Abstract
Studies on molecular co-crystal type materials are important in the design and preparation of easy-to-absorb drugs, non-centrosymmetric, and chiral crystals for optical performance, liquid crystals, or plastic phases. From a fundamental point of view, such studies also provide useful information on various supramolecular synthons and molecular ordering, including metric parameters, molecular matching, energetical hierarchy, and combinatorial potential, appealing to the rational design of functional materials through structure–properties–application schemes. Co-crystal salts involving anionic d-metallate coordination complexes are moderately explored (compared to the generality of co-crystals), and in this context, we present a new series of isomorphous co-crystalline salts (PPh4)3[M(CN)6](H3PG)2·2MeCN (M = Cr, 1; Fe, 2; Co 3; H3PG = phloroglucinol, 1,3,5-trihydroxobenzene). In this study, 1–3 were characterized experimentally using SC XRD, Hirshfeld analysis, ESI-MS spectrometry, vibrational IR and Raman, 57Fe Mössbauer, electronic absorption UV-Vis-NIR, and photoluminescence spectroscopies, and theoretically with density functional theory calculations. The two-dimensional square grid-like hydrogen-bond {[M(CN)6]3−;(H3PG)2}∞ network features original {[M(CN)6]3−;(H3PG)4} supramolecular cis-bis(chelate) motifs involving: (i) two double cyclic hydrogen bond synthons M(-CN⋅⋅⋅HO-)2Ar, {[M(CN)6]3−;H2PGH}, between cis-oriented cyanido ligands of [M(CN)6]3− and resorcinol-like face of H3PG, and (ii) two single hydrogen bonds M-CN⋅⋅⋅HO-Ar, {[M(CN)6]3−;HPGH2}, involving the remaining two cyanide ligands. The occurrence of the above tectonic motif is discussed with regard to the relevant data existing in the CCDC database, including the multisite H-bond binding of [M(CN)6]3− by organic species, mononuclear coordination complexes, and polynuclear complexes. The physicochemical and computational characterization discloses notable spectral modifications under the regime of an extended hydrogen bond network.
1. Introduction
Hexacyanidometallates of d block metal ions belong to the most versatile and common building blocks in the construction of functional molecular materials within the frame of various synthetic strategies. Based on their individual properties resulting from the valence electronic structure (magnetic, optical, redox reactivity, communicative molecular orbital system), polynuclear complexes are considered in the construction of switchable materials such as molecular magnets, nanomagnets, and photomagnets or solar energy converting units. These features are very often combined and enhanced with porosity, conductivity, or luminescence under the common flag of the Prussian Blue Analogues (PBAs) family and the related species of various dimensionality [1,2,3].
Nevertheless, going beyond the bimetallic or trimetallic building block approach toward coordination-based networks, another broad field has emerged, focusing on the development of an alternative molecular networks library. As in the case of PBAs, organic and inorganic parties in such networks meet together in shaping their properties and functionality; however, within the current scenery, the [M(CN)6]3− anions (being a representant of the broader family of anions) and “non-innocent” organic molecules are combined into hybrid supramolecular architectures through dominant non-covalent interactions. Several interesting groups of hybrid networks have been developed or at least suggested, which can be classified as real salts, double salts, or co-crystal salts, including their solvated analogues [4]. Firstly, a broad family of organic–inorganic perovskites was enriched with [AI][B]2[MIII(CN)6]·solv (A+ = alkali metal cation, B+ = alkyl organic cation) species, in which B+ are located within the more or less regular cavities formed by the framework of electrostatically bound A+ and [M(CN)6]3− [5,6,7,8,9]. The significant freedom of reorientation shown by B+ cations together with their symmetry and dipolar moment was exploited to imply the occurrence of thermal phase transitions between ordered/disordered states. As a result, notable switchable behavior might be observed for dielectric properties, non-centrosymmetry related properties (e.g., ferro-, pyro-, and piezoelectricity, second harmonic generation and even chirality), symmetry-independent third-harmonic generation (THG), and photoluminescent performance if appropriate species are involved [9]. Alternative molecular antiperovskite architectures were realized via reverse site occupation, hosting [Co(CN)6]3− anions at the cavities formed within {(MF6)(H2dabco)3}3+ (M = Al3+, Cr3+, or In3+; dabco = 1,4-diazabicyclo[2.2.2]octane) and {(MF6)(H2pip)3}3+ (M = Al3+ or Cr3+; pip = piperazine) frameworks [10]. Secondly, [M(CN)6]3− anions were combined with the specially preprogrammed bis(amidinium) organic dications of the dedicated directional distribution of N+-H hydrogen bond donors, which resulted in a series of tectonic networks stabilized by charge-assisted hydrogen bonds [11,12], some of them featuring reversible solvent uptake and post-crystallization structural transformations [13,14]. The underlaying crystal phases also gave rise to a series of epitaxially grown core–shell crystalline composites [15], considered molecular waveguides exploiting luminescent properties [16]. Thirdly, planar organic cations, e.g., tetrathiafulvalene (TTF) derivatives, engaged hexacyanidometallates into the switching of charge ordering and conductivity phenomena upon structural-phase transition [17,18]. Finally, selected co-crystalline type phases combining [M(CN)6]3− anions and planar neutral π-acidic species, e.g., naphtalenediimide (NDI) derivatives [19] and 1,4,5,8,9,12-hexaazatriphenylenehexacarbonitrile (HAT(CN)6), showed charge transfer properties [20], whereas specially preprogrammed pyrimidine derivatives [21,22] were studied from the standpoint of surface-enhanced anion binding.
The current study is dedicated to new d-metallate-based supramolecular synthons that might be of importance in the design of functional molecular platforms in solution and in the solid-state. As a counterpart, we selected phloroglucinol (H3PG; 1,3,5-trihydroxobenzene), a triangular hydrogen bond donor widely tested in the formation of hydrogen-bonded architectures [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Its resulting functional character is well documented in the context of topological reactivity [24], combinatorial or modular synthetic strategies [25,35], molecular recognition and selective binding [31,34,39], proton disorder [26], drugs fabrication improvement [27], luminescence switching [28], switchable magnetic properties [23], chiral properties and photonic materials [36], and general molecular organization [23,29,30,31,32,33,34,37,38]. In particular, molecular recognition and selective binding of H3PG and other associated resorcinol and naphtalenediol-type co-formers involved various polynuclear d-metal ions clusters serving as the molecular hosts [31,34,39,40,41,42,43,44,45,46]. However, anionic d-metallates or f-metallates were moderately represented in these studies, counting some examples of pyridine–dicarboxylate complexes forming the hydrogen-bonded networks with pyridinium and polypyridinium cations [34,43,45,46].
In this paper, we present a new series of hybrid organic–inorganic isomorphous co-crystal salts (PPh4)3[M(CN)6](H3PG)2·2MeCN (M = Cr, 1; Fe, 2; Co 3). As a novel contribution to the field of multicomponent molecular architectures, our structural, physicochemical, and computational studies describe the original {[M(CN)6]3−;(H3PG)4} supramolecular cis-bis(chelate) motifs involving typical single M-CN⋅⋅⋅HO-Ar and double cyclic M(-CN⋅⋅⋅HO-)2Ar hydrogen-bond synthons.
2. Results and Discussion
2.1. Structural Studies
Compounds 1–3 crystallized in the monoclinic system, space group C2/c (Table S1). The uniformity of the powder samples and the identity of the crystals examined with SC XRD were confirmed by PXRD (Figure S1, Supplementary Materials). The crystal structures consist of PPh4+ cations, hexacyanidometallate [M(CN)6]3− anions, phloroglucinol (H3PG) co-former molecules, and solvent molecules MeCN. Asymmetric units contain 1 and ½ PPh4+, 1 H3PG, ½ [M(CN)6]3− anion, and 1 MeCN molecule (Figure S2), and the most important bond lengths and angles are shown in Table S2. [M(CN)6]3− and H3PG form an exclusive hydrogen-bonded 2D subnetwork {[M(CN)6]3−;(H3PG)2}∞, which coexists with another subnetwork formed by organic cations and solvent molecules (Figure 1a,b). The {[M(CN)6]3−;(H3PG)2}∞ subnetwork reveals a rhombus square grid-like topology, where [M(CN)6]3− hydrogen-bond acceptors are located in its nodes, and H3PG hydrogen bond donors act as linear linkers. Each [M(CN)6]3− anion forms four hydrogen bond contacts with four neighboring H3PG molecules (Figure 1c): two double cyclic ring-type pattern synthons with two H3PG, {[M(CN)6]3−;H2PGH} and two single synthons of the linear pattern D with the remaining two H3PG molecules, {[M(CN)6]3−;HPGH2} [47]. Importantly, the original double synthon is formed owing to structural and electronic matching between the cis-dicyanido fragment of the complex and resorcinol-like [48] (1,3-dihydroxobenzene) fragment of H3PG, as a vivid example of the realization of the molecular tectonics concept [12]. The interatomic distances and the related angles within both synthons are collected in Table 1. The observed N⋅⋅⋅O and N⋅⋅⋅H separations, together with the related almost linear N⋅⋅⋅H-O angles, allow us to classify them as moderate-to-strong hydrogen-bonding interactions [49] (see also calculated interaction energies presented in the Section 2.4 below). Among the N⋅⋅⋅O and N⋅⋅⋅H distances, the shortest ones are noted in the single {[M(CN)6]3−;(HPGH2)} synthon along with the whole 1–3 series, whereas those in the {[M(CN)6]3−;(H2PGH)} synthon are slightly longer and notably diversified, which is most probably due to steric effects that might accompany the formation of such a complex motif. The double synthons are almost planar, with a ca. 10° angle between the H3PG plane and the plane formed by the cis-dicyanido fragment of [M(CN)6]3−. The described spatial orientation of all four synthons around the central [M(CN)6]3− within the {[M(CN)6]3−;(H2PGH)2(HPGH2)2} structural fragment shown in Figure 1c is identical to the distribution of ligands in canonical cis stereoisomers of bis(chelated) six-coordinate d metal ion complexes [ML2A2] (L = bidentate chelating ligands, A = monodentate ligands), and thus we suggest to describe such aggregation as the non-covalent cis-bischelation. Moreover, the formation of the double synthons leads to the notable deformation of the [M(CN)6]3− complex from octahedron following the spatial demands imposed by the distribution of the -OH groups in H3PG. The C2-M1-C3 and N2⋅⋅⋅M1⋅⋅⋅N3 angles in all compounds are close to 85° and 82°, respectively, notably smaller compared to other close-to-right angles; this feature resembles the standard biting angles of ca. 72–75° observed in the complexes with the flat chelating ligands (e.g., o-phenanthroline). Furthermore, the M1-C3-N3 angles involved in the double synthons are visibly deviated from linearity, unlike the M1-C1-N1 and M1-C2-N2 ones. The M-C and C-N bond lengths and M-C-N angles in [M(CN)6]3−, as well as all metric parameters of H3PG, are in good agreement with the statistical values observed in the CCDC database. The overall deformation of [M(CN)6]3− complexes might be represented by shape measures SOC-6 (Table S3) using the CShM method [50].
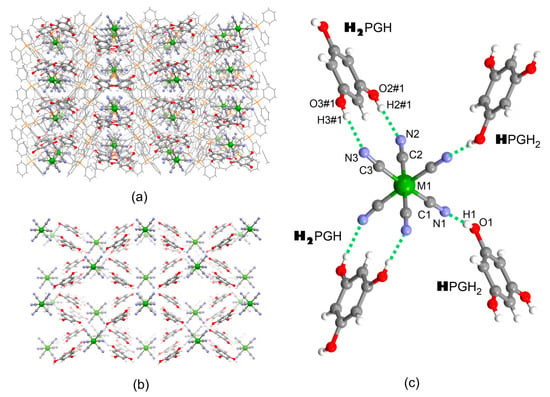
Figure 1.
Crystal structure of 1–3: (a) hydrogen-bonded {[M(CN)6]3−;{(H3PG)2} layers spread along the ab crystallographic plane; (b) perpendicular view of hydrogen-bonded {[M(CN)6]3−;(H3PG)2} layers along c crystallographic direction (PPh4+ cations and MeCN solvent molecules omitted for clarity); (c) supramolecular hydrogen-bonded cis-“bis(chelate)” {[M(CN)6]3−(H2PGH)2(HPGH2)2} as a part of the hydrogen-bonded single layer (for metric parameters see Table 1). Legend: green—Cr, Fe or Co, grey—C, blue—N, red—O, orange—P, white—H; protons involved in hydrogen bonds with one [M(CN)6]3− are indicated in bold.

Table 1.
The most important hydrogen-bonding parameters and related angles in 1–3 (in [Å] or in [deg]; see also Figure 1c).
The molecular environment of the tectons engaged in the hydrogen-bond networks is completed by the oligomeric arrays of PPh4+ cations accompanied by some MeCN molecules; these components form multiple weak C-H⋅⋅⋅A interactions (A = O atoms and ring system of H3PG, N atoms of [M(CN)6]3−) in the regions not involved in the typical hydrogen bonds (Figure S3). The {PPh4+}∞ 3D subnetwork itself provides substantial structural stabilization through so-called multiple phenyl embrace (MPE) motifs, here realized mainly by the sextuple phenyl embrace (SPE) or offset sextuple phenyl embrace (OSPE) and other hybrid patterns, with the shortest P⋅⋅⋅P distances of 6.2, 6.6, and 7.3 Å in all structures [51,52] (Figure S4).
The non-covalent cis-bis(chelated) {[M(CN)6]3−(H2PGH)2(HPGH2)2} motif observed in the crystal structures of 1–3 obeys the local C2 symmetry axis passing through the M1 center (this local axis converges with the lattice C2 axes). Again, as in the case of the cis-bis(chelated) [M(L2A2)] building blocks, this feature might be important from the standpoint of the molecular design of chiral and non-centrosymmetric molecular materials. Such local arrangement is a rare feature among architectures hosting non-bridged [M(CN)6]3− anions and might be realized with the tris(2-aminoethyl)amine or tris(2-pyridylmethyl)amine ligand of the neighboring cationic complex [53]. On the contrary, more examples of trans-bischelation were found in the structural database, including synthons with multiple hydrogen bond donors as large cyclic alkane-polyammonium organic cations [54] or alkane-polyamine ligands of other metal complexes in the structure [55,56,57]. Interestingly, the hydrogen-bonded trischelation involving bis(amidinium) dications and [M(CN)6]3− tectons might be distinguished along with the series of honeycomb 2D tectonic networks reported by Ferlay and Hosseini [12,13,14], important from the viewpoint of porosity design. However, these synthons were not considered in terms of local symmetry by these authors. Our 2D square-grid hydrogen-bonded arrangement provides a new topological solution, an alternative to the 2D honeycomb architecture based on H3PG and [Co2Fe2(CN)6(tp*)2(bpy*)4]2+ cationic block (tp* = hydrotris(3,5-dimethylpyrazol-1-yl)borate, bpy* = 4,4′-dimethyl-2,2′-bipyridine), and involved in the thermal and light-induced electron-transfer-coupled spin transitions (ETCST) [23].
It must be underlined that the described hydrogen-bonded network definitely dominates the overall architecture in 1–3, which results in the significant modification of the [M(CN)6]3− surroundings compared to that in the crystal structures of the (PPh4)3[Cr(CN)6]·2H2O (refcode SEGFAM) [58] and (PPh4)3[Fe(CN)6]·7H2O (refcode VOLVEZ) [59]. This modification consists of the saturation of hydrogen-bonding connections around [M(CN)6]3− exploiting much stronger Brønsted ring-OH acid compared to H-OH or ring-C-H groups (Figure S5), with a high potential to change the spectroscopic characteristics of 1–3 relative to their parent compounds (see below).
2.2. Hirshfeld Analysis
The square-grid-like anionic subnetwork is possible due to two synthons based on moderate-to-strong hydrogen bonds. These features can be observed on the Hirshfeld surfaces [60,61,62] generated for the [Fe(CN)6]3− anion and H3PG molecule (Figure 2a–d). They emerge as red spots marking distances shorter than a sum of van der Waals radii and also as spikes on corresponding fingerprints. This analysis also shows that in the case of the [Fe(CN)6]3− unit, N⋅⋅⋅H interactions prevail (74.4%) (Figure S6). For H3PG, it is only 14.7% of created contacts, but they are the shortest ones and correspond to hydrogen bonds, whereas O⋅⋅⋅H distances are more numerous (23.1%) but much longer, and the tiny spike at (1.4, 1.1) corresponds to two C-H⋅⋅⋅O hydrogen bonds (Figure S7). Both synthons can be clearly visible in Figure S8. The interface is formed by H⋅⋅⋅N and H⋅⋅⋅C atoms for double synthons and H⋅⋅⋅N for single ones. For H3PG, the most numerous (38.2%) H⋅⋅⋅H interactions are created with surrounding PPh4+ cations. The C-H⋅⋅⋅N hydrogen-bonds trap PPh4+ units in the anionic subnetwork, which supports the compartmentalization of the cations despite electrostatic repulsion. Dance et al. explained this effect by aromatic rings embraces [51] (see above), which can also be nicely identified on Hirshfeld surfaces (Figure 2e–h). These contacts are formed by hydrogen and carbon atoms of two interacting PPh4+ units (Figures S9 and S10). Due to asymmetric unit content, the column pattern is given as ⋅⋅⋅P1-P1-P2⋅⋅⋅ and there are two interfaces between two P1 moieties and P1 and P2 units. Figure S11 present all interfaces involved in these interactions with prevailing H⋅⋅⋅C and H⋅⋅⋅H contacts. Mutual orientation of phenyl rings involved in this embrace points to SPE or OSPE motifs. There are also some red spots on the Hirshfeld surface which were identified as C-H⋅⋅⋅N hydrogen bonds with [Fe(CN)6]3− anions and acetonitrile molecules (Figures S9g and S10g). They occur as small spikes at ca. (0.95, 1.35) and ca. (1, 1.4) (Figures S9h and S10h). Hence, these interactions are involved in the network stabilization and interactions between both sublattices (Figure 3). The positions of (PPh4)+ cations and the surface color indicate that weak interactions prevail, corresponding to X⋅⋅⋅H (X = H, C, N) created by the [Fe(CN)6]3− anion and H3PG involved in π-π interactions between strongly inclined rings.
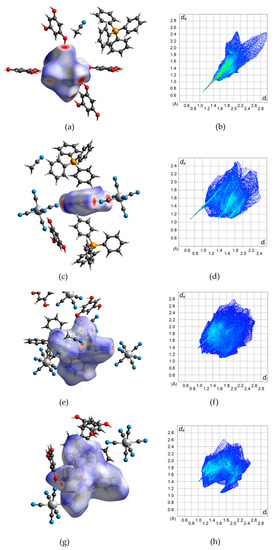
Figure 2.
Hirshfeld surfaces (left column) of the molecular building blocks in 2 and corresponding fingerprints (right column) for all interactions: (a,b)–[Fe(CN)6]3−; (c,d)–H3PG; (e,f)–P(1)Ph4+; (g,h)–P(2)Ph4+. The detailed images for all individual interactions are given in the Supplementary Materials (Figures S6, S7, S9 and S10).
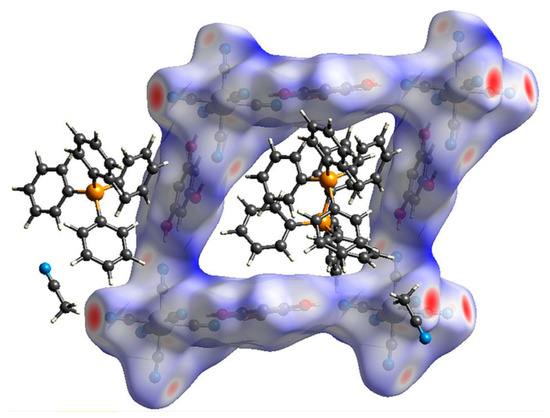
Figure 3.
Hirshfeld surface presenting contacts formed by the anion subnetwork grid.
2.3. ESI-MS
The ESI-MS spectrograms in the negative ionization mode measured for MeOH solutions of 1–3 do not differ significantly from each other. All of them consistently exhibit the presence of three mono-negative peak-sets assignable to the salt-like {(PPh4)2[M(CN)6]}– aggregates (m/z = 886.24– for 1, 890.23– for 2, and 893.23– for 3) (as a dominating feature), and to two progressive co-crystal-like {(PPh4)2[M(CN)6](H3PG)} – (m/z = 1012.27– for 1, 1016.26– for 2, and 1019.27– for 3) and {(PPh4)2[Fe(CN)6](H3PG)2}– (m/z = 1138.32– for 1, 1142.29– for 2, and 1145.30– for 3) aggregates (Figure 4 and Figure S12). A similar progression in positive ionization mode involves the mono-positive {(PPh4)4[M(CN)6]}+ (m/z = 1565.48+ for 1, 1569.47+ for 2, and 1572.45+ for 3) (as a dominating feature), {(PPh4)4[M(CN)6](H3PG)}+ (m/z = 1691.49+ for 1, 1695.52+ for 2, and 1698.48+ for 3), and {(PPh4)4[Fe(CN)6](H3PG)2}+ (m/z = 1821.50+ observed only for 2) aggregates (Figure S13). The absence of {(PPh4)4[Cr(CN)6](H3PG)2}+ and {(PPh4)4[Co(CN)6](H3PG)2}+ motifs might be attributed to competitive fragmentation events. Interestingly, in the spectral m/z range of 1980+–2850+, the Fe congener 2 shows numerous peak-sets of the isotopic patterns assignable to the aggregates of the general formula {(PPh4)x[Fe(CN)6]y(H3PG)z}n+, (x, y, z–small natural numbers; n = 2, 3). Among them, we recognized the distinct peak-sets located at every 63 m/z unit and showed the component lines separation of m/z = 0.5 units, attributable to Fe-containing aggregates of double positive charge. The other peak-sets were located at every 42 m/z unit and exhibited the component lines separation of m/z = 0.33, and were tentatively assigned to Fe-containing aggregates of triple-positive charge. This conforms with the possible enrichment of successive aggregates with an additional H3PG molecule (molecular mass of 126 D). The above observations indicate the notable tendency for the formation of the aggregates between [M(CN)6]3− anions and H3PG in the gas phase, which is in line with the structural data.
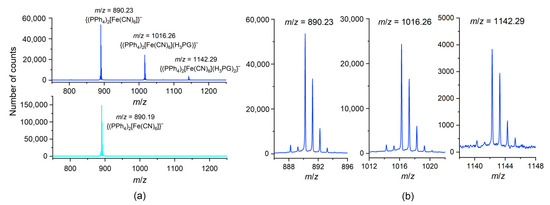
Figure 4.
The ESI–MS spectra in the negative ionization mode for 2 (blue) and for (PPh4)3[Fe(CN)6]·6H2O (cyan) as a reference: (a) the m/z range of 750––1250– showing the peak-sets assigned to {(PPh4)2[M(CN)6]}–, {(PPh4)2[M(CN)6](H3PG)}–, and {(PPh4)2[Fe(CN)6](H3PG)2}– aggregates; (b) the details of the relevant isotopic patterns. The spectra are fully representative for the whole series 1–3, based on the perfect fit of the individual isotopic patterns.
2.4. DFT Calculations
To shed some light on the strength and nature of the interaction between the [M(CN)6]3− anion and H3PG molecule(s) in 1–3, dispersion-corrected density functional theory (DFT + D4) [63] calculations were performed employing molecular cluster models, {[M(CN)6]3−;(H2PGH)2(HPGH2)2}, {[M(CN)6]3−;H2PGH} and {[M(CN)6]3−;HPGH2}, extracted from the corresponding crystal structures. For a comparison, analogous analyses were also carried out for 4,4′-bipyridyl/H3PG {4,4′bpy;HPGH2} and trans-1,2-bis(4-pyridyl)ethylene/H3PG {dpe;HPGH2} clusters extracted from crystal structures reported in Refs. [25,64], respectively. A full description of computational details used in these studies, along with additional calculated results, are provided in the Supplementary Materials (Table S4, Figures S14–S17).
Table 2 and Table S4 list values of interaction energy between the [M(CN)6]3− and neighboring H3PG molecule(s) (either four following the cis-bis(chelated) {[M(CN)6]3−;(H2PGH)2(HPGH2)2} structural fragment or one corresponding to the double hydrogen-bonded {[M(CN)6]3−;H2PGH} and single hydrogen-bonded {[M(CN)6]3−;HPGH2} motifs) and between 4,4′bpy or dpe and H3PG in {4,4′bpy;HPGH2} and {dpe;HPGH2} used as a reference. As can be seen, computed for a given motif does not show a strong dependence on the basis set nor the density functional employed in the calculations, although the double-hybrid functionals (expected to give the most accurate results [65]) systematically indicate somewhat stronger interactions between hydrogen-bond acceptor and hydrogen-bond donor molecules in the examined clusters compared to standard gradient and global hybrid functionals. Note also that tremendously decreased magnitude interaction energies were obtained for the motifs extracted from 1–3 when the acetonitrile continuum solvent model was employed in the calculations (see Table S4), in line with additional energetic stabilization of the charged [M(CN)6]3− fragment in such electrostatic medium; as in the crystal structures, the charge of the hexacyanidometallate anion is also screened to some extent by the surrounding moieties (cations in particular), and we expect the [M(CN)6]3−/H3PG interaction energies to be smaller in magnitude than those determined by the gas-phase calculations although definitely not so diminished as indicated by solvation ones. Nevertheless, all the methods uniformly demonstrate: (i) a slight increase in the magnitude of interaction energies between [M(CN)6]3− and H3PG molecule(s) in the cis-bis(chelated) {[M(CN)6]3−;(H2PGH)2(HPGH2)2} and double hydrogen-bonded {[M(CN)6]3−;H2PGH} when going from system 1 (M = Cr) through 3 (Co) to 2 (Fe), (ii) overall similar values of the interaction energy between [M(CN)6]3− and H3PG in the single hydrogen-bonded {[M(CN)6]3−;HPGH2} motif in all three compounds, and (iii) the pronounced (more than double) increase in the magnitude of these energies when compared with the corresponding single hydrogen-bonded reference clusters. We also note in passing that the computed interaction energies for {[M(CN)6]3−;H2PGH} and {[M(CN)6]3−;HPGH2} overall do not appear to be additive, that is, their respective sum does not reproduce (in fact, it generally exceeds) the value obtained for {[M(CN)6]3−;(H2PGH)2(HPGH2)2}. This is not surprising as, in such 1:1 motifs, the H-bonding component of the [M(CN)6]3−/H3PG interaction might be more effective compared to that in the motif with [M(CN)6]3− surrounded by four hydrogen-bond donor molecules. Even the interaction energy in the double {[M(CN)6]3−;H2PGH} synthon is overall slightly smaller (less negative) than the sum of interaction energies of two simple {[M(CN)6]3−;HPGH2} motifs. This small energetical penalty might be attributed to a minor steric hindrance that appears when two resorcinol-like H-O-ring groups accommodate the cis-oriented cyanido ligands in [M(CN)6]3−, which is in agreement with the hydrogen-bond distances observed for both motifs in the crystal structure. Nevertheless, this does not diminish the overall strength and significance of the interaction within the double cyclic synthons.

Table 2.
DFT-computed interaction energy values (in kcal mol−1) between [M(CN)6]3− (M = Cr, Fe, Co) anion and H3PG molecule(s) in molecular clusters {[M(CN)6]3−;(H2PGH)2(HPGH2)2}, {[M(CN)6]3−;H2PGH}, and {[M(CN)6]3−;HPGH2} extracted from the crystal structures of 1, 2, and 3. For comparison, the corresponding interaction energies between 4,4′-bipyridyl (4,4′bpy) and H3PG in molecular cluster {4,4′bpy;HPGH2} extracted from the crystal structure reported in Ref. [64] are listed.
To comment on the nature of [M(CN)6]3−/H3PG interaction in 1–3, extended transition state–natural orbitals for chemical valence (ETS-NOCV) [66] charge and bonding-energy decomposition analyses were then performed for double hydrogen-bonded {[Co(CN)6]3−;H2PGH} and single hydrogen-bonded {[Co(CN)6]3−;HPGH2} motifs, the results of which, along with those obtained for the reference systems, are presented in Figure 5 and Figure S15–S17. As expected, the dominant NOCV contributions to the differential electron density, that is, the redistribution of electron density around [Co(CN)6]3−, 4,4′bpy or dpe and H3PG in the considered molecular clusters, describe hydrogen-bonding interactions and clearly show its covalent nature via visible hydrogen-bond acceptor and donor charge-transfer (CT) interaction between the occupied lone-pair of nitrogen and the unoccupied σ* orbital of the O–H bond, accompanied in the case of [Co(CN)6]3−/H3PG by the participation of π-type orbitals of both moieties (see below) [66]. The orbital component of the hydrogen bonds present in {[Co(CN)6]3−;H2PGH} and {[Co(CN)6]3−;HPGH2} appears to be rather energetically similar with a slight enhancement as that in {[Co(CN)6]3−;HPGH2}, in line with its shortest distance. The one observed in {4,4′bpy;HPGH2}, of comparable hydrogen-bonding separation as in {[Co(CN)6]3−;HPGH2}, demonstrates a non-negligible decrease, indicating that nitrogen in negatively charged [M(CN)6]3− might be a better hydrogen-bond acceptor. The analysis of the interaction energy components obtained using the ETS energy decomposition scheme (see Figure 5 and Figure S15–S17) enabled us to elucidate further a large difference in the interaction between [Co(CN)6]3− and H3PG and between 4,4′bpy or dpe and H3PG in the considered molecular clusters. Namely, the results show that the strong interaction in {[Co(CN)6]3−;H2PGH} and {[Co(CN)6]3−;HPGH2} is determined by both electrostatic and orbital-interaction components [67], with the latter being represented not only by the σ-CT hydrogen-bonding channel but primarily, as indicated by the analysis of other energetically relevant NOCV contributions, by the polarization (intra-CT) of the π-electron system within H3PG, enhanced likely due to ion–dipole interaction imposed by the negative charge of the [Co(CN)6]3−. Such electron-transfer channels in {4,4′bpy;HPGH2} and {dpe;HPGH2} are visibly diminished, which seems to be directly responsible for a pronounced decrease in the magnitude of the orbital-interaction contribution (even for {dpe;HPGH2}, for which a shorter hydrogen-bond distance significantly strengthens its corresponding orbital component, see Figure S17). This decrease in along with less stabilizing provides less counterbalance for the repulsive Pauli interaction and, accordingly, a significant drop in the absolute values of for {4,4′bpy;HPGH2} and {dpe;HPGH2} was observed.
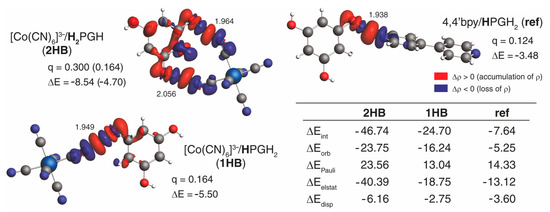
Figure 5.
Results of ETS-NOCV analysis of the interaction between [M(CN)6]3− anion and H3PG molecule in {[Co(CN)6]3−;H2PGH} and {[Co(CN)6]3−;HPGH2} molecular clusters extracted from the crystal structure of 3 and between 4,4′-bipyridyl (4,4′bpy) and H3PG in {4,4′bpy;HPGH2} molecular cluster extracted from the crystal structure reported in Ref. [64] used here as a reference. Isosurfaces (±0.0005 au) of dominant NOCV contributions to the differential electron density Δρ describing hydrogen bonding along with their charge (q in e) and orbital energy (ΔE in kcal mol−1) assessment. Two values of q and ΔE provided for the {[Co(CN)6]3−;H2PGH} motif correspond to the total assessment for both hydrogen bonds and to the shortest one only (given in parentheses). Numbers listed close to the O–H···N contacts are the hydrogen-bond distances, in Å. In the table: The corresponding interaction energy components (in kcal mol−1) as obtained using the ETS energy decomposition scheme are presented. Based on BLYP + D4//TZP calculations.
2.5. Spectroscopic Studies
2.5.1. Vibrational Studies
The IR spectra of 1–3 in the 4000–700 cm−1 and 600–100 cm−1 range (Figure 6a, Figures S18 and S19) combine the spectral features of all relevant structural components: ν(O-H) vibrations of H3PG; ν(C-H) vibrations of H3PG, PPh4+ and MeCN; ν(C≡N) vibrations of [M(CN)6]3− and MeCN, and a full set of skeletal vibrations of H3PG and PPh4+ (for details see Materials and methods). H3PG and all co-crystal salts 1–3 show a significant spread of the band assigned to the ν(O-H) vibrations in the 3600–2500 cm−1 range due to the presence of a hydrogen-bond network. However, for 1–3, the high wavenumber limit and the position of the band maximum are visibly shifted to lower energy (all in cm−1): from 3600 to ca. 3470 and from 3195 to ca. 3120, respectively. This can be interpreted in terms of the increased strength of hydrogen bonds (and decreased strength of O-H bonds) in 1–3 compared to those in H3PG [35,36,68], expected based on the difference in Brønsted acidity of H3PG and H2O and the basicity of H3PG and [M(CN)6]3−. The bands characteristic of ν(C≡N) are notably shifted to the higher wavenumber (ca. 20 cm−1) in 1–3 compared to the precursors (all in cm−1): from 2112 m towards 2126 m, 2129 m, and 2140 m for 1, from 2098 s, 2109 w, and 2116 vw towards 2116 s, 2129 w and 2135 vw for 2, and from 2106 m(sh), 2109 s and 2122 towards 2128 s and 2140 m for 3 (Figure 6a) [20,69,70,71]. The above spectral changes are consistent with the occurrence of CN−⋅⋅⋅H-OH3PG hydrogen bonds, notably stronger and extended, compared to rather local CN−⋅⋅⋅H-OH2O hydrogen bonds observed in the crystal structures of (PPh4)3[M(CN)6]·nH2O salts (Figure S5) [58,59]. While the fingerprint region is not diagnostic for the modification of the vibrational (and electronic) structure (Figure S18), the spectra in the FIR region 600–100 cm−1 indicate notable hipsochromic shifts of the skeletal δ(Cr-C-N), δ(Cr-C-N), and ν(Cr-C) vibrations, and bathochromic shifts of selected H3PG vibrations (Figure S19), being representative to the whole 1–3 series [9,69,72]. Modification of vibrational structure in 1 compared to the precursors, occurring due to a relocation of the electronic density as the result of the hydrogen-bonding architecture, was also confirmed by Raman spectra (Figures S20–S22) [69].
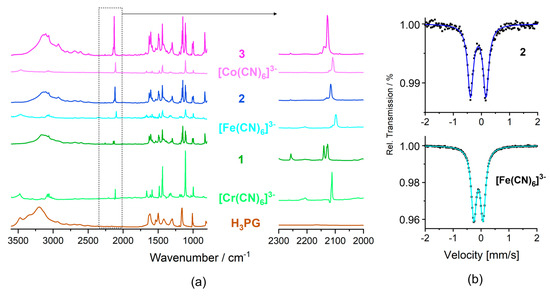
Figure 6.
(a) Infrared spectra of 1-3 in the absorption mode compared with the spectra of H3PG and (PPh4)3[M(CN)6]·nH2O precursor salts with enlargement of including the ν(C≡N) stretching vibration range shown on the right. (b) 57Fe Mössbauer spectra of 2 (top) and of the (PPh4)3[Fe(CN)6]·7H2O precursor (bottom).
2.5.2. 57Fe Mössbauer Spectra
The solid-state 57Fe Mössbauer spectra for 2 and the (PPh4)3[Fe(CN)6]·7H2O reference are presented in Figure 6b. Both spectra were reproduced using single doublets assignable to the low-spin (LS) FeIII state expected for the LS [Fe(CN)6]3− anion. Co-crystal salt 2 reveals the isomeric shift δ2 = –0.11 mm s−1, and quadrupole splitting QS2 = 0.55 mm s−1. δ2 is smaller than δref = –0.09 mm s−1 for the reference salt, which is in line with more significant electron density removal from the 3 d metal valence orbitals expected for more extended and stronger hydrogen bonds CNN⋅⋅⋅H-OH3PG in 2 compared to those present in the reference salt. QS2 is considerably larger than QSref = 0.33 mm s−1 for the reference, which might be related to a specific distribution of the cyanido ligands of the local C2 symmetry and slightly decreased degeneracy of the t2g orbital set due to the bis(chelate)-like arrangement of four H3PG hydrogen bond donors. The observed negative correlation between δ and QS change is in line with the tendency found for a set of hydrated and dehydrated PBAs [1,73].
2.5.3. UV-Vis Electronic Absorption Spectra
The colors of the starting materials are: white for H3PG, pale yellow for [Cr(CN)6]3− salt, yellow for [Fe(CN)6]3− salt, and white for [Co(CN)6]3− salt, whereas the obtained co-crystal salts are yellow (1), yellow–brownish (2), and pale yellow (3). The solid-state UV-Vis absorption spectra for 1–3, together with the spectra of H3PG and of the respective (PPh4)3[M(CN)6]·nH2O precursors in the 200–800 nm range, are presented in Figure 7. In general, the most important spectral features characteristic for [M(CN)6]3− and H3PG units were reproduced for 1–3, and below, we only discuss the directly detectable changes observed individually for each product compared to the spectra of the precursors.
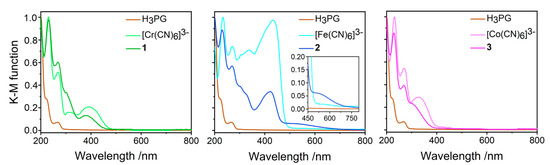
Figure 7.
Electronic absorption UV-Vis spectra of 1–3 in the solid-state compared to H3PG and respective (PPh4)3[M(CN)8]⋅nH2O precursors. The reflectance spectra were recalculated into the Kubelka-Munk finction. Colors: 1—green, 2—blue, and 3—pink.
Compounds 1 and 3 showed the hypsochromic shift of the bands assignable to the lowest energy spin-allowed transitions as compared to (PPh4)3[Cr(CN)6]·2H2O and (PPh4)3[Co(CN)6]·6H2O, respectively. For 1, the energies of the 4A2g → 4T2g (4F) and 4A2g → 4T1g (4F) transitions [9,69,74,75,76,77] were increased from 388 to 380 nm (ΔE = 550 cm−1) and from 315 to ca. 290 nm (ΔE of at least of 2700 cm−1; this estimation lacks exactness due to the bands’ overlap). For 3, the energy of the 1A1g → 1T1g transition [70,76,77,78,79] was shifted from 324 to ca. 297 nm (Δ of at least 2800 cm−1). For the Fe(CN)6]3− analogue, the lowest-energy range was dominated by ligand-to-metal charge-transfer (LMCT) σ(CN–) → π(2t2g) transitions with a possible admixture of one of the ligand-field (LF) spin-forbidden transitions [71,76,77,80]. While the whole band was also shifted to a higher energy from 433 nm for the reference towards 422 nm for 2 (ΔE = 600 cm−1), another feature centered at ca. 490 nm appeared for 2, covering the range up to 750 nm. All observed changes should be interpreted in terms of the stabilization or destabilization of the relevant metal and cyano-ligand orbitals involved in the transitions due to the relocation of electronic density along the σ- and π-channels under the impact of the electrostatic field provided by the species surrounding cyanido-ligands. For 1 and 3, the relative increase of ΔO splitting might be directly inferred due to the stronger electrostatic field imposed by six CN−⋅⋅⋅H-OH3PG hydrogen bonds, compared to the CN−⋅⋅⋅H-Owater hydrogen bonds observed in the crystal structures of (PPh4)3[M(CN)6]·nH2O salts [58,59]. The range of the ΔO change is comparable to those reported for [Cr(CN)6]3− and [Co(CN)6]3− anions in the solid matrices of alkali metal halides; however, a sign of this change might depend on the distance and geometry of CN⋅⋅⋅cation motif [69,70,75]. The theoretical consideration of the energy levels for the mono-ionized [Co(CN)6]4– anion suggests that our observations for 1 and 3 might be due to the relative stabilization of 2t2g orbitals, from where the electrons are excited in the LF states [79]. In the case of 2, the interpretation is not so straightforward as the 2t2g orbitals (the incomplete configuration 2t2g5) involved in the LMCT transitions are the electron recipient levels. Thus, in this case, one should also consider the relocation of electronic density on the relevant lower energy orbitals (of 3t1u and/or 2a1g type) or some splitting of the involved orbitally degenerated states under the electrostatic field of hydrogen bonds [77]. The low energy spin forbidden bands of [Cr(CN)6]3− and [Co(CN)6]3− were scarcely detectable in our setup and were not considered in the analysis. A more precise description of the electronic structure of our co-crystal salts might be obtained with the application of more advanced experimental methods based on X-ray absorption and emission, or ultrafast photoelectron spectroscopy coupled with transient infrared studies combined with modern computational methods [71,78,80,81,82,83].
2.5.4. Photoluminescence Studies
The photoluminescence spectra of 1 and the (PPh4)3[Cr(CN)6]⋅2H2O reference at 77 K are presented in Figure 8, both revealing three distinguishable bands of the location and maximum lines specified in Table 3. The photoluminescence pattern observed in the range 750–875 nm is assigned to the 2Eg → 4A2g phosphorescence characteristic of various inorganic solids and hybrid molecular solids involving [Cr(CN)6]3− moiety [9,69,75,84,85] and may be interpreted in terms of its vibronic properties. The positions of these bands are usually indicated in respect to the R1 (0′-0) emission line (here not measured, however, expected to be located at ca. 795–800 nm) and might be attributed to the specific fundamental modes involving some of the skeletal δ(C-Cr-C), δ(Cr-C-N) and δ(Cr-C) vibrations (below 450–500 cm−1; ν9, ν13, ν7, ν8, and ν12 in the increasing energy order) and combination modes (above 500 cm−1) [9,69,75]. The systematic bathochromic shift of ca. 5 nm was observed, going from (PPh4)3[Cr(CN)6]⋅2H2O to 1, which might be interpreted in terms of the modification of molecular surroundings described above, and correlates with the hypsochromic shift of the 4A2g → 4T2g (4F) and 4A2g → 4T1g (4F) transitions in the UV-Vis spectra. This nicely corresponds with the systematic increase of the relevant absorption energy and decrease of the 2Eg → 4A2g phosphorescence energy observed for the solid phases of (PPh4)3[Cr(CN)6]⋅2H2O, 1, and K3[Cr(CN)6], coming from the first one to the last one [69,75,84]. The emission lifetimes τ1 determined using the equation corresponding to the single decay process are (all in ms): 6.8 (77 K) and 5.5 (298 K) for (PPh4)3[Cr(CN)6]⋅2H2O, and 5.1 (77 K) and 4.5 (298 K) for 1 (λexc = 395 nm followed at the various accessible emission lines) (Figures S23 and S24). The observed slight decrease of τ1 for 1 might be tentatively attributed to the specific character of hydrogen bond architecture in 1 described above. The shortening of lifetimes coincides with the decrease of average luminescence quantum yields measured at room temperature, from 10.9(2)% for (PPh4)3[Cr(CN)6]⋅2H2O to 8.84(4)% for 1. More detailed information could be inferred from LHe measurements, supported by computational methods, which are beyond the scope of this study.
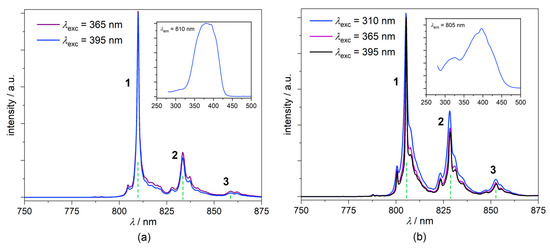
Figure 8.
2Eg → 4A2g emission spectra of 1 (a) and (PPh4)3[Cr(CN)6]⋅2H2O reference (b) at various excitation wavelengths in T = 77 K. The insets show the excitation spectra at λem related to the highest emission intensity in emission spectra.

Table 3.
The energy of 2Eg → 4A2g phosphorescence bands in 1 and (PPh4)3[Cr(CN)6]⋅2H2O in 77 K, in nm (cm−1).
3. Conclusions and Perspectives
The PPh4+ cation-assisted supramolecular self-assembly between the [M(CN)6]3− and triple-point H3PG hydrogen bond donor led to the formation of the new 2D hydrogen-bonded network {[M(CN)6]3−;(H3PG)2} of square-like topology alternative to the 2D hexagonal networks formed between H3PG and [Co2Fe2(CN)6(tp*)2(bpy*)4]2+ [23] or between [M(CN)6]3− and bisaminidnium dications [13]. [M(CN)6]3− complexes located in the nodes of this network are surrounded by four H3PG molecules that form two single M-CN⋅⋅⋅HO-Ar and two double cyclic M(-CN⋅⋅⋅HO-)2Ar synthons into an original non-covalent cis-bis(chelated) {[M(CN)6]3−;(H2PGH)2(HPGH2)2} motif, recognized by analogy with the cis-bis(chelated) 6-coordinate [ML2A2] complexes. Hirshfeld analysis indicated the notable intensity of the underlaying interactions. The strong mutual affinity of both tectons was confirmed by ESI-MS spectrometry and was nicely quantified by the substantial energies of interactions reaching ca. 23–27 kcal mol−1 for simple {[M(CN)6]3−;HPGH2} and ca. 45–50 kcal mol−1 for double cyclic {[M(CN)6]3−;H2PGH} motifs obtained based on the gas-phase DFT + D4 calculations for the molecular clusters extracted from the crystal structures. Thus, the H3PG proved to be an efficient double-point receptor unit for [M(CN)6]3− anions thanks to the molecular matching between the resorcinol-like face of H3PG and cis-oriented pairs of cyanido ligands of [M(CN)6]3−. The observed {[M(CN)6]3−;(H2PGH)2(HPGH2)2} motif revealed chiral (C2) local surroundings of the [M(CN)6]3− complex, which might be of importance in the broader context of supramolecular design towards interesting symmetry-related structure-properties schemes. The spectroscopic characteristics of 1–3 revealed notable modifications as compared to the (PPh4)3[M(CN)6]⋅nH2O references characterized by less saturated hydrogen-bond surroundings of [M(CN)6]3− involving relatively weaker hydrogen-bond donors; this also includes the photoluminescent properties of the [Cr(CN)6]3− anion.
The above findings might be important from the standpoint of the design of modular multisite anion receptors dedicated to binding of d-metallates and the development of alternative pathways towards the controlled synthesis of new multicomponent (coordination-based, hybrid organic-inorganic, etc.) architectures and materials of functional features. In particular, the targeted CCDC search indicates the existence of several very interesting complex molecular motifs exhibiting multiple resorcinol groups [86,87,88,89,90,91,92,93]; they may be definitely considered as potential platforms for multisite anions receptors exploiting the synthons described in this manuscript. Advanced research in this direction is underway in our group.
4. Materials and Methods
K3[Fe(CN)6], K3[Co(CN)6], K3[Cr(CN)6], PPh4Cl, PPh4Br, 1,3,5-trihydroxybenzene, and solvents were purchased from a commercial source (Sigma-Aldrich, Alfa Aesar, etc.) and used without further purification.
4.1. Synthetic Procedures
(PPh4)3[Fe(CN)6]·7H2O, (PPh4)3[Co(CN)6]·6H2O, and (PPh4)3[Cr(CN)6]·2H2O were prepared by metathesis of the corresponding potassium salts of the complexes with PPh4Cl.
Synthesis of 1. Acetonitrile solutions of (PPh4)3[Cr(CN)6]·2H2O (0.227 g, 0.18 mmol in 15 mL of CH3CN) and H3PG (0.0324 g, 0.2 mmol in 15 mL of CH3CN) were mixed to obtain a colorless solution, and the mixture was tightly closed in the vessel. After one day, pale yellow crystals of 1 appeared. The crystals were filtered and washed with cold acetonitrile (10 mL, 2 °C) and dried in air. The composition of (PPh4)3[Cr(CN)6](H3PG)2·2MeCN was defined by a single-crystal X-ray diffraction analysis. Phase purity was proved by XRD data. Yield: 0.168 g, 60.0%. Elemental analysis. Calc. for C94H78CrN8O6P3 (Mw = 1560.55 g·mol−1): C, 72.34%; H, 5.04%; N, 7.18%. Found: C, 72.1%; H, 5.0%; N, 7.1%. IR (KBr, cm−1): 3470–2500 v(O-H), 3059 v(C-H), 2257 v(C≡N) (MeCN), 2126, 2129, 2140 v(C≡N) ([Cr(CN)6]3−), other: 1585, 1481, 1438, 1186, 1109, and 995 (vibrations of PPh4+) 1626, 1606, 1491, 1419 br, 1297, 1155,1146, 1010, 1004, and 832 (vibrations of H3PG). Solubility: MeOH–good, CH3CN–poor. Stability: composition stable up to ca. 80 °C, then loses 2 crystallization MeCN molecules in the range 80–140 °C; above 190 °C massive decomposition occurs (Figure S25).
Synthesis of 2. Acetonitrile solutions of (PPh4)3[Fe(CN)6]·6H2O (0.240 g, 0.018 mmol in 15 mL of CH3CN) and H3PG (0.0324 g, 0.2 mmol in 15 mL of CH3CN) were mixed to obtain a pale yellow solution, and the mixture was tightly closed in the vessel. After one day, brown–yellow crystals of 2 appeared. The crystals were filtered and washed with cold acetonitrile (10 mL, 2 °C) and dried in air. The composition of (PPh4)3[Fe(CN)6](H3PG)2·2MeCN was defined by a single-crystal X-ray diffraction analysis. Phase purity was proved by XRD data. Yield: 0.180 g, 64.0%. Elemental analysis. Calc. for C94H78FeN8O6P3 (Mw = 1564.40 g·mol−1): C, 72.17%; H, 5.03%; N, 7.16%. Found: C, 72.2%; H, 5.0%; N, 7.2%. IR (KBr, cm−1): 3445–2500 v(O-H), 3063 v(C-H), 2257 v(C≡N) (MeCN), 2116, 2130, 1441 v(C≡N) ([Fe(CN)6]3−); other: 1585, 1481, 1438, 1186, 1109, and 995 (vibrations of PPh4+) 1626, 1606, 1491, 1419 br, 1297, 1155,1146, 1010, 1004, 832 (vibrations of H3PG). Solubility: MeOH–good, CH3CN–poor. Stability: composition stable up to ca. 70 °C, then loses 2 crystallization MeCN molecules in the range 70–130 °C; above 170 °C massive decomposition occurs (Figure S25).
Synthesis of 3. Acetonitrile solutions of (PPh4)3[Co(CN)6]·7H2O (0.244 g, 0.018 mmol in 15 mL of CH3CN) and H3PG (0.0324 g, 0.2 mmol in 15 mL of CH3CN) were mixed to obtain a colorless solution immediately, and the mixture was tightly closed in the vessel. After one day, colorless crystals of 3 appeared. The crystals were filtered and washed with cold acetonitrile (10 mL, 2 °C) and dried in air. The composition of (PPh4)3[Co(CN)6](H3PG)2·2MeCN was defined by a single-crystal X-ray diffraction analysis. Phase purity was proved by XRD data. Yield: 0.126 mg, 44.8%. Elemental analysis. Calc. for C94H78CoN8O6P3 (Mw = 1567.48 g·mol−1): C, 72.02%; H, 5.02%; N, 7.15%. Found: C, 72.12%; H, 4.9%; N, 7.1%. IR (KBr, cm−1): 3460–2500 v(O-H), 3066 v(C-H), 2257 v(C≡N) (MeCN), 2128, 2140 v(C≡N) ([Co(CN)6]3−), other: 1585, 1481, 1438, 1186, 1109, 995 (vibrations of PPh4+) 1626, 1606, 1491, 1419 br, 1297, 1155,1146, 1010, 1004, 832 (vibrations of H3PG). Solubility: MeOH–good, CH3CN–poor. Stability: composition stable up to ca. 70 °C, then loses 2 crystallization MeCN molecules in the range 70–120 °C; above 230 °C massive decomposition occurs (Figure S25).
4.2. X-ray Diffraction Analysis
Single crystal X-ray diffraction data for all compounds were collected using a Bruker D8 Quest Eco diffractometer equipped with a Photon II detector and a Mo Kα (λ = 0.71073 Å) radiation source with a graphite monochromator and Oxford Cryostream cooling system. Crystals for measurement were taken from the mother solution and covered by NVH immersion oil. All measurements were performed in 100.0 K. Data reduction and cell parameter refinement were performed using Apex software with included SAINT and SADABS programs. Intensities of reflections for the sample absorption were corrected using the multiscan method. Structures were solved by the intrinsic phasing method and refined anisotropically with weighted full-matrix least-squares on F2 using SHELXT [94] and SHELXL [95] programs with Olex 2 graphic interface [96].
Hydrogen atoms within structures were placed in idealized positions and refined using a riding coordinate model with isotropic displacement parameter set at 1.2–1.5 times Ueq of appropriate carrier atoms. Crystal data and structure refinement parameters are summarized in Table S1. The structural figures in the article were prepared using the latest Mercury software [97]. The crystal structures are deposited in the CCDC database. The deposition numbers are 2,175,600 (1), 2,175,601 (2), and 2,175,602 (3).
4.3. Physical Techniques and Calculations
Elemental analyses of CHNS were performed on the air-dried samples using the Elemental Vario Micro Cube CHNS analyzer. Powder X-ray diffraction patterns for 1, 2, and 3 in 0.5 mm glass capillary were collected on a D8 Advance Eco (Bruker) using a Cu−Kα radiation source. The thermogravimetric (TGA) curves for the polycrystalline samples were collected using TG209 F1 Libra thermogravimetric analyzer with aluminum pans as holders. The data were collected in the temperature range of 21–400 °C under a nitrogen atmosphere with a heating rate of 1 °C per minute. Infrared (IR) absorption spectra in the range 4000–675 cm−1 were measured on the selected single-crystals using a Nicolet iN10 MX Fourier transform infrared microscope. Far infrared (FIR) spectra in the range 600–100 cm−1 were measured on the powder samples dispersed Apiezon N grease using an FT-IR Bruker Vertex 70 V spectrometer. Raman spectra in the range 3200–100 cm−1 were recorded on the microcrystalline samples with a Renishaw inVia Raman spectrometer with the excitation line 514.5 nm of Ar laser. The transmission 57Fe Mössbauer spectra were collected in 1024 channels, with a 10 mCi 57Co source in an Rh matrix, at room temperature using a Wissel spectrometer. The velocity scale was calibrated using the α-Fe foil standard. The powder sample was directly placed in copper rings and sealed with Kapton foil. The background spectra of the sample holders did not reveal any significant contribution to the main spectra. Mössbauer spectra were fitted with the use of the WinNormos-for-Igor software package, assuming the Lorentzian shape of the resonance lines, i.e., the saturation effects were not included. For each compound, one quadrupole doublet was considered in the fitted model, assigned to the FeIII (LS) electron state. Diffuse reflectance spectra in the UV-Vis-NIR range were performed for the ground powder samples mixed with BaSO4 (2 mass %) using a Shimadzu UV-3600i Plus spectrophotometer equipped with the 50 mm integrating sphere. The spectra were recalculated according to the Kubelka–Munk equation. Solid-state photoluminescent characterization for all reported compounds was performed using an FS5 spectrofluorometer (Edinburgh Instruments) equipped with a Xe arc lamp (150 W, excitation spectra) or a 365 nm diode flashlight (10 W, emission spectra) serving as excitation sources, and a Hamamatsu photomultiplier of the R928P type as a detector. All lifetime measurements were conducted using the same spectrofluorometer employing a multichannel scaling module with a microsecond Xe flashlamp (5 W), while the collected data curves were fitted using a monoexponential decay function within a Fluoracle software (Edinburgh Instruments). Absolute quantum yields (QYs) were measured by a direct excitation method using the Xe arc lamp, an integrating sphere module (SC-30), and a Teflon pin as a reference, while the related calculations were performed within the implemented software. The Fluoracle program was also employed for the background corrections and a smoothing procedure, while for the data collected using the 365 nm flashlight, the application of a non-linear baseline was determined using the Asymmetric Least-Square Smoothing method (OriginPro 2021b) was found necessary.
4.4. Hirshfeld Analysis
The structural interaction analyses based on Hirshfeld surfaces were performed in CrystalExplorer [60,61,62].
4.5. Quantum-Chemical Calculations and Analyses
All computations were performed at the density functional theory (DFT) level employing molecular cluster models extracted from the corresponding crystal structures. A full description of computational details used in these studies is provided in the Supplementary Materials [63,66,98,99,100,101,102,103,104,105,106,107,108,109].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules27134111/s1: additional data for structural description (Figures S1–S5, Tables S1–S3); details of Hirshfeld analysis (Figures S6–S11); additional ESI-MS spectra (Figures S12 and S13); computational methodology description and additional calculated results for molecular clusters in the gas phase, and in continuum solvent model (Figures S14–S17, Table S4); additional IR spectra (fingerprint and FIR region) (Figures S18 and S19); Raman spectra (Figures S20–S22); additional emission spectra and emission lifetimes (Figures S23 and S24); TGA characteristics (Figure S25); related literature citations.
Author Contributions
K.J.: investigation—syntheses, measurements, data analysis and visualization of PXRD, TGA, IR spectroscopy, and UV-Vis electronic absorption spectroscopy, writing—original draft fragments preparation; J.K.: investigations—SC XRD structural measurements, crystal structure solution and refinement, data curation; D.G.: investigation–preliminary synthesis, structural data curation and description, participation in photoluminescence measurements, writing—original draft fragments preparation, review; E.K.-G.: participation in conceptualization, preliminary synthesis and investigation, writing—review; D.T.: investigation—DFT calculations, data analysis and visualization; M.S.-H.: conceptualization and investigation—DFT calculations, data analysis and visualization, writing—original draft fragments preparation, review; J.J.Z. investigation—photoluminescence measurements and data visualization, writing—review; K.D.-K.: investigation—measurements of 57Fe Mössbauer spectra, data visualization, writing—review; T.M.M.: investigation—Hirshfeld analysis, data visualization, writing—original draft fragments preparation, review; R.P.: funding acquisition, project administration, conceptualization, supervision, writing—original draft preparation, writing—review, corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the financial support from the National Science Centre (Poland) research project no. UMO-2019/35/B/ST5/01481 (PI R. Podgajny). Measurements were carried out with equipment funded by the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The insight into detailed data might be obtained after the contact with the corresponding author.
Acknowledgments
The authors acknowledge Jan J. Stanek for his support in accessing the 57Fe Mössbauer spectrometer, Szymon Chorazy for his support in accessing the photoluminescence spectrometer and data discussion, Łukasz Hetmańczyk for measurements of FIR spectra, Aleksandra Wesełucha-Birczyńska for her support in accessing the Raman spectrometer, and Paulina Moskal for measurements of Raman spectra. The computational part of the research was supported by PL-Grid Infrastructure and the ACC Cyfronet AGH in Krakow, Poland.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of compounds 1–3 are available from the authors.
References
- Avilaa, Y.; Acevedo-Peña, P.; Reguera, L.; Reguera, E. Recent progress in transition metal hexacyanometallates: From structure to properties and functionality. Coord. Chem. Rev. 2022, 453, 214274. [Google Scholar] [CrossRef]
- Zakrzewski, J.J.; Liberka, M.; Zychowicz, M.; Chorazy, S. Diverse physical functionalities of rare-earth hexacyanidometallate frameworks and their molecular analogues. Inorg. Chem. Front. 2021, 8, 452–483. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Magott, M.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Podgajny, R.; Sieklucka, B. Octacyanidometallates for multifunctional molecule-based materials. Chem. Soc. Rev. 2020, 49, 5945–6001. [Google Scholar] [CrossRef] [PubMed]
- Grothe, E.; Meekes, H.; Vlieg, E.; Ter Horst, J.H.; de Gelder, R. Solvates, salts, and cocrystals: A proposal for a feasible classification system. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, H.-Y.; Graf, R.; Spiess, H.W.; Yao, Y.-F.; Zhu, R.-Q.; Xiong, R.-G. Tunable and Switchable Dielectric Constant in an Amphidynamic Crystal. J. Am. Chem. Soc. 2013, 135, 5230–5233. [Google Scholar] [CrossRef]
- Qian, K.; Shao, F.; Yan, Z.; Pang, J.; Chen, X.; Yang, C. A perovskite-type cage compound as a temperature-triggered dielectric switchable material. CrystEngComm 2016, 18, 7671–7674. [Google Scholar] [CrossRef]
- Rok, M.; Moskwa, M.; Działowa, M.; Bieńko, A.; Rajnák, C.; Boča, R.; Bator, G. Multifunctional materials based on the double-perovskite organic—Inorganic hybrid (CH3NH3)2[KCr(CN)6] showing switchable dielectric, magnetic, and semiconducting behaviour. Dalton Trans. 2019, 48, 16650–16660. [Google Scholar] [CrossRef]
- Rok, M.; Bator, G.; Medycki, W.; Zamponi, M.; Balčiūnas, S.; Šimėnas, M.; Banys, J. Reorientational dynamics of organic cations in perovskite-like coordination polymers. Dalton Trans. 2018, 47, 17329–17341. [Google Scholar] [CrossRef]
- Ma̧czka, M.; Nowok, A.; Zarȩba, J.K.; Stefańska, D.; Ga̧gor, A.; Trzebiatowska, M.; Sieradzki, A. Near-Infrared Phosphorescent Hybrid Organic—Inorganic Perovskite with High-Contrast Dielectric and Third-Order Nonlinear Optical Switching Functionalities. ACS Appl. Mater. Interfaces 2022, 14, 1460–1471. [Google Scholar] [CrossRef]
- Shi, C.; Yu, H.; Wang, Q.-W.; Ye, L.; Gong, Z.-X.; Ma, J.-J.; Jiang, J.-Y.; Hua, M.-M.; Shuai, C.; Zhang, Y.; et al. Hybrid Organic–Inorganic Antiperovskites. Angew. Chem. Int. Ed. 2020, 59, 167–171. [Google Scholar] [CrossRef]
- Ferlay, S.; Félix, O.; Hosseini, M.W.; Planeix, J.-M.; Kyritsakas, N. Second sphere supramolecular chirality: Racemic hybrid H-bonded 2-D molecular networks. Chem. Commun. 2002, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Dechambenoit, P.; Ferlay, S.; Hosseini, M.W.; Planeix, J.-M.; Kyritsakas, N. Molecular tectonics: Control of packing of hybrid 1-D and 2-D H-bonded molecular networks formed between bisamidinium dication and cyanometallate anions. New J. Chem. 2006, 30, 1403–1410. [Google Scholar] [CrossRef]
- Dechambenoit, P.; Ferlay, S.; Kyritsakas, N.; Hosseini, M.W. Molecular Tectonics: Control of Reversible Water Release in Porous Charge-Assisted H-Bonded Networks. J. Am. Chem. Soc. 2008, 130, 17106–17113. [Google Scholar] [CrossRef] [PubMed]
- Dechambenoit, P.; Ferlay, S.; Kyritsakas, N.; Hosseini, M.W. In situ reduction of Fe(III) into Fe(II): An example of post-crystallisation transformation. Chem. Commun. 2009, 6798–6800. [Google Scholar] [CrossRef]
- Olmsted, B.K.; Ferlay, S.; Dechambenoit, P.; Hosseini, M.W.; Ward, M.D. Microscopic Topography of Heterocrystal Interfaces. Cryst. Growth Des. 2009, 9, 2841–2847. [Google Scholar] [CrossRef]
- Catalano, L.; Berthaud, J.; Dushaq, G.; Karothu, D.P.; Rezgui, R.; Rasras, M.; Ferlay, S.; Hosseini, M.W.; Naumov, P. Sequencing and Welding of Molecular Single-Crystal Optical Waveguides. Adv. Funct. Mater. 2020, 30, 2003443. [Google Scholar] [CrossRef]
- Ota, A.; Ouahab, L.; Golhen, S.; Yoshida, Y.; Maesato, M.; Saito, G.; Świetlik, R. Phase Transition from Mott Insulating Phase into the Charge Ordering Phase with Molecular Deformation in Charge-Transfer Salts κ-(ET)4[M(CN)6][N(C2H5)4]·2H2O (M = CoIII and FeIII). Chem. Mater. 2007, 19, 2455–2462. [Google Scholar] [CrossRef]
- Łapiński, A.; Świetlik, R.; Ouahab, L.; Golhen, S. Spectroscopic Studies of the Phase Transition from the Mott Insulator State to the Charge-Ordering State of κ-(ET)4[M(CN)6][N(C2H5)4]·2H2O (M = CoIII and FeIII) Salts. J. Phys. Chem. A 2013, 117, 5241–5250. [Google Scholar] [CrossRef]
- You, M.-H.; Li, M.-H.; Liu, Y.-F.; Li, H.-H.; Lin, M.-J. Unprecedented five-fold interpenetrated donor–acceptor hybrid heterostructure induced by anion–π interactions. CrystEngComm 2019, 21, 6688–6692. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Pinkowicz, D.; Srebro-Hooper, M.; Hooper, J.; Podgajny, R. Anion–π recognition between [M(CN)6]3− complexes and HAT(CN)6: Structural matching and electronic charge density modification. Dalton Trans. 2017, 46, 3482–3491. [Google Scholar] [CrossRef]
- Arranz-Mascarós, P.; Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Godino-Salido, M.-L.; Gutiérrez-Valero, M.-D.; Lopez-Garzón, R.; Savastano, M. Thermodynamics of Anion−π Interactions in Aqueous Solution. J. Am. Chem. Soc. 2013, 135, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; Arranz-Mascarós, P.; Bazzicalupi, C.; Bianchi, A.; Giorgi, C.; Godino-Salido, M.L.; Gutiérrez-Valero, M.D.; López-Garzón, R. Binding and removal of octahedral, tetrahedral, square planar and linear anions in water by means of activated carbon functionalized with a pyrimidine-based anion receptor. RSC Adv. 2014, 4, 58505–58513. [Google Scholar] [CrossRef]
- Sekine, Y.; Nihei, M.; Oshio, H. Dimensionally Controlled Assembly of an External Stimuli-Responsive [Co2Fe2] Complex into Supramolecular Hydrogen-Bonded Networks. Chem. Eur. J. 2017, 23, 5193–5197. [Google Scholar] [CrossRef] [PubMed]
- Santra, R.; Biradha, K. Solid state double [2 + 2] photochemical reactions in the co-crystal forms of 1,5-bis(4-pyridyl)-1,4-pentadiene-3-one: Establishing mechanism using single crystal X-ray, UV and 1H NMR. CrystEngComm 2011, 13, 3246–3257. [Google Scholar] [CrossRef]
- Dubey, R.; Desiraju, G.R. Combinatorial Crystal Synthesis: Structural Landscape of Phloroglucinol:1,2-bis(4-pyridyl)ethylene and Phloroglucinol:Phenazine. Angew. Chem. Int. Ed. 2014, 53, 13178–13182. [Google Scholar] [CrossRef]
- Thomas, L.H.; Craig, G.A.; Morrison, C.A.; Reilly, A.M.; Wilson, C.C. New Route to Local Order Models for Disordered Crystalline Materials: Diffuse Scattering and Computational Modeling of Phloroglucinol Dihydrate. Cryst. Growth Des. 2011, 11, 2045–2049. [Google Scholar] [CrossRef][Green Version]
- Guo, C.; Zhang, Q.; Zhu, B.; Zhang, Z.; Ma, X.; Dai, W.; Gong, X.; Ren, G.; Mei, X. Drug–Drug Cocrystals Provide Significant Improvements of Drug Properties in Treatment with Progesterone. Cryst. Growth Des. 2020, 20, 3053–3063. [Google Scholar] [CrossRef]
- Pal Singh, M.; Baruah, J.B. Detection of hydroxyaromatics in a superior manner by a water soluble fluorescent iron-complex. Inorg. Chim. Acta 2020, 504, 119467. [Google Scholar] [CrossRef]
- Sarma, B.; Sreenivas Reddy, L.; Nangia, A. The Role of π-Stacking in the Composition of Phloroglucinol and Phenazine Cocrystals. Cryst Growth Des. 2008, 8, 4546–4552. [Google Scholar] [CrossRef]
- Braun, D.E.; Tocher, D.A.; Price, S.L.; Griesser, U.J. The Complexity of Hydration of Phloroglucinol: A Comprehensive Structural and Thermodynamic Characterization. J. Phys. Chem. B 2012, 116, 3961–3972. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Bar, A.K.; Mukherjee, P.S. Ruthenium−Oxygen Coordination-Driven Self-Assembly of a RuII8 Incomplete Prism: Synthesis, Structure, and Shape-Selective Molecular Recognition Study. Inorg. Chem. 2010, 49, 10235–10237. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Lee, I.S.; Chung, Y.K. Crystal Engineering with Structurally Flexible 1,1′-Substituted Ferrocenes for Nonlinear Optical Materials. Eur. J. Inorg. Chem. 2003, 2003, 2311–2317. [Google Scholar] [CrossRef]
- Arora, K.K.; Talwelkar, M.S.; Pedireddi, V.R. Supramolecular synthesis of some molecular adducts of 4,4′-bipyridine N,N′-dioxide. New J. Chem. 2009, 33, 57–63. [Google Scholar] [CrossRef]
- Shankar, K.; Singh, M.P.; Baruah, J.B. Extent of protonation of 4,4′-bipyridinium cations and nature of host influences the amount of guest intake by cobalt(II) 2,6-pyridinedicarboxylate. Inorg. Chim. Acta 2018, 469, 440–446. [Google Scholar] [CrossRef]
- Pfletscher, M.; Wölper, C.; Gutmann, J.S.; Mezger, M.; Giese, M. A modular approach towards functional supramolecular aggregates—Subtle structural differences inducing liquid crystallinity. Chem. Commun. 2016, 52, 8549–8552. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.; Krappitz, T.; Dong, R.Y.; Michal, C.A.; Hamad, W.Y.; Patrick, B.O.; MacLachlan, M.J. Tuning the photonic properties of chiral nematic mesoporous organosilica with hydrogen-bonded liquid-crystalline assemblies. J. Mater. Chem. C 2015, 3, 1537–1545. [Google Scholar] [CrossRef]
- Pfletscher, M.; Hölscher, S.; Wölper, C.; Mezger, M.; Giese, M. Structure–Property Relationships in Hydrogen-Bonded Liquid Crystals. Chem. Mater. 2017, 29, 8462–8471. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Pakulski, P.; Potępa, I.; Podgajny, R. Binary and Ternary Core–Shell Crystals of Polynuclear Coordination Clusters via Epitaxial Growth. under revision.
- Shanmugaraju, S.; Bar, A.K.; Joshi, S.A.; Tatil, P.Y.P.; Mukherjee, P.S. Constructions of 2D-Metallamacrocycles Using Half-Sandwich RuII2 Precursors: Synthesis, Molecular Structures, and Self-Selection for a Single Linkage Isomer. Organometallics 2011, 30, 1951–1960. [Google Scholar] [CrossRef]
- Cho, Y.L.; Uh, H.; Chang, S.-Y.; Chang, H.-Y.; Choi, M.-G.; Shin, I.; Jeong, K.-S. A Double-Walled Hexagonal Supermolecule Assembled by Guest Binding. J. Am. Chem. Soc. 2001, 123, 1258–1259. [Google Scholar] [CrossRef]
- Sekiya, R.; Nishikiori, S.; Ogura, K. Crystalline Inclusion Compounds Constructed through Self-Assembly of Isonicotinic Acid and Thiocyanato Coordination Bridges. J. Am. Chem. Soc. 2004, 126, 16587–16600. [Google Scholar] [CrossRef]
- Trokowski, R.; Akine, S.; Nabeshima, T. Synthesis, characterization and molecular recognition of a bis-platinum terpyridine dimer. Chem. Commun. 2008, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Kirillov, A.M.; Baruah, J.B. A modular approach for molecular recognition by zinc dipicolinate complexes. Dalton Trans. 2015, 44, 14411–14423. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.; Abella, D.; Rama, T.; Alvarino, C.; García, M.D.; Peinador, C.; Quintela, J.M. Guest-induced stereoselective self-assembly of quinoline-containing PdII and PtII metallacycles. RSC Adv. 2016, 6, 80181–80192. [Google Scholar] [CrossRef]
- Singh, M.P.; Baruah, J.B. Stable host–guest complexes of bis-2,6-pyridinedicarboxylate iron(III) with dihydroxybenzenes. Polyhedron 2017, 138, 103–108. [Google Scholar] [CrossRef]
- Shankar, K.; Baruah, J.B. Inclusion of dihydroxyaromatics by a lanthanum(III) 2,6-dipicolinate complex. Polyhedron 2017, 126, 262–267. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Paul, M.; Desiraju, G.R. From a Binary to a Quaternary Cocrystal: An Unusual Supramolecular Synthon. Angew. Chem. Int. Ed. 2019, 58, 12027–12031. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Supramolecular Motifs: Concerted Multiple Phenyl Embraces between Ph4P+ Cations Are Attractive and Ubiquitous. Chem.-Eur. J. 1996, 2, 481–486. [Google Scholar] [CrossRef]
- Kuzniak, E.; Hooper, J.; Srebro-Hooper, M.; Kobylarczyk, J.; Dziurka, M.; Musielak, B.; Pinkowicz, D.; Raya, J.; Ferlay, S.; Podgajny, R. A concerted evolution of supramolecular interactions in a {cation; metal complex; π-acid;solvent} anion-π system. Inorg. Chem. Front. 2020, 7, 1851–1863. [Google Scholar] [CrossRef]
- Parker, R.J.; Spiccia, L.; Batten, S.R.; Cashion, J.D.; Fallon, G.D. The Encapsulation of Ferrocyanide by Copper(II) Complexes of Tripodal Tetradentate Ligands. Novel H-Bonding Networks Incorporating Heptanuclear and Pentanuclear Heterometallic Assemblies. Inorg. Chem. 2001, 40, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Bencini, A.; Bianchi, A.; Garcia-Espana, E.; Giusti, M.; Mangani, S.; Micheloni, M.; Orioli, P.; Paoletti, P. Anion coordination chemistry. 2. Electrochemical, thermodynamic, and structural studies on supercomplex formation between large polyammonium cycloalkanes and the two complex anions hexacyanoferrate(II) and hexacyanocobaltate(III). Inorg. Chem. 1987, 26, 3902–3907. [Google Scholar] [CrossRef]
- Brorson, M.; Galsbøl, F.; Simonsen, K.; Skov, L.K.; Søtofte, I. Preparation and Characterization of cis- and trans-[Ir(tn)2Cl2]CF3SO3 and of [Ir(tn)3]Cl3 (tn=propane-1,3-diamine). Acta Chem. Scand. 1998, 52, 1017–1023. [Google Scholar] [CrossRef][Green Version]
- Kou, H.Z.; Jiang, Y.-B.; Zhou, B.C.; Wang, R.J. Cyano-Bridged 2D CuII−CrIII Coordination Polymers: Structural Evidence for Formation of a Polymeric Macrocyclic Metallic Compound. Inorg. Chem. 2004, 43, 3271–3276. [Google Scholar] [CrossRef]
- Cvrtila, I.; Stilinović, V. New Tricks by Old Anions: Hydrogen Bonded Hexacyanoferrous Anionic Networks. Cryst. Growth Des. 2017, 17, 6793–6800. [Google Scholar] [CrossRef]
- Alborés, P.; Slep, L.D.; Weyhermüller, T.; Rentschler, E.; Baraldo, L.M. Exchange coupling across the cyanide bridge: Structural and DFT interpretation of the magnetic properties of a binuclear chromium(III) complex. Dalton Trans. 2006, 948–954. [Google Scholar] [CrossRef]
- Graham, M.J.; Zadrozny, J.M.; Shiddiq, M.; Anderson, J.S.; Fataftah, M.S.; Hill, S.; Freedman, D.E. Influence of Electronic Spin and Spin–Orbit Coupling on Decoherence in Mononuclear Transition Metal Complexes. J. Am. Chem. Soc. 2014, 136, 7623–7626. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A generally applicable atomic-charge dependent London dispersion correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef] [PubMed]
- Coupar, P.I.; Ferguson, G.; Glidewell, C. Hydrogen-Bonded Chains in 4,4′-Dihydroxybenzophenone-4,4′-Bipyridyl (1/1) and Chains of Rings in 1,3,5-Trihydroxybenzene-4,4′-Bipyridyl (2/3). Acta Cryst. 1996, C52, 2524–2528. [Google Scholar] [CrossRef]
- Goerigk, L.; Mehta, N. Trip to the Density Functional Theory Zoo: Warnings and Recommendations for the User. Aust. J. Chem. 2019, 72, 563–573. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef]
- van der Lubbe, S.C.C.; Guerra, C.F. The Nature of Hydrogen Bonds: A Delineation of the Role of Different Energy Components on Hydrogen Bond Strengths and Lengths. Chem.-Asian J. 2019, 14, 2760–2769. [Google Scholar]
- Perruchas, S.; Boubekeur, K.; Molinié, P. Radical cation salts of TTF(CH2OH)4 with cyanide anions: Synthesis, crystal structure and magnetic properties of [TTF(CH2OH)4][TCNQF4] and [TTF(CH2OH)4]3[18-crown-6 ⊂ K][M(CN)6]·DMF (M = Fe, Co). Polyhedron 2005, 24, 1555–1564. [Google Scholar] [CrossRef]
- Hanuza, J.; Strek, W.; Hermanowicz, K.; Jezowska-Trzebiatowska, B.; Trabjerg, I. Spectroscopic behaviour of Cr(CN)63− ion isolated in KCl host. J. Mol. Struct. 1986, 144, 141–153. [Google Scholar] [CrossRef]
- Jain, S.C.; Warrier, A.V.R.; Sehgal, H.K. Vibrational and electronic properties of Co(CN)63− doped in alkali halides. J. Phys. C Sol. St. Phys. 1972, 5, 1511–1518. [Google Scholar] [CrossRef]
- Ross, M.; Andersen, A.; Fox, Z.W.; Zhang, Y.; Hong, K.; Lee, J.-H.; Cordones, A.; March, A.M.; Doumy, G.; Southworth, S.H.; et al. Comprehensive Experimental and Computational Spectroscopic Study of Hexacyanoferrate Complexes in Water: From Infrared to X-ray Wavelengths. J. Phys. Chem. B 2018, 122, 5075–5086. [Google Scholar] [CrossRef]
- Kuzniak, E.; Pinkowicz, D.; Hooper, J.; Srebro-Hooper, M.; Hetmańczyk, Ł.; Podgajny, R. Molecular Deformation, Charge Flow, and Spongelike Behavior in Anion–π {[M(CN)4]2–;[HAT(CN)6]}∞ (M=Ni, Pd, Pt) Supramolecular Stacks. Chem. Eur. J. 2018, 24, 16302–16314. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, R.; Knobel, M.; Reguera, E. Modification of the magnetic properties in molecular magnets based on Prussian blue analogues through adsorbed species. J. Phys. Condens. Matter 2006, 18, 11243–11254. [Google Scholar] [CrossRef]
- Hendrickx, M.F.A.; Mironov, V.S.; Chibotaru, L.F.; Ceulemans, A. An Ab Initio Study of the Ligand Field and Charge-Transfer Transitions of Cr(CN)63− and Mo(CN)63−. J. Am. Chem. Soc. 2003, 125, 3694–3695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanuza, J.; Strek, W.; Hermanowicz, K.; Jezowska-Trzebiatowska, B.; Trabjerg, I. Spectroscopic properties of Cr(CN)63− doped in a KBr crystal. Chem. Phys. 1984, 86, 137–145. [Google Scholar] [CrossRef]
- Mitsuru, S.; Hirohiko, A.; Hideo, Y. The DV-Xα MO Study of the Electronic Structures of [M(CN)6]3− (M = Cr, Mn, Fe, and Co) and [Fe(CN)6]4−. Bull. Chem. Soc. Jap. 1981, 54, 2898–2903. [Google Scholar]
- Alexander, J.J.; Gray, H.B. Electronic structures of hexacyanometalate complexes. J. Am. Chem. Soc. 1968, 90, 4260–4271. [Google Scholar] [CrossRef]
- Sreekantan, S.; Lalithambika, N.; Atak, K.; Seidel, R.; Neubauer, A.; Brandenburg, T.; Xiao, J.; Winter, B.; Aziz, E.F. Chemical bonding in aqueous hexacyano cobaltate from photon- and electron-detection perspectives. Sci. Rep. 2017, 7, 40811. [Google Scholar]
- Sano, M.; Hatano, Y.; Kashiwagi, H.; Yamatera, H. An Ab Initio Calculations of the Electronic Structure of the [Co(CN)6]3− Ion. Bull. Chem. Soc. Jap. 1981, 54, 1523–1530. [Google Scholar] [CrossRef]
- Hahn, A.W.; Van Kuiken, B.E.; Chilkuri, V.G.; Levin, N.; Bill, E.; Weyhermüller, T.; Nicolaou, A.; Miyawaki, J.; Harada, Y.; DeBeer, S. Probing the Valence Electronic Structure of Low-Spin Ferrous and Ferric Complexes Using 2p3d Resonant Inelastic X-ray Scattering (RIXS). Inorg. Chem. 2018, 57, 9515–9530. [Google Scholar] [CrossRef]
- Ojeda, J.; Arrell, C.A.; Longetti, L.; Chergui, M.; Helbing, J. Charge-transfer and impulsive electronic-to-vibrational energy conversion in ferricyanide: Ultrafast photoelectron and transient infrared studies. Phys. Chem. Chem. Phys. 2017, 19, 17052–17062. [Google Scholar] [CrossRef]
- Yu, P.; Yang, F.; Zhao, J.; Wang, J. Hydration Dynamics of Cyanoferrate Anions Examined by Ultrafast Infrared Spectroscopy. J. Phys. Chem. B 2014, 118, 3104–3114. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, M.; Sun, Z.; Gaffney, K.J. Dynamics of Solvent-Mediated Electron Localization in Electronically Excited Hexacyanoferrate(III). J. Am. Chem. Soc. 2012, 134, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Sieklucka, B.; Ohkoshi, S. Near-Infrared Photoluminescence in Hexacyanido-Bridged Nd–Cr Layered Ferromagnet. Cryst. Growth Des. 2016, 16, 4918–4925. [Google Scholar] [CrossRef]
- Kitzmann, W.R.; Moll, J.; Heinze, K. Spin-fip luminescence. Photochem. Photobiol. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liang, Y.; Ma, P.; Li, S.; Wang, J.; Niu, J. Self assembly of carboxylate/alcoholate functionalized ring-shape phosphomolybdates. CrystEngComm 2014, 16, 8041–8046. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Liu, J.-L.; Yang, H.-M.; Jia, A.-Q.; Zhang, Q.-F. Inclusion of ferrocene within the deepened cavities formed from the hydrogen bonding self-assembly of calix[4]resorcinarenes with bis-pyridines. J. Incl. Phenom. Macro. 2016, 85, 105–110. [Google Scholar] [CrossRef]
- Papaefstathiou, G.S.; Darrow, B.G.; MacGillivray, L.R. Crystal and molecular structure of [Cu2(3,5-dihydroxybenzoate)4 (acetonitrile)2]·8H2O. J. Chem. Crystallogr. 2002, 32, 191–195. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Z.; Zeller, M.; Luck, R.L. Dicopper moieties stabilized by Fréchet-type dendrons: Syntheses and structural characterizations. Polyhedron 2014, 80, 206–215. [Google Scholar] [CrossRef]
- Chaumont, C.; Mobian, P.; Kyritsakas, N.; Henry, M. Synthesis, topology and energy analysis of crystalline resorcinol-based oligophenylene molecules with various symmetries. CrystEngComm 2013, 15, 6845–6862. [Google Scholar] [CrossRef]
- Dan, M.; Cheetham, A.K.; Rao, C.N.R. Diverse Structures and Dimensionalities in Hybrid Frameworks of Strontium and Lanthanum with Isomeric Dihydroxybenzoates. Inorg. Chem. 2006, 45, 8227–8238. [Google Scholar]
- Liwporncharoenvong, T.; Luck, R.L. Quadruply Bonded Dimolybdenum Atoms Surrounded by Dendrons: Preparation, Characterization, and Electrochemistry. J. Am. Chem. Soc. 2001, 123, 3615–3616. [Google Scholar] [CrossRef] [PubMed]
- Bhyrappa, P.; Wilson, S.R.; Suslick, K.S. View Author Information Hydrogen-Bonded Porphyrinic Solids: Supramolecular Networks of Octahydroxy Porphyrins. J. Am. Chem. Soc. 1997, 119, 8492–8502. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF 2019.304, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: https://www.scm.com (accessed on 21 April 2022).
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Santra, G.; Sylvetsky, N.; Martin, J.M.L. Minimally Empirical Double-Hybrid Functionals Trained against the GMTKN55 Database: RevDSD-PBEP86-D4, RevDOD-PBE-D4, and DOD-SCAN-D4. J. Phys. Chem. A 2019, 123, 5129–5143. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Schüürmann, G. COSMO: A New Approach to Dielectric Screening in Solvents with Explicit Expressions for the Screening Energy and Its Gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like Screening Model for Real Solvents: A New Approach to the Quantitative Calculation of Solvation Phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Regular Two-Component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Total Energy Using Regular Approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A. Natural Orbitals for Chemical Valence as Descriptors of Chemical Bonding in Transition Metal Complexes. J. Mol. Model. 2007, 13, 347–355. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).