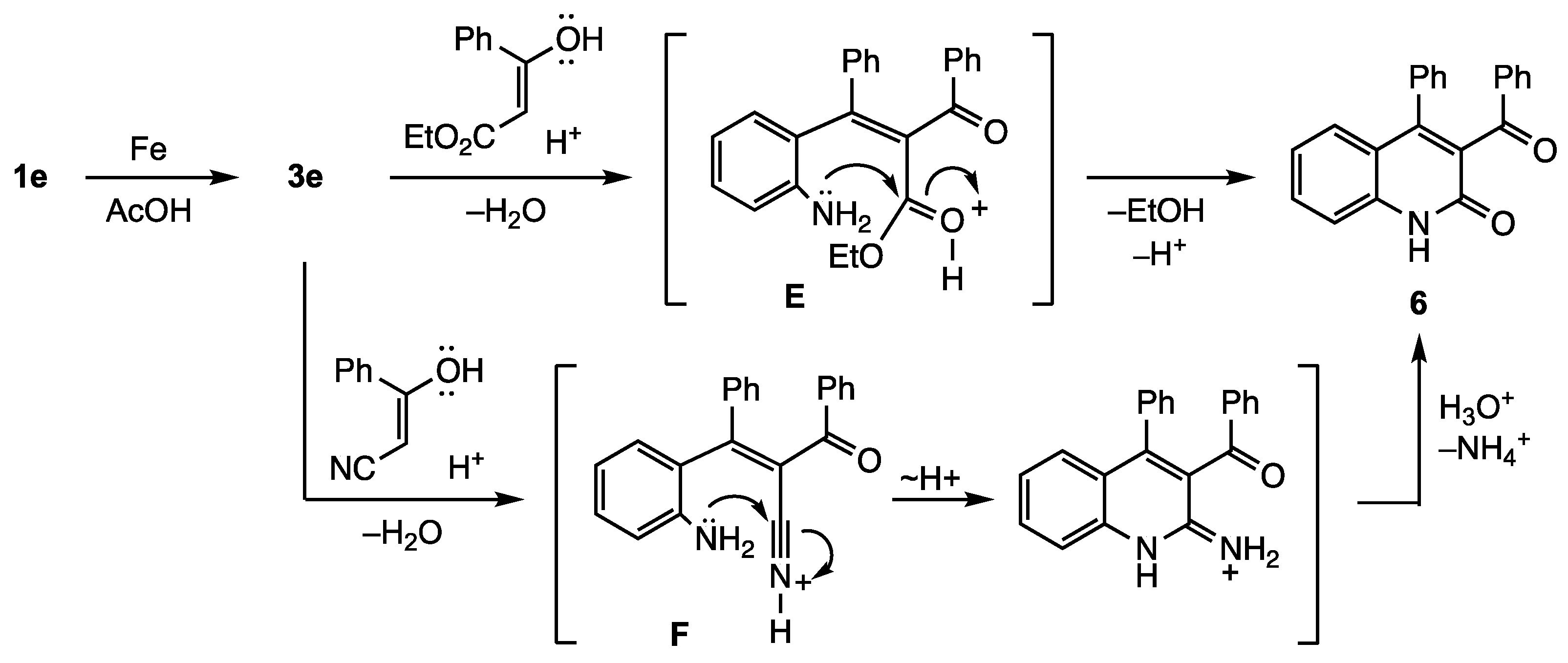
2. Results and Discussion
3. Experimental Section
3.1. General Methods
3.2. General Procedure for Domino Reduction-Heterocyclization
3.3. Reactions with 2-Nitrobenzaldehyde (1a)
3.3.1. Ethyl 2-Methylquinoline-3-carboxylate
3.3.2. Ethyl 2-(Trifluoromethyl)quinoline-3-carboxylate
3.3.3. Methyl 2-Ethylquinoline-3-carboxylate
3.3.4. Methyl 2-Pentylquinoline-3-carboxylate
3.3.5. Methyl 2-Isopropylquinoline-3-carboxylate
3.3.6. Methyl 2-(tert-Butyl)quinoline-3-carboxylate
3.3.7. Ethyl 2-Phenylquinoline-3-carboxylate
3.3.8. Methyl 2-Benzylquinoline-3-carboxylate
3.3.9. Methyl 2-Phenethylquinoline-3-carboxylate
3.3.10. Methyl 2-(Phenoxymethyl)quinoline-3-carboxylate
3.3.11. 2-Methyl-3-(phenylsulfonyl)quinoline
3.3.12. 2-Methylquinoline-3-carbonitrile
3.3.13. 2-Phenylquinoline-3-carbonitrile
3.3.14. 3,3-Dimethyl-3,4-dihydroacridine-1(2H)-one
3.3.15. 1-(2-Methylquinolin-3-yl)ethan-1-one
3.3.16. (2-Methylquinolin-3-yl)(phenyl)methanone
3.3.17. 2-Methyl-3-phenylquinoline
3.3.18. 2,3-Diphenylquinoline
3.3.19. 5,6-Dihydrobenzo[a]acridine
3.4. Reactions with 5-Fluoro-2-nitrobenzaldehydes (1b)
3.4.1. Ethyl 6-Fluoro-2-(trifluoromethyl)quinoline-3-carboxylate
3.4.2. Methyl 6-Fluoro-2-isopropylquinoline-3-carboxylate
3.4.3. Ethyl 6-Fluoro-2-phenylquinoline-3-carboxylate
3.4.4. Methyl 2-Benzyl-6-fluoroquinoline-3-carboxylate
3.4.5. 6-Fluoro-2-Methyl-3-(phenylsulfonyl)quinoline
3.4.6. 6-Fluoro-2-phenylquinoline-3-carbonitrile
3.4.7. 7-Fluoro-3,3-dimethyl-3,4-dihydroacridine-1(2H)-one
3.5. Reactions with 5-Methoxy-2-nitrobenzaldehyde (1c)
3.5.1. Ethyl 6-Methoxy-2-(trifluoromethyl)quinoline-3-carboxylate
3.5.2. Methyl 2-Isopropyl-6-methoxyquinoline-3-carboxylate
3.5.3. Ethyl 6-Methoxy-2-phenylquinoline-3-carboxylate
3.5.4. Methyl 2-Benzyl-6-methoxyquinoline-3-carboxylate
3.5.5. 6-Methoxy-2-methyl-3-(phenylsulfonyl)quinoline
3.5.6. 6-Methoxy-2-phenylquinoline-3-carbonitrile
3.5.7. 7-Methoxy-3,3-dimethyl-3,4-dihydroacridine-1(2H)-one
3.6. Reactions with 2-Nitroacetophenone (1d)
3.6.1. 4-Methyl-3-propionylquinolin-2(1H)-one
3.6.2. 3-Benzoyl-4-methylquinolin-2(1H)-one
3.6.3. 4-Methyl-2-phenylquinoline-3-carbonitrile
3.6.4. 1-(2,4-Dimethylquinolin-3-yl)ethan-1-one
3.6.5. 3,3,9-Trimethyl-3,4-dihydroacridin-1(2H)-one
3.6.6. 2,4-Dimethyl-3-phenylquinoline
3.6.7. 12-Methyl-5,6-dihydrobenzo[a]acridine
3.7. Reactions with 2-Nitrobenzophenone (1e)
3.7.1. Methyl 2-Ethyl-4-phenylquinoline-3-carboxylate
3.7.2. 3-Benzoyl-4-phenylquinolin-2(1H)-one
3.7.3. 3-Benzoyl-4-phenylquinolin-2(1H)-one
3.7.4. (2-Methyl-4-phenylquinolin-3-yl)ethan-1-one
3.7.5. 3,3-Dimethyl-9-phenyl-3,4-dihydroacridin-1(2H)-one
3.7.6. 2-Methyl-3,4-diphenylquinoline
3.7.7. 3-Butyl-2,4-diphenylquinoline
3.7.8. 12-Phenyl-5,6-dihydrobenzo[a]acridine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. Handbook of Heterocyclic Chemistry, 3rd ed.; Elsevier: New York, NY, USA, 2010; p. 816. [Google Scholar]
- Boger, D.L.; Chen, J.-H. A modified Friedländer condensation for the synthesis of 3-hydroxyquinoline-2-carboxylates. J. Org. Chem. 1995, 60, 7369–7371. [Google Scholar] [CrossRef]
- Na, J.E.; Lee, K.Y.; Park, D.Y.; Kim, J.N. Modified Friedländer synthesis of quinolines from N-phenyl cyclic enaminones. Bull. Korean Chem. Soc. 2005, 26, 323–326. [Google Scholar] [CrossRef][Green Version]
- Munday, B.P.; Ellerd, M.G. Name Reactions and Reagents in Organic Synthesis; Wiley: New York, NY, USA, 1988; pp. 86–87. [Google Scholar]
- Cheng, C.-C.; Yan, S.-J. The Friedländer synthesis of quinolines. Org. React. 1982, 28, 37–201. [Google Scholar] [CrossRef]
- Cho, C.S.; Kim, B.T.; Kim, T.-J.; Shi, S.C. Ruthenium-catalysed oxidative cyclisation of 2-aminobenzyl alcohol with ketones: Modified Friedlaender quinoline synthesis. Chem. Commun. 2001, 2576–2577. [Google Scholar] [CrossRef]
- Motojura, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Multifunctional catalysis of a ruthenium-grafted hydrotalcite: One-pot synthesis of quinolines from 2-aminobenzyl alcohol and various carbonyl compounds via aerobic oxidation and aldol reaction. Tetrahedron Lett. 2004, 45, 6029–6032. [Google Scholar] [CrossRef]
- Xing, R.-G.; Li, Y.-N.; Liu, Q.; Han, Y.-F.; Wei, X.; Li, J.; Zhou, B. Selective reduction of nitroarenes by a Hantzsch 1,4-dihydropyridine: A facile and efficient approach to substituted quinolines. Synthesis 2011, 2066–2072. [Google Scholar] [CrossRef]
- Rajawinslin, R.R.; Gawande, S.D.; Kavala, V.; Huang, Y.-H.; Kuo, C.-W.; Kuo, T.-S.; Chen, M.-L.; He, C.H.; Yao, C.-F. Iron/acetic acid mediated intermolecular tandem C–C and C–N bond formation: And easy access to acridone and quinolione derivatives. RSC Adv. 2014, 4, 37806–37811. [Google Scholar] [CrossRef]
- Zhu, Z.; Seidel, D. Acetic acid promoted redox annulations with dual C–H functionalization. Org. Lett. 2017, 19, 2841–2844. [Google Scholar] [CrossRef]
- Augustine, R.L.; Gustavsen, A.J.; Wanat, S.F.; Pattison, I.C.; Houghton, K.S.; Koletar, G. Synthesis of α-monosubstituted indoles. J. Org. Chem. 1973, 38, 3004–3011. [Google Scholar] [CrossRef]
- Bunce, R.A.; Herron, D.M.; Ackerman, M.L. Aryl-fused nitrogen heterocycles by a tandem reduction-Michael addition reaction. J. Org. Chem. 2000, 65, 2847–2850. [Google Scholar] [CrossRef]
- Bunce, R.A.; Schammerhorn, J.E. Dibenzo-fused seven-membered nitrogen heterocycles by a tandem reduction-lactamization reaction. J. Heterocycl. Chem. 2006, 43, 1031–1035. [Google Scholar] [CrossRef]
- Bunce, R.A.; Nammalwar, B. 1,2,3,9-Tetrahydro-4H-carbazol-4-one and 8,9-dihydropyrido[1,2-a]indol-6(7H)-one from 1H-indole-2-butanoic acid. J. Heterocycl. Chem. 2009, 46, 172–177. [Google Scholar] [CrossRef]
- Labadie, S.S.; Parmer, C. Efficient synthesis of hexahydrocarbazoles. Synth. Commun. 2011, 41, 1752–1758. [Google Scholar] [CrossRef]
- Embrey, S.J.; Barrios-Perez, C.; Bunce, R.A. (±)-cis-4a-Alkyl-1,3,4,4a,9,9a-hexahydro-2H-carbazol-2-ones by domino nitro reduction-aza-Michael addition to enones. J. Heterocycl. Chem. 2022, 59, 750–759. [Google Scholar] [CrossRef]
- Kempter, G.; Hirschberg, S. Heterocycles from amino ketones. V. The Friedlaender synthesis with nitrogen-, oxygen-, or sulfur-containing five- and six-membered ring ketones. Chem. Ber. 1965, 98, 419–427. [Google Scholar] [CrossRef]
- Da Settimo, A.; Primofiore, G.; Livi, O.; Ferrarini, P.L.; Spinelli, S. Synthesis of some 3-substituted quino[3,2-c][1,8]naphthyridines. A new heterocyclic system. J. Heterocycl. Chem. 1979, 16, 169–174. [Google Scholar] [CrossRef]
- Suzuki, M.; Tanikawa, K.; Sakoda, R. Practical synthesis of quinoline nucleus of NK-104. Heterocycles 1999, 50, 479–483. [Google Scholar] [CrossRef]
- Nammalwar, B.; Murie, M.; Fortenberry, C.; Bunce, R.A. Synthesis of quinoline and 1,8-naphthyridine-3-carboxylic acids using a self-catalyzed Friedländer approach. Tetrahedron Lett. 2014, 55, 3181–3183. [Google Scholar] [CrossRef]
- Bawa, S.; Kumar, S.; Drabu, S.; Kumar, R. Structural modifications of quinoline-based anti-malarial agents: Recent developments. J. Pharm. Bioallied Sci. 2010, 2, 64–71. [Google Scholar] [CrossRef]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar] [CrossRef]
- Vanderckhove, S.; D’hooghe, M. Quinoline-based antimalarial hybrid compounds. Bioorg. Med. Chem. 2015, 23, 5098–5119. [Google Scholar] [CrossRef] [PubMed]
- Nqoro, X.; Tobeka, N.; Aderibigbe, B.A. Quinoline-based hybrid compounds with antimalarial activity. Molecules 2017, 22, 2268. [Google Scholar] [CrossRef] [PubMed]
- Parada, L.K.L.; Méndez, L.Y.V.; Kousnetzov, V.V. Quinoline-substituted 1,2,3-triazole-based molecules, as promising conjugated hybrids in biomedical research. Org. Med. Chem. 2018, 8, 555708. [Google Scholar] [CrossRef]
- Uddin, A.; Chawla, M.; Irfan, I.; Mahajan, S.; Singh, S.; Abid, M. Medicinal chemistry updates on quinoline- and endoperoxide-based hybrids with potent antimalarial activity. RSC Med. Chem. 2021, 12, 24–42. [Google Scholar] [CrossRef]
- Nyamwihura, R.J.; Zhang, H.; Collins, J.T.; Crown, O.; Ogungbe, I.V. Nopol-based quinoline derivatives as antiplasmodial agents. Molecules 2021, 26, 1008. [Google Scholar] [CrossRef]
- Kousnetzov, V.V.; Meléndez-Gómez, C.M.; Valencia Peña, J.L.; Vargas-Méndez, L.Y. Discover and development of therapeutics from natural products against neglected tropical diseases. Nat. Prod. Drug Discov. 2019, 87–164. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, S.A. Quinoline: A promising antitubercular target. Biomed. Pharmacother. 2014, 68, 1161–1175. [Google Scholar] [CrossRef]
- Mohamed, M.F.A.; Abou-Rahma, G.A. Molecular targets and anticancer activity of quinoline-chalcone hybrids: Literature review. RSC Adv. 2020, 10, 31139–31155. [Google Scholar] [CrossRef]
- Martorana, A.; LaMonica, G.; Lauria, A. Quinoline-based molecules targeting c-Met, EGF, and VEGF receptors and the proteins involved in related carcinogenic pathways. Molecules 2020, 25, 4279. [Google Scholar] [CrossRef]
- Desai, N.C.; Patel, B.Y.; Jadeja, K.A.; Dave, B.P. Landscaping of quinoline based heterocycles as potential antimicrobial agents: A mini review. Nov. Approaches Drug Des. Dev. 2017, 1, 555570. [Google Scholar] [CrossRef]
- Moore, G.G.I.; Harrington, J.K.; Swingle, K.F. Antiinflammatory fluoroalkanesulfonanilides. 3. Fluoroalkanesulfonamido diaryl system. J. Med. Chem. 1975, 18, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.C.E.; Atkinson, C.M.; Schofield, K.; Stephenson, O. o-Amino-ketones of the acetophenone and benzophenone type. J. Chem. Soc. 1945, 646–657. [Google Scholar] [CrossRef]
- House, H.O. Modern Synthetic Reactions, 2nd ed.; W. A. Benjamin: Menlo Park, CA, USA, 1972; p. 211. [Google Scholar]
- Sridharan, V.; Ribelles, P.; Ramos, M.T.; Menéndez, J.C. Cerium(IV) ammonium nitrate is an excellent, general catalyst for the Friedländer and Friedländer−Borsche quinoline syntheses: Very efficient access to the antitumor alkaloid luotonin A. J. Org. Chem. 2009, 74, 5715–5718. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yang, W.; Yan, T.; Cai, M. An efficient heterogeneous gold(I) catalyzed intermolecular cycloaddition of 2-aminoarylcarbonyls and internal alkynes leading to polyfunctionalized quinolines. Synth. Commun. 2019, 49, 799–813. [Google Scholar] [CrossRef]
- Bunce, R.A.; Nago, T.; Sonobe, N. (±)-2-Alkyl-1,2,3,4-tetrahydroquinoline-3-carboxylic esters by catalyst and pressure dependent reductive cyclizations. J. Heterocycl. Chem. 2007, 44, 1059–1064. [Google Scholar] [CrossRef]
- Mukai, C.; Kobayashi, M.; Kubota, S.; Takahashi, Y.; Kitagaki, S. Construction of azacycles based on endo-mode cyclization of allenes. J. Org. Chem. 2004, 69, 2128–2136. [Google Scholar] [CrossRef]
- Moon, M.P.; Komin, A.P.; Wolfe, J.F.; Morris, G.F. Photostimulated reactions of 2-bromopyridine and 2-chloroquinoline with nitrile-stabilized carbanions and certain other nucleophiles. J. Org. Chem. 1983, 48, 2392–2399. [Google Scholar] [CrossRef]
- Fomum, Z.T.; Nkengfack, A.E.; Landor, S.R.; Landor, P.D. Allenes. Part 46. Synthesis of 1,2-dihydro-4H-3,1-benzoxazines, 4H-3,1-benzoxazines, and 3-cyanoquinolines from allenic and acetylenic nitriles. J. Chem. Soc. Perkin Trans. 1988, 277–281. [Google Scholar] [CrossRef]
- Armesto, D.; Gallego, M.G.; Horspool, W.M. Photochemical synthesis of quinoline derivatives by cyclization of 4-aryl-N-benzoyloxy-2,3-diphenyl-1-azabuta-1,3-dienes. J. Chem. Soc. Perkin Trans. 1989, 1, 1623–1626. [Google Scholar] [CrossRef]
- Boyer, F.; Decombe, J. Synthesis of quinoline bases. Bull. de la Société Chim. de Fr. 1967, 2373–2376. [Google Scholar]
- Troger, J.; Cohaus, C. Quinoline syntheses carried out with 6-amino-3-methoxybenzaldehyde and a condensation product resulting from this aldehyde. J. Prakt. Chem. 1927, 117, 97–116. [Google Scholar]
- Jia, C.-S.; Dong, Y.-W.; Tu, S.J.; Wang, G.W. Microwave-assisted solvent-free synthesis of substituted 2-quinolones. Tetrahedron 2007, 63, 892–897. [Google Scholar] [CrossRef]
- Ryabukhin, S.V.; Volochnyuk, D.M.; Plaskon, A.S.; Naumchik, V.S.; Tolmachev, A.A. Chlorotrimethylsilane-mediated Friedländer Synthesis of polysubstituted quinolines. Synthesis 2007, 1214–1224. [Google Scholar] [CrossRef]
- Bose, D.S.; Indrees, M.; Jakka, N.M.; Rao, J.V. Diversity-oriented synthesis of quinolines via Friedländer annulation reaction under mild catalytic conditions. J. Comb. Chem. 2010, 12, 100–110. [Google Scholar] [CrossRef]
- Shaabani, A.; Soleimani, E.; Mofakham, H. Microwave-assisted synthesis of the quinolin-2(1H)-one derivatives. Lett. Org. Chem. 2007, 4, 515–518. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hou, R.-S.; Cheng, H.-T.; Chen, L.-C. An efficient protocol for the Friedländer synthesis of quinolines using the Lewis acidic ionic liquid choline chloride·2ZnCl2. Heterocycles 2009, 78, 487–493. [Google Scholar] [CrossRef]
- Fehnel, E.A. Friedländer syntheses with o-aminoaryl ketones. I. Acid-catalyzed condensations of o-amino-benzophenone with ketones. J. Org. Chem. 1966, 31, 2899–2902. [Google Scholar] [CrossRef]
| Expt No | Nitro Cpd | Ketone | Product | Yield (%) |
|---|---|---|---|---|
| 3.3.1 | 1a | 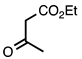 |  | 98 |
| 3.3.2 | 1a | 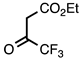 |  | 90 |
| 3.3.3 | 1a |  | 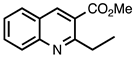 | 87 |
| 3.3.4 | 1a | 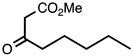 | 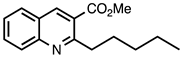 | 82 |
| 3.3.5 | 1a | 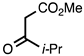 |  | 99 |
| 3.3.6 | 1a | 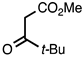 | 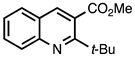 | 90 |
| 3.3.7 | 1a | 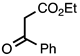 | 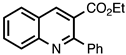 | 99 |
| 3.3.8 | 1a | 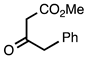 | 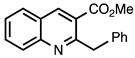 | 99 |
| 3.3.9 | 1a | 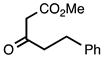 | 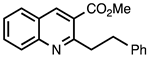 | 93 |
| 3.3.10 | 1a | 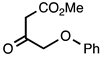 | 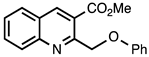 | 96 |
| 3.3.11 | 1a | 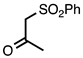 | 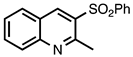 | 99 |
| 3.3.12 | 1a | 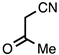 |  | 68 |
| 3.3.13 | 1a |  | 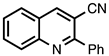 | 70 |
| 3.3.14 | 1a |  | 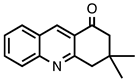 | 99 |
| 3.3.15 | 1a | 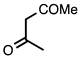 | 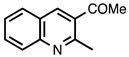 | 79 |
| 3.3.16 | 1a | 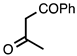 |  | 86 |
| 3.3.17 | 1a | 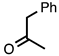 | 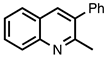 | 79 |
| 3.3.18 | 1a |  |  | 61 |
| 3.3.19 | 1a | 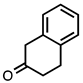 |  | 73 |
| Expt No | Nitro Cpd | Ketone | Product | Yield (%) |
|---|---|---|---|---|
| 3.4.1 | 1b | 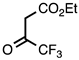 | 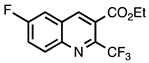 | 70 |
| 3.4.2 | 1b |  | 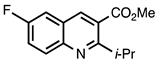 | 67 |
| 3.4.3 | 1b |  | 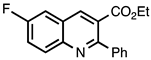 | 82 |
| 3.4.4 | 1b |  | 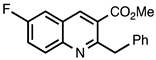 | 85 |
| 3.4.5 | 1b | 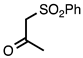 | 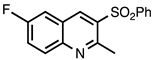 | 80 |
| 3.4.6 | 1b | 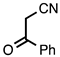 | 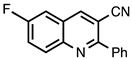 | 68 |
| 3.4.7 | 1b |  | 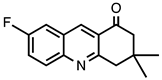 | 80 |
| Expt No | Nitro Cpd | Ketone | Product | Yield (%) |
|---|---|---|---|---|
| 3.5.1 | 1c |  |  | 65 |
| 3.5.2 | 1c |  | 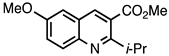 | 68 |
| 3.5.3 | 1c | 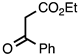 | 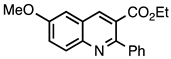 | 80 |
| 3.5.4 | 1c |  | 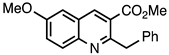 | 80 |
| 3.5.5 | 1c |  |  | 82 |
| 3.5.6 | 1c | 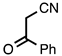 |  | 74 |
| 3.5.7 | 1c | 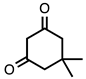 | 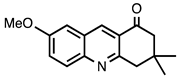 | 75 |
| Expt No | Nitro Cpd | Ketone | Product | Yield (%) |
|---|---|---|---|---|
| 3.6.1 | 1d | 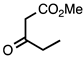 |  | 83 |
| 3.6.2 | 1d | 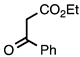 | 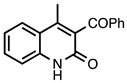 | 93 |
| 3.6.3 | 1d | 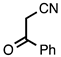 | 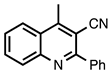 | 68 |
| 3.6.4 | 1d | 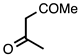 | 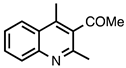 | 63 |
| 3.6.5 | 1d |  | 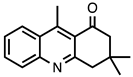 | 79 |
| 3.6.6 | 1d | 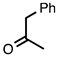 | 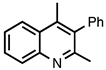 | 57 |
| 3.6.7 | 1d | 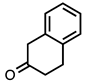 |  | 68 |
| Expt No | Nitro Cpd | Ketone | Product | Yield (%) |
|---|---|---|---|---|
| 3.7.1 | 1e | 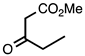 | 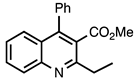 | 78 |
| 3.7.2 | 1e | 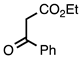 | 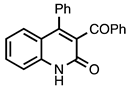 | 85 |
| 3.7.3 | 1e | 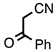 | 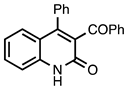 | 82 |
| 3.7.4 | 1e | 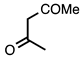 | 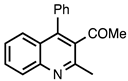 | 88 |
| 3.7.5 | 1e |  | 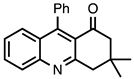 | 85 |
| 3.7.6 | 1e | 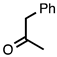 | 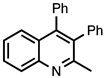 | 85 |
| 3.7.7 | 1e | 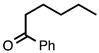 |  | 58 |
| 3.7.8 | 1e |  | 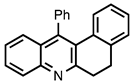 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fobi, K.; Bunce, R.A. Domino Nitro Reduction-Friedländer Heterocyclization for the Preparation of Quinolines. Molecules 2022, 27, 4123. https://doi.org/10.3390/molecules27134123
Fobi K, Bunce RA. Domino Nitro Reduction-Friedländer Heterocyclization for the Preparation of Quinolines. Molecules. 2022; 27(13):4123. https://doi.org/10.3390/molecules27134123
Chicago/Turabian StyleFobi, Kwabena, and Richard A. Bunce. 2022. "Domino Nitro Reduction-Friedländer Heterocyclization for the Preparation of Quinolines" Molecules 27, no. 13: 4123. https://doi.org/10.3390/molecules27134123
APA StyleFobi, K., & Bunce, R. A. (2022). Domino Nitro Reduction-Friedländer Heterocyclization for the Preparation of Quinolines. Molecules, 27(13), 4123. https://doi.org/10.3390/molecules27134123