Protective Effect of Resveratrol against Hexavalent Chromium-Induced Genotoxic Damage in Hsd:ICR Male Mice
Abstract
:1. Introduction
2. Results
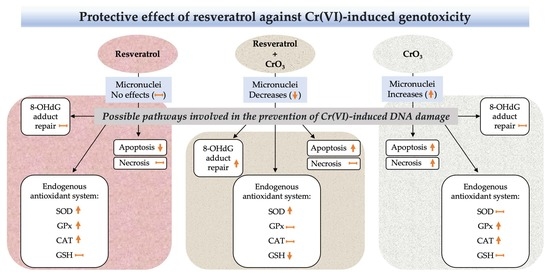
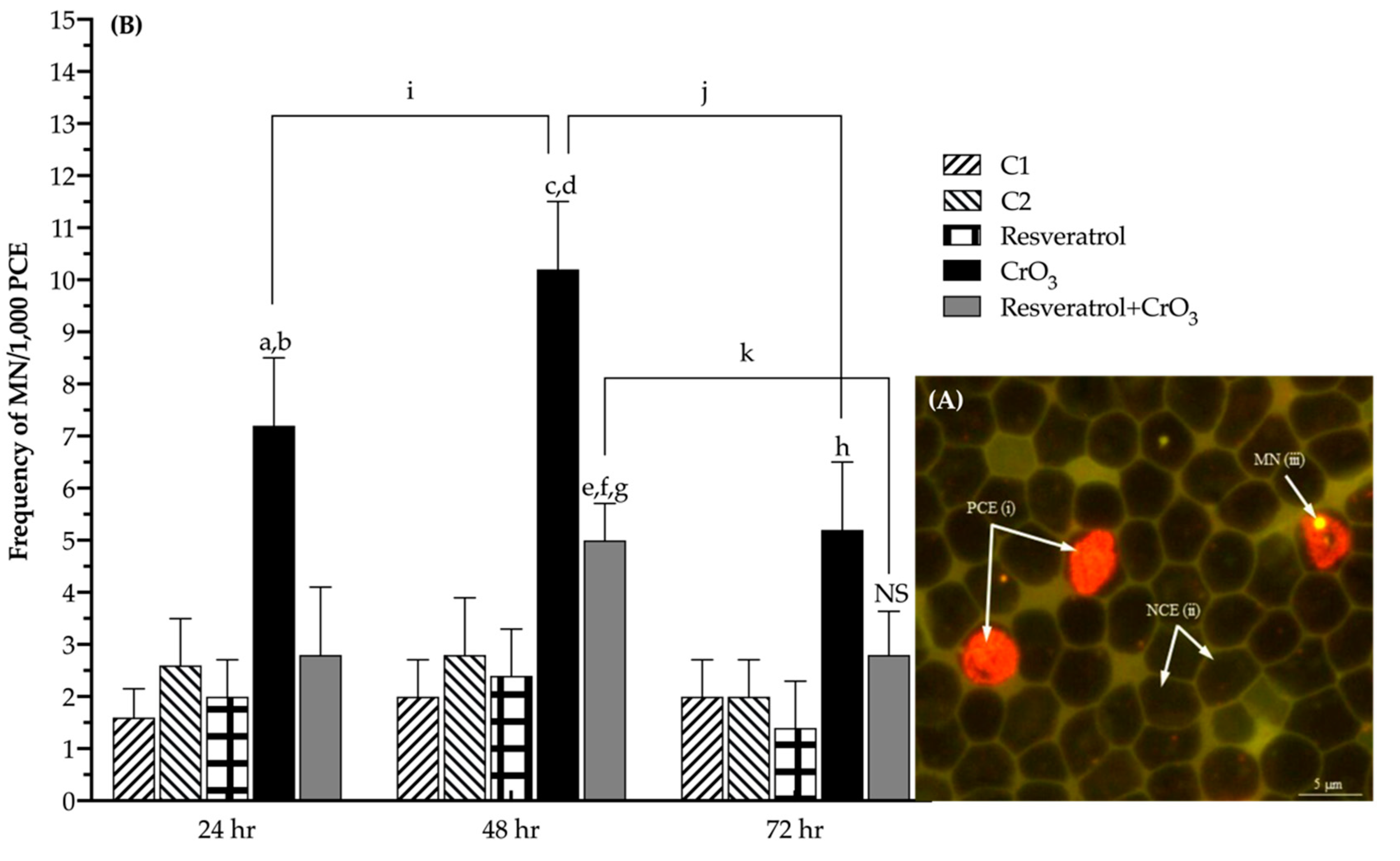
2.1. Effect of Resveratrol on MN Induced by CrO3
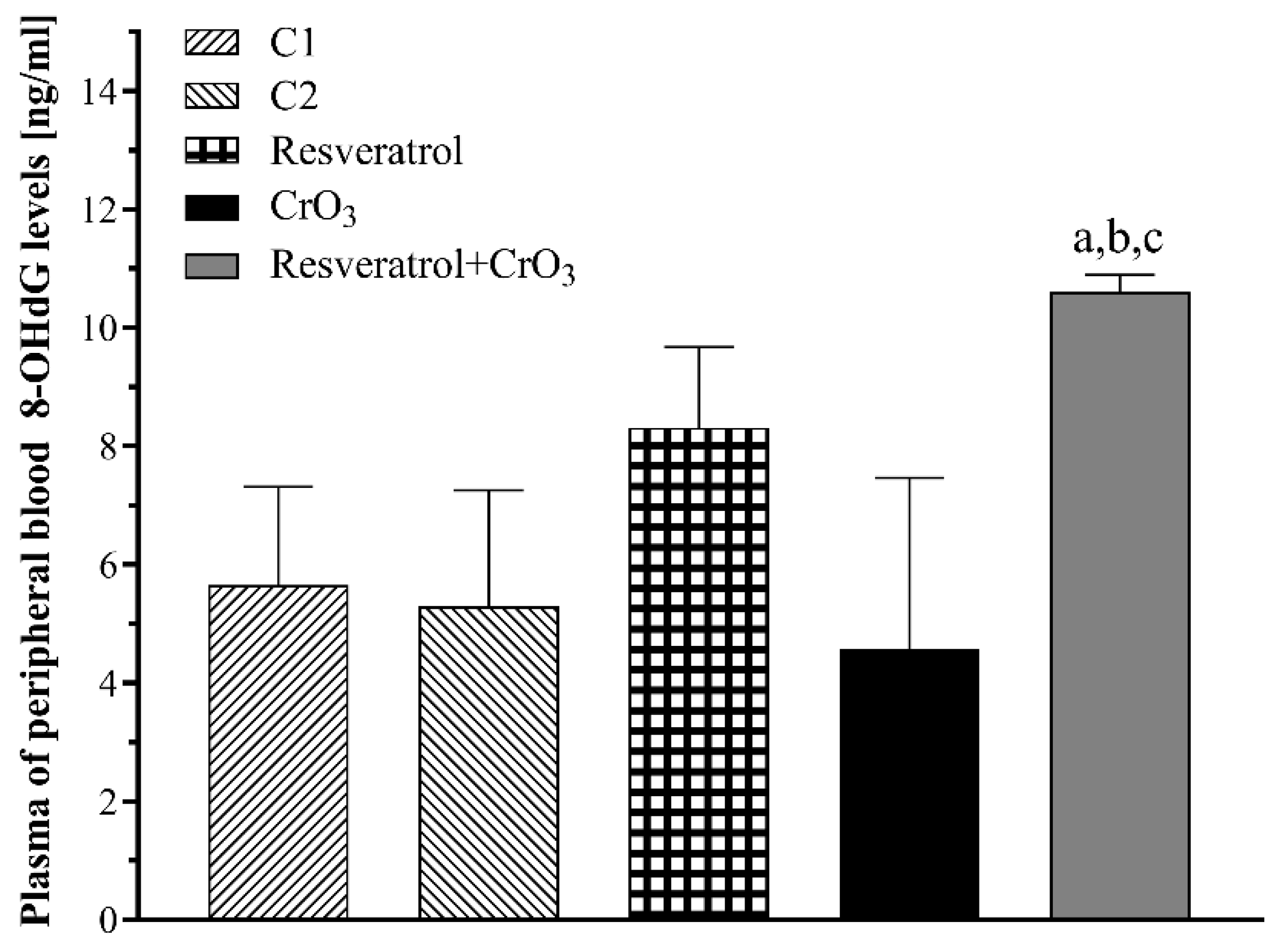
2.2. Effect of Resveratrol and CrO3 on 8-OHdG Adduct Levels
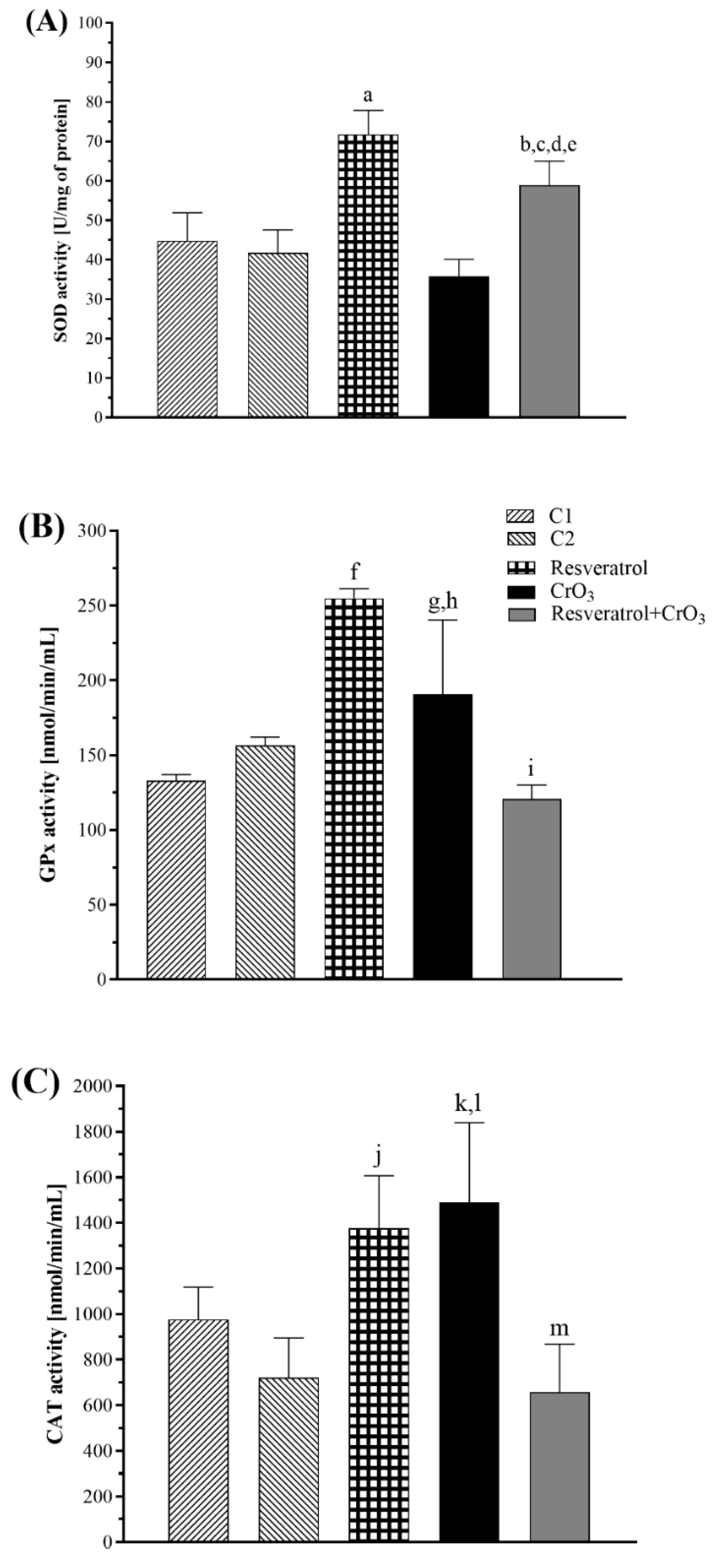
2.3. Effect of Resveratrol and CrO3 on the Antioxidant System
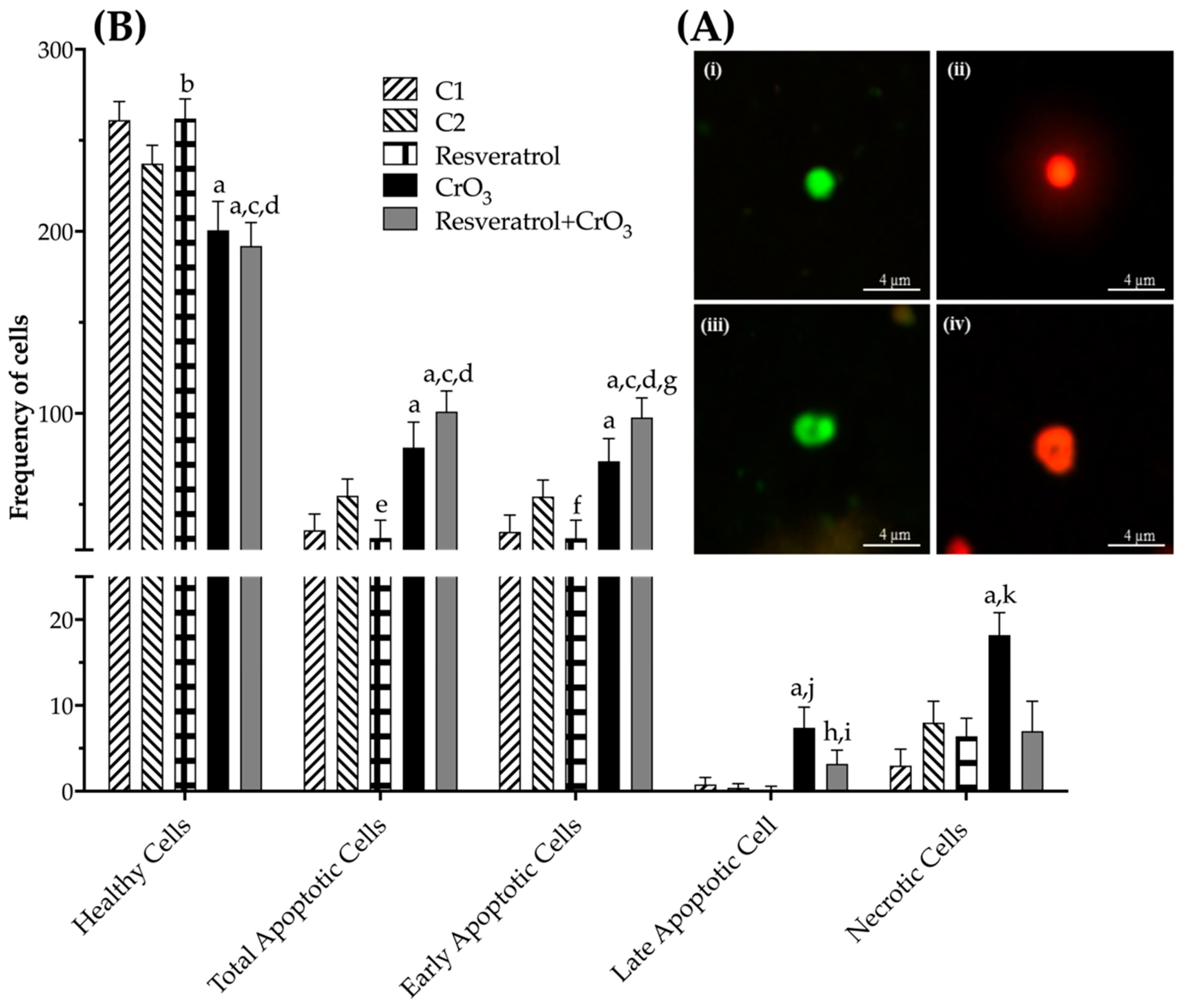
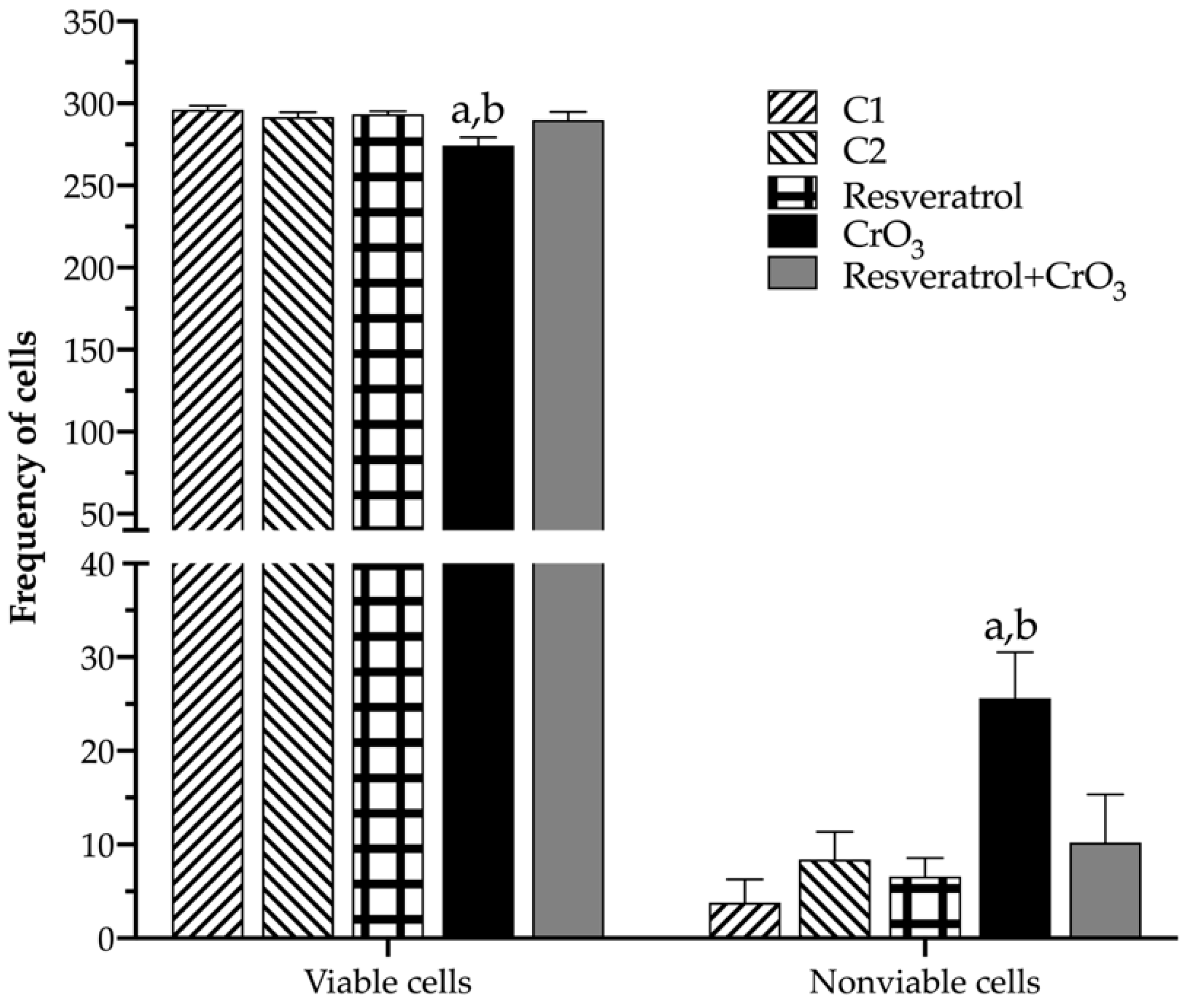
2.4. Effect of Resveratrol and CrO3 on Apoptotic and Necrotic Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Micronuclei Assay
4.4. Plasma 8-Hydroxydeoxyguanosine Levels
4.5. Antioxidant System
4.5.1. Superoxide Dismutase Activity
4.5.2. Glutathione Peroxidase Activity
4.5.3. Catalase Activity
4.5.4. Glutathione Levels
4.6. Apoptosis and Cell Viability
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Méndez, T.; Ortiz-Muñiz, A.R.; Mendoza-Núñez, V.M.; García-Rodríguez, M.C. The role of resveratrol on heavy metal-induced oxidative stress. Nutr. Hosp. 2020, 37, 374–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Repossi, G.; Das, U.N.; Eynard, A.R. Molecular Basis of the Beneficial Actions of Resveratrol. Arch. Med. Res. 2020, 51, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Andreazza, A.C.; Nardin, P.; Funchal, C.; Gonçalves, C.A.; Gottfried, C. Resveratrol attenuates oxidative-induced DNA damage in C6 Glioma cells. Neurotoxicology 2007, 28, 886–891. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Law, B.Y.; Liang, X.; Ma, N.; Xu, G.; Wang, X.; Yuan, X.; Tang, H.; Chen, Q.; et al. Resveratrol decreases cell apoptosis through inhibiting DNA damage in bronchial epithelial cells. Int. J. Mol. Med. 2020, 45, 1673–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhardt, S.; Reiter, R.J.; Tan, D.X.; Hardeland, R.; Cabrera, J.; Karbownik, M. DNA oxidatively damaged by chromium(III) and H2O2 is protected by the antioxidants melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int. J. Biochem. Cell Biol. 2001, 33, 775–783. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, X.; Zhao, W.; Zhang, Z. Dual role of resveratrol in modulation of genotoxicity induced by sodium arsenite via oxidative stress and apoptosis. Food Chem. Toxicol. 2013, 59, 8–17. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, K.P.; Huang, T.; Yan, C.C.; Liu, L.R.; Zhu, Q.L.; Guo, F.F.; Liu, C.; Li, B.X. The rescuable function and mechanism of resveratrol on As2O3-induced hERG K+ channel deficiency. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 1079–1089. [Google Scholar] [CrossRef]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Burghardt, R.C. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol. 2016, 303, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Khalid, S.; Afzal, N.; Khan, J.A.; Hussain, Z.; Qureshi, A.S.; Anwar, H.; Jamil, Y. Antioxidant resveratrol protects against copper oxide nanoparticle toxicity in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, A.L.; Levy, L.S.; Shuker, L.K. Chromium in the environment: An evaluation of exposure of the UK general population and possible adverse health effects. J. Toxicol. Environ. Health B Crit. Rev. 2000, 3, 145–178. [Google Scholar] [CrossRef] [PubMed]
- EPA. Environmental Protection Agency. Toxicological review of hexavalent chromium. CAS No. 18540-29-9. In Support of Summary Information on the Integrated Risk Information System (IRIS). External Review Draft; EPA/635/R-10/004ª; Office of Research and Development: Washington, DC, USA, 2010; Volume 635. Available online: https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=221433# (accessed on 1 January 2022).
- Shi, X.; Chiu, A.; Chen, C.T.; Halliwell, B.; Castranova, V.; Vallyathan, V. Reduction of chromium(VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health B Crit. Rev. 1999, 2, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Ceryak, S.; Patierno, S.R. Complexities of chromium carcinogenesis: Role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003, 533, 3–36. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 1–32. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Barnes, C.; Yedjou, C.; Velma, V.R.; Tchounwou, P.B. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ. Toxicol. 2009, 24, 66–73. [Google Scholar] [CrossRef] [Green Version]
- García-Rodríguez, M.C.; Nicolás-Méndez, T.; Montaño-Rodríguez, A.R.; Altamirano-Lozano, M.A. Antigenotoxic effects of (-)-epigallocatechin-3-gallate (EGCG), quercetin, and rutin on chromium trioxide-induced micronuclei in the polychromatic erythrocytes of mouse peripheral blood. J. Toxicol. Environ. Health A 2014, 77, 324–336. [Google Scholar] [CrossRef]
- de Freitas, K.S.; Squarisi, I.S.; Acésio, N.O.; Nicolella, H.D.; Ozelin, S.D.; Reis Santos de Melo, M.; Guissone, A.; Fernandes, G.; Silva, L.M.; da Silva Filho, A.A.; et al. Licochalcone A, a licorice flavonoid: Antioxidant, cytotoxic, genotoxic, and chemopreventive potential. J. Toxicol. Environ. Health A 2020, 83, 673–686. [Google Scholar] [CrossRef]
- Sousa, H.G.; Uchôa, V.T.; Cavalcanti, S.; de Almeida, P.M.; Chaves, M.H.; Lima Neto, J.S.; Nunes, P.; da Costa Júnior, J.S.; Rai, M.; Do Carmo, I.S.; et al. Phytochemical screening, phenolic and flavonoid contents, antioxidant and cytogenotoxicity activities of Combretum leprosum Mart. (Combretaceae). J. Toxicol. Environ. Health A 2021, 84, 399–417. [Google Scholar] [CrossRef]
- Gu, H.F.; Mao, X.Y.; Du, M. Prevention of breast cancer by dietary polyphenols-role of cancer stem cells. Crit. Rev. Food Sci. Nutr. 2020, 60, 810–825. [Google Scholar] [CrossRef]
- Majolo, F.; Bitencourt, S.; Wissmann Monteiro, B.; Viegas Haute, G.; Alves, C.; Silva, J.; Pinteus, S.; Santos, R.; Torquato, H.; Paredes-Gamero, E.J.; et al. Antimicrobial and antileukemic effects: In vitro activity of Calyptranthes grandifolia aqueous leaf extract. J. Toxicol. Environ. Health A 2020, 83, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Wang, Y.; Chen, J.; Liu, J.; He, X.; Huang, D.; Wu, Y.; Chen, Y.; Weng, Z. Protective effects of (-)-epigallocatechin gallate and curcumin against acrylamide toxicity. Toxicol. Environ. Chem. 2021, 103, 199–218. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Nadal, L.L.; Broedbaek, K.; Nielsen, P.E.; Weimann, A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim. Biophys. Acta 2014, 1840, 801–808. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, M.C.; López-Santiago, V.; Altamirano-Lozano, M.A. Effect of chlorophyllin on chromium trioxide-induced micronuclei in polychromatic erythrocytes in mouse peripheral blood. Mutat. Res. 2001, 496, 145–151. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Sutou, S.; Shimada, H.; Sato, S.; Sasaki, Y.F.; Wakata, A. Difference between intraperitoneal and oral gavage application in the micronucleus test. The 3rd collaborative study by CSGMT/JEMS.MMS. Collaborative Study Group for the Micronucleus Test/Mammalian Mutagenesis Study Group of the Environmental Mutagen Society of Japan. Mutat. Res. 1989, 223, 329–344. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2016. [Google Scholar] [CrossRef] [Green Version]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- EPA. Environmental Protection Agency. Health Effects Test Guidelines OPPTS 870.5395. Mammalian Erythrocyte Micronucleus Test, Office of Prevention, Pesticides and Toxic Substances (7101). US. 1998. EPA 712-C-98-226. Available online: https://www.regulations.gov/document/EPA-HQ-OPPT-2009-0156-0032 (accessed on 1 January 2022).
- Hu, G.; Long, C.; Hu, L.; Xu, B.P.; Chen, T.; Gao, X.; Zhang, Y.; Zheng, P.; Wang, L.; Wang, T.; et al. Circulating lead modifies hexavalent chromium-induced genetic damage in a chromate-exposed population: An epidemiological study. Sci. Total Environ. 2021, 752, 141824. [Google Scholar] [CrossRef]
- Maeng, S.H.; Chung, H.W.; Yu, I.J.; Kim, H.Y.; Lim, C.H.; Kim, K.J.; Kim, S.J.; Ootsuyama, Y.; Kasai, H. Changes of 8-OH-dG levels in DNA and its base excision repair activity in rat lungs after inhalation exposure to hexavalent chromium. Mutat. Res. 2003, 539, 109–116. [Google Scholar] [CrossRef]
- Thompson, C.M.; Fedorov, Y.; Brown, D.D.; Suh, M.; Proctor, D.M.; Kuriakose, L.; Haws, L.C.; Harris, M.A. Assessment of Cr(VI)-induced cytotoxicity and genotoxicity using high content analysis. PLoS ONE 2012, 7, e42720. [Google Scholar] [CrossRef] [PubMed]
- Urbano, A.M.; Ferreira, L.M.; Alpoim, M.C. Molecular and cellular mechanisms of hexavalent chromium-induced lung cancer: An updated perspective. Curr. Drug Metab. 2012, 13, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.S.; Wise, J.P.S. Chromium and genomic stability. Mutat. Res. 2012, 733, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Ying, S.; Feng, L.; Wang, H.; Yao, C.; Li, T.; Zhang, Y.; Fu, S.; Ding, D.; Guo, X.; et al. Decreased 8-oxoguanine DNA glycosylase 1 (hOGG1) expression and DNA oxidation damage induced by Cr (VI). Chem. Biol. Interact. 2019, 299, 44–51. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.Y.; Mou, Y.H.; Wang, L.H.; Zhou, Y.N.; Wu, C.F. Differences in the activities of resveratrol and ascorbic acid in protection of ethanol-induced oxidative DNA damage in human peripheral lymphocytes. Food Chem. Toxicol. 2012, 50, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Kuczmarska, A.; Rubiś, B.; Filas, V.; Murias, M.; Zieliński, P.; Piwocka, K.; Książek, K. Resveratrol delays replicative senescence of human mesothelial cells via mobilization of antioxidative and DNA repair mechanisms. Free Radic. Biol. Med. 2012, 52, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- García-Rodríguez, M.C.; Serrano-Reyes, G.; Hernández-Cortés, L.M.; Altamirano-Lozano, M.A. Antigenotoxic effects of (-)-epigallocatechin-3-gallate (EGCG) and its relationship with the endogenous antioxidant system, 8-hydroxydeoxyguanosine adduct repair (8-OHdG), and apoptosis in mice exposed to chromium(VI). J. Toxicol. Environ. Health A 2021, 84, 331–344. [Google Scholar] [CrossRef]
- O’Flaherty, E.J. A pharmacokinetic model for chromium. Toxicol. Lett. 1993, 68, 145–158. [Google Scholar] [CrossRef]
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, M.C.; Carvente-Juárez, M.M.; Altamirano-Lozano, M.A. Antigenotoxic and apoptotic activity of green tea polyphenol extracts on hexavalent chromium-induced DNA damage in peripheral blood of CD-1 mice: Analysis with differential acridine orange/ethidium bromide staining. Oxidative Med. Cell Longev. 2013, 2013, 486419. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.J.; Suwalsky, M.; Ramírez, D.; Tapia, J.; Sepulveda, B. Antioxidant effect of resveratrol in single red blood cells measured by thermal fluctuation spectroscopy. Arch. Biochem. Biophys. 2019, 665, 30–35. [Google Scholar] [CrossRef]
- Nwose, E.U.; Jelinek, H.F.; Richards, R.S.; Kerr, P.G. Erythrocyte oxidative stress in clinical management of diabetes and its cardiovascular complications. Br. J. Biomed. Sci. 2007, 64, 35–43. [Google Scholar] [CrossRef]
- Zeitz, J.O.; Mohrmann, S.; Fehse, L.; Most, E.; Helmbrecht, A.; Saremi, B.; Eder, K. Tissue and plasma antioxidant status in response to dietary methionine concentration and source in broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Hussaini, N.A.; Garba, S.H.; Wahid, S.; Damilola, F.K.; Adeola, A.A.; Wasiu, I.A. Prospective of chromium (VI) reduction under in vitro and in vivo conditions and stimulation of antioxidant defense of cowpea under the exposure of Cr (VI). Appl. Soil Ecol. 2018, 132, 187–193. [Google Scholar] [CrossRef]
- Wang, X.F.; Xing, M.L.; Shen, Y.; Zhu, X.; Xu, L.H. Oral administration of Cr(VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicology 2006, 228, 16–23. [Google Scholar] [CrossRef]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J.A. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, G.; Singh, A.K.; Kumar, A.; Prakash, O.; Singh, M.P. Resveratrol modulates pyrogallol-induced changes in hepatic toxicity markers, xenobiotic metabolizing enzymes and oxidative stress. Eur. J. Pharmacol. 2008, 596, 146–152. [Google Scholar] [CrossRef]
- Chow, H.H.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cao, Z.; Zhu, H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol. Res. 2006, 53, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Gil-Becerra, D. Implications of polyphenols on endogenous antioxidant defense systems in human diseases. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 201–217. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.Y.; Liu, L.; Li, Y.X.; Xun, A.Y.; Zeng, W.S.; Jia, C.H.; Wei, X.X.; Feng, J.L.; Zhao, L.; et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 2010, 41, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Liu, S.M.; Pahlevan, S.; Yuan, J.; Khazaeli, M.; Ni, Z.; Chan, J.Y.; Vaziri, N.D. Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption. Dig. Dis. Sci. 2015, 60, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Orosz, Z.; Rivera, A.; Labinskyy, N.; Xiangmin, Z.; Olson, S.; Podlutsky, A.; Csiszar, A. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2417–H2424. [Google Scholar] [CrossRef]
- Krishna, G.; Hayashi, M. In vivo rodent micronucleus assay: Protocol, conduct and data interpretation. Mutat. Res. 2000, 455, 155–166. [Google Scholar] [CrossRef]
- Hu, G.; Zheng, P.; Feng, H.; Jia, G. Imbalance of oxidative and reductive species involved in chromium(VI)-induced toxic effects. React. Oxyg. Species 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Chiu, A.; Shi, X.L.; Lee, W.K.; Hill, R.; Wakeman, T.P.; Katz, A.; Xu, B.; Dalal, N.S.; Robertson, J.D.; Chen, C.; et al. Review of chromium (VI) apoptosis, cell-cycle-arrest, and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 188–230. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.H.; Lin, J.C.; Wang, T.Y.; Lin, T.J.; Yen, M.C.; Liu, Y.H.; Wu, P.L.; Chen, F.W.; Shih, Y.L.; Yeh, I.J. Hexavalent chromium intoxication induces intrinsic and extrinsic apoptosis in human renal cells. Mol. Med. Rep. 2020, 21, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Takashina, M.; Inoue, S.; Tomihara, K.; Tomita, K.; Hattori, K.; Zhao, Q.L.; Suzuki, T.; Noguchi, M.; Ohashi, W.; Hattori, Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int. J. Oncol. 2017, 50, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Mirzapur, P.; Khazaei, M.R.; Moradi, M.T.; Khazaei, M. Apoptosis induction in human breast cancer cell lines by synergic effect of raloxifene and resveratrol through increasing proapoptotic genes. Life Sci. 2018, 205, 45–53. [Google Scholar] [CrossRef]
- Martins, L.A.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.F.; Souza, I.C.; Moreira, J.C.; Borojevic, R.; Gottfried, C.; Guma, F.C. Resveratrol induces pro-oxidant effects and time-dependent resistance to cytotoxicity in activated hepatic stellate cells. Cell Biochem. Biophys. 2014, 68, 247–257. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [Green Version]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Yu, M.; Xue, J.; Li, Y.; Zhang, W.; Ma, D.; Liu, L.; Zhang, Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch. Toxicol. 2013, 87, 1025–1035. [Google Scholar] [CrossRef]
- Asadi, S.; Moradi, M.N.; Khyripour, N.; Goodarzi, M.T.; Mahmoodi, M. Resveratrol Attenuates Copper and Zinc Homeostasis and Ameliorates Oxidative Stress in Type 2 Diabetic Rats. Biol. Trace Elem. Res. 2017, 177, 132–138. [Google Scholar] [CrossRef]
- Marques, F.Z.; Morris, J.B. Commentary on resveratrol and hormesis: Resveratrol—A hormetic marvel in waiting? Hum. Exp. Toxicol. 2010, 29, 1026–1028. [Google Scholar] [CrossRef]
- Petrella, C.; Carito, V.; Carere, C.; Ferraguti, G.; Ciafrè, S.; Natella, F.; Bello, C.; Greco, A.; Ralli, M.; Mancinelli, R.; et al. Oxidative stress inhibition by resveratrol in alcohol-dependent mice. Nutrition 2020, 79–80, 110783. [Google Scholar] [CrossRef]
- Hayashi, M.; Morita, T.; Kodama, Y.; Sofuni, T.; Ishidate, M., Jr. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat. Res. 1990, 245, 245–249. [Google Scholar] [CrossRef]

| Treatment | Dose (mg/kg) | Time Analysis (h) | n | PCE/NCE 1000 Cells (mean ± SD) |
|---|---|---|---|---|
| C1 | 0 | 0 | 5 | 48.5 ± 8.6 |
| 24 | 46.7 ± 4.6 | |||
| 48 | 48.2 ±11.9 | |||
| 72 | 52.0 ± 8.2 | |||
| C2 | 60 | 0 | 5 | 46.7 ± 7.0 |
| 24 | 47.2 ± 9.6 | |||
| 48 | 53.0 ± 7.5 | |||
| 72 | 43.4 ±13.2 | |||
| Resveratrol | 50 | 0 | 5 | 54.8 ±12.2 |
| 24 | 47.5 ± 9.5 | |||
| 48 | 48.7 ± 5.9 | |||
| 72 | 50.5 ± 6.4 | |||
| CrO3 | 20 | 0 | 5 | 46.5 ±10.0 |
| 24 | 45.8 ± 7.0 | |||
| 48 | 51.1 ±12.8 | |||
| 72 | 39.8 ±12.9 | |||
| Resveratrol + CrO3 | 50 + 20 | 0 | 5 | 45.6 ± 7.9 |
| 24 | 46.4 ± 2.8 | |||
| 48 | 53.8 ±12.7 | |||
| 72 | 44.6 ± 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolás-Méndez, T.; Kacew, S.; Ortiz-Muñiz, A.R.; Mendoza-Núñez, V.M.; García-Rodríguez, M.d.C. Protective Effect of Resveratrol against Hexavalent Chromium-Induced Genotoxic Damage in Hsd:ICR Male Mice. Molecules 2022, 27, 4028. https://doi.org/10.3390/molecules27134028
Nicolás-Méndez T, Kacew S, Ortiz-Muñiz AR, Mendoza-Núñez VM, García-Rodríguez MdC. Protective Effect of Resveratrol against Hexavalent Chromium-Induced Genotoxic Damage in Hsd:ICR Male Mice. Molecules. 2022; 27(13):4028. https://doi.org/10.3390/molecules27134028
Chicago/Turabian StyleNicolás-Méndez, Tonancy, Sam Kacew, Alda Rocío Ortiz-Muñiz, Víctor Manuel Mendoza-Núñez, and María del Carmen García-Rodríguez. 2022. "Protective Effect of Resveratrol against Hexavalent Chromium-Induced Genotoxic Damage in Hsd:ICR Male Mice" Molecules 27, no. 13: 4028. https://doi.org/10.3390/molecules27134028
APA StyleNicolás-Méndez, T., Kacew, S., Ortiz-Muñiz, A. R., Mendoza-Núñez, V. M., & García-Rodríguez, M. d. C. (2022). Protective Effect of Resveratrol against Hexavalent Chromium-Induced Genotoxic Damage in Hsd:ICR Male Mice. Molecules, 27(13), 4028. https://doi.org/10.3390/molecules27134028