Abstract
Background: Heterocyclic compounds and their fused analogs, which contain pharmacophore fragments such as pyridine, thiophene and pyrimidine rings, are of great interest due to their broad spectrum of biological activity. Chemical compounds containing two or more pharmacophore groups due to additional interactions with active receptor centers usually enhance biological activity and can even lead to a new type of activity. The search for new effective neurotropic drugs in the series of derivatives of heterocycles containing pharmacophore groups in organic, bioorganic and medical chemistry is a serious problem. Methods: Modern methodology of drugs involves synthesis, physicochemical study, molecular modeling and selection of active compounds through virtual screening and experimental evaluation of the biological activity of new chimeric compounds with pharmacophore fragments. For the synthesis of new compounds, classical organic methods were used and developed. For the evaluation of neurotropic activity of new synthesized compounds, some biological methods were used according to indicators characterizing anticonvulsant, sedative and antianxiety activity as well as side effects. For docking analysis, various soft ware packages and methods were used. Results: As a result of multistep reactions, 11 new, tri- and tetracyclic heterocyclic systems were obtained. The studied compounds exhibit protection against pentylenetetrazole (PTZ) seizures as well as some psychotropic effects. The biological assays evidenced that nine of the eleven studied compounds showed a high anticonvulsant activity by antagonism with pentylenetetrazole. The toxicity of the compounds is low, and they do not induce muscle relaxation in the studied doses. According to the study of psychotropic activity, it was found that the selected compounds have an activating behavior and anxiolytic effects on the “open field” and “elevated plus maze” (EPM) models. The data obtained indicate the anxiolytic (antianxiety) activity of the derivatives of tricyclic thieno[2,3-b]pyridines and tetracyclic pyridothieno[3,2-d]pyrimidin-8-ones, especially pronounced in compounds 3b–f and 4e. The studied compounds increase the latent time of first immobilization on the “forced swimming” (FS) model and exhibit antidepressant effects; compounds 3e and 3f especially exhibit these effects, similarly to diazepam. Docking studies revealed that compounds 3c and 4b bound tightly in the active site of γ-aminobutyric acid type A (GABAA) receptors with a value of the scoring function that estimates free energy of binding (∆G) at −10.0 ± 5 kcal/mol. Compound 4e showed the best affinity ((∆G) at −11.0 ± 0.54 kcal/mol) and seems to be an inhibitor of serotonin (SERT) transporter. Compounds 3c–f and 4e practically bound with the groove of T4L of 5HT_1A and blocked it completely, while the best affinity observed was in compound 3f ((∆G) at −9.3 ± 0.46 kcal/mol). Conclusions: The selected compounds have an anticonvulsant, activating behavior and anxiolytic effects and at the same time exhibit antidepressant effects.
1. Introduction
In recent years, there has been an increase in the level of natural and social disasters in the world, which has affected people’s health. Patients of the COVID-19 pandemic have respiratory problems, severe pneumonia and neuropsychiatric disorders. At the same time, the number of malignant neoplasms and infectious diseases is increasing year by year. Pharmacological practice now uses drugs, particularly psychotropics, which are not without flaws; these drugs are endowed with toxicity and have an adverse effect on the body. Synthesis of pharmaceuticals for the treatment of neuropsychiatric disorders, in particular epilepsy, is a serious challenge for synthetic organic chemistry. The antiepileptic agents most commonly used in medicine often cause toxic side responses from different organs and systems, emotional disturbances, impaired memory, etc. In this regard, the search for and study of anticonvulsants possessing combined psychotropic properties are of unquestionable interest. In experimental psychopharmacology, while searching for new neurotrophic compounds, it is important and relevant to model both the pathology itself and its individual manifestations in animals. Such an approach of differentiated (application of interoceptive stimuli, such as corazole) and integrative (for example, “open field”) modelling, biostatistical evaluation of the spectrum of pharmacological action of substances and comparison of the major and side effects make it possible to carry out a more detailed selection of promising pharmaceutical agents among newly synthesized compounds.
Currently, molecular modeling methods and, in particular, molecular docking are intensively used in modern pharmaceutical chemistry for the study and primary assessment of the bioactivity of newly synthesized and/or modified compounds [1]. Moreover, one of the promising strategies in creating new pharmaceuticals is the design and synthesis of hybrid compounds consisting of two or more different bioactive fragments acting through the activation of several mechanisms of action [2].Docking analysis is basically used for the conformational search for the best and most reliable orientation of ligand during complexation of the ligand target [3]. To achieve the maximal result (approximate to real conditions), pair docking is usually used for predicting the interaction of the ligand with the target.
The derivatives of condensed pyridines are of interest as biologically active substances [4]. Thus, a large number of substituted pyrazolopyridine derivatives have been found to possess various biological properties such as A(1) adenosine receptor antagonism [5], antimicrobial properties [6], nonanionic antiplatelet agents [7] and psychotropic effects [8]. Some alkaloids of the pyrano[3,4-c]pyridine series exert universal effects: hypotensive, anticonvulsant, antipsychotic, anti-inflammatory and antitumor effects [9,10,11]. The derivatives of fused pyrimidines have also attracted a considerable interest in medicinal chemistry research due to their versatility and a broad bioactive potential [12]. Thieno[3,2-d]pyrimidines are structural analogues of purines. Purines, as endogenous scaffolds, play an important biochemical role in a variety of regular physiological functions. As bioisosteres to purines, thieno[3,2-d]pyrimidines were also found to exhibit numerous biological activities, probably due to their interaction with various physiological factors [13,14]. On the other hand, tetracyclic fused systems containing pyran, pyridine, thiophene and pyrimidine rings can be considered analogues of heterosteroids, which are known to display varied biological effects [15,16,17]. Moreover, literature data evidenced that bi-, tri-, tetra- and pentacyclic systems containing pyridine and pyrimidine rings were endowed with neurotropic properties [18,19,20], and the addition of a cycle does not reduce biological activity.
In a previous paper [21], we described the synthesis as well as the neurotropic activity of a series of derivatives of pyrazol-1-yl-substituted pyrano[3,4-c]pyridine. The results of these previous studies have enabled us to identify several compounds that exhibited potent and wide-spectrum anticonvulsant properties in maximal electroshock seizure (MES) and subcutaneous pentylentetrazole (PTZ) seizure tests. Furthermore, the compounds were studied for their anxiolytic and antidepressant activities in some psychotropic models, such as “open field”, ″elevated plus-maze″ (EPM) and ″forced swimming″ (FS). Moreover, our studies have shown that three derivatives among the pyrazol-1-yl-substituted pyrano[3,4-c]pyridines 1 and 2 exhibited anticonvulsant, anxiolytic, some antidepressant and sedative activities similar to the commercial drug diazepam; this also indicates activity (Figure 1).
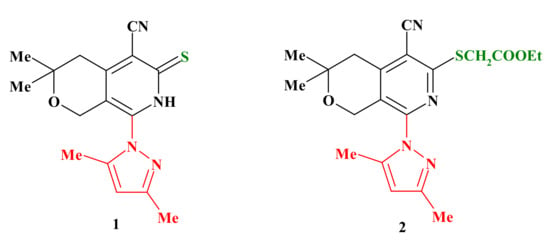
Figure 1.
The structure of starting pyrazol-1-yl-substituted thioxopyrano[3,4-c]pyridine 1 and ethyl thioacetatepyrano[3,4-c]pyridine 2.
Based on above considerations we now synthesized new compounds with the aim of evaluating neurotropic activity and probable mechanisms of action and to study the influence of the structure of compounds on biological activity. Continuing our studies in the field, in this paper, we present the synthesis of new pyrazol-1-yl-substituted tricyclic and tetracyclic heterocyclic systems—N-alkyl(aryl) derivatives of pyrano[4,3-d]thieno[2,3-b]pyridine and pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d] pyrimidin-8(9H)-one. We also study the neurotropic properties of the synthesized compounds and perform molecular docking to evaluate probable mechanisms of action.
2. Results and Discussion
2.1. Chemistry
For the synthesis of the target compounds, we used as starting materials 8-(3,5-dimethyl-1H-pyrazol-1-yl)-3,3-dimethyl-6-thioxo-3,4,6,7-tetrahydro-1H-pyrano[3,4-c]pyridine-5-carbonitrile (1) and ethyl {[5-cyano-8-(3,5-dimethyl-1H-pyrazol-1-yl)-3,3-di methyl-3,4-dihydro-1H-pyrano[3,4-c]pyridin-6-yl]thio}acetate (2) (Figure 1).
Both starting materials 1 and 2 are versatile substrates: in fact, both of them are decorated on nearby carbon atoms of the pyridine ring by two functional groups (nitrile and thioxo-/thioalkyl) able to open the way to compounds with a new fused ring (a thiophene ring), which, in turn, will still contain other reactive groups useful for further chemical transformation.
Additionally, 8-(3,5-Dimethyl-1H-pyrazol-1-yl)-3,3-dimethyl-6-thioxo-3,4,6,7-tetra hydro-1H-pyrano[3,4-c]pyridine-5-carbonitrile (1) and ethyl {[5-cyano-8-(3,5-dimethyl-1H-pyrazol-1-yl)-3,3-dimethyl-3,4-dihydro-1H-pyrano[3,4-c]pyridin-6-yl]thio}acetate(2)were used as key intermediates for the synthesis of a new tricyclic condensed system—N-alkyl(aryl) derivatives of pyrano[4,3-d]thieno[2,3-b]pyridines 3a-f (yield 78−98%). The physicochemical characterizations of compounds 1 and 2 [21] were already reported.
Compound 2 was subjected to intramolecular cyclization by the action of sodium ethoxide to obtainsubstituted pyrano[4,3-d]thieno[2,3-b]pyridine 3a (method A). The latest was also synthesized in one pot from pyridinethione1 and ethyl chloroacetate in the presence of sodium ethoxide (method B). In the case of alkylation of compound 1 with the chloroacetic acid derivatives which contain an active methylene group, the closure of the thiophenic ring proceeds simultaneously with the alkylation. As a result of one-pot reaction, new tricyclic condensed system compounds 3a–f were synthesized with good yields (Scheme 1, Table 1).
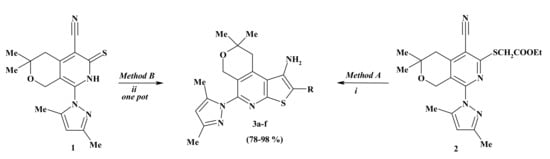
Scheme 1.
Reagents and conditions: i EtONa, stirred 2h, 60 °C (Method A); ii EtONa, AlkHal, stirred 2 h, 60 °C (Method B).

Table 1.
1-Amino-N-alkyl(aryl)-5-pyrazol-1-yl-pyrano[4,3-d]thieno[2,3-b]pyridines 3.
The structures of newly synthesized compounds 3a–f were confirmed by NMR, IR spectroscopy and by elemental analysis. Thus, in the 1H-NMR spectra of these new compounds, the presence of the NH2 group proton at 6.25−7.00 ppm was observed (see Supplementary Materials). The IR spectra of 3a–f show the amino group (NH, NH2) absorptions near 3238−3504 cm−1.
The newly synthesized 1-amino-N-alkyl(aryl)-5-pyrazol-1-yl-pyrano[4,3-d] thieno[2,3-b]pyridines 3a,c–f still contain amino and carboxy functional groups which can undergo new cyclization reactions. Thus, by interaction of compound 3a with formamide pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8-one, 4a was synthesized. By condensation of compounds 3c–f with triethylorthoformate in the presence of acetic anhydride, new derivatives of pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d] pyrimidin-8-ones 4b–e were synthesized with high yields (Scheme 2, Table 2).
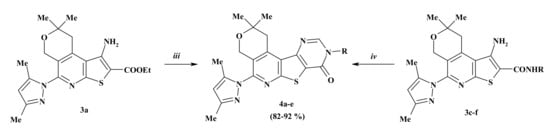
Scheme 2.
Reagents and conditions: iii HCONH2, refluxed 4 h; iv HC(OEt)3, (MeCO)2O, refluxed 2 h.

Table 2.
Pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8-ones 4.
The structures of newly synthesized pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5] thieno[3,2-d] pyrimidin-8-ones 4a–e were supported by NMR and IR spectroscopy. Thus, in the 1H-NMR spectra, the signals of the NH2 group characteristic of the initial compounds 3 were absent, while the pyrimidine ring CH group signals appeared, indicating the cyclization of compounds 4. The structure of compounds 4a–e was also supported by 13C-NMR data. In 13C-NMR spectra, the signals of CH and C=O groups of the pyrimidine cycle are observed at 146.4–149.0 and 157.5–161.2 ppm, respectively (see Supplementary Materials). The IR spectra of compounds 4a–e did not show the characteristic bands of the amino group but showed bands in the range of ν 1668–1675 cm−1, typical for the carbonyl group.
2.2. Biological Assays
The neurotropic activity of 11 newly synthesized heterocyclic compounds—5-pyrazol-1-yl substituted pyrano[4,3-d]thieno[2,3-b]pyridines 3a–f and pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8-ones 4a–e—was carried out according to indicators characterizing anticonvulsant, sedative and antianxiety activity and side effects.
The anticonvulsive action of the tested compounds was assessed by evaluating the antagonism between the convulsive pentylenetetrazole (PTZ) action and maximal electroshock seizures (MES) [22,23,24,25,26]. PTZ-induced testing is considered an experimental model for the clonic component of epilepsy seizures and prognostic anxiolytic [27] activities of the compounds. The MES test is used as an animal model for the generalized tonic seizures of epilepsy. Ethosuximide was used as a control [28]. The side effects of the compounds—neurotoxicity (movement coordination disorder, myorelaxation and ataxia) with the test of ″rotating rod″ [22,29] and maximal tolerated dose (MTD)—were also studied on mice. To determine the 50% effective and 50% neurotoxic doses, a statistical method of probit analysis by Litchfield and Wilcoxon was used [30,31]. From a practical point of view, the active compound’s protective index (PI) was identified (Protective Index = Toxic Dose of 50% (TD50)/Effective Dose of 50% (ED50)).
Evaluation of the anticonvulsant activity of all the synthesized compounds revealed that they, to varying degrees, exhibit PTZ antagonism. Thus, the compounds, at a dose of 50 mg/kg, prevented PTZ clonic seizures in 40–80% of animals. However, compounds 3b–f and 4b–e had a pronounced anticonvulsant action. Intraperitoneal injections of these compounds into mice, starting with a dose of 20 mg/kg, were accompanied by the prevention of PTZ seizures, and the ED50 ranged from 24 mg/kg to 44 mg/kg (Table 3). It should be mentioned that tested compounds are more active than ethosuximideaccording to the test on PTZ, but less so than diazepam. The effective dose of ethosuximide (ED50, mg/kg) in the antagonism with PTZ in mice was 155.0 mg/kg, while for diazepam it was 0.5 mg/kg. The compounds, tested by the ″rotating rod″ method, in doses of 50–100 mg/kg in mice did not violate the coordination of movements; no signs of muscle relaxation were observed. TD50 of the studied compounds ranged from 505 mg/kg to 600 mg/kg. Ethosuximide in the studied doses of 100–200 mg/kg in mice also does not cause muscle relaxation.

Table 3.
Anticonvulsant activity by pentylentetrazol antagonism, myorelaxation and maximal tolerated doses (MTD) of the examined all compounds (intraperitoneal injection).
The structure–activity relationship study revealed that the presence of 3,4-dichlorophenyl substituent on the second position of thiophene ring of tricyclic pyranothienopiridine moiety (3e) is beneficial for anticonvulsant activity. The replacement of 3,4-dichlorophenyl substituent by carbonitrile (3b), phenyl (3c), 2,4-dimethoxyphenyl (3d) or phenylethyl (3f) groups slightly decreased the activity, while replacement of the 3,4-dichlorophenyl group with an ethyl carboxylate group (3a) decreased the activity much more. Similar activity is observed in the case of tetracyclic thienopyrimidines.
According to the maximal tolerated doses (MES) test, the compounds studied, as well as reference drugs, did not exhibit an anticonvulsant effect. They did not protect from tonic and clonic seizures caused by MES. Maximal tolerated doses of studied compounds and ethosuximide are within the limits of 1000−1500 mg/kg. Protective indexes of the selected compounds were large, especially for compound 3e, and far exceed indexes of ethosuximide and diazepam.
The most effective 9 compounds—3b–f and 4b–e—were studied on the ″open field″, ″elevated plus maze″ (EPM) and ″forced swimming″ tests at a dose of 50 mg/kg since the ED50s of these compounds are within 50 mg/kg at the confidence intervals.
In the ″open field″ behavioral model, [32,33,34] in rats of the control group, the number of horizontal displacements, vertical displacements and examined cells were 25.8, 6.1 and 0.5, respectively (Table 4).

Table 4.
Effect of compounds (3b, 3c, 3d, 3e, 3f, 4b, 4c, 4d and 4e) in rats in the ″open field″ test.
The compounds under study cause changes in the behavioral indices in comparison with the control—with the injection of the compounds, marked changes in the horizontal and vertical movements of animals were observed. Compounds 3c–f and 4b–e statistically significantly increase the horizontal movements of animals; others do not result in any behavior changes associated with horizontal movements. As for vertical movements, compounds 3b and 3e statistically significantly reduce them, exhibiting some sedative effect. In contrast, compound 3d increases vertical movements and thereby leads to activation of behavior. However, all selected compounds statistically significantly, compared with the control, increase the number of sniffing cell examinations, which may be due to the manifestation of the antianxiety activity of the compounds (Table 4), which was especially pronounced in compounds 3b, 3d, 3e, 3f and 4e.Ethosuximide at the effective dose of 200 mg/kg has no effect on all indicators of research activities, while diazepam (2 mg/kg), in comparison with the control group of mice, causes a significant increase in the number of cells examined, i.e., pronounced antianxiety effect. The studied compounds 3c, 3d, 3f, 4b–e and diazepam, by increasing horizontal movements, exhibit an activating effect on behavior.
In order to assess fears, the methodology of ″elevated plus maze″ (EPM) developed by Pellow was used [35]. The ″elevated plus maze″ is a behavioral assay (fear) used to estimate the antianxiety effects of pharmacological agents, synthetic compounds, etc. [36,37,38]. In brief, rats or mice are placed at the junction of the four arms of a maze with their face to an open arm, followed by the recording of their entries/duration in each arm by a video tracking system and observer simultaneously for 5 min. On the EPM model, control animals are predominantly in closed sleeves (Table 5).

Table 5.
Effect of compounds (3b, 3c, 3d, 3e, 3f, 4b, 4c, 4d and 4e), on the state of ″fear and despair″ of mice in the EPM model (observation time 5 min).
After administration, compounds 3b–f and 4b–e statistically reliably decrease the time spent in closed arms, and after intraperitoneal injection of compounds 3d, 3f and 4d, a decrease in the number of entries into the closed arms was observed. All selected compounds were statistically significant and, compared to the control, increased the time spent by experienced animals in the center, which indicates sedative activity, especially in compounds 3e, 3f and 4c. After injection of the compounds, experimental animals went to the open arms and were located there for 3 (4c), 9.8 (3e), 10 (4d) and 11.4 (3d) s in contrast to the control.
The time spent in the open arms by mice after administration of compound 3d was 11.4 s; at the same time, control mice which received ethosuximide at a dose of 200 mg/kg did not enter the open arms due to fear. Animals that received diazepam at a dose of 2 mg/kg also entered the open arms and stayed there for 57 s. The data obtained indicate anxiolytic activity in all of the selected compounds, especially expressed in compounds 3e, 3d, 3f and 4d.
The structure–activity relationships study revealed that the presence of phenyl (3c), 2,4-dimethoxyphenyl (3d) and phenethyl (3f) substituent on the second position of the thiophene ring of tricyclic pyranothienopiridine moiety and all tetracyclic pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8-ones (4b-e) except compound 4a increased the horizontal movements of animals, exhibiting an activating effect. Tricyclic thienopyridines containing nitrile (3b) or 3,4-dichlorophenyl (3e) substituents on the second position of the thiophene ring decreased the vertical movements of animals, exhibiting some sedative effect. The compound (3d) 2,4-dimethoxyphenyl substituent is beneficial for its activating effects of both horizontal and vertical movements.
The ″forced swimming″ test (FS) is one of the most commonly used assays [39]. The FST is used to monitor depressive-like behavior and is based on the assumption that immobility reflects a measure of behavioral despair.
On the ″forced swimming″ model in control mice, the first immobilization occurs after 92 s (Table 6). Some selected compounds (3e, 3f) tested at a dose of 50 mg/kg statistically significantly increased the duration of the latent period of the first immobilization and decreased the total immobilization duration.

Table 6.
Effect of compounds 3b, 3c, 3d, 3e, 3f, 4b, 4c, 4d and 4e on the ″forced swimming″ (observation time 6 min).
For compounds 3e and 3f, the duration of the latent period of the first immobilization increased to 172 and 121 s, respectively, while the total time of immobilization decreased to 48 and 20 s, respectively. This suggests that compounds 3e and 3f studied at a dose of 50 mg/kg show some antidepressant effect in the same manner as diazepam. These compounds and diazepam increase the total time of active swimming, but not statistically significantly. The remaining compounds decrease the total time of active swimming. The data obtained with the use of ethosuximide at a dose of 200 mg/kg coincide with the control data.
According to the structure–activity relationship study, the presence of 3,4-dichlorophenyl (3e) or phenylethyl (3f) substituent on the second position of the thiophene ring of tricyclic thienopyridine moiety as well as 3,4-dichlorophenyl (4d) substituent on the ninth position of the pyrimidine ring of tetracyclic pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidinone moiety is favorable for antidepressant activity.
2.3. Molecular Docking
Typically, antiepileptic drugs block sodium channels or enhance γ-aminobutyric acid type A (GABA) function by general targeting of GABAA receptors and, therefore, new compounds docked with the GABAA receptor. The serotonin transporter (SERT or 5HT_1A) is implicated in a number of neurobehavioral disorders (e.g., depression, anxiety, autism). The results of in vivo investigation showed that some of the newly synthesized compounds were endowed with antidepression and antianxiety properties and therefore docked with the SERT or 5HT_1A receptors.
2.3.1. Docking and Conformational Analysis of GABAA Receptor Complexation
Docking analysis of the studied compounds revealed that of 11 compounds, the interaction of seven (3a, 3c, 3d, 3e, 4b, 4c and 4d) with the GABAA receptor was observed. To compare the effects of the test compounds with GABAA, diazepam was used as a control. The results of docking analysis indicate that diazepam interacts by hydrophobic and electrostatic forces with GABAA in subsite 1 of the ECD (extracellular domain) interface with an energy of −7.5 kcal/mol (Table 7).

Table 7.
Biophysical calculation of complexations of compounds (3a, 3c–e and 4b–d) and diazepam with GABAA.
Compounds 3b, 3f, 4a and 4e do not bind to this receptor. Complexation is observed both in the active center of the protein and in allosteric sites, which are evidenced by the obtained spatial interaction parameters. Energy values of complexation were calculated for seven compounds. The lowest binding energy was found for compounds 3c and 4b, followed by 3d, but compound 3a had the highest binding energy (Table 7). The mean square deviation during the complexation did not exceed RMSD ≤ 2 Å. Obtaining the spatial parameters of complexation is evidence that all the investigated compounds bind to the ligand binding domain in two basic sites of the protein. A conformational map of the binding of diazepam to GABAA indicates that the amino acid residues Phe200, Tyr205, Ala201, Tyr 97, Glu155 and Tyr202 in chain C and Asp 43, Tyr176, Tyr66, Asn41, Gln64 and Tyr 62 in chain B are involved in the complexation process (Figure 2).
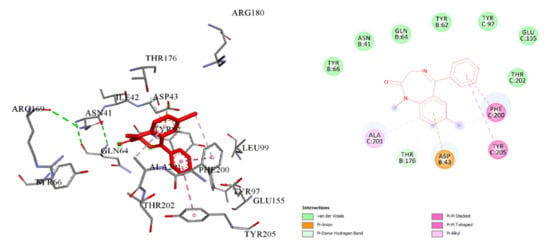
Figure 2.
Visualization of the interaction of diazepam with GABAA spatial orientation and conformational map of complexation.
The first is the benzamidine site of subsite 1 of the ECD interface [40]. This loop is formed due to amino acid residues included in chains B and C, as well as in E and D [41]. Compounds 3a, 3c and 3e are binding at this site. A study of the interaction of 3a with GABAA revealed that complexation is due to hydrophobic interactions and hydrogen bonds (Figure 3). Hydrogen bonds are observed with the amino acid residues in the chain C by Tyr 202 and Tyr97 and in the B chain by Tyr 62. The length of the observed hydrogen bonds does not exceed 3.0 Å.
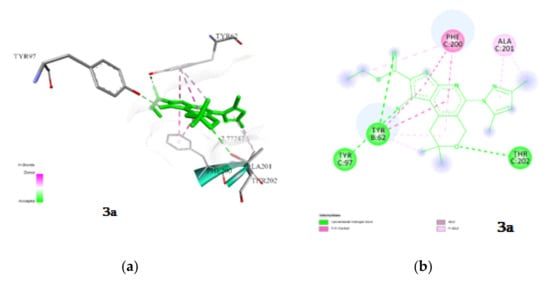
Figure 3.
Visualization of the interaction of 3a with GABAA; (a) spatial orientation and character of the interaction; (b) conformational map of complexation. Green lines are hydrogen bonds, violet−hydrophobic interactions.
In the case of 3c, the hydrogen bonds were not detected at the interaction. The interaction is carried out at the expense of electrostatic and hydrophobic interactions (Figure 4).
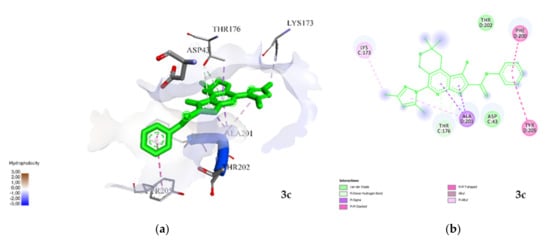
Figure 4.
Visualization of the interaction of 3c with GABAA; (a) spatial orientation and character of the interaction; (b) conformational map of complexation. Green lines are hydrogen bonds, violet−hydrophobic interactions.
Compound 3e binds in chain B and C through both hydrogen and electrostatic interactions; hydrophobic interactions are also present. From the point of view of spatial arrangement, it practically repeats the position of 3a, while hydrogen bonds are formed with Tyr97 in chain C and Tyr 62 in chain B, not exceeding a value of 3.3 Å. The values of the binding constants can be explained by the difference in electrostatic forces and the amount of amino acid residue in favor of 3e (Figure 5).
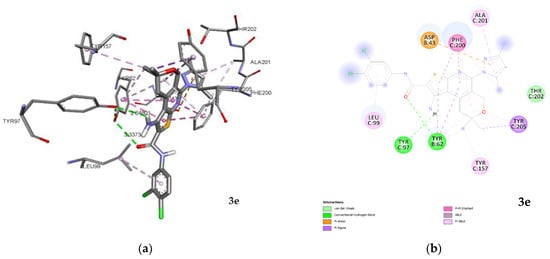
Figure 5.
Visualization of the interaction of 3e with GABAA; (a) spatial orientation and character of the interaction; (b) conformational map of complexation. Green lines are hydrogen bonds, orange—electrostatic and violet−hydrophobic interactions.
The second site of the investigated compounds is subsite 3 in the ECD interface, which is specific for cations and responsible for inhibition. This site is usually formed between the α+/β subunit of chains with the involvement of amino acid residues in the positions of 137, 127 and 182 [42]. Complexation at this site is observed by four compounds (3d, 4b, 4c and 4d). The interaction of 3d is due to the formation of a hydrogen bond with Met 137 with a distance of 3.3 Å as well as electrostatic interaction. In other cases, there is a hydrophobic interaction (Figure 6).
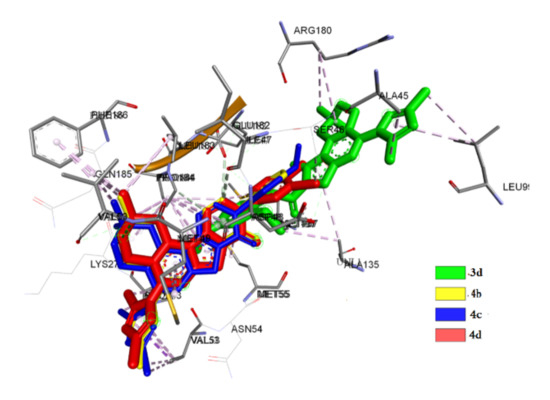
Figure 6.
Superposition of 3d, 4b, 4c and 4d in subsite 3 of GABAA.
The spatial orientation of 4b is different from position 3d, with a deviation of 5.8 Å, herewith there is observed a hydrogen bond with Val 50, linked by the binding site of the subsite 3. Compound 4b binds with Met137 from the key residues and exhibits electrostatic and hydrophobic interaction with Glu182. Despite the absence of hydrogen bonds with the key residues of subsite 3, the advantage of 4b is obvious compared to 3d in terms of spatial-energy characteristics.
Obtained spatial characteristics of 4c and 4d have shown that they practically repeated the position of 4b with a deviation of 0.8 Å and the same types of interaction. The difference is the absence of hydrogen bonds with the residue in subsite 3. The interaction is carried out by the electrostatic and hydrophobic interactions with identical amino acid residues. From this point of view, it can be predicted that compounds 4b, 4c and 4d will be identical; at the same time, the highest-binding constant values are exhibited by compounds 4b and 4c.
2.3.2. Docking and Conformational Analysis of SERT Complexation
A study of the interaction of the compounds with SERT (serotonin transporter) revealed that the eight compounds interact with SERT, with the exception of 2, 3a, 4a and 4c. The biophysical properties of interaction for eight compounds have been calculated and are presented in Table 8.

Table 8.
Biophysical calculation of complexations of compounds (2, 3c–f, 4b, 4d and 4e) with SERT.
Obtained spatial and conformation parameters of complexation are evidence that the studied compounds’ positions can be divided into two classes. The first class includes compounds for which binding occurs especially at the central site of the active center of SERT [43], such as 2, 3e and 4e. The second class is formed by compounds that bind predominantly in the allosteric site [44], such as 3c, 3d, 3f, 4b and 4d. Compounds that bind at the central site of SERT exhibit different energy indices and binding constants. Compound 4e possesses the highest values, with the involvement of key amino acid residues Ile172, Tyr176, Phe335, Tyr95, Phe341 and Val501 [45]. The spatial arrangement of 2 corresponds to the position of 4e with a deviation at a38° angle. At the same time, the amino acid residues involved in the process of complexation correspond to each other with a difference of just one residue. Compound 2, instead of Val501, has Tyr497, which is also included in the central site [46]. The conformational maps of complexation obtained indicate that interactions carried out by hydrogen bonds and electrostatic forces. Hydrophobic interactions are also involved in the complexation. In compound 2, the hydrogen bonds are formed with two residues, the Tyr497 and Glu 493, which do not reach the central binding site with distances of 3.3 and 3.0 Å, respectively. In 4e one hydrogen bond is visualized with Tyr175, which is not included in the active site. It should be noted that the interaction of 4e in the central site of the active center is predominantly carried out due to electrostatic and hydrophobic forces. The basically spatial position of 3e is different from the rest of other members of the group. The quantity of amino acid residues involved in the complexation is less than that of 2 and 4e. Compound 3e interacts with Ile172, Tyr176, Phe335 and Tyr497 and formed a hydrogen bond with Tyr497 with a distance of 3.0 Å. In other cases, hydrophobic interaction predominates. It should be noted that 3e also interacts hydrophobically with the amino acid residues Phe556 and Pro561 of the allosteric site [47]. Single hydrophobic interactions with Arg104 are also observed in 2 and 4e. The results of superposition of 2, 3e and 4e in the central site are presented in Figure 7.
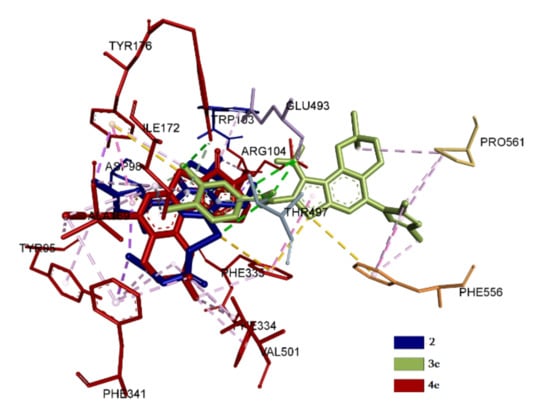
Figure 7.
Superposition of 2, 3e and 4e in the central site of the active center of SERT.
The spatial positions and binding types of compounds 3c, 3d, 3f, 4b and 4d in the allosteric site can be divided into three sub-classes. The first sub-class includes 3d and 4d; the second sub-class includes 3c and 3f; the third sub-class includes only 4b. The last is unique because it exhibits a directed action without affecting the central binding site of SERT (Figure 8).
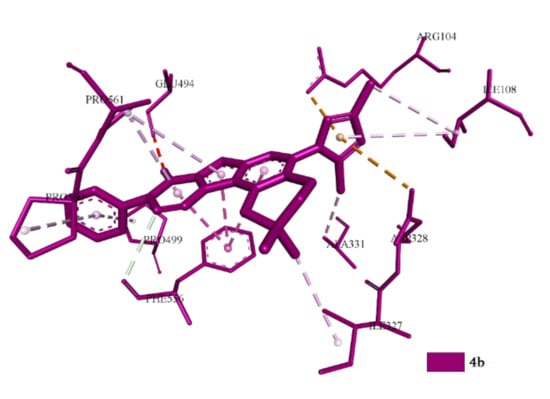
Figure 8.
Superstation of 4b in the central site of the active center of SERT.
In this case, predominantly hydrophobic interactions with practically all residues are observed, forming an allosteric binding site. Such residues include Arg104, Asp328, Ala331, Phe556, Pro561 and Glu494. The second sub-class compounds (3c, 3f), by the values of conformation, are identical and interactions are carried out due to hydrophobic and electrostatic interactions involving amino acid residues Arg104, Ala33, Glu494 and Phe556. In addition to the allosteric site, hydrophobic interactions with Phe335 are observed (Figure 9).
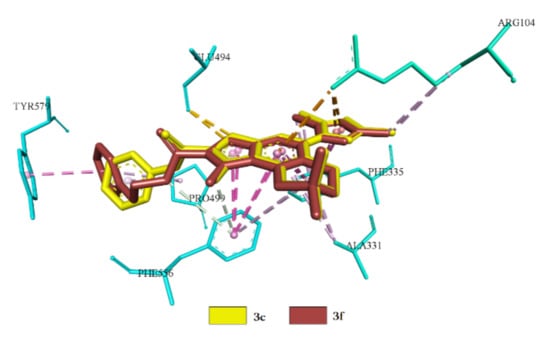
Figure 9.
Superpositions of 3c, 3f in the central site of the active center of SERT.
The positions in the allosteric site of the best conformers of the first subclass are located mirrored relative to each other. The difference of root-mean-square deviation (RMSD) is 0.5 Å with an angle of 36°. Amino acid residues Arg104, Asp328, Ala331 and Phe556 are included in the complexation for both representatives of this subclass. The interactions are carried out at the expense of hydrophobic interaction. Apart from that, hydrophobic interaction of Phe335 and Tyr176 with the central binding site is observed in 4d, and in 3d, it is observed forTyr176 and Ile172 (Figure 10).
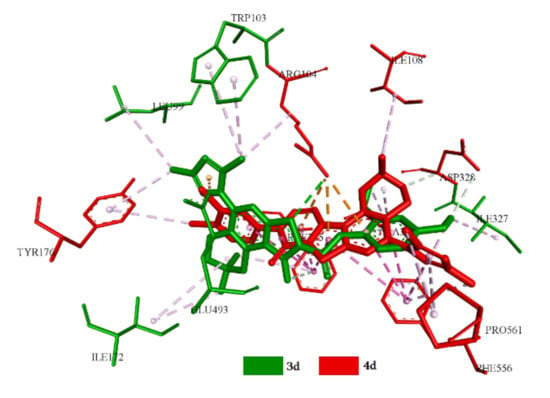
Figure 10.
Superstation position of 3d and 4d in the central site of the active center of SERT.
2.3.3. Docking and Conformational Analysis of 5HT_1A Complexation
The results of docking analysis for investigated compounds with 5HT_1A exhibited that interactions are observed for seven compounds (Table 9).

Table 9.
Biophysical calculation of complexations of compounds (3a, 3c–f, 4a and 4e) with 5HT_1A.
For compounds 3b, 4b, 4c and 4d no interaction was observed. All compounds except 3a and 4a show similar results of biophysical indicators of interaction. The obtained conformational maps indicate that the interaction is carried out in two clusters with the involvement of the ICL (intracellular loop) of TM5 and TM6 helices [48], where interaction is observed for 3a and 4a (Figure 11).
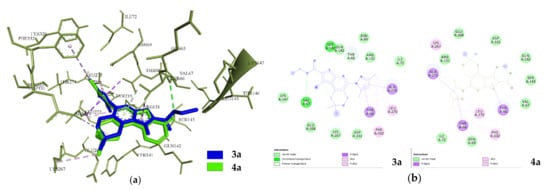
Figure 11.
Visualization of interaction of 3a and 4a with 5HT_1A; (a) superposition in the ICL interaction interface; (b) conformation maps of interaction.
The second is a cluster in the T4L (T4-lysozyme) domain [49], where compounds 3c, 3d, 3e, 3f and 4e bind. The spatial positions of 3a and 4a in the ICL are practically identical to RMSD 0.5 Å with an angle of 7.2°. The interaction of 3a and 4a is due to the van der Waals forces and hydrophobic interactions with the key residues of Arg131, Phe332, Glu268and Tyr66, although 3a has two hydrogen bonds with Ser143 and Val 67 (Figure 11).
For compounds included in the second cluster, the interactions in the T4L domain are carried out basically due to hydrophobic force with the involvement of amino acid residues Glu1011 and Leu1032. It should be noted that compound 3f forms ahydrogen bond with Glu1011 with a distance of 3.2 Å. All compounds also interact with Asp1020 due to hydrophobic forces, except for3e. In the same way, interactions are observed with Tyr1018, except for 3c. Compounds 3e and 3f form hydrophobic bonds with Arg1014 and Glu1022 (Figure 12).
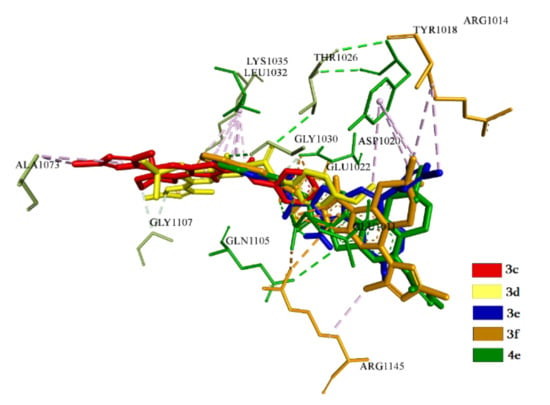
Figure 12.
Superposition of compounds (3c–f and 4e) in the groove of T4L domain of 5HT_1A.
The second cluster compounds practically bind with the groove of T4L and block it completely [50]. At the same time, they show similar results of energy parameters. The best compound, judging by the indicators, is 3f.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
All chemicals and solvents were of commercially high purity grade purchased from Sigma-Aldrich (Saint Louis, MO, USA). Melting points (m.p.) were determined on a Boetius microtable. They are expressed in degrees centigrade (°C). 1H NMR and 13C NMR spectra were recorded in DMSO-d6/CCl4, 1/3, v/v solution (300MHz for 1H and 75.462 MHz for 13C) on a Varian mercury spectrometer (Varian Inc., Palo Alto, CA, USA). Chemical shifts are reported as δ (parts per million) relative to TMS (tetramethylsilane) as the internal standard. IR spectra were recorded on Nicolet Avatar 330-FTIR spectrophotometer (Thermo Nicolet, Foster, CA, USA) and the reported wave numbers are given in cm−1. Elemental analyses were performed on a Euro EA 3000 Elemental Analyzer (EuroVector, Pavia, Italy).
3.1.2. Methods for the Synthesis of Ethyl 1-Amino-5-(3,5-dimethyl-1H-pyrazol-1-yl)-8,8-dimethyl-8,9-dihydro-6H-pyrano[4,3-d]thieno[2,3-b]pyridine-2-carboxylate (3a)
Method A: To a solution of sodium ethoxide prepared from sodium (0.046 g, 2 mmol) and ethanol (20 mL), compound 2 (0.8 g, 2.0 mmol) was added. The mixture was stirred for 2 h at 60 °C and cooled; the white precipitate was filtered off and washed with water, dried and recrystallized from EtOH. Yield 81%, m.p. 160–161 °C.IR ν/cm−1: 3504, 3370 (NH2), 1664 (C=O). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH: 1.29 (t, J = 7.1 Hz, 3H, OCH2CH3), 1.30 (s, 6H, C(CH3)2), 2.19 (s, 3H, CH3), 2.27 (s, 3H, CH3), 3.32 (s, 2H, 9-CH2), 4.29 (q, J = 7.1 Hz, 2H, OCH2), 4.67 (s, 2H, 6-CH2), 6.11 (s, 1H, CH), 6.87 (s, 2H, NH2). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 11.8, 13.3, 14.3, 26.3, 36.4, 59.4, 60.2, 69.1, 107.2, 121.1, 123.1, 141.1, 144.3, 148.2, 148.7, 149.5, 156.4, 164.5. Anal. calcd for C20H24N4O3S: C 59.98; H 6.04; N 13.99; S 8.01%. Found: C 60.13; H 5.98; N 14.13; S 7.85%.
Method B: To a solution of sodium ethoxide prepared from sodium (0.09 g, 4.0 mmol) and anhydrous ethanol (30 mL), compound 1 (0.63 g, 2.0 mmol) was added. The mixture was stirred until it became homogeneous, then ethyl chloroacetate (0.25 g, 2 mmol) was added, the mixture was stirred for 2 h at 60 °C and cooled and the white precipitate was filtered off, washed with water and recrystallized from EtOH. Yield 78%.
3.1.3. General Method for the Preparation of Compounds 3b–f
To a solution of anhydrous potassium carbonate (0.69 g, 5.0 mmol) in ethanol (20 mL), compound 1 (0.63 g, 2.0 mmol) and the appropriate alkylating agent (2.0 mmol) were added. The mixture was refluxed for 3 h. After cooling, the obtained crystals were filtered off, washed with water, dried and recrystallized from ethanol/dioxane mixture (1:1).
1-Amino-5-(3,5-dimethyl-1H-pyrazol-1-yl)-8,8-dimethyl-8,9-dihydro-6H-pyrano[4,3-d]thieno[2,3-b]pyridine-2-carbonitrile (3b). It was obtained as white crystals; yield 89%, m.p. 260–261 °C. IR ν/cm−1: 3478, 3332, 3238 (NH2), 2198 (CN). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH:1.34 (s, 6H, C(CH3)2), 2.23 (s, 3H, CH3), 2.35 (s, 3H, CH3), 3.29 (t, J = 1.3 Hz, 2H, 9-CH2), 4.71 (t, J = 1.3 Hz, 2H, 6-CH2), 5.96 (s, 1H, CH), 6.25 (br s, 2H, NH2). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 11.9, 13.1, 26.2, 36.2, 59.5, 68.7, 75.5, 106.9, 114.7, 121.2, 121.5, 140.6, 143.6, 147.9, 148.2, 151.0, 156.4. Anal. calcd for C18H19N5OS: C 61.17; H 5.42; N 19.81; S 9.07%. Found: C 61.32; H 5.34; N 19.67; S 9.25%.
1-Amino-5-(3,5-dimethyl-1H-pyrazol-1-yl)-8,8-dimethyl-N-phenyl-8,9-dihydro-6H-pyrano[4,3-d]thieno[2,3-b]pyridine-2-carboxamide (3c). It was obtained as white crystals; yield 98%, m.p. 220–221 °C.IR ν/cm−1: 3453–3281 (NH2, NH), 1643 (C=O).1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH: 1.36 (s, 6H, C(CH3)2), 2.25 (s, 3H, CH3), 2.35 (s, 3H, CH3), 3.35 (t, J = 1.2 Hz, 2H, 9-CH2), 4.69 (t, J = 1.2 Hz, 2H, 6-CH2), 5.96 (s, 1H, CH), 6.90 (br s, 2H, NH2), 6.98-7.05 (m, 1H, CH), 7.22-7.30 (m, 2H, 2CH), 7.69-7.74 (m, 2H, 2CH), 9.14 (s, 1H, NH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 11.8, 13.1, 26.3, 36.4, 59.5, 68.7, 99.3, 106.5, 120.7, 120.9, 122.7, 123.7, 127.6, 138.7, 140.4, 143.2, 147.4, 148.0, 148.3, 155.1, 163.5. Anal. calcd for C24H25N5O2S: C 64.41; H 5.63; N 15.65; S 7.16%. Found: C 64.57; H 5.70; N 15.79; S 7.34%.
1-Amino-N-(2,4-dimethoxyphenyl)-5-(3,5-dimethyl-1H-pyrazol-1-yl)-8,8-dimethyl-8,9-dihydro-6H-pyrano[4,3-d]thieno[2,3-b]pyridine-2-carboxamide (3d). It was obtained as white crystals; yield 92%, m.p. 204–205 °C.IR ν/cm−1: 3455–3293 (NH2, NH), 1640 (C=O). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH: 1.36 (s, 6H, C(CH3)2), 2.25 (s, 3H, CH3), 2.37 (s, 3H, CH3), 3.34 (t, J = 1.2 Hz, 2H, 9-CH2), 3.80 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 4.72 (t, J = 1.2 Hz, 2H, 6-CH2), 5.97 (s, 1H, CH), 6.45 (dd, J = 8.8, 2.6 Hz, 1H, CH), 6.54 (d, J = 2.6 Hz, 1H, CH), 6.84 (s, 2H, NH2), 7.87 (s, 1H, NH), 8.05 (d, J = 8.8 Hz, 1H, CH).13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 11.9, 13.1, 26.3, 36.3, 54.7, 55.4, 59.5, 68.7, 98.1, 99.0, 103.5, 106.7, 120.6, 121.0, 121.1, 123.9, 140.5, 143.4, 147.4, 147.9, 148.0, 149.5, 154.3, 156.0, 162.4. Anal. calcd for C26H29N5O4S: C 61.52; H 5.76; N 13.80; S 6.32%. Found: C 61.62; H 5.81; N 13.66; S 6.45%.
1-Amino-N-(3,4-dichlorophenyl)-5-(3,5-dimethyl-1H-pyrazol-1-yl)-8,8-dimethyl-8,9-dihydro-6H-pyrano[4,3-d]thieno[2,3-b]pyridine-2-carboxamide (3e). It was obtained as white crystals; yield 90%, m.p. 226–227 °C. IR ν/cm−1: 3450–3287 (NH2, NH), 1643 (C=O). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH: 1.36 (s, 6H, C(CH3)2), 2.24 (s, 3H, CH3), 2.35 (s, 3H, CH3), 3.34 (t, J = 1.2 Hz, 2H, 9-CH2), 4.69 (t, J = 1.2 Hz, 2H, 6-CH2), 5.96 (s, 1H, CH), 7.00 (br s, 2H, NH2), 7.36 (d, J = 8.8 Hz, 1H, CH), 7.70 (dd, J = 8.8, 2.5 Hz, 1H, CH), 8.12 (d, J = 2.5 Hz, 1H, CH),9.43 (s, 1H, NH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 11.8, 13.1, 26.3, 36.4, 59.5, 68.7, 98.2, 106.6, 120.0, 121.0, 121.9, 123.4, 124.9, 129.2, 130.8, 138.9, 140.4, 143.5, 147.6, 148.0, 149.1, 155.3, 163.7. Anal. calcd for C24H23Cl2N5O2S: C 55.82; H 4.49; N 13.56; S 6.21%. Found: C 55.98; H 4.41; N 13.74; S 6.07%.
1-Amino-5-(3,5-dimethyl-1H-pyrazol-1-yl)-8,8-dimethyl-N-(2-phenylethyl)-8,9-dihydro-6H-pyrano[4,3-d]thieno[2,3-b]pyridine-2-carboxamide (3f). It was obtained as white crystals; yield 89%, m.p. 97–98 °C. IR ν/cm−1: 3409–3285 (NH2, NH), 1628 (C=O). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH:1.35 (s, 6H, C(CH3)2), 2.24 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.83-2.91 (m, 2H, CH2), 3.33 (t, J = 1.2 Hz, 2H, 9-CH2), 3.43-3.52 (m, 2H, NHCH2), 4.66 (t, J = 1.2 Hz, 2H, 6-CH2), 5.94 (s, 1H, CH), 6.71 (br s, 2H, NH2), 7.11-7.29 (m, 5H, 5CH), 7.42 (t, J = 5.6 Hz, 1H, NH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 11.7, 13.1, 26.3, 35.4, 36.3, 40.5, 59.4, 68.7, 100.0, 106.3, 120.9, 124.1, 125.4, 127.7, 128.3, 139.2, 140.3, 143.0, 146.9, 147.0, 147.9, 154.6, 164.6. Anal. calcd for C26H29N5O2S: C 65.66; H 6.15; N 14.73; S 6.74%. Found: C 65.49; H 6.24; N 14.57; S 6.86%.
3.1.4. Method for the Synthesis of 5-(3,5-Dimethyl-1H-pyrazol-1-yl)-2,2-dimethyl-1,4-dihydro-2H-pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8(9H)-one (4a)
The mixture of compound 3a (0.8 g, 2.0 mmol) and formamide (5 mL) was refluxed for 4 h. After cooling, the obtained brown crystals were filtered off, washed with water, dried and recrystallized from dimethyl sulfoxide (DMSO). Yield 82%, m.p. > 340 °C. IR ν/cm−1: 3340 (NH), 1670 (C=O).1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH:1.38 (s, 6H, C(CH3)2), 2.25 (s, 3H, CH3), 2.43 (s, 3H, CH3), 3.54 (t, J = 1.2 Hz, 2H, 1-CH2), 4.80 (t, J = 1.2 Hz, 2H, 4-CH2), 5.99 (s, 1H, CH), 8.15 (s, 1H, NH), 12.82 (s, 1H, CH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 12.0, 13.1, 26.4, 36.9, 59.6, 68.7, 107.2, 122.3, 122.9, 124.5, 140.9, 144.9, 146.4, 147.8, 148.4, 151.7, 156.9, 157.5. Anal. calcd for C19H19N5O2S: C 59.82; H 5.02; N 18.36; S 8.41%. Found: C 59.98; H 4.95; N 18.51; S 8.57%.
3.1.5. General Method for the Preparation of Compounds 4b–f
A mixture of the appropriate compounds 3c–f (2.0 mmol), triethylorthoformate (2.0 mL) and acetic anhydride (2.0 mL) was refluxed for 2 h. The obtained crystals were filtered off, washed with water, dried and recrystallized from a mixture of chloroform/ethanol 3:1.
5-(3,5-Dimethyl-1H-pyrazol-1-yl)-2,2-dimethyl-9-phenyl-1,4-dihydro-2H-pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8(9H)-one (4b). It was obtained as white crystals; yield 92%, m.p. 238–239 °C. IR ν/cm−1: 1671 (C=O). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH:1.40 (s, 6H, C(CH3)2), 2.27 (s, 3H, CH3), 2.47 (s, 3H, CH3), 3.56 (t, J = 1.2 Hz, 2H, 1-CH2), 4.84 (t, J = 1.2 Hz, 2H, 4-CH2), 6.01 (s, 1H, CH), 7.50-7.65 (m, 5H, 5CH), 8.40 (s, 1H, CH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 12.2, 13.1, 26.4, 37.0, 59.8, 68.6, 107.3, 122.4, 122.8, 124.1, 126.7, 128.6, 128.9, 136.4, 140.9, 144.8, 147.7, 148.1, 148.4, 150.5, 155.6, 157.9. Anal. calcd for C25H23N5O2S: C 65.63; H 5.07; N 15.31; S 7.01%. Found: C 65.81; H 5.13; N 15.47; S 6.88%.
9-(2,4-Dimethoxyphenyl)-5-(3,5-dimethyl-1H-pyrazol-1-yl)-2,2-dimethyl-1,4-dihydro-2H-pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8(9H)-one (4c). It was obtained as white crystals; yield 91%, m.p. 285–286 °C. IR ν/cm−1: 1668 (C=O). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH:1.40 (s, 6H, C(CH3)2), 2.27 (s, 3H, CH3), 2.45 (s, 3H, CH3), 3.57 (t, J = 1.1 Hz, 2H, 1-CH2), 3.84 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 4.84 (t, J = 1.1 Hz, 2H, 4-CH2), 6.01 (s, 1H, CH), 6.65 (dd, J = 8.7, 2.6 Hz, 1H, CH), 6.73 (d, J = 2.6 Hz, 1H, CH), 7.29 (dd, J = 8.7 Hz, 1H, CH), 8.15 (s, 1H, CH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 12.1, 13.1, 26.4, 36.9, 55.0, 55.5, 59.8, 68.6, 99.1, 104.8, 107.2, 117.7, 122.3, 122.9, 124.3, 129.1, 140.8, 144.8, 147.9, 148.3, 149.0, 150.5, 155.0, 155.7, 157.9, 161.2. Anal. calcd for C27H27N5O4S: C 62.65; H 5.26; N 13.53; S 6.20%. Found: C 62.79; H 5.17; N 13.37; S 6.33%.
9-(3,4-Dichlorophenyl)-5-(3,5-dimethyl-1H-pyrazol-1-yl)-2,2-dimethyl-1,4-dihydro-2H-pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8(9H)-one (4d). It was obtained as white crystals; yield 89%, m.p. 191–192 °C. IR ν/cm−1: 1675 (C=O). 1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH:1.40 (s, 6H, C(CH3)2), 2.26 (s, 3H, CH3), 2.47 (s, 3H, CH3), 3.56 (t, J = 1.1 Hz, 2H, 1-CH2), 4.85 (t, J = 1.1 Hz, 2H, 4-CH2), 6.01 (s, 1H, CH), 7.55 (dd, J = 8.6, 2.5 Hz, 1H, CH), 7.75 (d, J = 8.6 Hz, 1H, CH), 7.84 (d, J = 2.5 Hz, 1H, CH), 8.45 (s, 1H, CH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 12.2, 13.1, 26.4, 37.0, 59.8, 68.6, 107.3, 107.4, 122.5, 124.0, 126.8, 129.1, 130.6, 132.0, 132.4, 135.7, 141.0, 144.9, 147.4, 148.2, 148.5, 150.5, 155.4, 157.9. Anal. calcd for C25H21Cl2N5O2S: C 57.04; H 4.02; N 13.30; S 6.09%. Found: C 57.23; H 3.96; N 13.16; S 6.26%.
5-(3,5-Dimethyl-1H-pyrazol-1-yl)-2,2-dimethyl-9-(2-phenylethyl)-1,4-dihydro-2H-pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-8(9H)-one (4e). It was obtained as white crystals; yield 84%,m.p. 175–176 °C. IR ν/cm−1: 1673 (C=O).1H NMR (300 MHz, DMSO-d6/CCl4, 1/3, v/v) δH:1.38 (s, 6H, C(CH3)2), 2.25 (s, 3H, CH3), 2.44 (s, 3H, CH3), 3.11 (t, J = 7.2 Hz, 2H, NCH2CH2), 3.48 (t, J = 1.2 Hz, 2H, 1-CH2), 4.35 (t, J = 7.2 Hz, 2H, NCH2CH2), 4.81 (t, J = 1.2 Hz, 2H, 4-CH2), 6.00 (s, 1H, CH), 7.17–7.31 (m, 5H, 5CH), 8.20 (s, 1H, CH). 13C NMR (75.462 MHz, DMSO-d6/CCl4, 1/3, v/v) δC: 12.1, 13.1, 26.3, 34.4, 36.9, 47.6, 59.7, 68.5, 107.1, 122.2, 124.2, 126.2, 128.0, 128.4, 137.0, 140.8, 144.7, 147.9, 148.3, 148.4, 150.9, 156.0, 157.8. Anal. calcd for C27H27N5O2S: C 66.78; H 5.60; N 14.42; S 6.60%. Found: C 66.94; H 5.67; N 14.28; S 6.76%.
3.2. Biological Evaluation
Compounds were studied for their possible neurotropic activities (anticonvulsant, sedative and antianxiety activity) as well as side effects on 450 white mice of both sexes weighing 18–24 g and 50 male rats of the Wistar line weighing 120–140 g. All groups of animals were maintained at 25 ± 2 °C in the same room on a common food ration. All the biological experiments were carried out in full compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of “(ETS No 123, Strasbourg, 03/18/1986): Strasbourg (France). European Treaty Series—No 123, 18 March 1986. 11 P”. University and Experiments were approved by the Animal Ethics Committee of the Scientific Technological Center of Organic and Pharmaceutical Chemistry of the National Academy of Sciences of the Republic of Armenia. P.N5 from 24 March 2016.
3.2.1. Evaluation of the Anticonvulsant Activity of the Synthesized Compounds
The anticonvulsant effect of the new synthesized compounds was investigated by PTZ convulsion tests (Acros organics, New Jersey, USA), MES [22,23,24,25,26]. The PTZ test is an experimental model for inducing myoclonic seizures as well as for predicting the anxiolytic properties of compounds. Outbred mice (weight 18–22 g) were used for the study. The PTZ test was carried out in mice by subcutaneous administration of analeptic at a dose of 90 mg/kg and the effectiveness of the preparations was determined by the prevention of clonic seizures. The anticonvulsant activity of the compounds was also carried out to prevent the tonicextensor phase of the convulsive seizure caused by maximal electroshock (MES). The parameters of the maximal electroshock were 50 mA, the duration was 0.2 s, the oscillation frequency was 50 imp/s, and the evaluation criterion was the warning of the tonicextensor phase of a convulsive seizure. Substances were administered intraperitoneally in doses of 10, 25, 50, 75 and 100 mg/kg in suspension with carboxymethylcellulose (“Viadi—Ingredients”) with Tween-80 (“Ferak Berlin”) 45 min before the injection of the convulsive agent PTZ causing electrical irritation. The control animals were administered an emulsifier. Each dose of compounds for each test was studied in 8 animals. Analogues for comparison were an anticonvulsant drug from the group of succinimide ethosuximide (neuraxpharmArzneimittel GmbH (Germany) [28]. Thecomparison drug, ethosuximide, was administered intraperitoneally in doses from 100 to 300 mg/kg.
3.2.2. Evaluation of the Psychotropic Properties of the Synthesized Compounds
The psychotropic properties of selected compounds (3b–f and 4b–e) were studied by tests: ″open field″, ″elevated plus maze—EPM″ and ″forced swimming″.
Open field test. The motor behavior of rats was studied on a modified ″open field″ model [32,33,34]. For this purpose, an installation was used, the bottom of which was dvided into squares with holes (cells). Experiments were performed in the daytime with natural light. Within 5 min of the experiment, the indicators of sedative and activating behavior were determined—the number of horizontal movements, standing on the hind legs (vertical movements), sniffing of the cells. The number of animals on this model was 8 for each compound, control and reference drug. The studied compounds were administered to rats in the most effective dose of 50 mg/kg intraperitoneally as a suspension with methylcarboxycellulose with Tween-80.
Elevated plus maze—EPM test. Antianxiety and sedative effects were studied in mice on an ″elevated plus maze″ model [35]. The labyrinth is a cruciform machine raised above the floor, having a pair of open and closed sleeves opposed to each other. Normal animals prefer to spend most of their time in the closed (dark) sleeves of the labyrinth. The anxiolytic effect of the compounds was estimated by the increase in the number of entries into open (light) sleeves and the time spent in them without increasing total motor activity. This model records the time spent in the closed sleeve and the number of attempts to enter the installation center. In the above model, the test compounds and the reference drug were injected intraperitoneally before the experiments. The control animals were administered an emulsifier. Results were processed statistically (p ≤ 0.05).
Forced swimming test. To assess ″despair and depression″, the model ″compelling swimming″ [39] was used. Experimental animals were forced to swim in a glass container (height 22 cm, diameter 14 cm), filled 1/3 with water. Intact mice swim very actively, but soon they will be forced to immobilize. The latent period of immobilization, the total duration of active swimming and the period of immobilization are fixed for 6 min. The experiments were conducted under natural light.
3.2.3. Evaluation of Incoordination of Movements in the Rotating Rod Test
The adverse neurotoxic (muscle relaxant) effect of compounds was studied in doses of 50 to 100 mg/kg when administered intraperitoneally, as were reference drugs in effective anticonvulsant doses. Myorelaxation was investigated by the test of a ″rotating rod″ in mice [22,29]. To this end, mice were planted on a metal rod with a corrugated rubber coating, which rotated at a speed of 5 revolutions per minute. The number of animals that could not stay on it for 2 min was determined. To determine the ED50 and neurotoxic TD50, the statistical method of penetration by Litchfield and Wilcoxon was used [30,31]. Maximal tolerated doses (MTD) were also studied. The compounds, as administered by intraperitoneal injection in doses from 500–1800 mg/kg, were investigated.
3.3. Docking Studies
3.3.1. Design of Molecular Models
To create three-dimensional molecular models of the studied compounds, the ChemOffice program version 13.0 was used [51]. Minimization and stabilization of the obtained 3D structures were performed by using force fields MM2, which are used to optimize small molecule models [52]. The molecular models of the studied compounds were saved in *.PDB format after optimization. Molecular models of the studied targets were taken from the RCSB [53] with identification numbers SERT transporter [PDBID:5I6X], GABAA receptor [PDBID:4COF] and 5-HT1A receptor [PDBID:3NYA].
3.3.2. Molecular Docking
The AutoDock Vina and AutoDock Tools software packages were used for carrying out molecular docking using the “blind method” [54]. The statistical reliability of the docking results was provided by 10-fold repeatability of 20 initial conformations for each compound with a spatial search volume not exceeding 27,000 Å. The exhaustiveness equally is 200. The choice of the best conformers was carried out on the base of mean square deviation during complexation RMSD ≤ 2Å.
3.3.3. Binding Constant Calculation
To determine the binding constant [55] of the studied compounds with targets, the following equations were used:
where ΔGexp—interaction energy, R—gas constant, T—absolute temperature, K—binding constant.
3.3.4. Conformational Analysis and Visualization
To identify the types of binding during complexation of the studied compounds with targets, the Discovery Studio Visualizer [56] was used. The calculated criteria for the radius of interaction were calculated according to the standard: hydrogen bond length 3.4 Å, for Coulomb interactions—9Å and for van der Waals interactions—14 Å.
3.3.5. Statistical Analysis
The cluster analysis of the spatial and energy values of the ligand target complexation was carried out by the k-means method using the ClastVis online instrument [57]. Statistical analysis of the results of the investigation was conducted on the basis of the complexed application of standard statistical methods, including the calculation of standard deviations, average values and standard average errors.
4. Conclusions
Pyrazolo substituted new heterocyclic systems—tricyclic N-alkyl(aryl)derivatives of pyrano[4,3-d]thieno[2,3-b]pyridines and tetracyclic pyrano[4″,3″:4′,5′]pyrido[3′,2′:4,5] thieno[3,2-d]pyrimidin-8(9H)-ones were synthesized by newly developed methods and their neurotropic activity was studied. Compounds were tested for their anticonvulsive action by evaluating the antagonism between the PTZ convulsive action and maximal electrical shock seizures. The evaluation of anticonvulsant activity of all the synthesized compounds revealed that among all tested, compounds 3b–f and 4b–e had a pronounced anticonvulsant action. In the case of tricyclic pyranothienopyridines, the activity depends on the alkylated groups with activity that are ordered as: 3e ≥ 3b ≥ 3d ≥ 3c ≥ 3f > 3a. Similar activity is observed in the case of tetracyclic thienopyrimidines. The selected nine compounds appeared to be more active than ethosuximide according to the test on PTZ. These compounds were also studied for their possible anxiolytic and antidepressant activities by ″open field″, ″elevated plus maze″ and ″forced swimming″ tests. According to the open field behavioral model, all selected compounds, especially compounds 3b, 3d, 3e, 3f and 4e, in a statistically significant manner compared to the control, increase the number of sniffing cell examinations, which may be an indication of the antianxiety activity of the compounds. According to the EPM model, all selected compounds increase the time spent by experienced animals in the center, which indicates sedative activity. This is especially is expressed in compounds 3e, 3f and 4c. The results on the EPM model also confirmed the anxiolytic effect of all tested compounds. This effect was especially expressed in compounds 3e, 3d, 3f and 4d. According to the ″forced swimming″ model, some of the selected compounds (3e, 3f and 4d) statistically significantly increase the duration of the latent period of the first immobilization and show some antidepressant effect in the same manner as diazepam. The remaining compounds decrease the total time of active swimming. In terms of therapeutic effect, in vivo and in silico results are in accordance.
On the basis of obtained results, ″lead compounds″, which exhibit the best results of interaction with the studied targets according to the initial assessment, were selected. Docking analysis and obtained biophysics properties of complexations show that ″lead compounds″ (3a, 3c, 3d, 3e, 4b, 4c and 4d) interact with the GABAA receptor more strongly than comparative drug diazepam. Moreover, 3d, 4b, 4c and 4d interact in subsite 3 of the ECD, unlike diazepam. Compound 4b exhibits a directed action without affecting the central binding site of SERT and predominantly hydrophobic interactions with practically all residues are observed, which form an allosteric binding site. Compounds 3c, 3d, 3e, 3f and 4e practically bind with the groove of T4L dopamine of 5HT_1A and block it completely.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27113380/s1. The copies of 1H-NMR and 13C-NMR spectra for all newly synthesized compounds have been submitted along with the manuscript.
Author Contributions
S.S.D. and E.G.P. conceptualized and performed experiments on the synthesis of all compounds and analyzed the results. A.G.A. performed spectroscopic experiments of synthesized compounds. R.G.P. performed the neurotropic activity experiments. L.S.H. performed the molecular docking calculations. E.V.B. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Science Committee of RA in the frames of the research project № 20TTWS-1D032.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Yerevan State Medical University (YSMU, Yerevan, Armenia) (protocol code 7-5/2021 and date of approval 18.03.2021). The study followed the “Principles of laboratory animal care” and was carried out in accordance with the European Council Directive (2010/63/EU).
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of compounds 1–4 are available from the authors.
References
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Shiri, P. Novel Hybrid Molecules Based on triazole-β-lactam as Potential Biological Agents. Mini-Rev.Med. Chem. 2021, 21, 536–553. [Google Scholar] [CrossRef] [PubMed]
- Rayevsky, O.M. Modeling the Structure-Property Relationships; Poroikov, V.V., Ed.; Dobrosvet: Moscow, Russia, 2008; p. 288. (In Russian) [Google Scholar]
- Shiri, P.; Ramezanpour, S.; Amani, A.M.; Dehaen, W. A patent review on efficient strategies for the total synthesis of pazopanib, regorafenib and lenvatinib as novel anti-angiogenesis receptor tyrosine kinase inhibitors for cancer therapy. Mol. Divers. 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tuccinardi, T.; Schenone, S.; Bondavalli, F.; Brullo, C.; Bruno, O.; Mosti, L.; Zizzari, A.T.; Tintori, C.; Manetti, F.; Ciampi, O.; et al. Substituted Pyrazolo[3,4-b]pyridines as Potent A1 Adenosine Antagonists: Synthesis, Biological Evaluation, and Development of an A1 Bovine Receptor Model. ChemMedChem 2008, 3, 898–913. [Google Scholar] [CrossRef]
- Salem, M.S.; Ali, M.A.M. Novel Pyrazolo[3,4-b]pyridine Derivatives: Synthesis, Characterization, Antimicrobial and Antiproliferative Profile. Biol. Pharm. Bull. 2016, 39, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, A.L.; Salvador, R.R.; Silva, L.A.; Saito, M.S.; Mello, J.F.; Cabral, L.M.; Rodrigues, C.R.; Vera, M.A.; Muri, E.M.; de Souza, A.M.; et al. Synthesis and mechanistic evaluation of novel N′-benzylidene-carbohydrazide-1 H -pyrazolo[3,4-b]pyridine derivatives as non-anionic antiplatelet agents. Eur. J. Med. Chem. 2017, 135, 213–229. [Google Scholar] [CrossRef]
- Kasabov, K.A.; Kudryashov, N.V.; Volkova, A.; Shimshirt, A.A.; Kalinina, T.S.; Zhmurenko, L.A.; Voronina, T.A. Psychotropic Effects of a New Pyrazolo[C]Pyridine Derivate GIZh-72 are Related to Functional Activity of Atp-Sensitive Potassium Channels. Bull. Exp. Biol. Med. 2020, 168, 449–452. [Google Scholar] [CrossRef]
- Samatov, A.; Akramov, S.T.; Yunusov, S.Y. Alkaloids of Gentiana. Structure of gentianadine and gentianamine. Chem. Nat. Compd. 1967, 3, 150–154. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosal, S.; Chaudhuri, R.; Singh, A.; Sharma, P. Chemical Constituents of Gentianaceae XI: Antipsychotic Activity of Gentianine. J. Pharm. Sci. 1974, 63, 1341–1342. [Google Scholar] [CrossRef]
- Yu, S.; Huang, Q.-Q.; Luo, Y.; Lu, W. Total Synthesis of Camptothecin and SN-38. J. Org. Chem. 2011, 77, 713–717. [Google Scholar] [CrossRef]
- Jubeen, F.; Iqbal, S.Z.; Shafiq, N.; Khan, M.; Parveen, S.; Iqbal, M.; Nazir, A. Eco-friendly synthesis of pyrimidines and its derivatives: A review on broad spectrum bioactive moiety with huge therapeutic profile. Synth. Commun. 2018, 48, 601–625. [Google Scholar] [CrossRef]
- Disch, J.S.; Evindar, G.; Chiu, C.H.; Blum, C.A.; Dai, H.; Jin, L.; Schuman, E.; Lind, K.E.; Belyanskaya, S.L.; Deng, J.; et al. Discovery of Thieno[3,2-d]pyrimidine-6-carboxamides as Potent Inhibitors of SIRT1, SIRT2, and SIRT3. J. Med. Chem. 2013, 56, 3666–3679. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, Z.; Hui, J.; Zhao, Y.; Zhu, L. Synthesis and anticancer activities of thieno[3,2-d]pyrimidines as novel HDAC inhibitors. Bioorganic Med. Chem. 2013, 22, 358–365. [Google Scholar] [CrossRef]
- Barthakur, M.G.; Gogoi, S.; Dutta, M.; Boruah, R.C. A facile three-component solid phase synthesis of steroidal A-ring fused pyrimidines under microwave irradiation. Steroids 2009, 74, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Malhota, M.; Dhingra, R.; Sharma, R.; Bhadway, T.R. 17-Aza Steroids as 5α-Reductase Inhibitors: A Review. Int. J. Pharmacogn. Phytochem. Res. 2013, 5, 134–142. [Google Scholar]
- Kaur, S.; Kapoor, V.K.; Bhardwa, T.K.; Kaur, M. A facile route for the synthesis of bisquartenary azasteroids. Int. J. Pharmacogn. Phytochem. Res. 2013, 5, 728–732. [Google Scholar]
- Dashyan, S.; Paronikyan, E.G.; Noravyan, A.S. Synthesis and Anticonvulsive Activity of 5-Pyrrolidin-1-Ylpyrano[4″,3″:4′,5′]Pyrido-3′,2′:4,5]Thieno[3,2-D]Pyrimidine Derivatives. Pharm. Chem. J. 2016, 50, 221–225. [Google Scholar] [CrossRef]
- Babaev, E.V.; Koval, Y.I.; Rybakov, V.B.; Paronikyan, E.G.; Stepanyan, G.M.; Dashyan, S.S.; Rzhevskii, S.A.; Shadrin, I.A. 2-Allyloxy/propargyloxypyridines: Synthesis, structure, and biological activity. Bull. Acad. Sci. USSR Div. Chem. Sci. 2018, 67, 313–320. [Google Scholar] [CrossRef]
- Paronikyan, E.G.; Dashyan, S.S.; Dzhagatspanyan, I.A.; Nazaryan, I.M.; Akopyan, A.G.; Minasyan, N.S. Synthesis and neurotropic activity of the derivatives of fused triazolo[4,3-c]- and triazolo[1,5-c]pyrimidines. Russ. J. Bioorganic Chem. 2017, 43, 595–603. [Google Scholar] [CrossRef]
- Dashyan, S.S.; Paronikyan, R.G.; Mamyan, S.S.; Paronikyan, E.G. Synthesis, neurotropic activity and SAR of new S-alkyl derivatives of 8- pyrazol-1-yl pyrano[3,4-c]pyridines. Arkivoc 2022, 2022, 43–53. [Google Scholar] [CrossRef]
- Vogel, H.G.; Vogel, V.H. Psychotropic and Neurotropic Activity. In Drug Discovery and Evaluation: Pharmacological Assays; Vogel, H.E., Ed.; Springer: Berlin, Germany, 2008; Volume 58, pp. 569–874. Available online: https://scholar.google.com/scholar_lookup?title=Drug+Discovery+and+Evaluation (accessed on 28 May 2021).
- Löscher, W.; Schmidt, D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988, 2, 145–181. [Google Scholar] [CrossRef]
- Swinyard, E.A. Experimental Models of Epilepsy; Purpura, D.P., Penry, J.K., Tower, D., Woodbury, D.M., Walter, R., Eds.; Raven Press: New York, NY, USA, 1992; pp. 433–458. Available online: https://europepmc.org/article/med/4007184 (accessed on 28 May 2021).
- Gevorkyan, K.A.; Papayan, G.L.; Chshmarityan, S.G.; Paronikyan, R.G.; Akopyan, N.E.; Engoyan, A.P. Synthesis and anticonvulsant activity of oximes of 1-R-3-acetonyl-3-hydroxyoxindole and its derivatives. Pharm. Chem. J. 1987, 21, 95–98. [Google Scholar] [CrossRef]
- Katzung, B.G. Drugs Used in Generalized Seizures. In Basic and Clinical Pharmacology, 9th ed.; McGraw-Hill: New York, NY, USA, 2003; Large Medical Books. [Google Scholar]
- Patsalos, P.N. Properties of Antiepileptic Drugs in the Treatment of Idiopathic Generalized Epilepsies. Epilepsia 2005, 46, 140–148. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Löscher, W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004, 5, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Dunham, N.W.; Miya, T.S.; Edwards, L.D. The Pharmacological Activity of a Series of Basic Esters of Mono- and Dialkylmalonic Acids. J. Am. Pharm. Assoc. 1957, 46, 64–66. [Google Scholar] [CrossRef]
- Litchfield, J.T., Jr.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Mironov, A.H. The 1th Part. In Manual for Preclinical Studies of Drugs; Medicine: Moscow, Russia, 2012; pp. 235–250. (In Russian) [Google Scholar]
- File, S.E. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav. Brain Res. 2001, 125, 151–157. [Google Scholar] [CrossRef]
- Stanford, S.C. The Open Field Test: Reinventing the wheel. J. Psychopharmacol. 2007, 21, 134–135. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Jardim, M.C.; Nogueira, R.L.; Graeff, F.G.; Nunes-De-Souza, R.L. Evaluation of the elevated T-maze as an animal model of anxiety in the mouse. Brain Res. Bull. 1999, 48, 407–411. [Google Scholar] [CrossRef]
- Graeff, F.G.; Netto, C.F.; Zangrossi, H., Jr. The elevated T-maze as an experimental model of anxiety. Neurosci. Biobehav. Rev. 1998, 23, 237–246. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioral despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Puthenkalam, R.; Hieckel, M.; Simeone, X.; Suwattanasophon, C.; Feldbauer, R.; Ecker, G.F.; Ernst, M. Structural Studies of GABAA Receptor Binding Sites: Which Experimental Structure Tells us What? Front. Mol. Neurosci. 2016, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Sieghart, W. Allosteric Modulation of GABAA Receptors via Multiple Drug-Binding Sites. Adv. Pharmacol. 2014, 72, 53–96. [Google Scholar] [CrossRef]
- Coleman, J.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Jha, P.; Ragnarsson, L.; Lewis, R.J. Structure-Function of the High Affinity Substrate Binding Site (S1) of Human Norepinephrine Transporter. Front. Pharmacol. 2020, 11, 217. [Google Scholar] [CrossRef]
- Rannversson, H.; Andersen, J.; Bang-Andersen, B.; Strømgaard, K. Mapping the Binding Site for Escitalopram and Paroxetine in the Human Serotonin Transporter Using Genetically Encoded Photo-Cross-Linkers. ACS Chem. Biol. 2017, 12, 2558–2562. [Google Scholar] [CrossRef]
- Möller, I.R.; Slivacka, M.; Nielsen, A.K.; Rasmussen, S.G.F.; Gether, U.; Loland, C.J.; Rand, K.D. Conformational dynamics of the human serotonin transporter during substrate and drug binding. Nat. Commun. 2019, 10, 1687. [Google Scholar] [CrossRef]
- Chen, J.-G.; Sachpatzidis, A.; Rudnick, G. The Third Transmembrane Domain of the Serotonin Transporter Contains Residues Associated with Substrate and Cocaine Binding. J. Biol. Chem. 1997, 272, 28321–28327. [Google Scholar] [CrossRef] [Green Version]
- Freddolino, P.L.; Kalani, M.Y.S.; Vaidehi, N.; Floriano, W.B.; Hall, S.E.; Trabanino, R.J.; Kam, V.W.T.; Goddard, W.A. Predicted 3D structure for the human β2 adrenergic receptor and its binding site for agonists and antagonists. Proc. Natl. Acad. Sci. USA 2004, 101, 2736–2741. [Google Scholar] [CrossRef] [Green Version]
- Bang, I.; Choi, A.H.-J. Structural Features of β2 Adrenergic Receptor: Crystal Structures and Beyond. Mol. Cells 2014, 38, 105–111. [Google Scholar] [CrossRef]
- Thorsen, T.S.; Matt, R.; Weis, W.I.; Kobilka, B.K. Modified T4 Lysozyme Fusion Proteins Facilitate G Protein-Coupled Receptor Crystallogenesis. Structure 2014, 22, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Zielesny, A. Chemistry Software Package ChemOffice Ultra 2005. J. Chem. Inf. Model. 2005, 45, 1474–1477. [Google Scholar] [CrossRef]
- Evans, M.J.; Moore, J.S. A Collaborative, Wiki-Based Organic Chemistry Project Incorporating Free Chemistry Software on the Web. J. Chem. Educ. 2011, 88, 764–768. [Google Scholar] [CrossRef]
- Rose, P.W.; Prlić, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Di Costanzo, L.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2016, 45, D271–D281. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- FFogolari, F.; Brigo, A.; Molinari, H. The Poisson-Boltzmann equation for biomolecular electrostatics: A tool for structural biology. J. Mol. Recognit. 2002, 15, 377–392. [Google Scholar] [CrossRef]
- Jejurikar, B.L.; Rohane, S.H. Drug designing in discovery studio. Asian J. Res. Chem. 2021, 14, 135–138. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).