Determination of the Phenolic Profile by Liquid Chromatography, Evaluation of Antioxidant Activity and Toxicity of Moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae)
Abstract
:1. Introduction
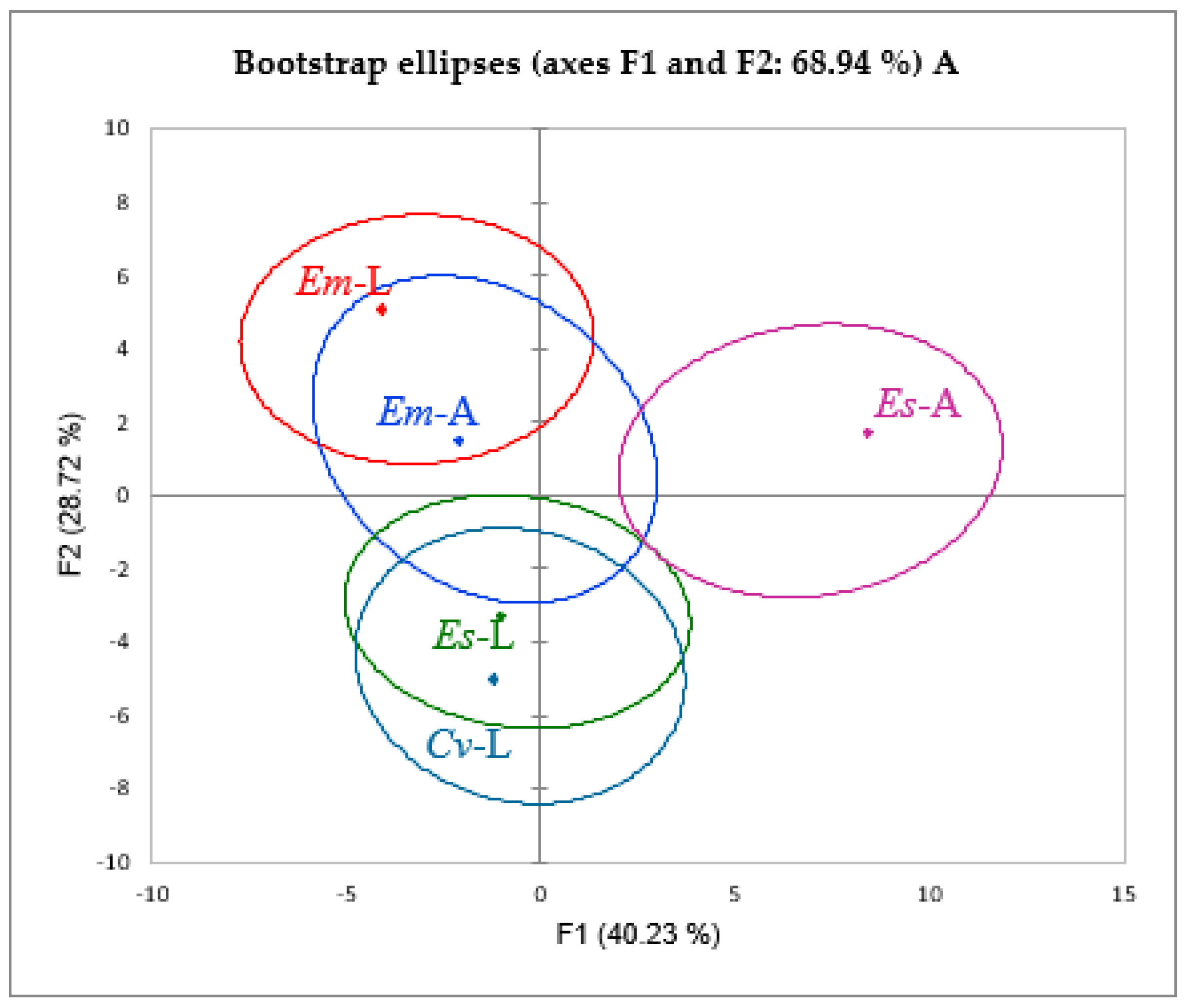
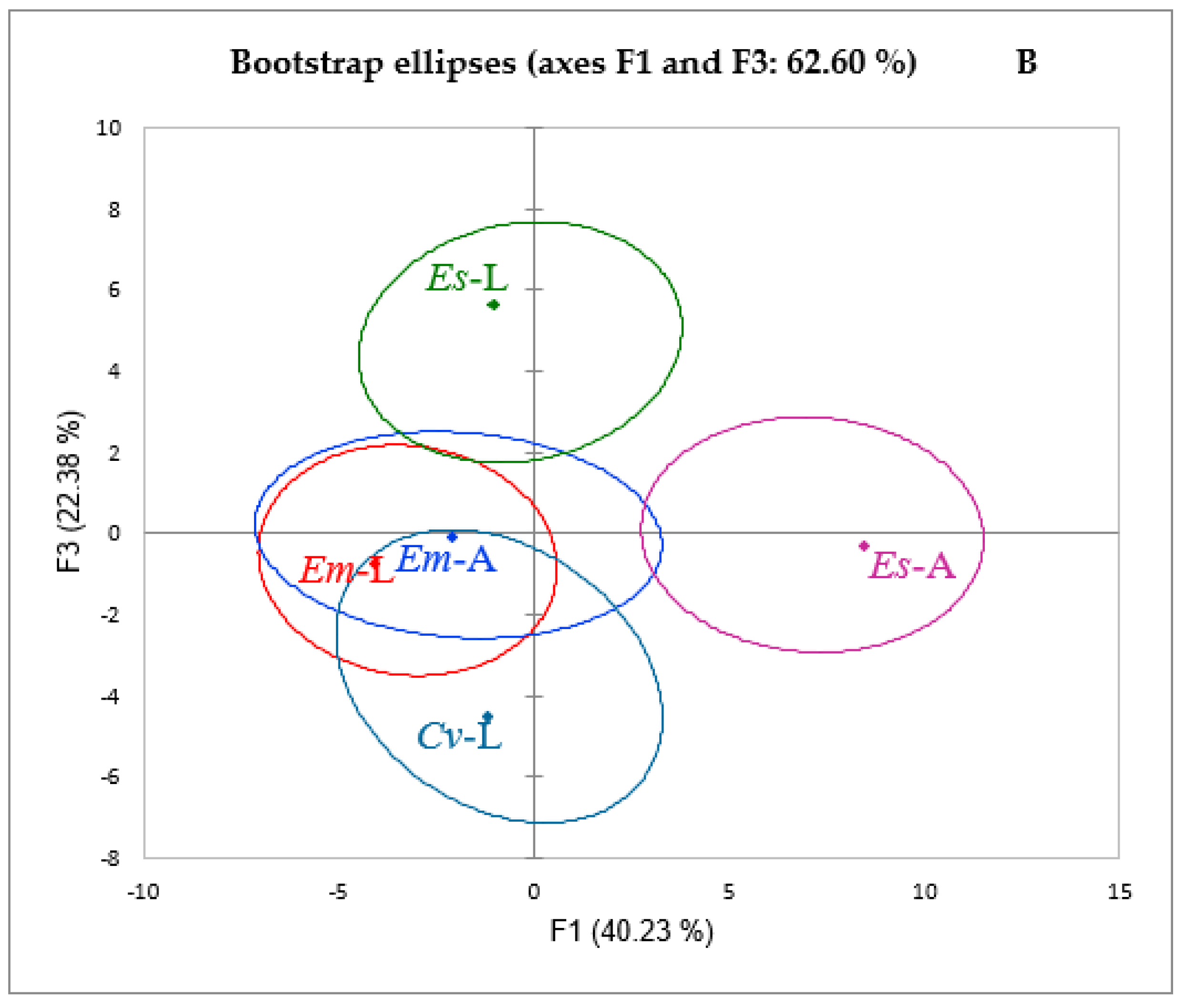
2. Results and Discussion
2.1. Polyphenol Composition
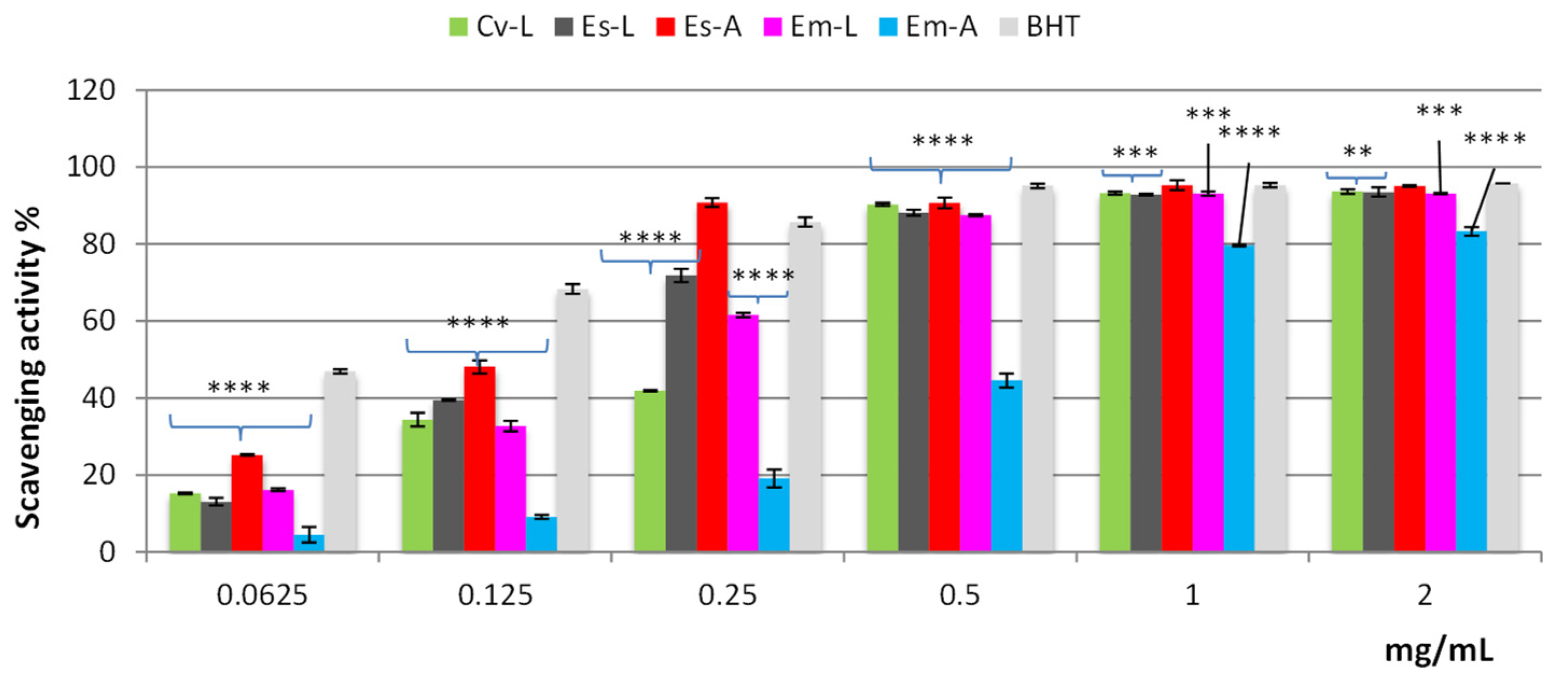
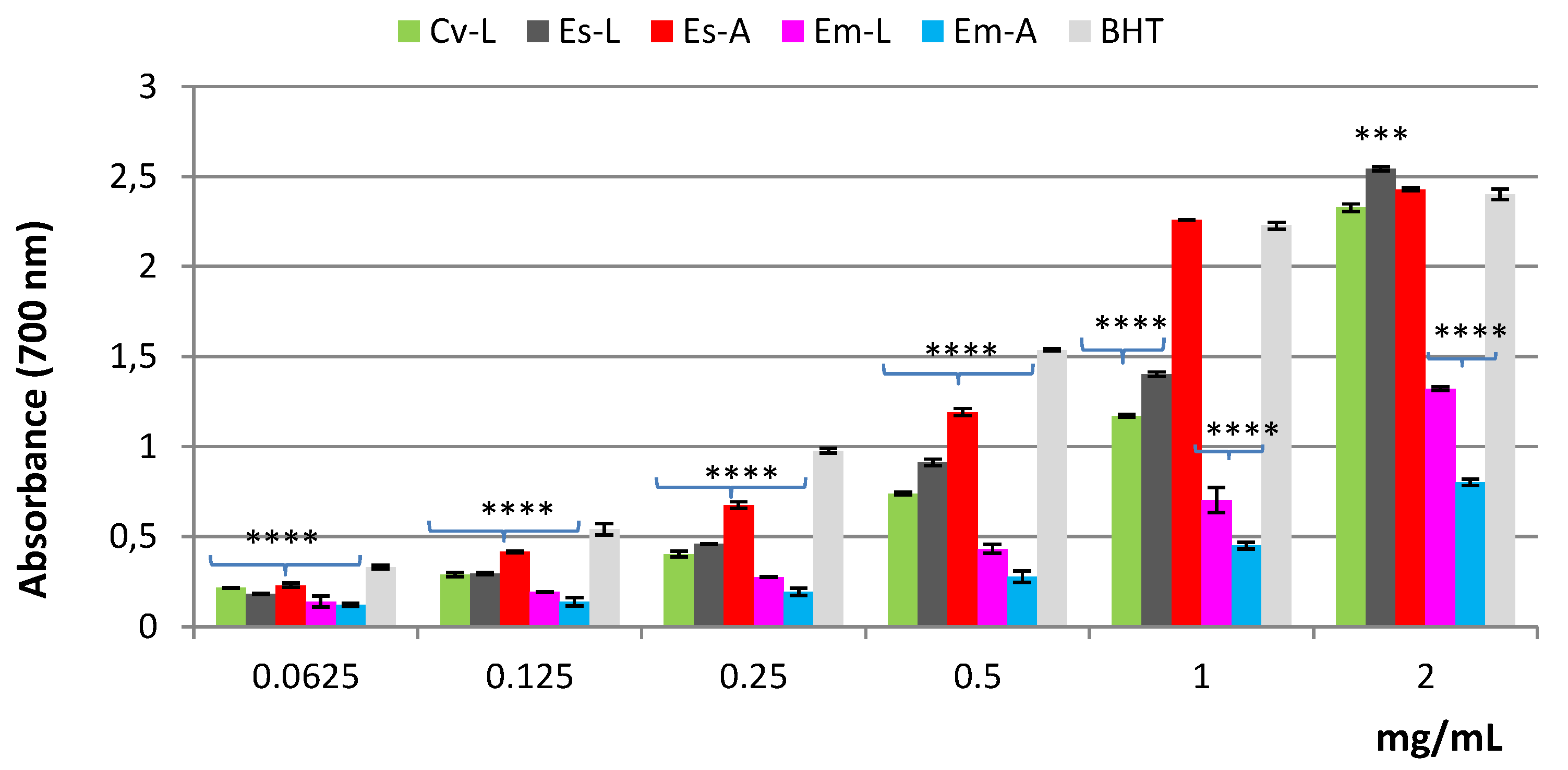
2.2. Antioxidant and Cytotoxic Activities
2.2.1. Antioxidant Activity
2.2.2. Artemia salina Lethality Bioassay
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Extraction Procedure
3.4. LC–DAD/ESI–MS Analyses
3.5. Preparation of Calibration Curves
3.6. Antioxidant and Cytotoxic Activities
3.6.1. Free Radical Scavenging Activity
3.6.2. Reducing Power Assay
3.6.3. Ferrous Ion (Fe2+) Chelating Activity
3.6.4. Artemia salina Lethality Bioassay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amezouar, F.; Badri, W.; Hsaine, M.; Bourhim, N.; Fougrach, H. Évaluation des activités antioxydante et anti-inflammatoire de Erica arborea L. du Maroc. Pathol. Biol. 2013, 61, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Guendouze-Bouchefa, N.; Madani, K.; Chibane, M.; Boulekbache-Makhlouf, L.; Hauchard, D.; Kiendrebeogo, M.; Stévigny, C.; Okusa, P.N.; Duez, P. Phenolic compounds, antioxidant and antibacterial activities of three Ericaceae from Algeria. Ind. Crops Prod. 2015, 70, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Ștefănescu, B.E.; Szabo, K.; Mocan, A.; Crişan, G. Phenolic compounds from five Ericaceae species leaves and their related bioavailability and health benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamim, A.; Miché, L.; Douaik, A.; Mrabet, R.; Ouhammou, A.; Duponnois, R.; Hafidi, M. Diversity of fungal assemblages in roots of Ericaceae in two Mediterranean contrasting ecosystems. Comptes Rendus Biol. 2017, 340, 226–237. [Google Scholar] [CrossRef]
- Sadki, C.; Hacht, B.; Souliman, A.; Atmani, F. Acute diuretic activity of aqueous Erica multiflora flowers and Cynodon dactylon rhizomes extracts in rats. J. Ethnopharmacol. 2010, 128, 352–356. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Zidane, L. Ethnobotanical Study Aimed at Investigating the Use of Medicinal Plants to Treat Nervous System Diseases in the Rif of Morocco. J. Chiropract. Med. 2020, 19, 70–81. [Google Scholar] [CrossRef]
- Djahafi, A.; Taïbi, K.; Abderrahim, L.A. Aromatic and medicinal plants used in traditional medicine in the region of Tiaret, North West of Algeria. Medit. Bot. 2021, 42, e71465. [Google Scholar] [CrossRef]
- Harnafi, H.; Bouanani, N.E.H.; Aziz, M.; Serghini-Caid, H.; Ghalim, N.; Amrani, S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in triton WR-1339 induced hyperlipidaemic rats: A comparison with fenofibrate. J. Ethnopharmacol. 2007, 109, 156–160. [Google Scholar] [CrossRef]
- Khlifi, R.; Lahmar, A.; Dhaouefi, Z.; Kalboussi, Z.; Maatouk, M.; Kilani-Jaziri, S.; Ghedira, K.; Chekir-Ghedira, L. Assessment of hypolipidemic, anti-inflammatory and antioxidant properties of medicinal plant Erica multiflora in triton WR-1339-induced hyperlipidemia and liver function repair in rats: A comparison with fenofibrate. Regul. Toxicol. Pharmacol. 2019, 107, 104404. [Google Scholar] [CrossRef]
- Sadki, C.; Atmani, F. Évaluation de l’effet antilithiasique, oxalo-calcique et phospho-ammoniaco-magnésien d’extrait aqueux d’Erica multiflora L. Prog. Urol. 2017, 27, 1058–1067. [Google Scholar] [CrossRef]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) hull) at different growth stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Moreira, T.; Pinto, D.; Pimentel, F.B.; Costa, A.S.G.; Nunes, M.A.; Gonçalves Albuquerque, T.; Costa, H.S.; Palmeira-de-Oliveira, A.; Oliveira, A.I.; et al. The phytochemical and bioactivity profiles of wild Calluna vulgaris L. flowers. Food Res. Int. 2018, 111, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monschein, M.; Iglesias Neira, J.; Kunert, O.; Bucar, F. Phytochemistry of heather (Calluna Vulgaris (L.) Hull) and its altitudinal alteration. Phytochem. Rev. 2010, 9, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Dezminieran, D.; Al Marghitas, L.; Fit, N.; Chirilaz, F.; Gherman, B.; Margaouan, R.; Aurori, A.; Bobis, O. Antibacterial Effect of Heather Honey (Calluna vulgaris) against Different Microorganisms of Clinical Importance. Bull. UASVM Anim. Sci. Biotechnol. 2015, 72, 72–77. [Google Scholar]
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of Scottish plants for antibacterial activity. J. Ethnopharmacol. 2002, 1–2, 73–77. [Google Scholar] [CrossRef]
- Kiruba, S.; Mahesh, M.; Nisha, S.R.; Miller Paul, Z.; Jeeva, S. Phytochemical analysis of the flower extracts of Rhododendron arboreum Sm. ssp. nilagiricum (Zenker) Tagg. Asian Pac. J. Trop. Biomed. 2011, 1, S284–S286. [Google Scholar] [CrossRef]
- Shamilov, A.A.; Bubenchikova, V.N.; Chernikov, M.V.; Pozdnyakov, D.I.; Garsiya, E.R. Vaccinium Vitis-Idaea L.: Chemical contents, pharmacological activities. Pharm. Sci. 2020, 26, 344–362. [Google Scholar] [CrossRef]
- Madhvi, S.K.; Sharma, M.; Iqbal, J.; Younis, M. Phytochemistry, traditional uses and pharmacology of Rhododendron Arboreum: A review. Res. J. Pharm. Technol. 2019, 12, 4565. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arigò, A.; Česla, P.; Šilarová, P.; Calabrò, M.L.; Česlová, L. Development of extraction method for characterization of free and bonded polyphenols in barley (Hordeum vulgare L.) grown in Czech Republic using liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 245, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Česla, P.; Fischer, J.; Jandera, P. Improvement of the sensitivity of 2D LC-MEKC separation of phenolic acids and flavonoids natural antioxidants using the on-line preconcentration step. Electrophoresis 2012, 33, 2464–2473. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In Phytochemicals—Source of Antioxidants and Role in Disease Prevention; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Khlifi, R.; Dhaouefi, Z.; Toumia, I.B.; Lahmara, A.; Siouda, F.; Bouhajeba, R.; Bellalah, A.; Chekir-Ghedira, L. Erica multiflora extract rich in quercetin-3-O-glucoside and kaempferol-3-O-glucoside alleviates high fat and fructose diet-induced fatty liver disease by modulating metabolic and inflammatory pathways in Wistar rats. J. Nutr. Biochem. 2020, 86, 108490. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, F.; Ben Hadj, A.S.; Landoulsi, A. Investigation of Extracts from Tunisian Ethnomedicinal Plants as Antioxidants, Cytotoxins, and Antimicrobials. Biomed. Environ. Sci. 2017, 30, 811–824. [Google Scholar]
- Mandìm, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Khan, M.I.; Khan, M.R.; Muhammad, N.; Khan, A.U.; Khan, R.A. Role of medicinal plants in oxidative stress and cancer. Open Access Sci. Rep. 2013, 2, 641. [Google Scholar]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Mariotte, A.M.; Ghedira, K.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-O-galactoside and myricetin-3-O-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol. In Vitro 2008, 22, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Protective effect of myricetin derivatives from Syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Mol. Vis. 2019, 25, 47–59. [Google Scholar]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Morishita, H.; Iwahashi, H.; Shitzuo, T.; Yoshiaki, S.; Kimura, M.; Kido, R. Inhibitory effects of chlorogenic acid on linoleic acid peroxidation and haemolysis. Phytochemistry. 1994, 36, 579–583. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Park, K.-S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Peak No | Compound | tR (min) | UV max (nm) | [M-H]- | E. multiflora (mg/Kg ± RSD%) | E. scoparia (mg/Kg ± RSD%) | C. vulgaris (mg/Kg ± RSD%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Aerial Parts | Leaves | Aerial Parts | Leaves | |||||
| 1 | Taxifolin-O-hexoside | 4.11 | 288 | 465, 303, 313 | 332.96 ± 0.68 | ||||
| 2 | Taxifolin-O-hexoside isomer | 4.24 | 284 | 465, 303, 313 | 214.93 ± 1.49 | ||||
| 3 | Digalloyl-quinic acid | 4.61 | 274 | 495 | Nq | ||||
| 4 | Caffeoylquinic acid | 4.81 | 297sh, 326 | 353, 191, 179 | 53.93 ± 0.11 | 61.11 ± 0.18 | |||
| 5 | 4-O-Caffeoylquinic acid | 4.91 | 297sh, 326 | 353, 191, 179 | 83.75 ± 0.74 | ||||
| 6 | Caffeoylquinic acid | 4.99 | 290, 325 | 353, 191,137 | 626.40 ± 0.77 | ||||
| 7 | Myricetin-O-hexoside | 5.38 | 258, 358 | 479, 317 | 2130.25 ± 0.78 | ||||
| 8 | Eriodictyol-O-hexoside | 5.42 | 297, 321 | 449, 287 | Nq | ||||
| 9 | Caffeoylquinic acid | 5.42 | 290, 325 | 353, 191,137 | 138.37 ± 0.23 | ||||
| 10 | Caffeoylquinic acid | 5.47 | 290, 325 | 353, 191,137 | 231.54 ± 1.68 | ||||
| 11 | Quercetin derivative | 5.63 | 260, 356 | 615, 463, 301 | 2.89 ± 0.83 | ||||
| 12 | Myricetin-O-hexoside isomer | 5.67 | 356 | 479, 317 | 43.46 ± 0.35 | ||||
| 13 | Myricetin-O-pentoside | 5.70 | 259, 357 | 449, 317 | 852.85 ± 1.97 | ||||
| 14 | Myricetin-O-rhamnoside | 5.74 | 260, 357 | 463, 317 | 1625.89 ± 0.39 | ||||
| 15 | Quercetin-O-hexoside | 5.83 | 255, 353 | 463, 301 | 213.14 ± 0.43 | ||||
| 16 | Rutin | 5.87 | 257, 354 | 609, 301 | 55.44 ± 2.59 | 14.16 ± 0.18 | |||
| 17 | Caffeoylquinic acid | 5.87 | 290, 325 | 353, 191,137 | 184.69 ± 0.95 | ||||
| 18 | Methoxy-myricetin-O-rhamnoside | 5.88 | 254, 358 | 493 | 810.78 ± 0.43 | ||||
| 19 | p-Coumaroylquinic acid | 6.07 | 312 | 337 | Nq | ||||
| 20 | Quercetin-O-hexoside | 6.08 | 255, 355 | 463, 301 | 117.43 ± 0.48 | 29.48 ± 1.76 | |||
| 21 | Quercetin-O-hexoside | 6.13 | 354 | 463, 301 | 4.78 ± 0.67 | 0.10 ± 2.51 | |||
| 22 | Kaempferol-O-(6″-galloyl)hexoside | 6.17 | 253, 358 | 599, 285 | 564.64 ± 0.19 | ||||
| 23 | Myricetin-O-rhamnoside | 6.20 | 358 | 463 | 268.52 ± 0.08 | ||||
| 24 | Myricetin-O-hexoside | 6.21 | 356 | 479, 317 | 184.38 ± 0.26 | ||||
| 25 | Kaempferol-rhamnosyl-hexoside | 6.24 | 264, 347 | 593, 447, 285 | 90.76 ± 1.19 | 15.24 ± 0.21 | |||
| 26 | Myricetin-O-hexoside | 6.25 | 356 | 479, 317 | 41.66 ± 1.88 | ||||
| 27 | Isorhamnetin-O-hexoside | 6.32 | 252, 357 | 477 | 683.43 ± 0.93 | ||||
| 28 | Kaempferol-hexoside | 6.51 | 264, 348 | 447, 285 | 4.83 ± 1.27 | 5.55 ± 2.06 | |||
| 29 | Myricetin-O-pentoside | 6.55 | 260, 357 | 449, 317 | 72.79 ± 0.05 | ||||
| 30 | Quercetin galloyl hexoside derivative | 6.56 | 357 | 615 | 160.67 ± 1.25 | ||||
| 31 | Myricetin-O-pentoside | 6.59 | 281, 349 | 449, 317 | 48.81 ± 2.22 | ||||
| 32 | Kaempferol-hexoside isomer | 6.61 | 264, 348 | 447, 285 | 17.08 ± 0.35 | 14.53 ± 0.44 | |||
| 33 | Myricetin-O-rhamnoside | 6.65 | 260, 357 | 463, 317 | 153.65 ± 1.13 | ||||
| 34 | Quercetin-O-hexoside | 6.68 | 255, 353 | 463, 301 | 64.25 ± 1.47 | 2.82 ± 3.24 | |||
| 35 | Myricetin-O-(6″-benzoyl)hexoside | 6.70 | 265,316, 358 | 583, 316 | 200.83 ± 0.20 | ||||
| 36 | Methyl-ellagic acid hexoside | 6.72 | 283 | 477 | Nq | ||||
| 37 | Myricetin-O-rhamnoside | 6.72 | 260, 357 | 463, 317 | 232.98 ± 0.35 | ||||
| 38 | Unknown | 6.97 | 344 | 649 | Nq | ||||
| 39 | Quercetin-O-(malonyl)hexoside | 7.03 | 356 | 549 | 18.52 ± 0.27 | ||||
| 40 | Quercetin-O-pentoside | 7.06 | 255, 354 | 433, 301 | 9.44 ± 0.28 | ||||
| 41 | Unknown | 7.11 | 358 | 599, 507, 463 | Nq | ||||
| 42 | Quercetin-O-(6″-p-hydroxybenzoyl) hexoside | 7.17 | 269, 356 | 583, 316 | 91.34 ± 1.22 | ||||
| 43 | Unknown | 7.22 | 350 | 723, 677, 477 | Nq | ||||
| 44 | Quercetin-O-rhamnoside | 7.22 | 255, 342 | 447, 301 | 32.30 ± 0.02 | ||||
| 45 | Kaempferol-O-rhamnoside | 7.77 | 263, 341 | 431, 285 | 18.77 ± 0.55 | ||||
| 46 | Unknown | 7.89 | 312 | 731 | Nq | ||||
| 47 | Myricetin-O-(6″-cinnamoyl)hexoside | 8.14 | 265, 359 | 609, 317, 301 | 757.33 ± 1.96 | ||||
| 48 | Unknown | 8.22 | 288, 308 | 289 | Nq | ||||
| 49 | Unknown | 8.30 | 309 | 483, 289 | Nq | ||||
| 50 | Quercetin-O-(6″-cinnamoyl)hexoside | 8.41 | 281 | 593, 447, 301 | 3.72 ± 1.10 | ||||
| 51 | Quercetin | 8.66 | 268, 370 | 301 | 3.34 ± 2.11 | 1.39 ± 5.97 | |||
| 52 | Myricetin-O-(6″-p-coumaroyl)hexoside | 8.77 | 265, 360 | 624 | 509.39 ± 0.94 | ||||
| 53 | Isorhamnetin-O-(6″-caffeoyl)hexoside | 9.45 | 264, 359 | 639 | 111.98 ± 0.50 | ||||
| 54 | Dimethylquercetin | 9.46 | 227, 344 | 329, 301 | 0.86 ± 8.95 | 1.38 ± 0.62 | |||
| 55 | Myricetin-O-(6″-cinnamoyl)hexoside | 9.53 | 264, 359 | 609, 317, 301 | 23.46 ± 1.49 | ||||
| 56 | Kaempferol | 10.22 | 366 | 285 | 0.49 ± 1.89 | 0.91 ± 1.84 | |||
| 57 | Isorhamnetin-O-hexoside-O-rhamnoside | 10.22 | 264, 359 | 623 | 9.76 ± 0.34 | ||||
| 58 | Quercetin-O-(6″-cinnamoyl)hexoside | 10.40 | 356 | 593, 447, 301 | 0.79 ± 1.12 | ||||
| 59 | Unknown | 10.97 | 356 | 637, 347 | Nq | ||||
| Total | 399.01 ± 1.46 | 227.6 ± 0.15 | 527.6 ± 1.55 | 9528.93 ± 54.32 | 1567.78 ± 13.01 | ||||
| Ericaceae Taxa | DPPH Test IC50 (mg/mL) | Reducing Power ASE/mL | Fe2+ Chelating Activity IC50 (mg/mL) |
|---|---|---|---|
| Cv-L | 0.212 ± 0.061 a | 2.790 ± 0.100 a | NA |
| Es-L | 0.189 ± 0.051 a | 2.721 ± 0.062 a | NA |
| Es-A | 0.142 ± 0.014 b | 1.898 ± 0.056 b | >2 |
| Em-L | 0.200 ± 0.001 a | 3.814 ± 0.091 c | NA |
| Em-A | 0.611 ± 0.017 c | 5.538 ± 0.148 d | >2 |
| Standard | BHT 0.154 ± 0.001 b | BHT 1.131 ± 0.037 e | EDTA 0.0067 ± 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekkai, D.; Oulad El Majdoub, Y.; Bekkai, H.; Cacciola, F.; Miceli, N.; Taviano, M.F.; Cavò, E.; Errabii, T.; Laganà Vinci, R.; Mondello, L.; et al. Determination of the Phenolic Profile by Liquid Chromatography, Evaluation of Antioxidant Activity and Toxicity of Moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae). Molecules 2022, 27, 3979. https://doi.org/10.3390/molecules27133979
Bekkai D, Oulad El Majdoub Y, Bekkai H, Cacciola F, Miceli N, Taviano MF, Cavò E, Errabii T, Laganà Vinci R, Mondello L, et al. Determination of the Phenolic Profile by Liquid Chromatography, Evaluation of Antioxidant Activity and Toxicity of Moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae). Molecules. 2022; 27(13):3979. https://doi.org/10.3390/molecules27133979
Chicago/Turabian StyleBekkai, Douaa, Yassine Oulad El Majdoub, Hamid Bekkai, Francesco Cacciola, Natalizia Miceli, Maria Fernanda Taviano, Emilia Cavò, Tomader Errabii, Roberto Laganà Vinci, Luigi Mondello, and et al. 2022. "Determination of the Phenolic Profile by Liquid Chromatography, Evaluation of Antioxidant Activity and Toxicity of Moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae)" Molecules 27, no. 13: 3979. https://doi.org/10.3390/molecules27133979
APA StyleBekkai, D., Oulad El Majdoub, Y., Bekkai, H., Cacciola, F., Miceli, N., Taviano, M. F., Cavò, E., Errabii, T., Laganà Vinci, R., Mondello, L., & L’Bachir El Kbiach, M. (2022). Determination of the Phenolic Profile by Liquid Chromatography, Evaluation of Antioxidant Activity and Toxicity of Moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae). Molecules, 27(13), 3979. https://doi.org/10.3390/molecules27133979









