Insight into the Nucleation Mechanism of p-Methoxybenzoic Acid in Ethanol-Water System from Metastable Zone Width
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials
2.2. Measurement of Solubility
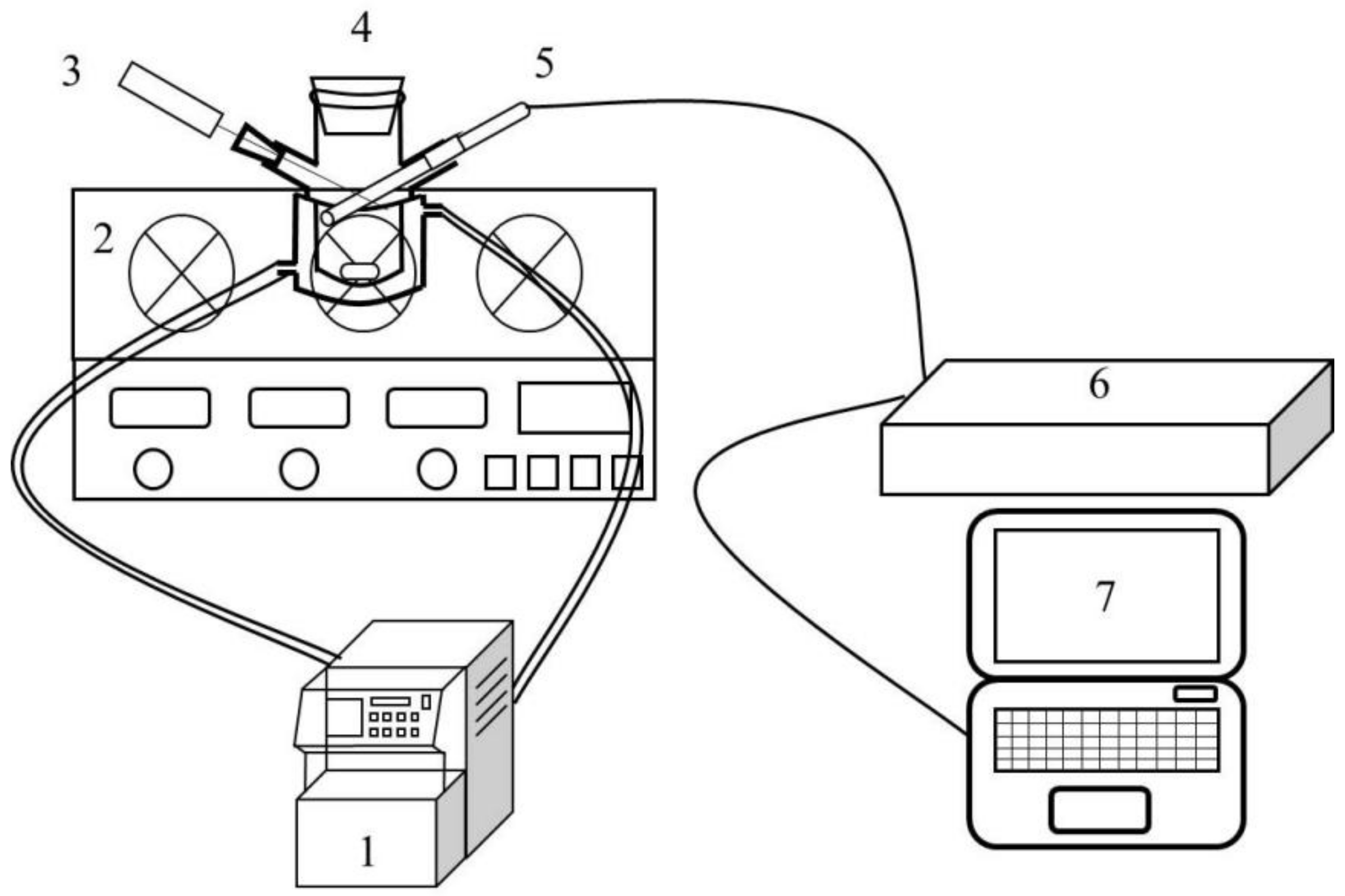
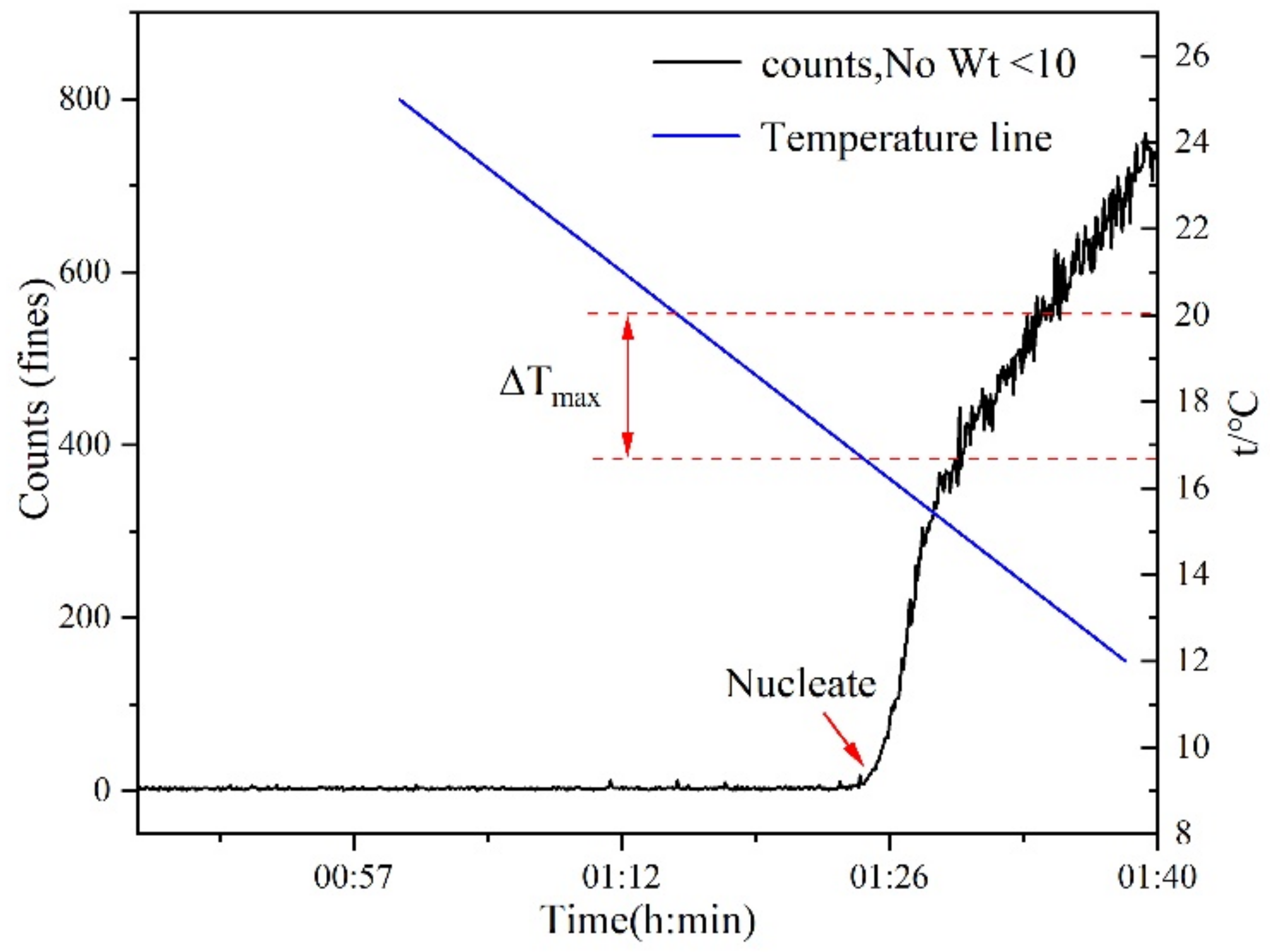
2.3. Metastable Zone Measurement Experiment for PMBA
2.4. X-ray Powder Diffraction
3. Theory
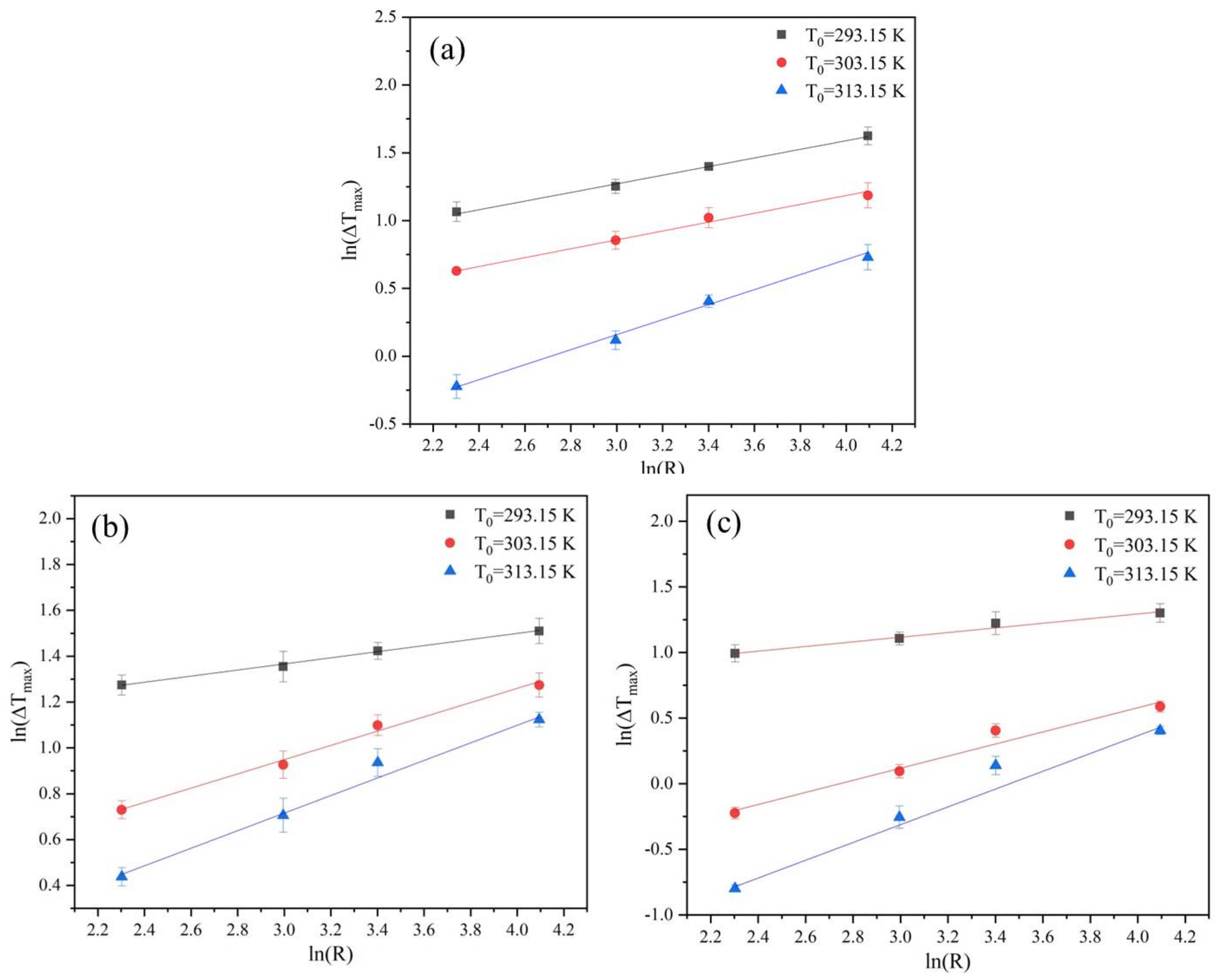
3.1. Nývlt’s Metastable Zone Model
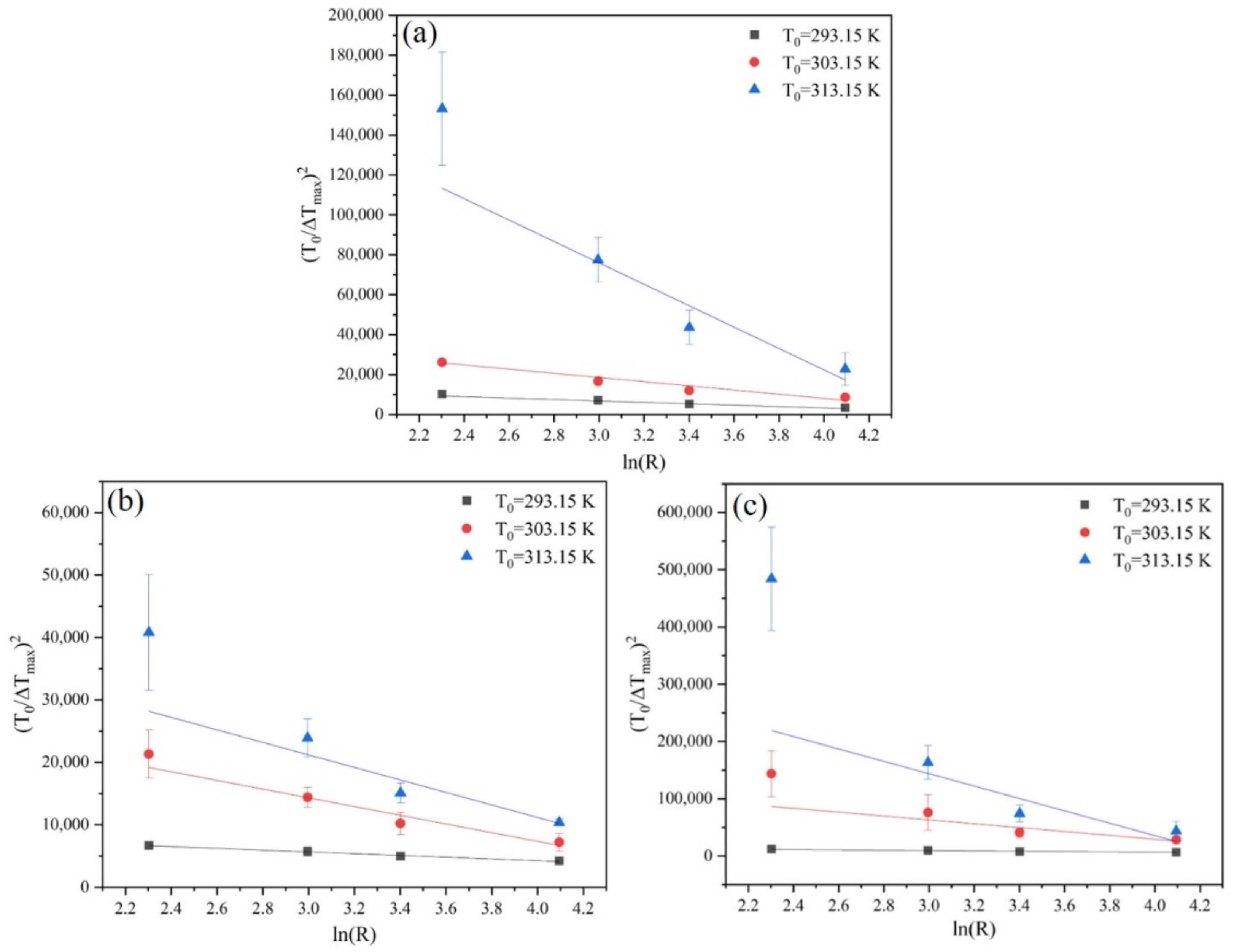
3.2. Sangwal Metastable Zone Model
3.3. Modified Sangwal Metastable Zone Model
4. Results and Discussion
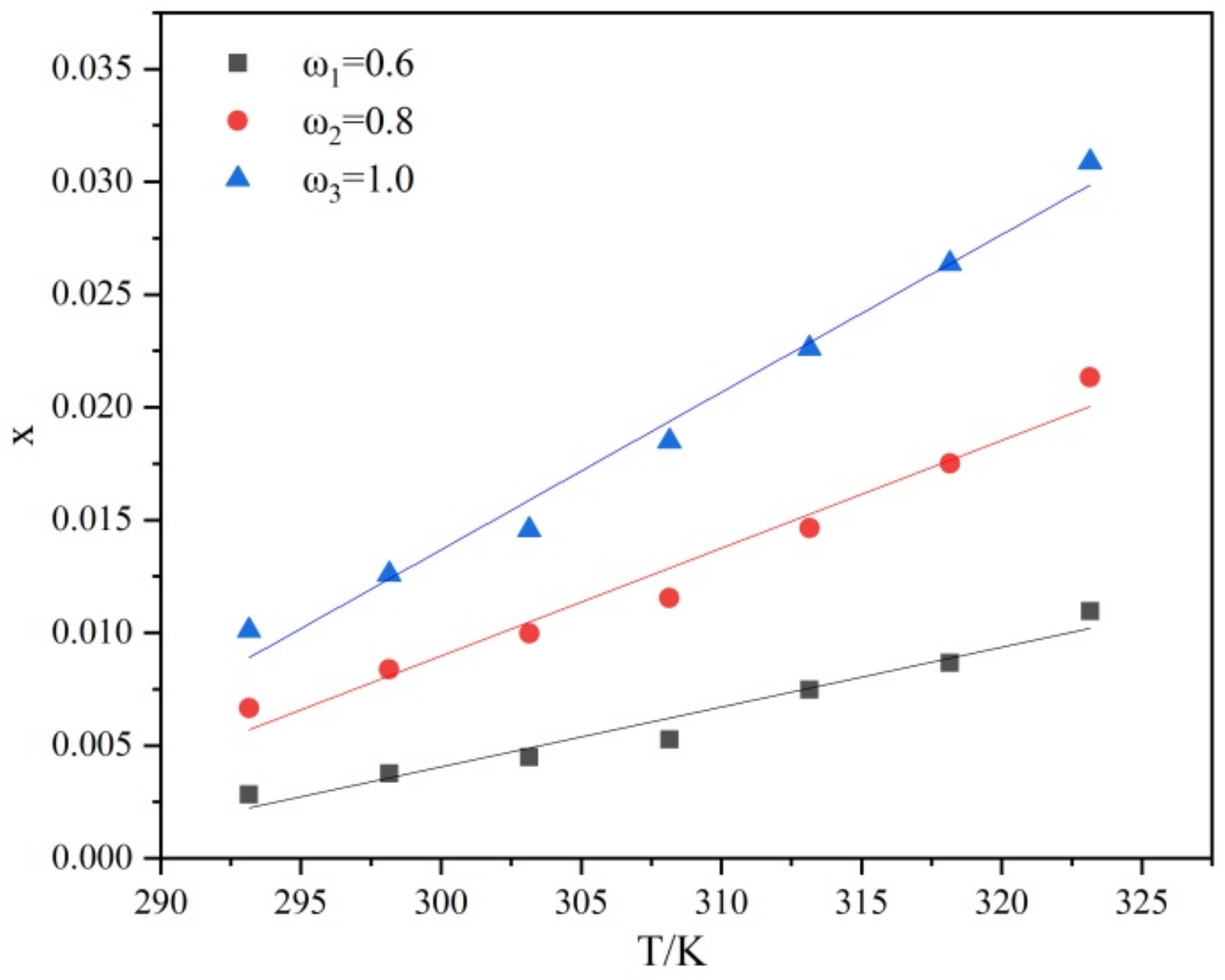
4.1. Solubility of PMBA in Ethanol Solution
4.2. Solid-State Characterization of PMBA
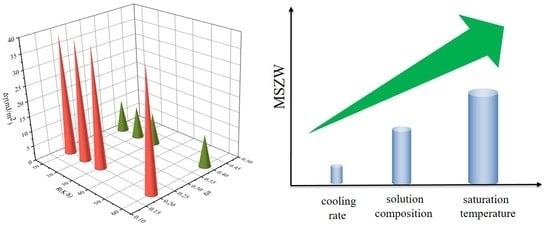
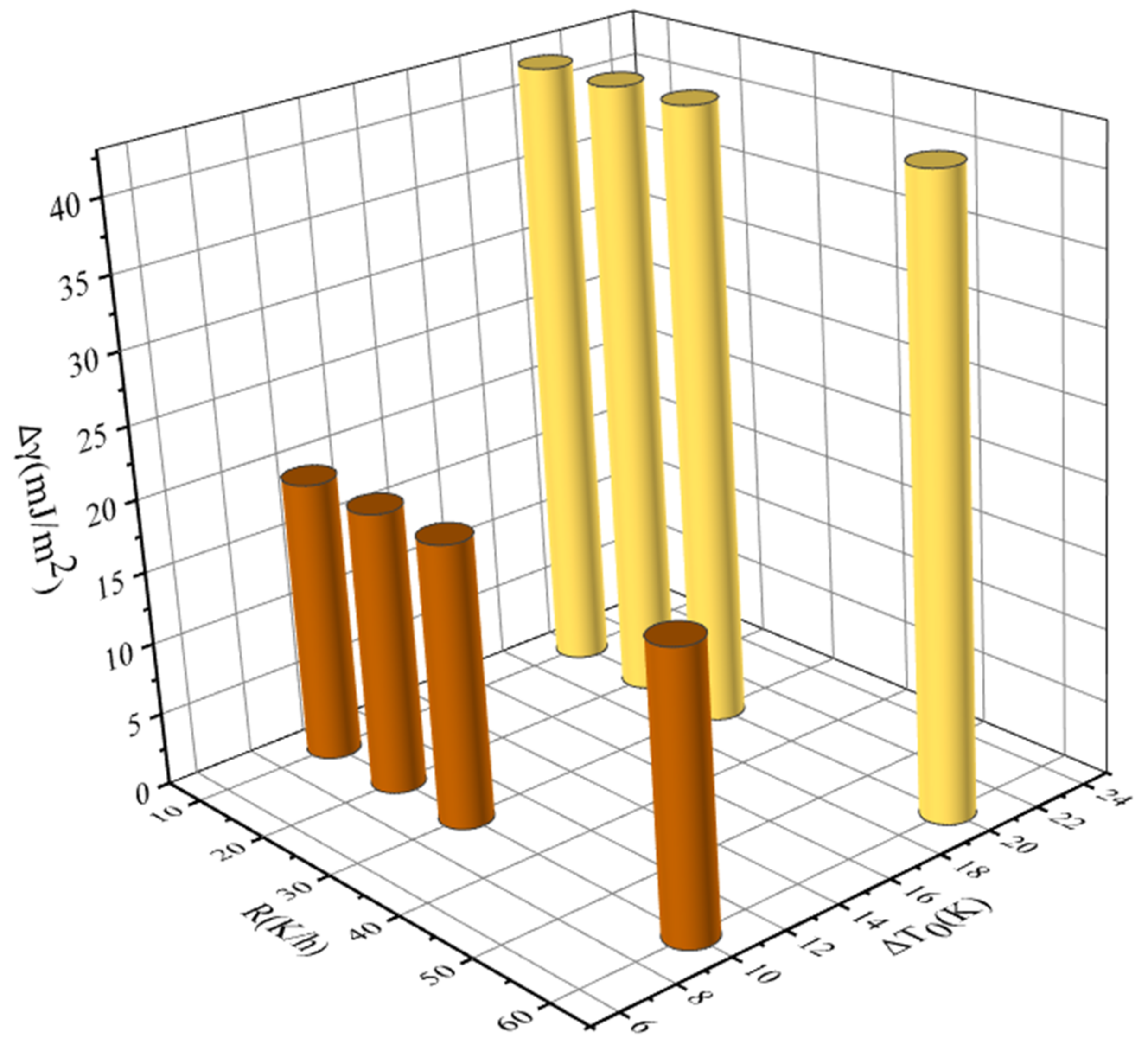
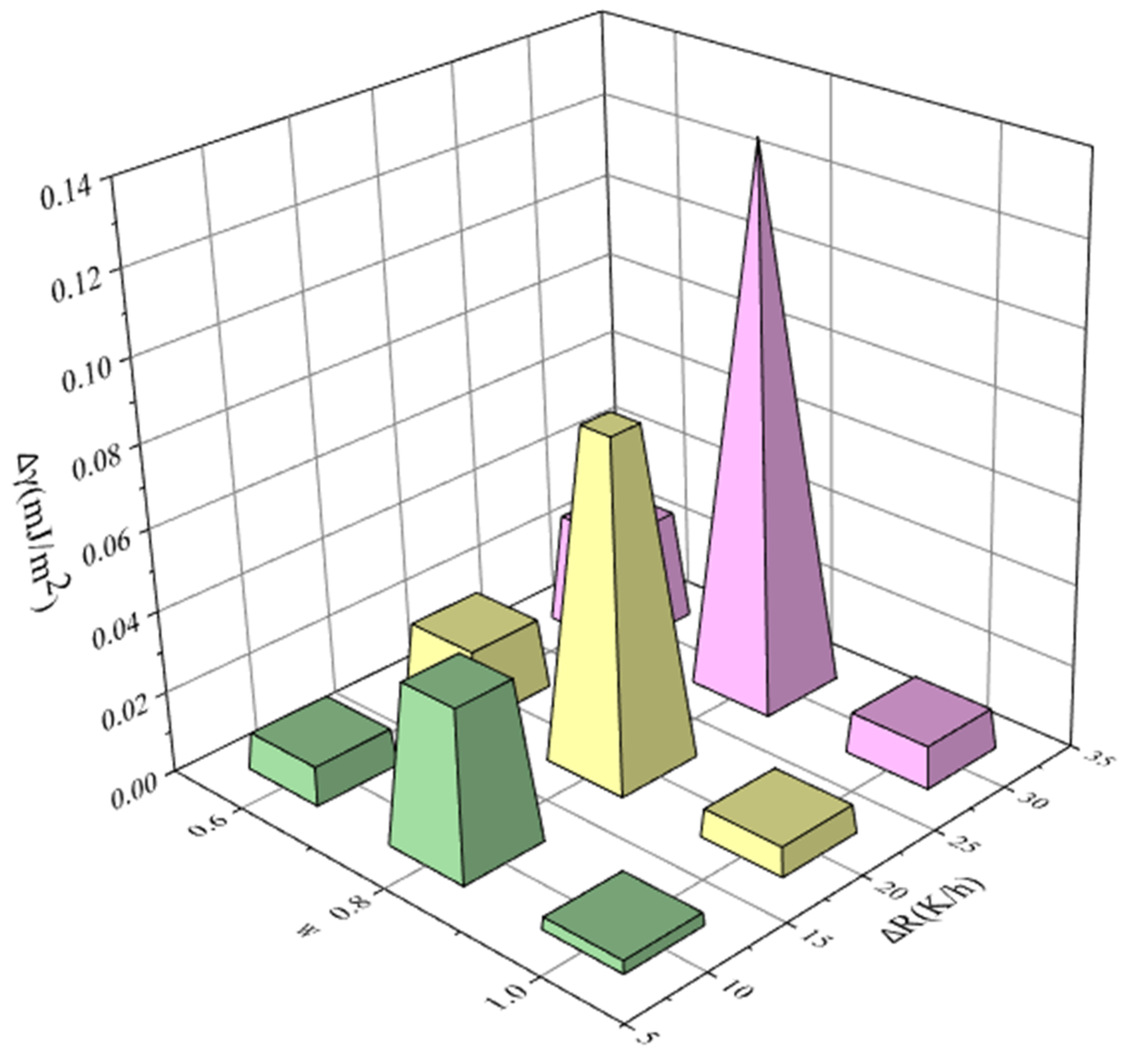
4.3. Effects of Saturation Temperature, Cooling Rate, and Ethanol Mass Fractions on MSZW
4.4. Nucleation Kinetic Parameters and Crystal Habit
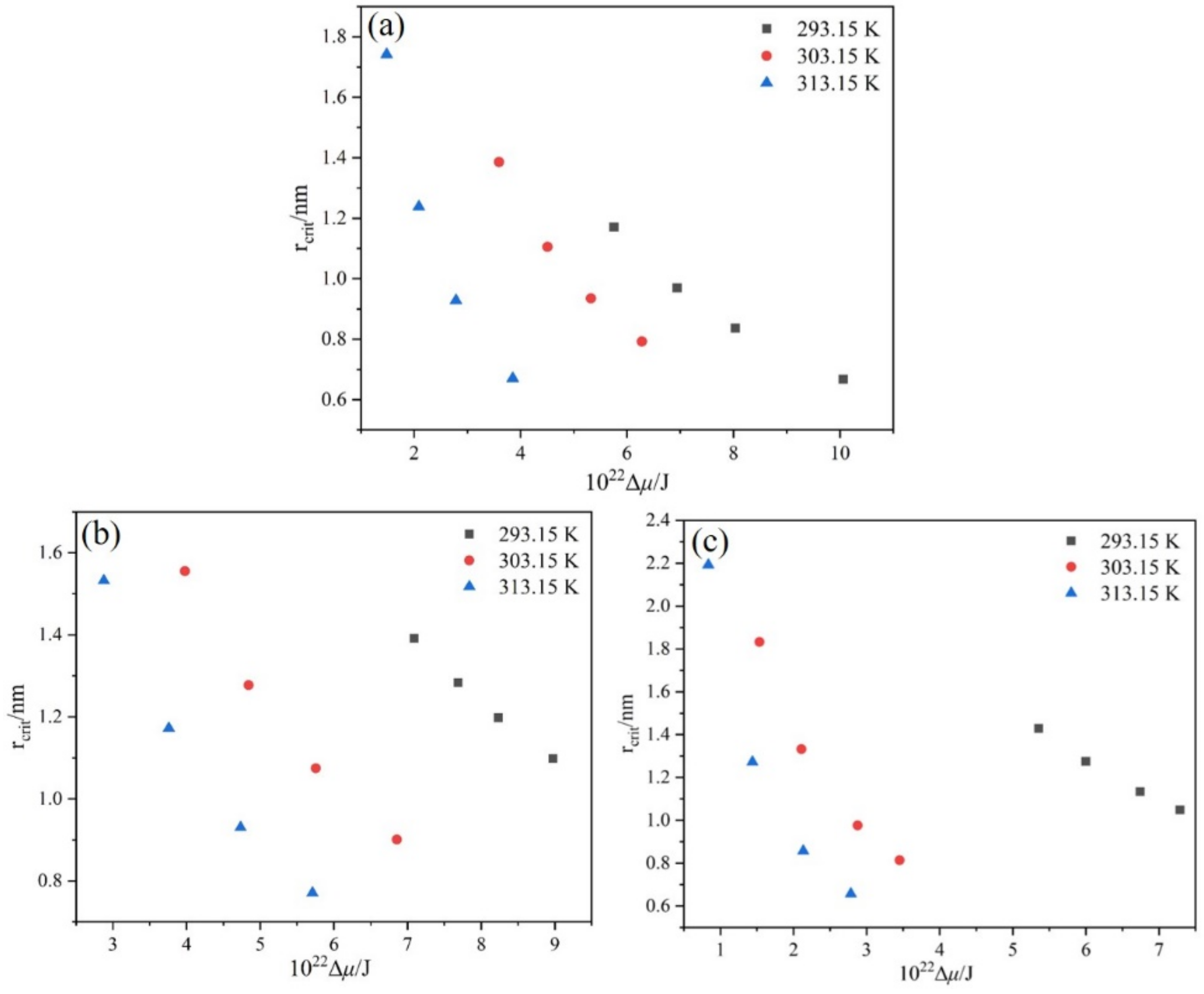
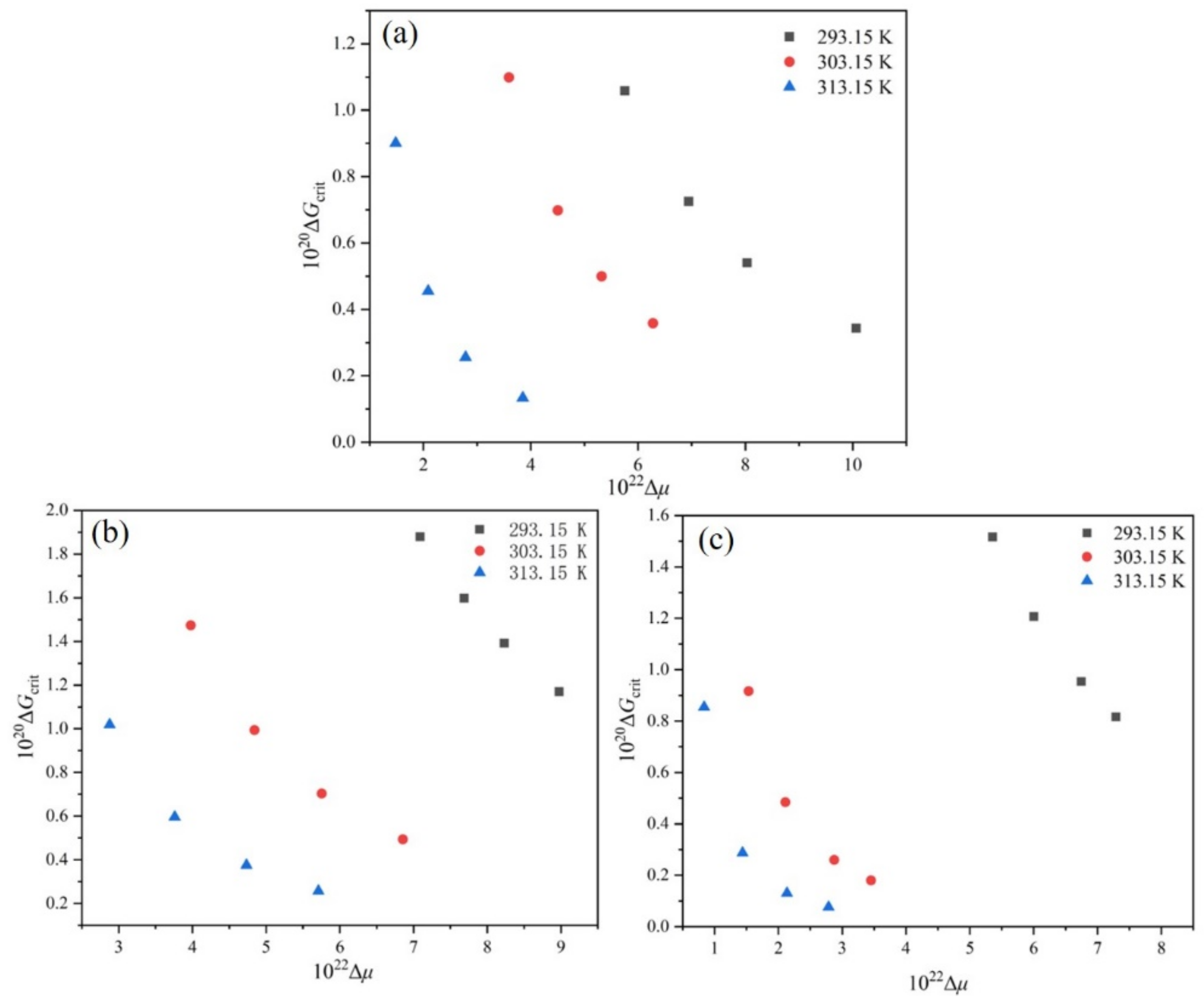
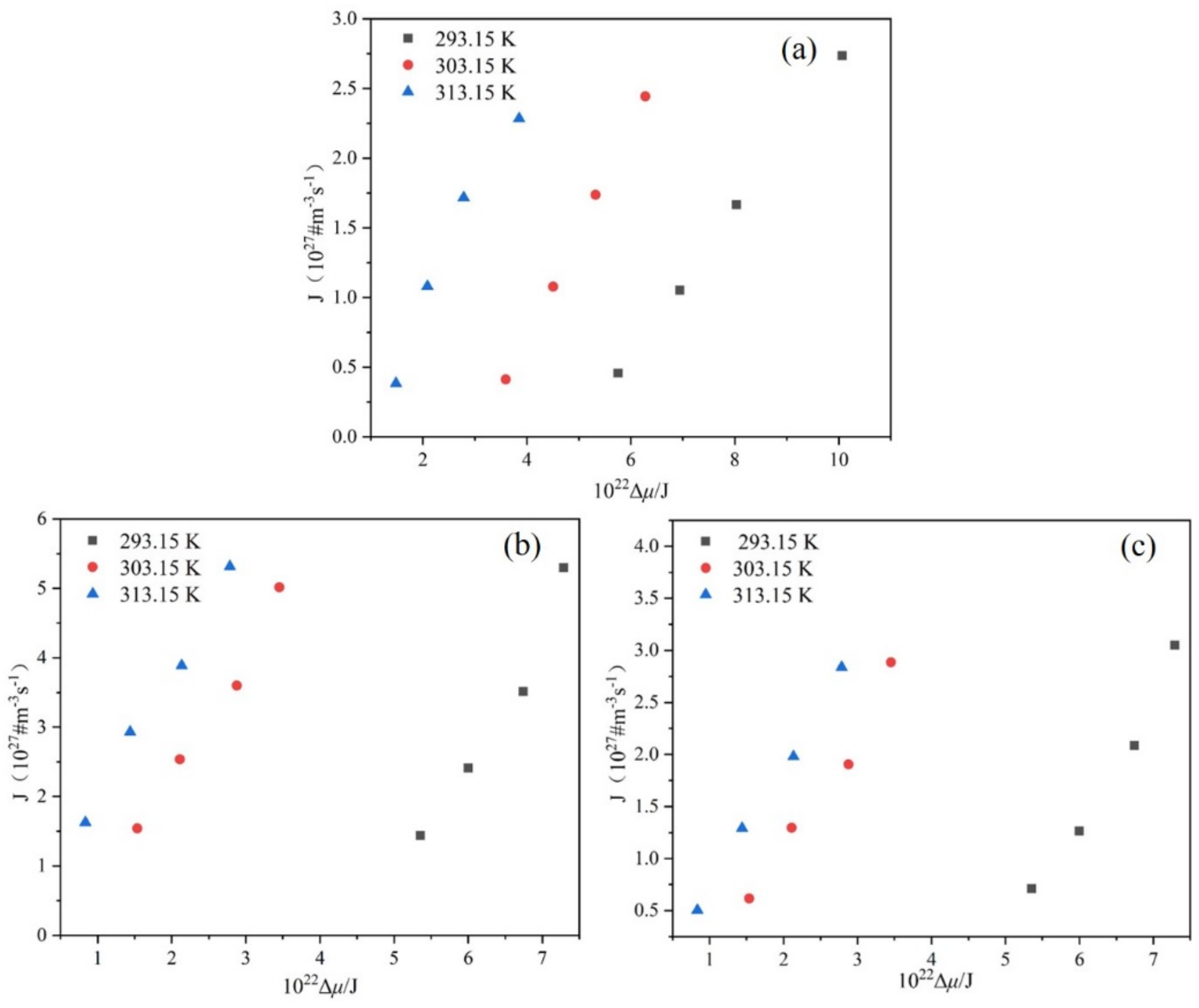
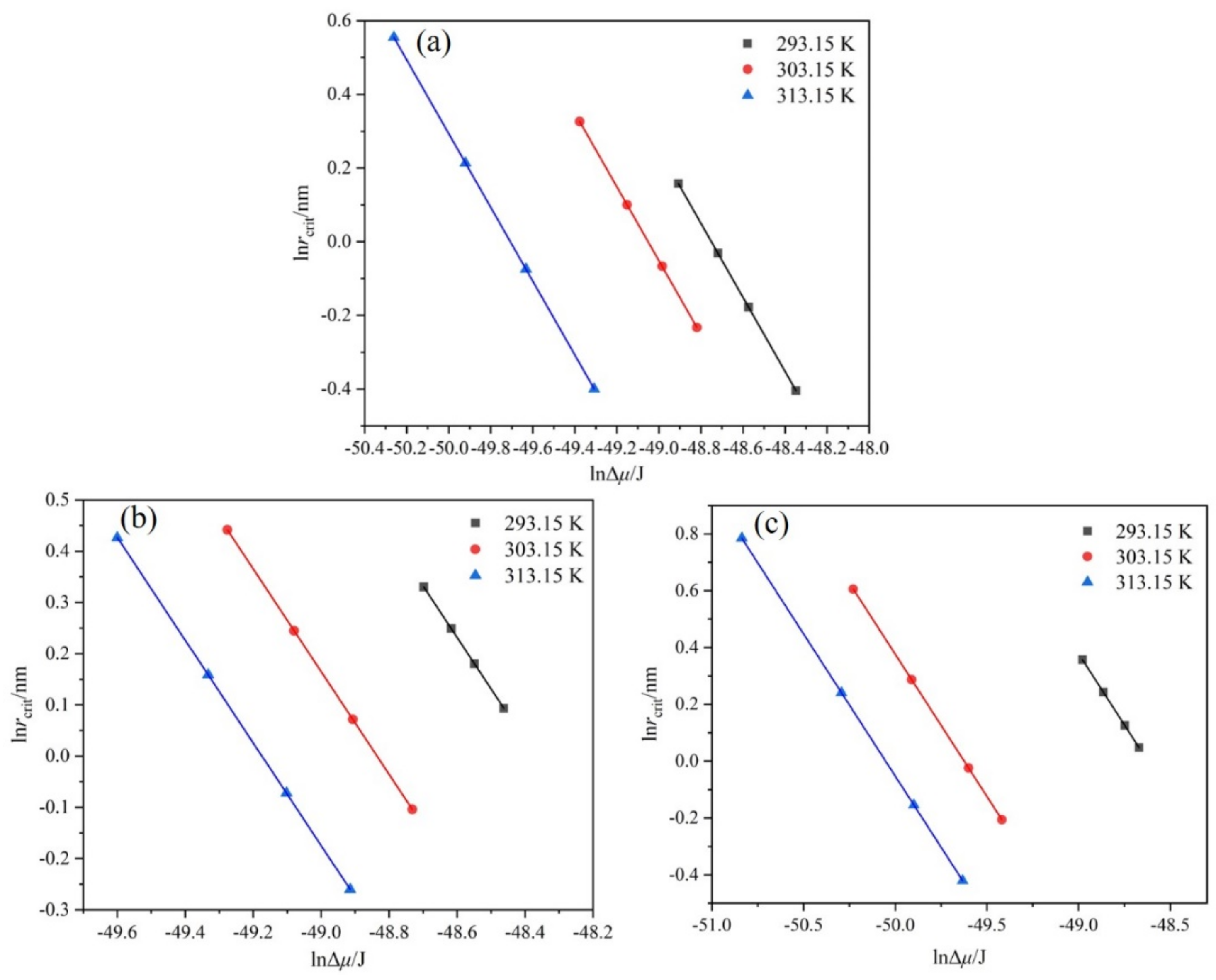
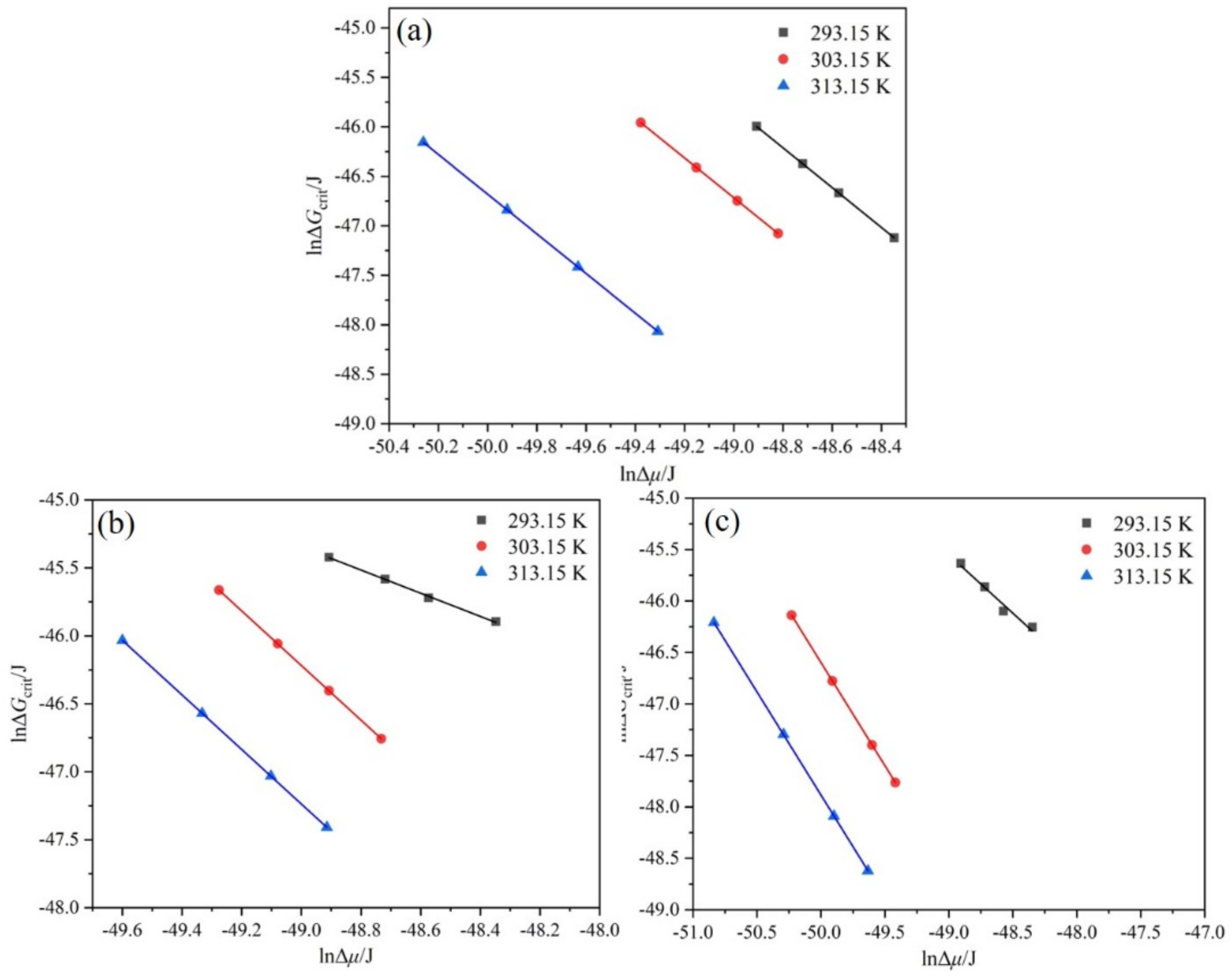
4.5. Critical Nucleation Parameters and Nucleation Kinetics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Derdoura, L.; Skliarb, D. A review of the effect of multiple conformers on crystallization from solution and strategies for crystallizing slow inter-converting conformers. Chem. Eng. Sci. 2014, 106, 275–292. [Google Scholar] [CrossRef]
- Nangia, A.K.; Desiraju, G.R. Crystal Engineering: An Outlook for the Future. Angew. Chem. Int. Ed. 2019, 58, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.D. Ions from the Hofmeister series and osmolytes: Effects on proteins in solution and in the crystallization process. Methods 2004, 34, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D.; Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Mitchell, N.A.; Frawley, P.J. Nucleation kinetics of paracetamol-ethanol solutions from metastable zone widths. J. Cryst. Growth 2010, 312, 2740–2746. [Google Scholar] [CrossRef]
- Lenka, M.; Sarkar, D. Determination of metastable zone width, induction period and primary nucleation kinetics for cooling crystallization of l-asparaginenohydrate. J. Cryst. Growth 2014, 408, 85–90. [Google Scholar] [CrossRef]
- Shiau, L.D.; Lu, T.S. A model for determination of the interfacial energy from the induction time or metastable zone width data based on turbidity measurements. CrysEngComm 2014, 16, 9743–9752. [Google Scholar] [CrossRef]
- Liang, K.P.; White, G.; Wilkinson, D.; Ford, L.J.; Roberts, K.J.; Wood, W.M.L. An examination into the effect of stirrer material and agitation rate on the nucleation of l-glutamic acid batch crystallized from supersaturated aqueous solutions. Cryst. Growth Des. 2004, 4, 1039–1044. [Google Scholar] [CrossRef]
- Peters, B. Supersaturation rates and schedules: Nucleation kinetics from isothermal metastable zone widths. J. Cryst. Growth 2011, 317, 79–83. [Google Scholar] [CrossRef]
- Yuan, Y.; Leng, Y.; Huang, C.; Yue, M.; Tan, Q. Effects of cooling rate, saturation temperature, and agitation on the metastable zone width of dl-malic acid-water system. Russ. J. Phys. Chem. A 2015, 89, 1567–1571. [Google Scholar] [CrossRef]
- Rajesh, N.P.; Perumal, C.K.L.; Raghavan, P.S.; Ramasamy, P. Effect of urea on metastable zone width, induction time and nucleation parameters of ammonium dihydrogen orthophosphate. Cryst. Res. Technol. 2001, 36, 55–63. [Google Scholar] [CrossRef]
- Yang, J.; Xu, S.; Wang, J.; Gong, J. Nucleation behavior of ethyl vanillin: Balance between chemical potential difference and saturation temperature. J. Mol. Liq. 2020, 303, 112609. [Google Scholar] [CrossRef]
- Xu, S.; Bu, Y.; Jiang, S.; Yang, P.; Wang, Y. Insights into the Role of Solvents in Nucleation Kinetics of Glutaric Acid from Metastable Zone Widths. Ind. Eng. Chem. Res. 2021, 60, 3073–3082. [Google Scholar] [CrossRef]
- Wang, Y.; Chuai, X.; Li, Y.; Guo, J.; Yang, J.; Liu, Z.; Xu, S. Nucleation Behaviors of Adipic Acid in Different Polarity Solvent Based on Metastable Zone Width. Crystals 2022, 12, 202. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, G.; Kamaraju, V.K.; He, Y.; Power, G.; Glennon, B. Primary Nucleation of Benzoic Acid in Aqueous Ethanol Solution. Ind. Eng. Chem. Res. 2020, 59, 484–490. [Google Scholar] [CrossRef]
- Gandhi, P.J.; Murthy., Z.V.P. Transmission of p-anisic acid through nanofiltration and goat membranes. Desalination 2013, 315, 46–60. [Google Scholar] [CrossRef]
- Nývlt, J. Kinetics of nucleation in solutions. J. Cryst. Growth 1968, 3–4, 377–383. [Google Scholar] [CrossRef]
- Kashchiev, D.; Borissova, A.; Hammond, R.B.; Robertsb, K.J. Effect of cooling rate on the critical undercooling for crystallization. J. Cryst. Growth 2010, 312, 698–704. [Google Scholar] [CrossRef]
- Sangwal, K. A novel self-consistent Nývlt-like equation for metastable zone width determined by the polythermal method. Cryst. Res. Technol. 2009, 44, 231–247. [Google Scholar] [CrossRef]
- Sangwal, K. Novel approach to analyze metastable zone width determined by the polythermal method: Physical interpretation of various parameters. Cryst. Growth Des. 2009, 9, 942–950. [Google Scholar] [CrossRef]
- Sangwal, K. Some features of metastable zone width of various systems determined by polythermal method. CrysEngComm 2011, 13, 489–501. [Google Scholar] [CrossRef]
- Mullin, J.W. Nucleation. In Crystallization, 4th ed.; Mullin, J.W., Ed.; Butterworth-Heinemann: Oxford, UK, 2001; pp. 181–215. [Google Scholar]
- Xu, S.; Wang, J.; Zhang, K.; Wu, S.; Liu, S.; Li, K.; Yu, B.; Gong, J. Nucleation behavior of eszopiclone-butyl acetate solutions from metastable zone widths. Chem. Eng. Sci. 2016, 155, 248–257. [Google Scholar] [CrossRef]
- Xiong, L.; Zhou, L.; Zhang, X.; Zhang, M.; Hou, B.; Bao, Y.; Du, W.; Su, W.; Zhang, S.; Yin, Q. Determination of metastable zone widths and nucleation behavior of aspirin in acetic acid and acetic anhydride binary solvent mixture. J. Mol. Liq. 2018, 269, 805–815. [Google Scholar] [CrossRef]
- Peng, H.; Tian, N.; Yu, C.; Gao, Y.; Li, K.; Yan, H.; Zhao, P.; Wu, S.; Chen, M.; Gong, J. Insights into the Role of Dipentaerythritol in the Thermodynamics and Nucleation Behavior of a Pentaerythritol-Water System. Cryst. Growth Des. 2022, 22, 449–460. [Google Scholar] [CrossRef]
- Sangwal, K. Recent developments in understanding of the metastable zone width of different solute-solvent systems. J. Cryst. Growth 2011, 318, 103–109. [Google Scholar] [CrossRef]
- Ramakers, L.A.I.; McGinty, J.; Beckmann, W.; Beckmann, W.; Levilain, G.; Lee, M.; Wheatcroft, H.; Houson, I.; Sefcik, J. Investigation of Metastable Zones and Induction Times in Glycine Crystallisation across Three Different Antisolvents. Cryst. Growth Des. 2020, 20, 4935–4944. [Google Scholar] [CrossRef]
- Zou, F.; Zhuang, W.; Chen, Q.; Yang, P.; Lin, C.; Jiao, P.; Zhou, J.; Wu, J.; Ying, H. Solvent effects on nucleation of disodium guanosine 5′-monophosphate in anti-solvent/water mixtures. CrystEngComm 2016, 18, 6653–6663. [Google Scholar] [CrossRef]
- Granberg, R.A.; Ducreux, C.; Gracin, S.; Rasmuson, A.C. Primary nucleation of paracetamol in acetone-water mixtures. Chem. Eng. Sci. 2001, 56, 2305–2313. [Google Scholar] [CrossRef]
- Sullivan, R.A.; Davey, R.J.; Sadiq, G.; Dent, G.; Back, K.R.; ter Horst, J.H.; Toroz, D.; Hammond, R.B. Revealing the Roles of Desolvation and Molecular Self-Assembly in Crystal Nucleation from Solution: Benzoic and p-Aminobenzoic Acids. Cryst. Growth Des. 2014, 14, 2689–2696. [Google Scholar] [CrossRef] [Green Version]





| ∆HS (J/mol) | ∆S (J/mol/K) | R2 | |
|---|---|---|---|
| 0.6 | 35032.33 | 70.75 | 0.9919 |
| 0.8 | 30061.06 | 60.87 | 0.9966 |
| 1.0 | 29637.00 | 62.92 | 0.9974 |
| R = 10 K/h | R = 20 K/h | R = 30 K/h | R = 60 K/h | ||
|---|---|---|---|---|---|
| 0.6 | 293.15 | 2.900 | 3.350 | 3.650 | 4.075 |
| 0.6 | 303.15 | 1.875 | 2.350 | 2.776 | 3.275 |
| 0.6 | 313.15 | 0.800 | 1.125 | 1.500 | 2.075 |
| 0.8 | 293.15 | 3.575 | 3.875 | 4.150 | 4.525 |
| 0.8 | 303.15 | 2.075 | 2.525 | 3.000 | 3.575 |
| 0.8 | 313.15 | 1.550 | 2.025 | 2.550 | 3.075 |
| 1 | 293.15 | 2.700 | 3.025 | 3.400 | 3.675 |
| 1 | 303.15 | 0.800 | 1.100 | 1.500 | 1.800 |
| 1 | 313.15 | 0.450 | 0.775 | 1.150 | 1.500 |
| Nucleation Order m | |||
|---|---|---|---|
| T0 = 293.15 K | T0 = 303.15 K | T0 = 313.15 K | |
| 0.6 | 3.18 | 3.16 | 1.85 |
| 0.8 | 7.50 | 3.23 | 2.55 |
| 1.0 | 5.63 | 2.14 | 1.46 |
| T0/K | (mJ/m2) | ||||
|---|---|---|---|---|---|
| R = 10 K/h | R = 20 K/h | R = 30 K/h | R = 60 K/h | ||
| 0.6 | 293.15 | 1.8450 | 1.8437 | 1.8426 | 1.8404 |
| 0.6 | 303.15 | 1.3658 | 1.3650 | 1.3644 | 1.3636 |
| 0.6 | 313.15 | 0.7090 | 0.7088 | 0.7085 | 0.7080 |
| 0.8 | 293.15 | 2.3195 | 2.3187 | 2.3179 | 2.3169 |
| 0.8 | 303.15 | 1.4552 | 1.4545 | 1.4537 | 1.4528 |
| 0.8 | 313.15 | 1.0368 | 1.0363 | 1.0357 | 1.0351 |
| 1.0 | 293.15 | 1.774 | 1.7733 | 1.7726 | 1.7720 |
| 1.0 | 303.15 | 0.6518 | 0.6516 | 0.6513 | 0.6511 |
| 1.0 | 313.15 | 0.4246 | 0.4244 | 0.4243 | 0.4241 |
| T0/K | A/f (s−1) | ||||
|---|---|---|---|---|---|
| R = 10 K/h | R = 20 K/h | R = 30 K/h | R = 60 K/h | ||
| 0.6 | 293.15 | 6.4345 | 6.4478 | 6.4601 | 6.4830 |
| 0.6 | 303.15 | 5.7808 | 5.7899 | 5.7981 | 5.8078 |
| 0.6 | 313.15 | 3.1034 | 3.1066 | 3.1104 | 3.1161 |
| 0.8 | 293.15 | 45.8461 | 45.8936 | 45.9373 | 45.9970 |
| 0.8 | 303.15 | 26.4174 | 26.4570 | 26.4989 | 26.5497 |
| 0.8 | 313.15 | 3.5892 | 3.5947 | 3.6008 | 3.6069 |
| 1.0 | 293.15 | 16.9758 | 16.9949 | 17.0169 | 17.0330 |
| 1.0 | 303.15 | 2.9710 | 2.9740 | 2.9779 | 2.9809 |
| 1.0 | 313.15 | 2.0819 | 2.0841 | 2.0866 | 2.0890 |
| Ethanol Mass Fraction | ||||||
|---|---|---|---|---|---|---|
| 293.15 | 303.15 | 313.15 | 293.15 | 303.15 | 313.15 | |
| N | M | |||||
| 0.6 | −13.22 | −32.68 | −234.00 | −85.41 | −213.90 | −1671.07 |
| 0.8 | −4.90 | −20.13 | −55.03 | −21.57 | −99.58 | −380.02 |
| 1.0 | −10.66 | −215.71 | −780.63 | −57.51 | −1535.38 | −5809.89 |
| (mJ/m2) | A/f | |||||
| 0.6 | 1.8498 | 1.3681 | 0.7098 | 6.5891 | 6.0545 | 3.3360 |
| 0.8 | 2.3254 | 1.4519 | 1.0384 | 44.3009 | 25.6925 | 3.6232 |
| 1.0 | 1.7777 | 0.6524 | 0.4249 | 16.1821 | 2.8894 | 2.0881 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Shang, Z.; Liu, M.; Dong, W.; Li, H.; Yin, H.; Gong, J.; Wu, S. Insight into the Nucleation Mechanism of p-Methoxybenzoic Acid in Ethanol-Water System from Metastable Zone Width. Molecules 2022, 27, 4085. https://doi.org/10.3390/molecules27134085
Wang G, Shang Z, Liu M, Dong W, Li H, Yin H, Gong J, Wu S. Insight into the Nucleation Mechanism of p-Methoxybenzoic Acid in Ethanol-Water System from Metastable Zone Width. Molecules. 2022; 27(13):4085. https://doi.org/10.3390/molecules27134085
Chicago/Turabian StyleWang, Guangle, Zeren Shang, Mingdi Liu, Weibing Dong, Haichao Li, Haiqing Yin, Junbo Gong, and Songgu Wu. 2022. "Insight into the Nucleation Mechanism of p-Methoxybenzoic Acid in Ethanol-Water System from Metastable Zone Width" Molecules 27, no. 13: 4085. https://doi.org/10.3390/molecules27134085
APA StyleWang, G., Shang, Z., Liu, M., Dong, W., Li, H., Yin, H., Gong, J., & Wu, S. (2022). Insight into the Nucleation Mechanism of p-Methoxybenzoic Acid in Ethanol-Water System from Metastable Zone Width. Molecules, 27(13), 4085. https://doi.org/10.3390/molecules27134085