Cell Fate following Irradiation of MDA-MB-231 and MCF-7 Breast Cancer Cells Pre-Exposed to the Tetrahydroisoquinoline Sulfamate Microtubule Disruptor STX3451
Abstract
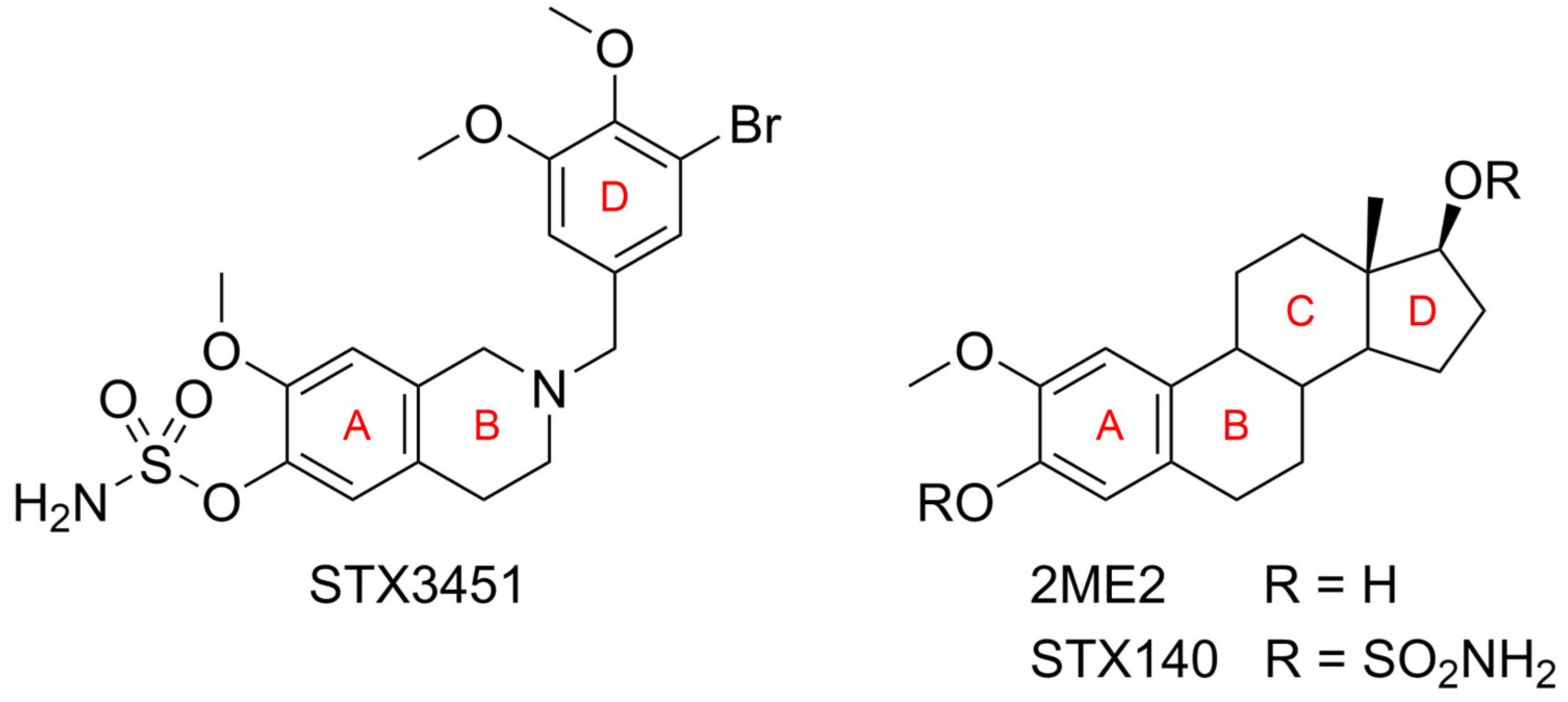
1. Introduction
2. Results
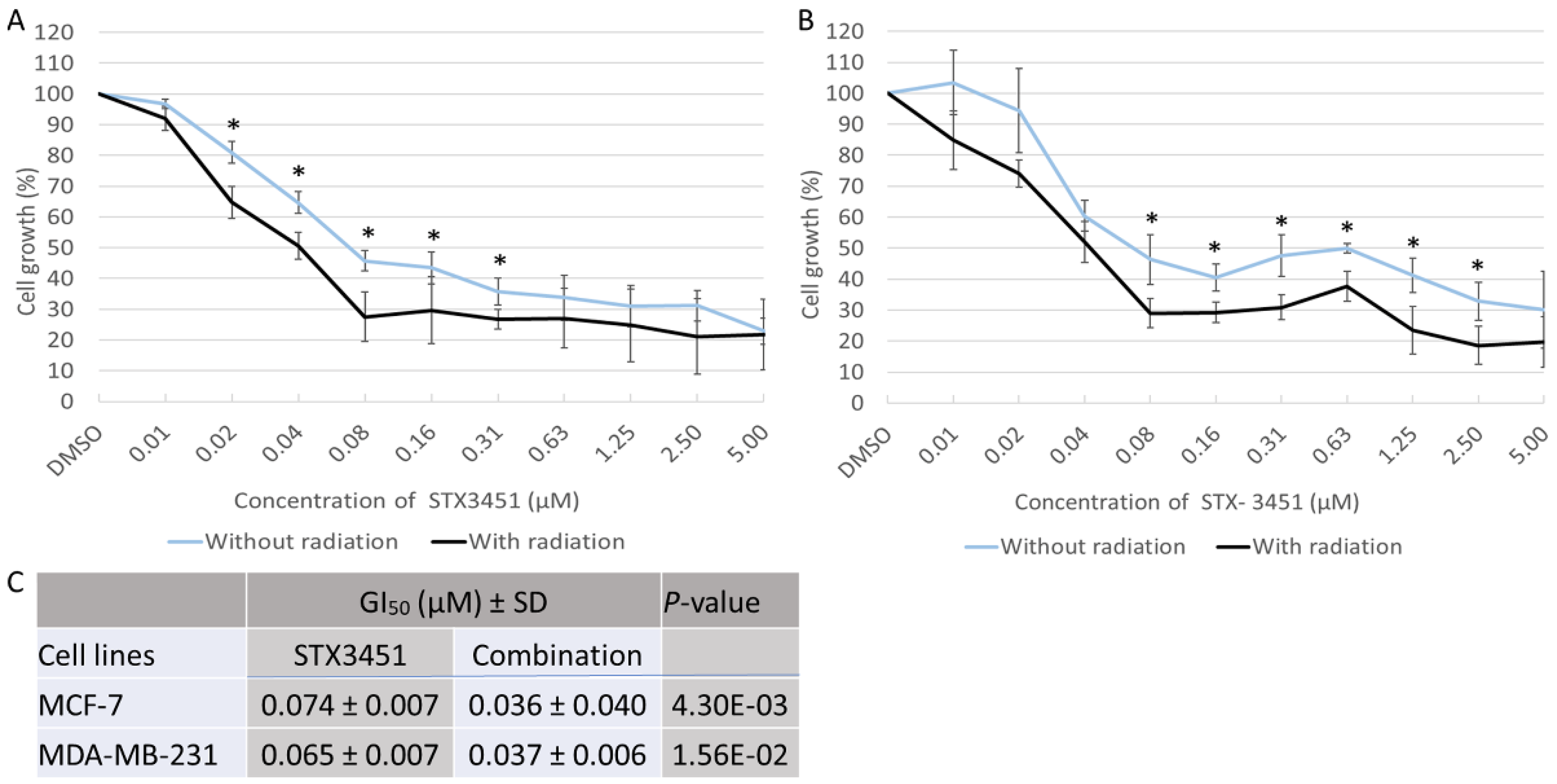
2.1. STX3451 Pre-Exposure Significantly Increases the Cytotoxicity of Radiation
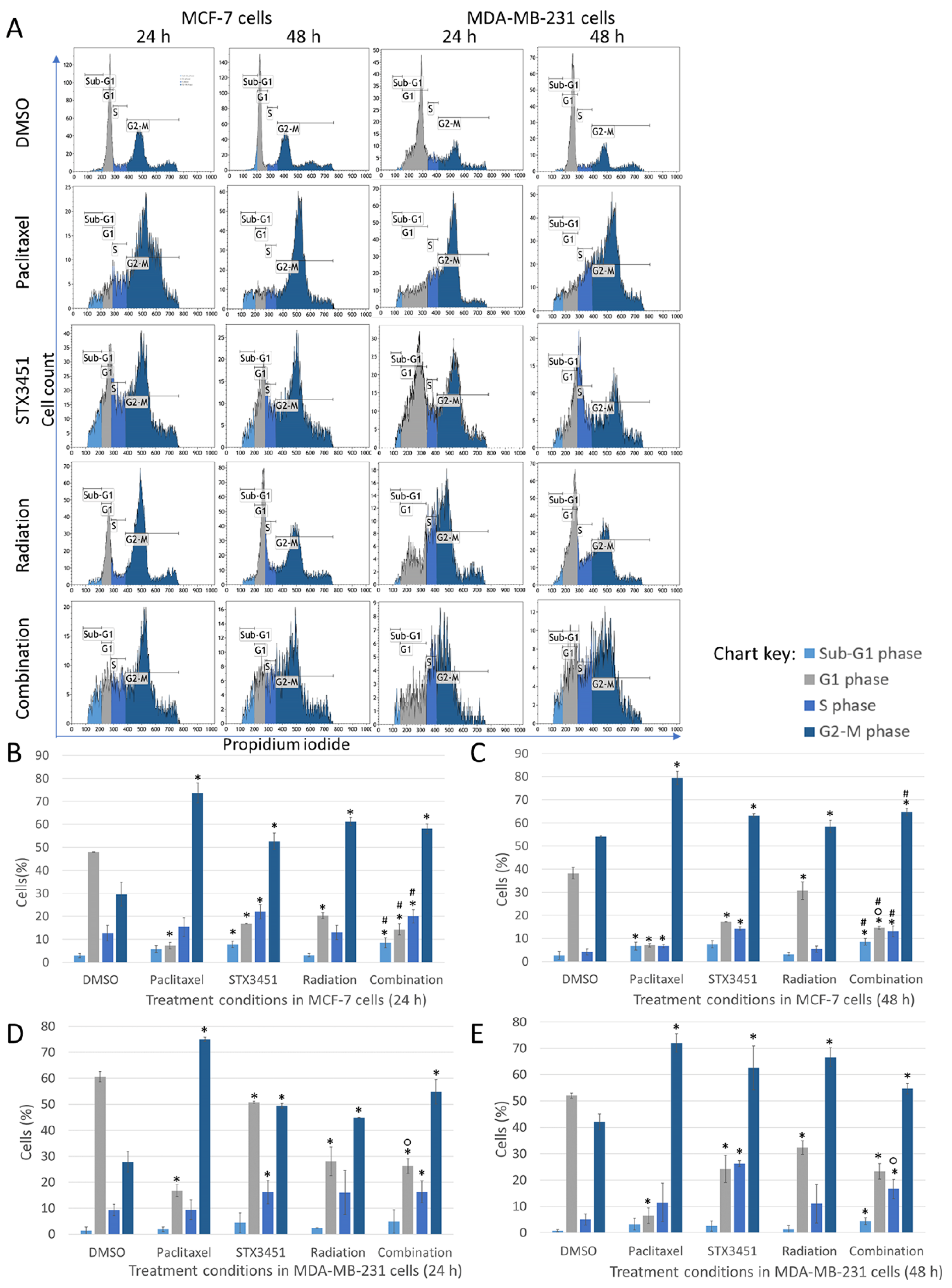
2.2. STX3451 and Radiation Induce a Metaphase Block, as Both Individual Modalities and in Combination
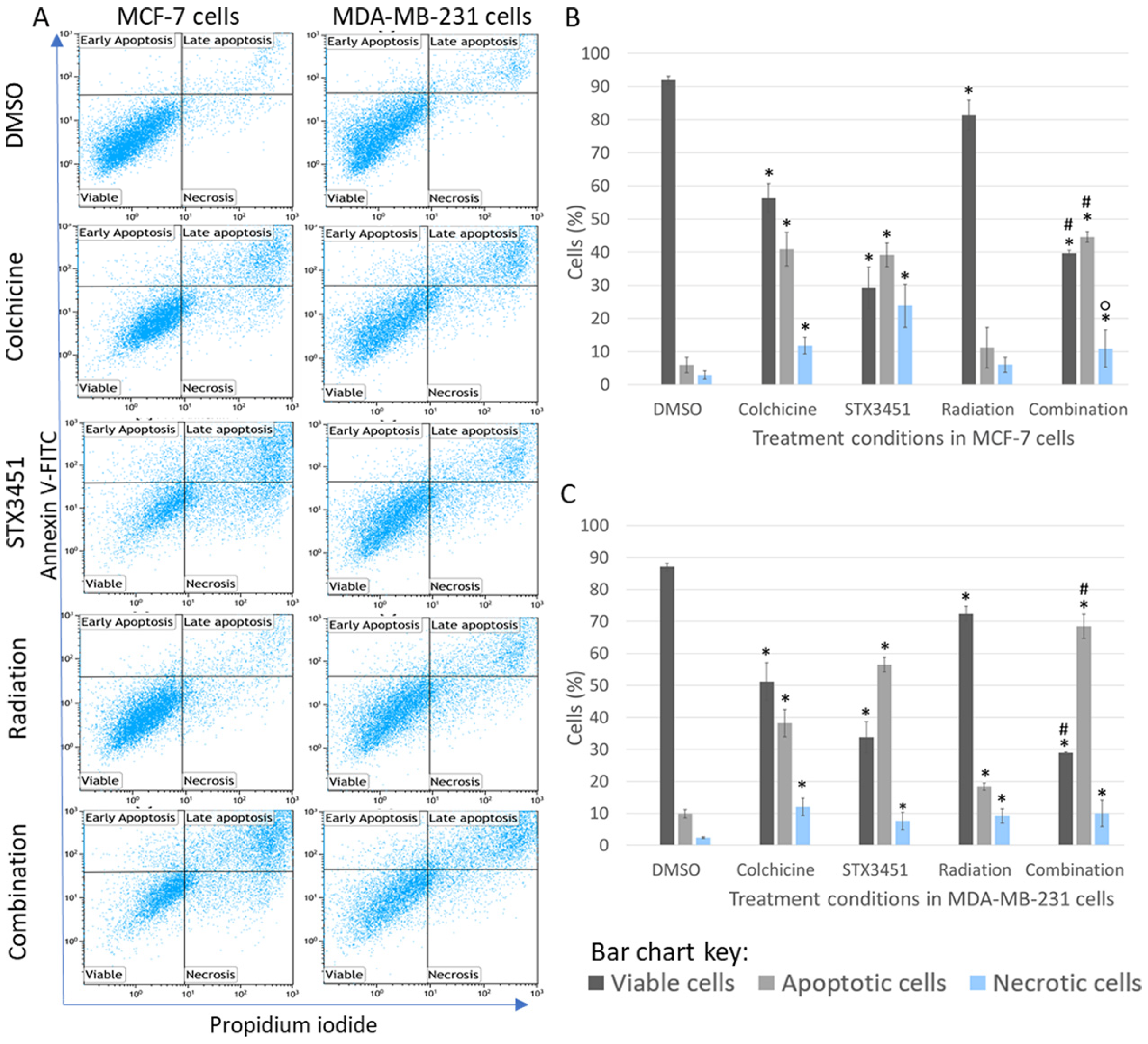
2.3. Early Apoptosis Induction Appears to Be Mainly Attributed to the Drug Exposure
2.4. Long-Term Viability Studies Indicated That STX3451 and 6 Gy Radiation Display a Synergistic Effect in Inhibiting Colony Formation
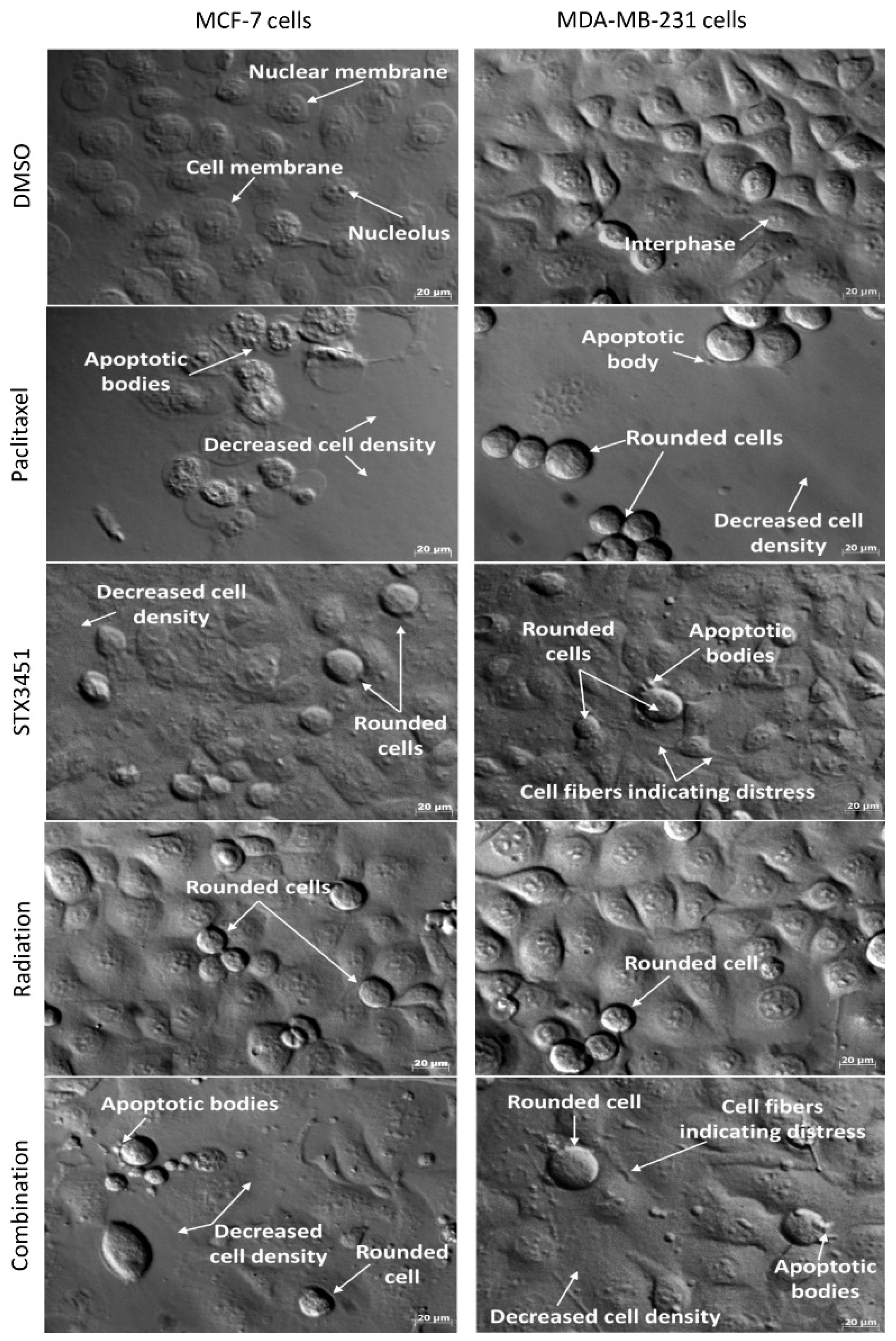
2.5. Radiated Cells Pre-Treated with STX3451 Display Cell Rounding, Decreased Cell Density and Evidence of Apoptosis
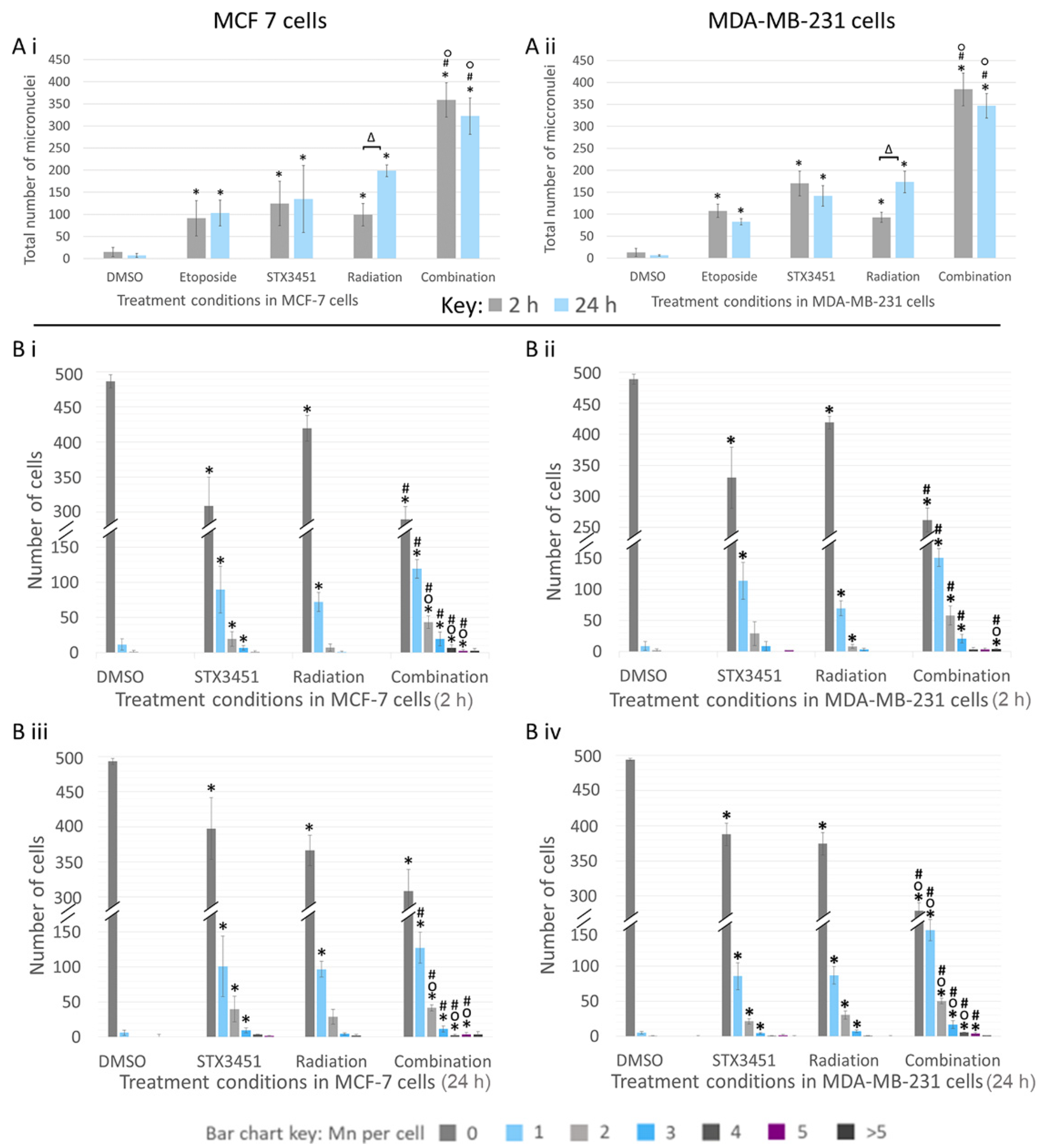
2.6. Chemo-Radiation Induces Greater DNA Damage Than the Individual Modalities
2.7. Increase in Reactive Oxygen Species Is Attributed Largely to STX3451 Exposure
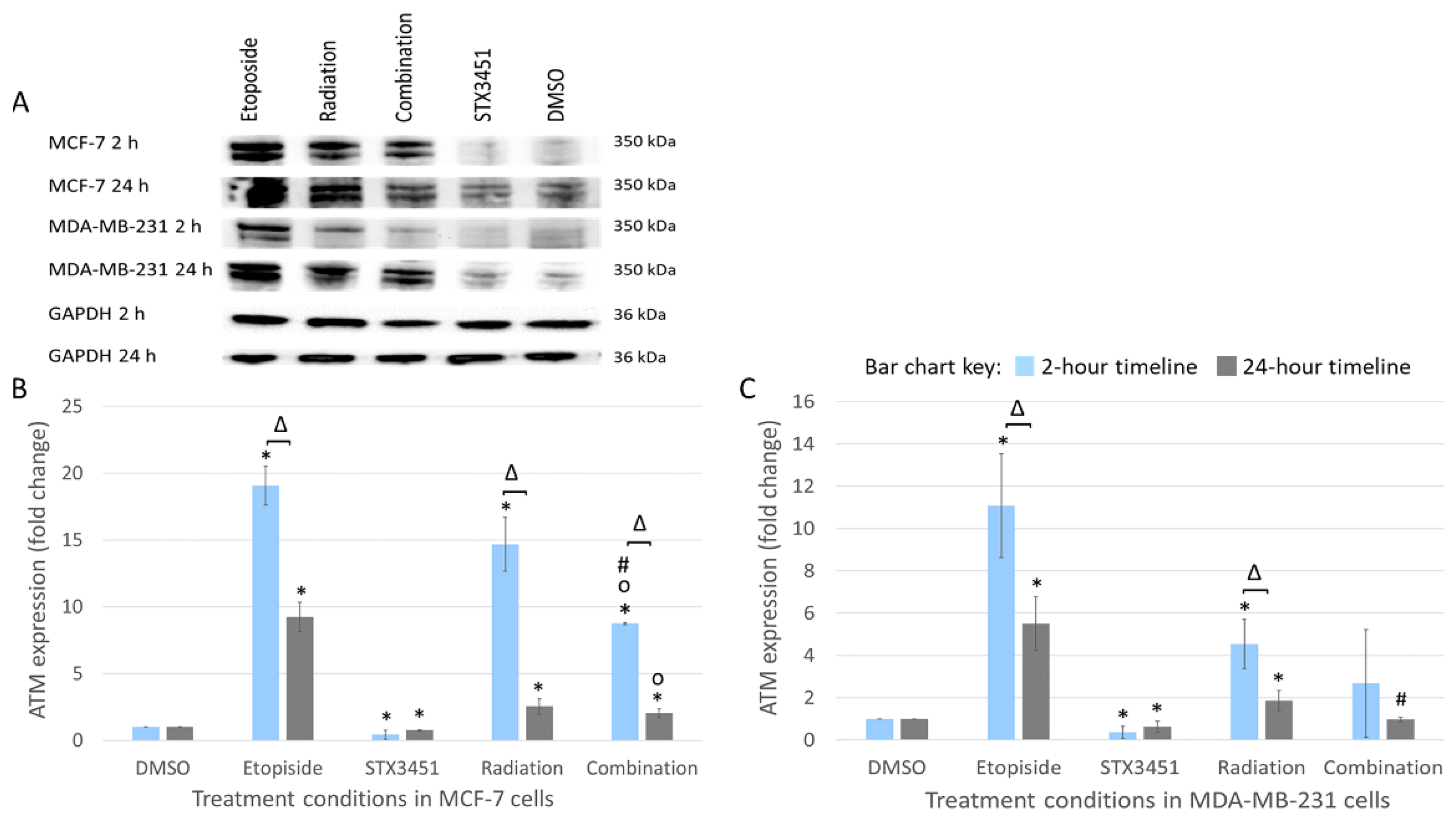
2.8. STX3451 Suppressed ATM Expression as Part of the DNA Damage Response
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cell Culture
4.2.2. Treatment Protocol
4.2.3. Experimental and Method Controls
4.2.4. Spectrophotometric Quantification of Crystal Violet Staining
4.2.5. Flow Cytometric Analysis of Cell Cycle Progression
4.2.6. Annexin V Apoptosis Detection by Flow Cytometry
4.2.7. Long-Term Survival Analysis Using Clonogenic Studies
4.2.8. Morphological Analysis Using Polarization-Optical Transmitted Light Differential Interference Contrast Microscopy (PlasDIC)
4.2.9. Quantification of DNA Damage via Assessment of Micronuclei Formation
4.2.10. Quantification of Reactive Oxygen Species (ROS) Using Flow Cytometry
4.2.11. Analysis of ATM Expression as Part of the DNA Damage Response Signaling Using Western Blot
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 2ME2 | 2-methoxyestradiol |
| AKT | protein kinase |
| ANOVA | analysis of variance |
| ATCC | American Type Culture Collection |
| ATM | ataxia telangiectasia mutated |
| BCA | bicinchoninic acid |
| BSA | bovine serum albumin |
| CA | California |
| CANSA | Cancer Association of South Africa |
| CBMN | cytokinesis-block micronucleus |
| CDK | cyclin-dependent kinases |
| CO | Colorado |
| ddH2O | double-distilled water |
| DDR | DNA damage response |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| DR3 | death receptor 3 |
| dsDNA | double-strand DNA |
| ESE-15-ol | 2-ethyl-3-O-sulfamoyl-estra-1,3,5(10),15-tetraen-17-ol |
| ESE-16 | 2-ethyl-3-O-sulfamoyl-estra-1,3,5(10)16-tetraene |
| FCS | fetal calf serum |
| FITC | fluorescein isothiocyanate |
| G1 phase | gap phase 1 |
| G2 phase | gap phase 2 |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GI50 | half-maximal growth inhibition concentration |
| Gy | gray |
| H2AX | histone 2A variant X |
| HE | hydroethidine |
| HIF-1α | hypoxia-inducible factor 1-αJHB—Johannesburg |
| KCl | potassium chloride |
| MA | Massachusetts |
| MCF-7 | Michigan Cancer Foundation-7 |
| MD | Maryland |
| MDA | microtubule disrupting agent |
| MDA-MB-231 | metastatic human breast adenocarcinoma cells |
| M | mitosis |
| Mn | micronuclei |
| MO | Missouri |
| MOPS | 3-morpholinopropane-1-sulfonic acid |
| MRN | meiotic recombination 11-double strand break repair protein-nibrin (MRE11-RAD50-NBS1) |
| mRNA | messenger ribonucleic acid |
| MDAs | microtubule disrupting agents |
| MW | molecular weight |
| NF-KB | nuclear factor kappa light chain enhancer of activated B cells |
| NIH | National Institute of Health |
| nM | nanomolar |
| NRF | National Research Foundation |
| O2·− | superoxide |
| PBS | phosphate-buffered saline |
| PE | plating efficiency |
| PI | propidium iodide |
| PlasDIC | polarization-optical transmitted light differential interference contrast microscopy |
| PVDF | polyvinylidene fluoride |
| RESCOM | School of Medicine Research Committee of the University of Pretoria |
| RIPA | radioimmunoprecipitation assay |
| RNase A | ribonuclease A |
| ROS | reactive oxygen species |
| RT | room temperature |
| SD | standard deviation |
| SF | surviving fraction |
| S phase | synthesis phase |
| STX 3451 | 2-(3-bromo-4,5-dimethoxybenzyl)-7-methoxy-6-sulfamoyloxy-1,2,3,4-tetrahydroisoquinoline |
| THIQ | tetrahydroisoquinoline |
| UK | United Kingdom |
| USA | United States of America |
| VT | Vermont |
| γ-H2AX | gamma-histone 2A variant X |
References
- Van Vuuren, R.J.; Visagie, M.H.; Theron, A.E.; Joubert, A.M. Antimitotic drugs in the treatment of cancer. Cancer Chemother. Pharmacol. 2015, 76, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.J.; Lin, C.M.; Flynn, E.; Folkman, J.; Hamel, E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc. Natl. Acad. Sci. USA 1994, 91, 3964–3968. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, S.L. Mechanism of action of 2-methoxyestradiol: New developments. Drug Resist. Updat. 2004, 6, 355–361. [Google Scholar] [CrossRef] [PubMed]
- LaVallee, T.M.; Zhan, X.H.; Johnson, M.S.; Herbstritt, C.J.; Swartz, G.; Williams, M.S.; Hembrough, W.A.; Green, S.J.; Pribluda, V.S. 2-methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res. 2003, 63, 468–475. [Google Scholar]
- Kato, S.; Sadarangani, A.; Lange, S.; Delpiano, A.M.; Vargas, M.; Brañes, J.; Carvajal, J.; Lipkowitz, S.; Owen, G.I.; Cuello, M.A. 2-Methoxyestradiol mediates apoptosis through caspase-dependent and independent mechanisms in ovarian cancer cells but not in normal counterparts. Reprod. Sci. 2008, 15, 878–894. [Google Scholar] [CrossRef]
- Sweeney, C.; Liu, G.; Yiannoutsos, C.; Kolesar, J.; Horvath, D.; Staab, M.J.; Fife, K.; Armstrong, V.; Treston, A.; Sidor, C.; et al. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin. Cancer Res. 2005, 11, 6625–6633. [Google Scholar] [CrossRef]
- Dubey, R.K.; Jackson, E.K. Potential vascular actions of 2-methoxyestradiol. Trends Endocrinol. Metab. TEM 2009, 20, 374–379. [Google Scholar] [CrossRef]
- Verenich, S.; Gerk, P.M. Therapeutic Promises of 2-Methoxyestradiol and Its Drug Disposition Challenges. Mol. Pharm. 2010, 7, 2030–2039. [Google Scholar] [CrossRef]
- Ireson, C.R.; Chander, S.K.; Purohit, A.; Perera, S.; Newman, S.P.; Parish, D.; Leese, M.P.; Smith, A.C.; Potter, B.V.L.; Reed, M.J. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br. J. Cancer 2004, 90, 932–937. [Google Scholar] [CrossRef]
- Tinley, T.L.; Leal, R.M.; Randall-Hlubek, D.A.; Cessac, J.W.; Wilkens, L.R.; Rao, P.N.; Mooberry, S.L. Novel 2-methoxyestradiol analogues with antitumor activity. Cancer Res. 2003, 63, 1538–1549. [Google Scholar] [CrossRef]
- Cushman, M.; He, H.-M.; Katzenellenbogen, J.A.; Varma, R.K.; Hamel, E.; Lin, C.M.; Ram, S.; Sachdeva, Y.P. Synthesis of Analogs of 2-Methoxyestradiol with Enhanced Inhibitory Effects on Tubulin Polymerization and Cancer Cell Growth. J. Med. Chem. 1997, 40, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P.; Ireson, C.R.; Tutill, H.J.; Day, J.M.; Parsons, M.F.C.; Leese, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A. The role of 17β-hydroxysteroid dehydrogenases in modulating the activity of 2-methoxyestradiol in breast cancer cells. Cancer Res. 2006, 66, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Leese, M.P.; Leblond, B.; Smith, A.; Newman, S.P.; Di Fiore, A.; De Simone, G.; Supuran, C.T.; Purohit, A.; Reed, M.J.; Potter, B.V.L. 2-Substituted estradiol bis-sulfamates, multitargeted antitumor agents: Synthesis, in vitro SAR, protein crystallography, and in vivo activity. J. Med. Chem. 2006, 49, 7683–7696. [Google Scholar] [CrossRef] [PubMed]
- Vorster, C.J.; Joubert, A.M. In vitro effects of 2-methoxyestradiol-bis-sulphamate on the non-tumorigenic MCF-12A cell line. Cell Biochem. Funct. 2010, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Visagie, M.H.; Joubert, A.M. 2-Methoxyestradiol-bis-sulphamate refrains from inducing apoptosis and autophagy in a non-tumorigenic breast cell line. Cancer Cell Int. 2012, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Potter, B.V.L. Estrogen O-sulfamates and their analogues: Clinical steroid sulfatase inhibitors with broad potential. J. Steroid Biochem. Mol. Biol. 2015, 153, 160–169. [Google Scholar] [CrossRef]
- Potter, B.V.L. SULFATION PATHWAYS: Steroid sulphatase inhibition via aryl sulphamates: Clinical progress, mechanism and future prospects. J. Mol. Endocrinol. 2018, 61, T233–T252. [Google Scholar] [CrossRef]
- Leese, M.P.; Jourdan, F.L.; Major, M.R.; Dohle, W.; Hamel, E.; Ferrandis, E.; Fiore, A.; Kasprzyk, P.G.; Potter, B.V.L. Tetrahydroisoquinolinone-based steroidomimetic and chimeric microtubule disruptors. ChemMedChem 2014, 9, 85–108. [Google Scholar] [CrossRef]
- Dohle, W.; Leese, M.P.; Jourdan, F.L.; Major, M.R.; Bai, R.; Hamel, E.; Ferrandis, E.; Kasprzyk, P.G.; Fiore, A.; Newman, S.P.; et al. Synthesis, antitubulin, and antiproliferative SAR of C3/C1-substituted tetrahydroisoquinolines. ChemMedChem 2014, 9, 350–370. [Google Scholar] [CrossRef]
- Dohle, W.; Leese, M.P.; Jourdan, F.L.; Chapman, C.J.; Hamel, E.; Ferrandis, E.; Potter, B.V.L. Optimisation of tetrahydroisoquinoline-based chimeric microtubule disruptors. ChemMedChem 2014, 9, 1783–1793. [Google Scholar] [CrossRef]
- Nel, M.; Joubert, A.M.; Dohle, W.; Potter, B.V.L.; Theron, A.E. Modes of cell death induced by tetrahydroisoquinoline-based analogs in MDA-MB-231 breast and A549 lung cancer cell lines. Drug Des. Dev. Ther. 2018, 12, 1881–1904. [Google Scholar] [CrossRef] [PubMed]
- Steel, G.G.; Peckham, M.J. Exploitable mechanisms in combined radiotherapy-chemotherapy: The concept of additivity. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 85–91. [Google Scholar] [CrossRef]
- Tofilon, P.J.; Camphausen, K. Molecular targets for tumor radiosensitization. Chem. Rev. 2009, 109, 2974–2988. [Google Scholar] [CrossRef][Green Version]
- Linam, J.; Yang, L.-X. Recent Developments in Radiosensitization. Anticancer Res. 2015, 35, 2479–2485. [Google Scholar] [PubMed]
- Amorino, G.P.; Freeman, M.L.; Choy, H. Enhancement of radiation effects in vitro by the estrogen metabolite 2-methoxyestradiol. Radiat. Res. 2000, 153, 384–391. [Google Scholar] [CrossRef]
- Casarez, E.V.; Dunlap-Brown, M.E.; Conaway, M.R.; Amorino, G.P. Radiosensitization and modulation of p44/42 mitogen-activated protein kinase by 2-Methoxyestradiol in prostate cancer models. Cancer Res. 2007, 67, 8316–8324. [Google Scholar] [CrossRef] [PubMed]
- Nolte, E.; Joubert, A.; Lakier, R.; van Rensburg, A.; Mercier, A. Exposure of breast and lung cancer cells to a novel estrone analog prior to radiation enhances Bcl-2-mediated cell death. Int. J. Mol. Sci. 2018, 19, 2887. [Google Scholar] [CrossRef]
- Helena, J.; Joubert, A.; Mabeta, P.; Coetzee, M.; Lakier, R.; Mercier, A. Intracellular Signaling Responses Induced by Radiation within an In Vitro Bone Metastasis Model after Pre-Treatment with an Estrone Analogue. Cells 2021, 10, 2105. [Google Scholar] [CrossRef]
- Mercier, A.E.; Prudent, R.; Pepper, M.S.; De Koning, L.; Nolte, E.; Peronne, L.; Nel, M.; Lafanechère, L.; Joubert, A.M. Characterization of signalling pathways that link apoptosis and autophagy to cell death induced by estrone Analogues which reversibly depolymerize microtubules. Molecules 2021, 26, 706. [Google Scholar] [CrossRef]
- Carrassa, L.; Broggini, M.; Erba, E.; Damia, G. Chk1, but not Chk2, is involved in the cellular response to DNA damaging agents: Differential activity in cells expressing or not p53. Cell Cycle 2004, 3, 1177–1181. [Google Scholar] [CrossRef]
- Lahtz, C.; Bates, S.E.; Jiang, Y.; Li, A.X.; Wu, X.; Hahn, M.A.; Pfeifer, G.P. Gamma irradiation does not induce detectable changes in DNA methylation directly following exposure of human cells. PLoS ONE 2012, 7, e44858. [Google Scholar] [CrossRef] [PubMed]
- Theron, A.; Nolte, E.; Lafanechere, L.; Joubert, A. Molecular crosstalk between apoptosis and autophagy induced by a novel 2-methoxyestradiol analogue in cervical adenocarcinoma cells. Cancer Cell Int. 2013, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Poruchynsky, M.S.; Komlodi-Pasztor, E.; Trostel, S.; Wilkerson, J.; Regairaz, M.; Pommier, Y.; Zhang, X.; Kumar Maity, T.; Robey, R.; Burotto, M.; et al. Microtubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteins. Proc. Natl. Acad. Sci. USA 2015, 112, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Hintelmann, K.; Kriegs, M.; Rothkamm, K.; Rieckmann, T. Improving the Efficacy of Tumor Radiosensitization Through Combined Molecular Targeting. Front. Oncol. 2020, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Nolte, E. Pro-Apoptotic and Radiosensitizing Potential of Four Candidate Microtubule Regulators in Breast Cancer Cells; Université Grenoble Alpes: Gières, France; University of Pretoria: Pretoria, South Africa, 2019. [Google Scholar]
- Weaver, B.A. How taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, e2597. [Google Scholar] [CrossRef] [PubMed]
- Stander, B.A.; Joubert, F.; Tu, C.; Sippel, K.H.; McKenna, R.; Joubert, A.M. Signaling pathways of ESE-16, an antimitotic and anticarbonic anhydrase estradiol analog, in breast cancer cells. PLoS ONE 2013, 8, e53853. [Google Scholar] [CrossRef]
- Markowitz, D.; Ha, G.; Ruggieri, R.; Symons, M. Microtubule-targeting agents can sensitize cancer cells to ionizing radiation by an interphase-based mechanism. OncoTargets Ther. 2017, 10, 5633–5642. [Google Scholar] [CrossRef]
- Shen, Y.C.; Upadhyayula, R.; Cevallos, S.; Messick, R.J.; Hsia, T.; Leese, M.P.; Jewett, D.M.; Ferrer-Torres, D.; Roth, T.M.; Dohle, W.; et al. Targeted NF1 cancer therapeutics with multiple modes of action: Small molecule hormone-like agents resembling the natural anticancer metabolite, 2-methoxyoestradiol. Br. J. Cancer 2015, 113, 1158–1167. [Google Scholar] [CrossRef][Green Version]
- Shen, Y.-c.; Arellano-Garcia, C.; Menjivar, R.E.; Jewett, E.M.; Dohle, W.; Karchugina, S.; Chernoff, J.; Potter, B.V.L.; Barald, K.F. Nonsteroidal sulfamate derivatives as new therapeutic approaches for Neurofibromatosis 2 (NF2). BMC Pharmacol. Toxicol. 2019, 20, 67. [Google Scholar] [CrossRef]
- Mabjeesh, N.J.; Escuin, D.; LaVallee, T.M.; Pribluda, V.S.; Swartz, G.M.; Johnson, M.S.; Willard, M.T.; Zhong, H.; Simons, J.W.; Giannakakou, P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 2003, 3, 363–375. [Google Scholar] [CrossRef]
- Dohle, W.; Prota, A.E.; Menchon, G.; Hamel, E.; Steinmetz, M.O.; Potter, B.V.L. Tetrahydroisoquinoline Sulfamates as Potent Microtubule Disruptors: Synthesis, Antiproliferative and Antitubulin Activity of Dichlorobenzyl-Based Derivatives, and a Tubulin Cocrystal Structure. ACS Omega 2019, 4, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wang, X.; Shen, Z.; Chen, S.; Yu, H.; Williams, N.; Wang, G. Anti-mitotic chemotherapeutics promote apoptosis through TL1A-activated death receptor 3 in cancer cells. Cell Res. 2018, 28, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.S.; Gozuacik, D.; Joubert, A.M. The in vitro effects of a novel estradiol analog on cell proliferation and morphology in human epithelial cervical carcinoma. Cell. Mol. Biol. Lett. 2018, 23, 10. [Google Scholar] [CrossRef]
- Theron, A.; Prudent, R.; Nolte, E.; van den Bout, I.; Punchoo, R.; Marais, S.; du Toit, P.; Hlophe, Y.; van Papendorp, D.; Lafanechère, L.; et al. Novel in silico-designed estradiol analogues are cytotoxic to a multidrug-resistant cell line at nanomolar concentrations. Cancer Chemother. Pharmacol. 2015, 75, 431–437. [Google Scholar] [CrossRef]
- Blajeski, A.L.; Phan, V.A.; Kottke, T.J.; Kaufmann, S.H. G1 and G2 cell-cycle arrest following microtubule depolymerization in human breast cancer cells. J. Clin. Investig. 2002, 110, 91–99. [Google Scholar] [CrossRef]
- Vidal, A.; Koff, A. Cell-cycle inhibitors: Three families united by a common cause. Gene 2000, 247, 1–15. [Google Scholar] [CrossRef]
- Limbo, O.; Chahwan, C.; Yamada, Y.; de Bruin, R.A.; Wittenberg, C.; Russell, P. Ctp1 is a cell-cycle-regulated protein that functions with Mre11 complex to control double-strand break repair by homologous recombination. Mol. Cell 2007, 28, 134–146. [Google Scholar] [CrossRef]
- Hopkins, M.; Tyson, J.J.; Novak, B. Cell-cycle transitions: A common role for stoichiometric inhibitors. Mol. Biol. Cell 2017, 28, 3437–3446. [Google Scholar] [CrossRef]
- Foppoli, C.; De Marco, F.; Blarzino, C.; Perluigi, M.; Cini, C.; Coccia, R. Biological response of human diploid keratinocytes to quinone-producing compounds: Role of NAD(P)H:quinone oxidoreductase 1. Int. J. Biochem. Cell Biol. 2005, 37, 852–863. [Google Scholar] [CrossRef]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. γH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fatah, T.M.; Arora, A.; Moseley, P.; Coveney, C.; Perry, C.; Johnson, K.; Kent, C.; Ball, G.; Chan, S.; Madhusudan, S. ATM, ATR and DNA-PKcs expressions correlate to adverse clinical outcomes in epithelial ovarian cancers. BBA Clin. 2014, 2, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, Y.; Ziv, Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, A.-H.; Khanbabaei, H.; Calin, G.A. Therapeutic potential of the miRNA–ATM axis in the management of tumor radioresistance. Cancer Res. 2020, 80, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Pazolli, E.; Alspach, E.; Milczarek, A.; Prior, J.; Piwnica-Worms, D.; Stewart, S.A. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 2012, 72, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Riballo, E.; Kühne, M.; Rief, N.; Doherty, A.; Smith, G.C.; Recio, M.a.-J.; Reis, C.; Dahm, K.; Fricke, A.; Krempler, A. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to γ-H2AX foci. Mol. Cell 2004, 16, 715–724. [Google Scholar] [CrossRef]
- Guo, Z.; Deshpande, R.; Paull, T.T. ATM activation in the presence of oxidative stress. Cell Cycle 2010, 9, 4805–4811. [Google Scholar] [CrossRef]
- Bakkenist, C.J.; Kastan, M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003, 421, 499–506. [Google Scholar] [CrossRef]
- Bodgi, L.; Foray, N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: Resolution of the linear-quadratic model. Int. J. Radiat. Biol. 2016, 92, 117–131. [Google Scholar] [CrossRef]
- Reynolds, P.; Anderson, J.A.; Harper, J.V.; Hill, M.A.; Botchway, S.W.; Parker, A.W.; O’Neill, P. The dynamics of Ku70/80 and DNA-PKcs at DSBs induced by ionizing radiation is dependent on the complexity of damage. Nucleic Acids Res. 2012, 40, 10821–10831. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hargrave, S.D.; Joubert, A.M.; Potter, B.V.L.; Dohle, W.; Marais, S.; Mercier, A.E. Cell Fate following Irradiation of MDA-MB-231 and MCF-7 Breast Cancer Cells Pre-Exposed to the Tetrahydroisoquinoline Sulfamate Microtubule Disruptor STX3451. Molecules 2022, 27, 3819. https://doi.org/10.3390/molecules27123819
Hargrave SD, Joubert AM, Potter BVL, Dohle W, Marais S, Mercier AE. Cell Fate following Irradiation of MDA-MB-231 and MCF-7 Breast Cancer Cells Pre-Exposed to the Tetrahydroisoquinoline Sulfamate Microtubule Disruptor STX3451. Molecules. 2022; 27(12):3819. https://doi.org/10.3390/molecules27123819
Chicago/Turabian StyleHargrave, Scott D., Anna M. Joubert, Barry V. L. Potter, Wolfgang Dohle, Sumari Marais, and Anne E. Mercier. 2022. "Cell Fate following Irradiation of MDA-MB-231 and MCF-7 Breast Cancer Cells Pre-Exposed to the Tetrahydroisoquinoline Sulfamate Microtubule Disruptor STX3451" Molecules 27, no. 12: 3819. https://doi.org/10.3390/molecules27123819
APA StyleHargrave, S. D., Joubert, A. M., Potter, B. V. L., Dohle, W., Marais, S., & Mercier, A. E. (2022). Cell Fate following Irradiation of MDA-MB-231 and MCF-7 Breast Cancer Cells Pre-Exposed to the Tetrahydroisoquinoline Sulfamate Microtubule Disruptor STX3451. Molecules, 27(12), 3819. https://doi.org/10.3390/molecules27123819






