Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2
Abstract
:1. Introduction
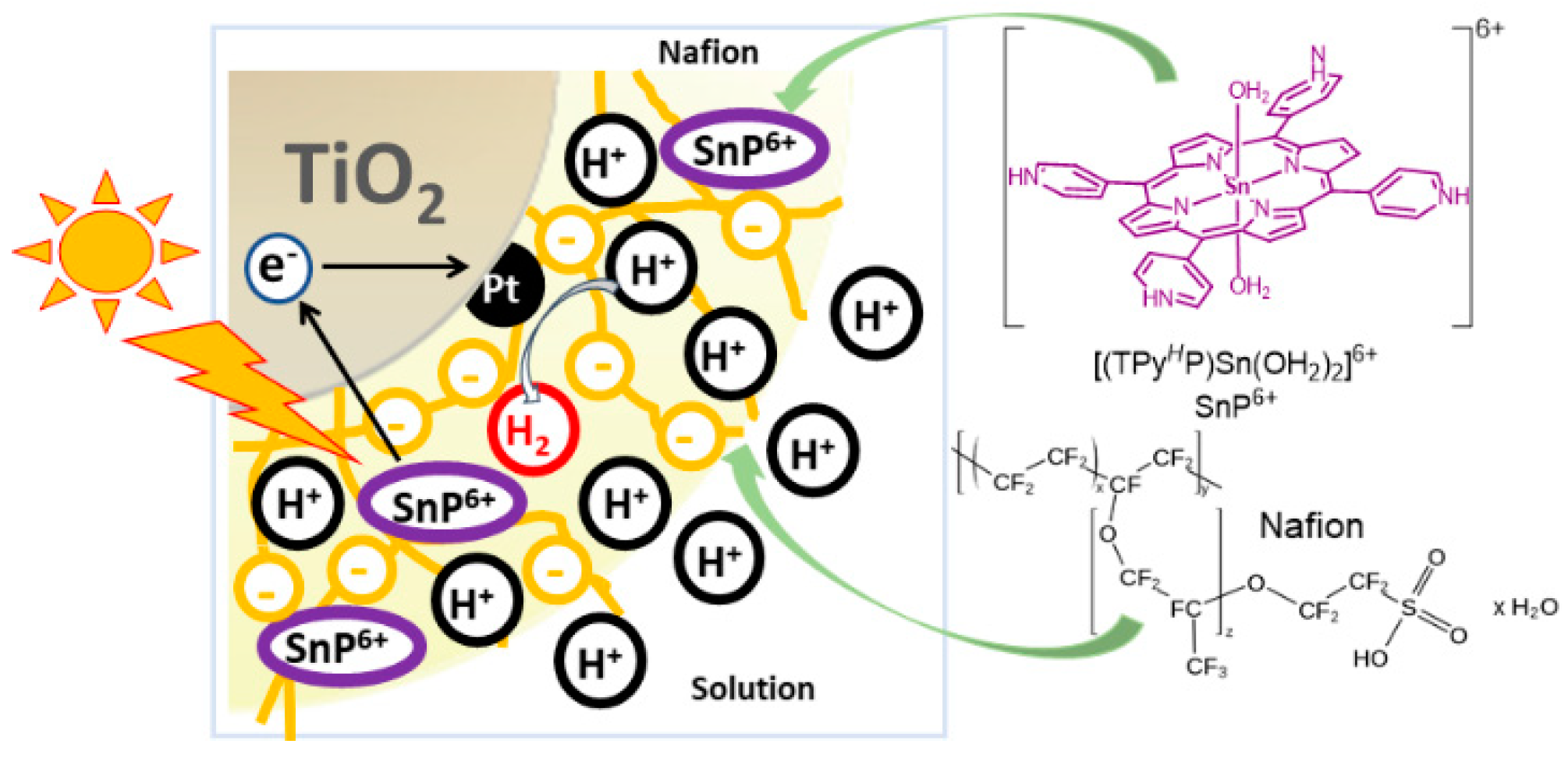
2. Results and Discussion
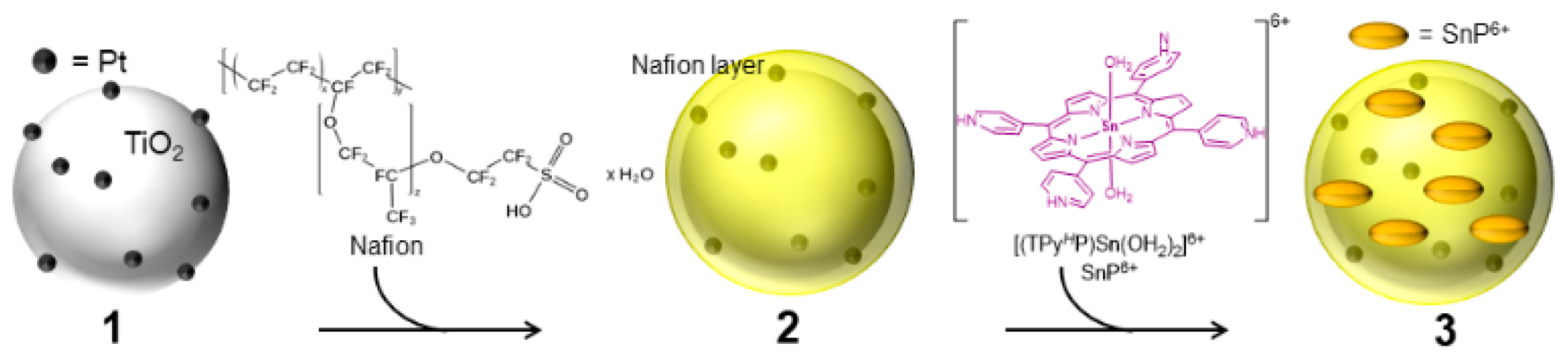
2.1. Fabrication of Photocatalyst
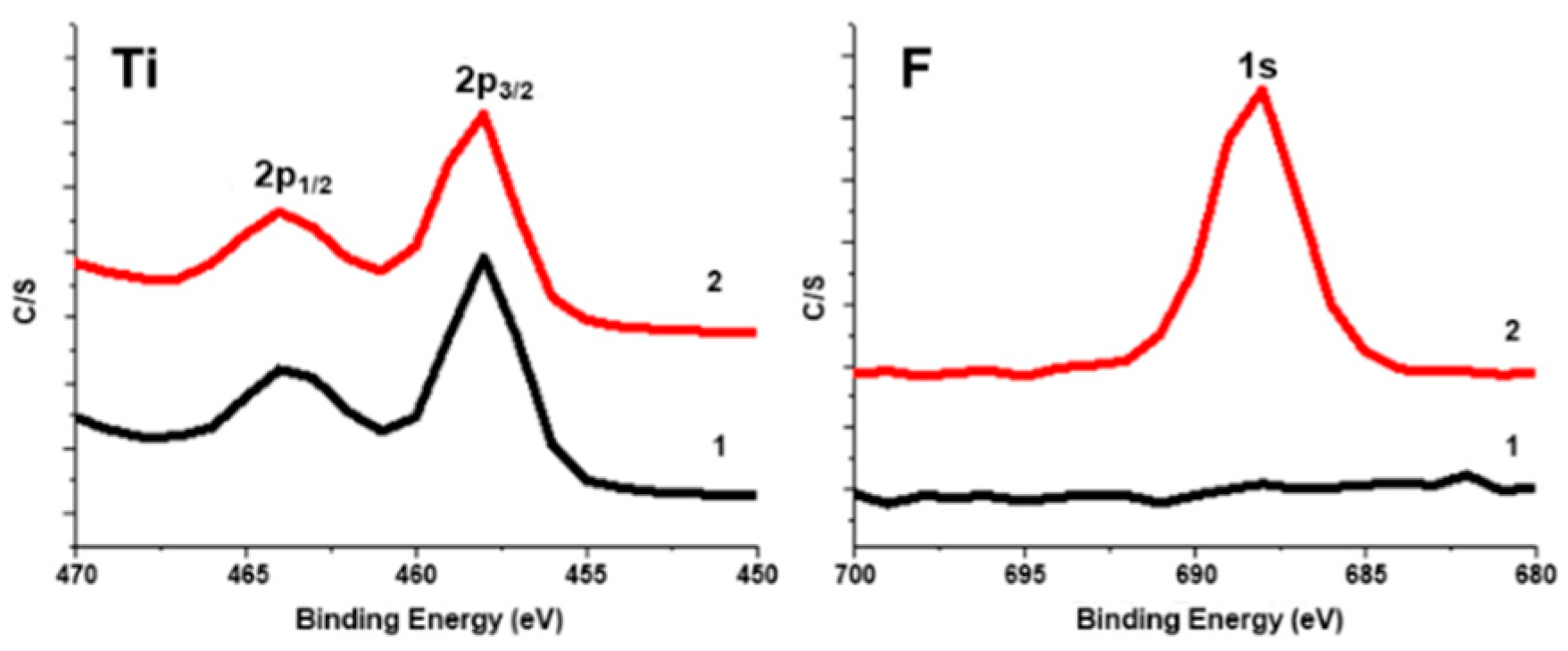
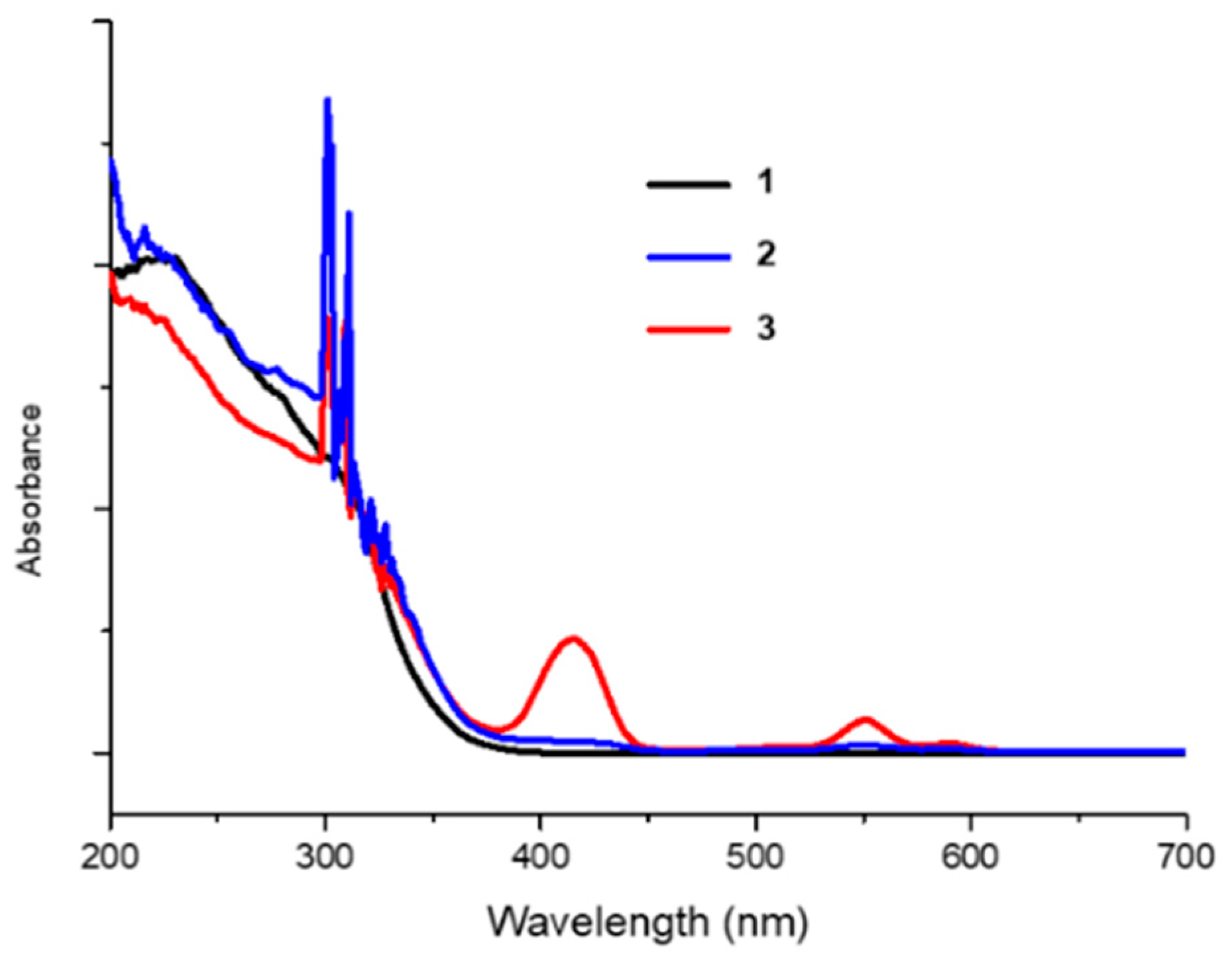
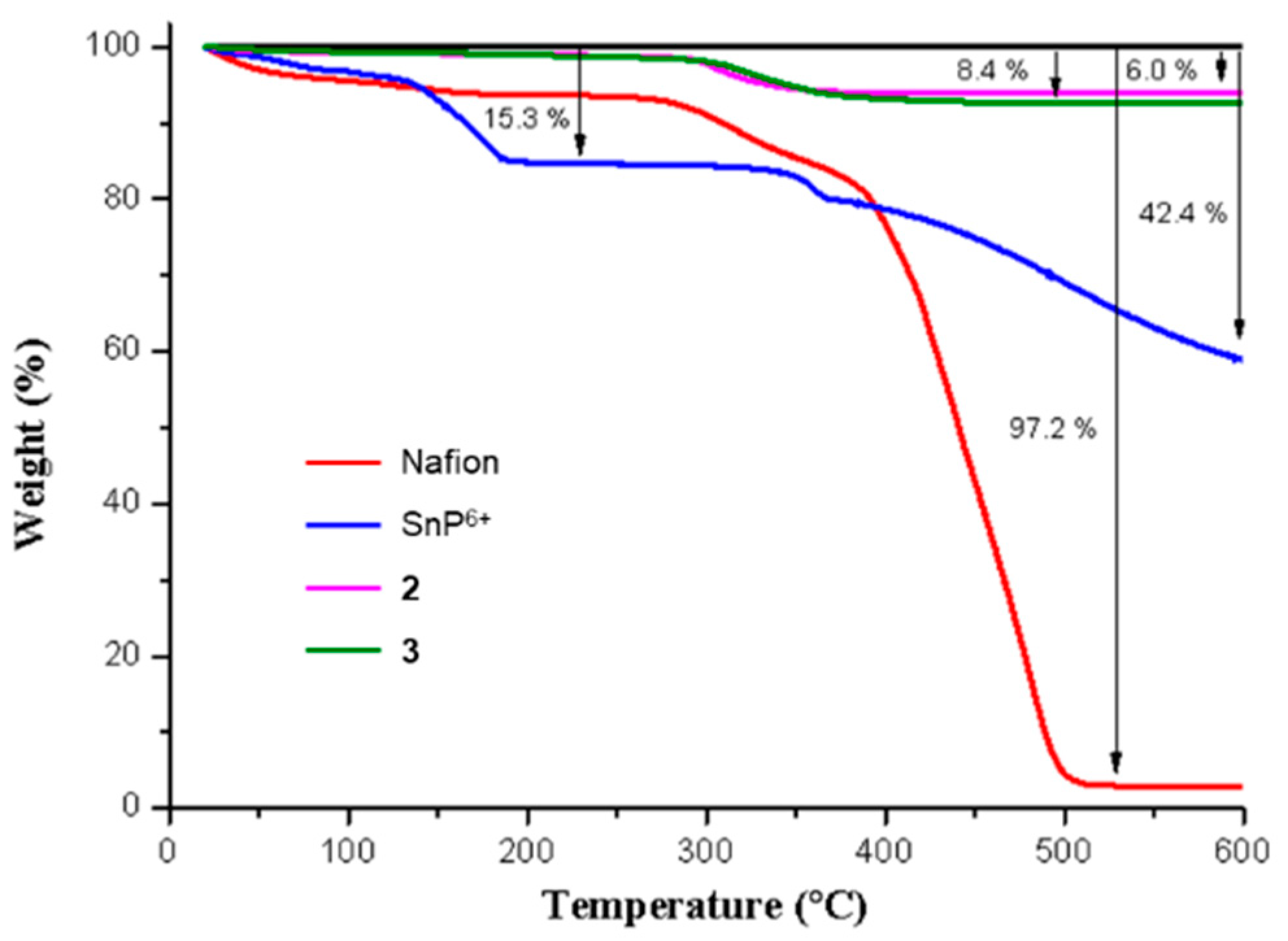
2.2. Embedding of Sn(IV)-Porphyrin Cations into Nafion-Coated Photocatalyst
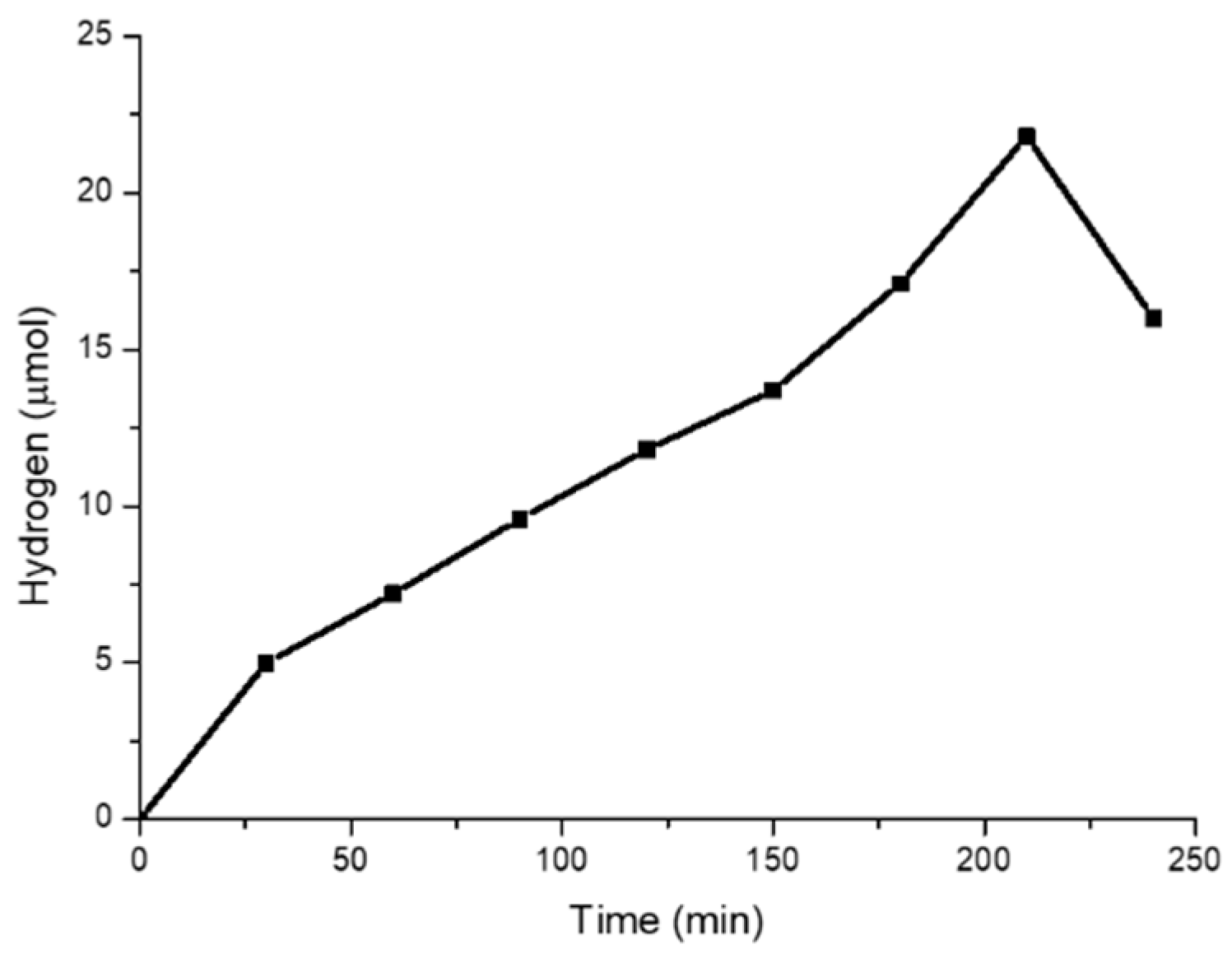
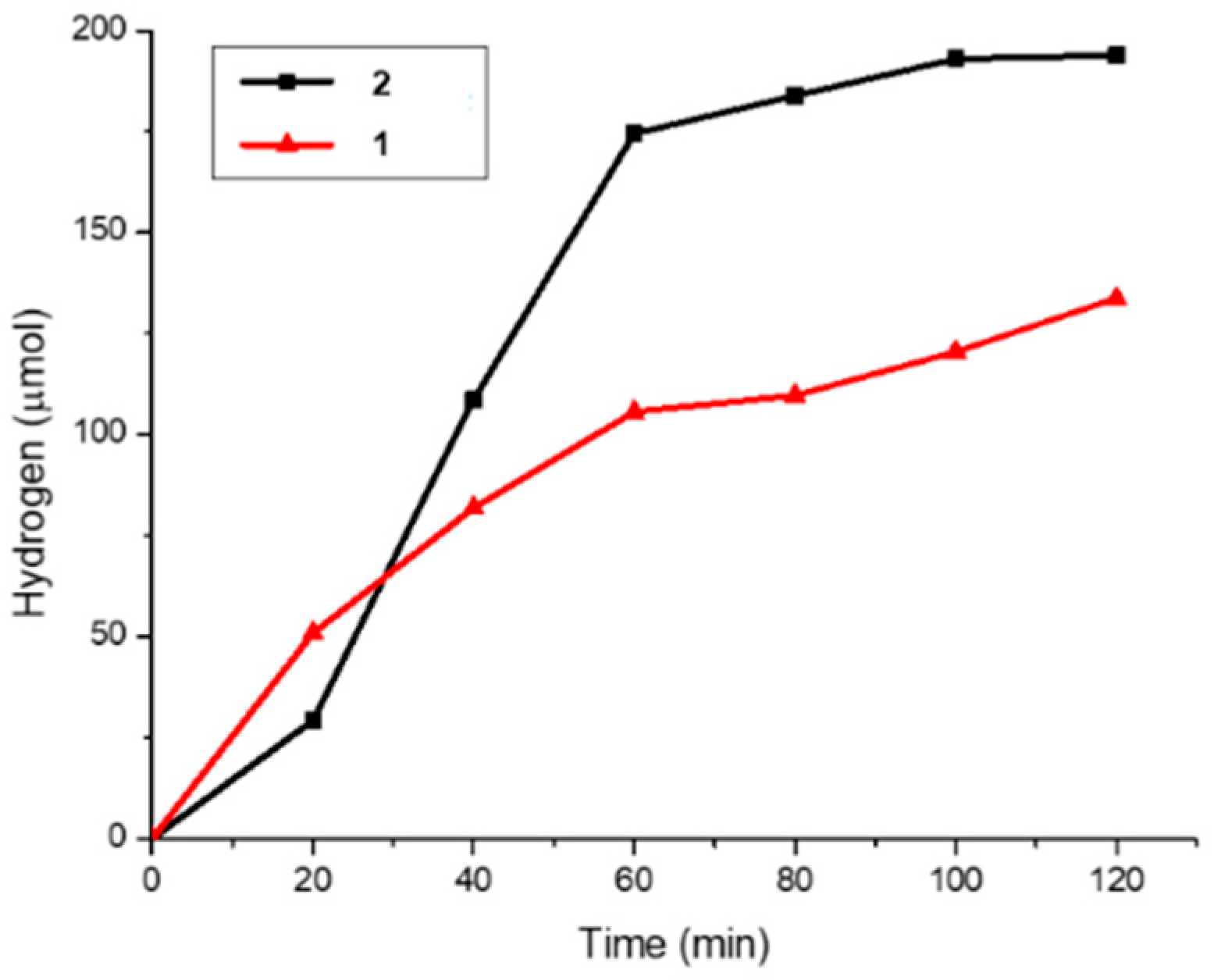
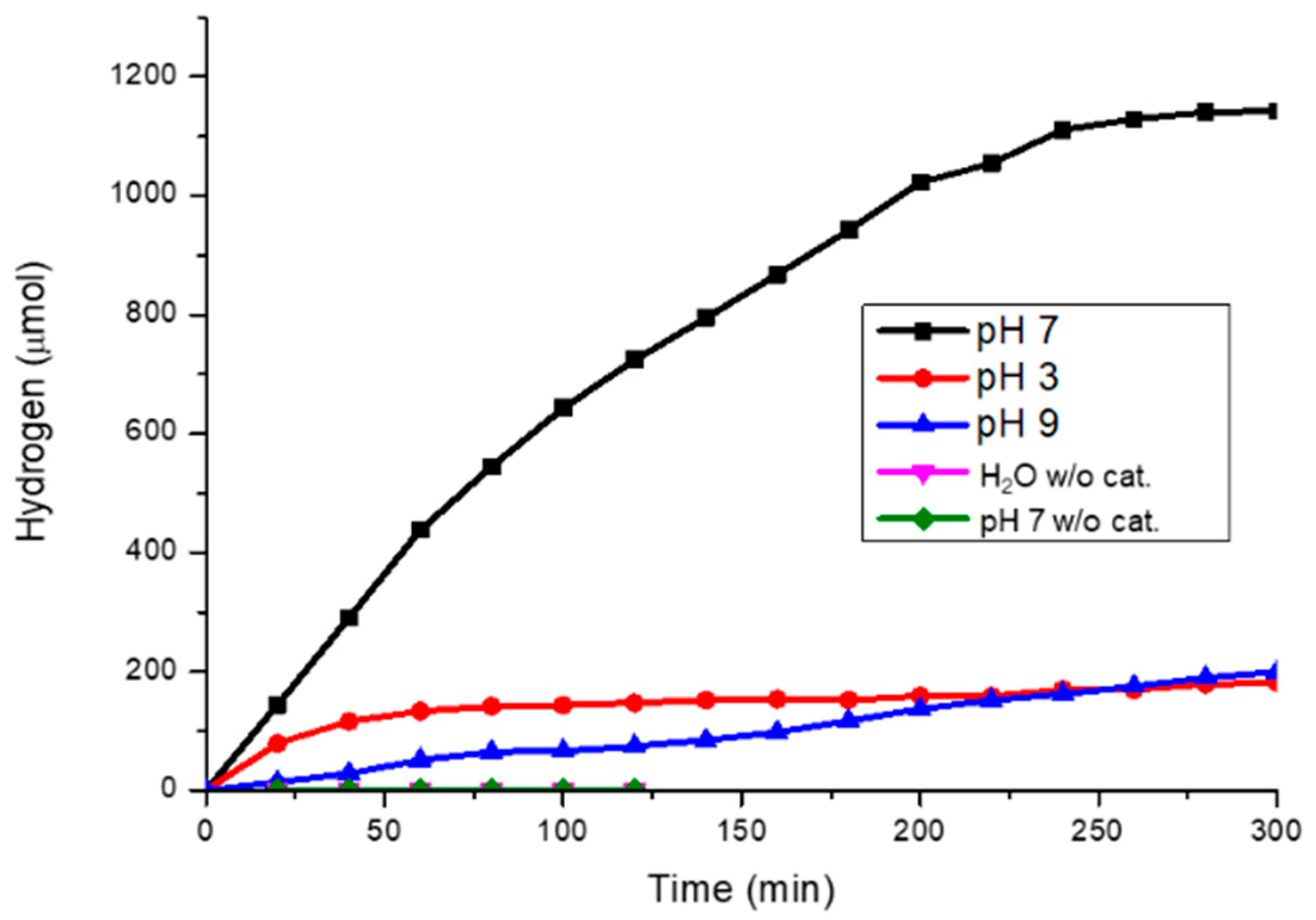
2.3. Photocatalytic Hydrogen Generation
3. Materials and Methods
3.1. Preparation of Photocatalyst
3.1.1. Platinized TiO2 (1)
3.1.2. Nafion-Coated Pt-TiO2 (2)
3.1.3. Sn(IV)-porphyrin cations-embedded Nafion/Pt-TiO2 (3)
3.2. Photocatalytic Hydrogen Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzani, V.; Credi, A.; Venturi, M. Photochemical Conversion of Solar Energy. Chem. Sustain. Chem. 2008, 1, 26–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Andreiadis, E.S.; Chavarot-Kerlidou, M.; Fontecave, M.; Artero, V. Artificial Photosynthesis: From Molecular Catalysts for Light-driven Water Splitting to Photoelectrochemical Cells. Photochem. Photobiol. 2011, 87, 946–964. [Google Scholar] [CrossRef] [PubMed]
- Young, K.J.; Martini, L.A.; Milot, R.L.; Snoeberger III, R.C.; Batista, V.S.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W. Light-driven water oxidation for solar fuels. Coord. Chem. Rev. 2012, 256, 2503–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Imahori, H.; Umeyama, T.; Kurotobi, K.; Takanom, Y. Self-assembling porphyrins and phthalocyanines for photoinduced charge separation and charge transport. Chem. Commun. 2012, 48, 4032–4045. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Trukhina, O.; Ince, M.; Torres, T. Towards artificial photosynthesis: Supramolecular, donor–acceptor, porphyrin- and phthalocyanine/carbon nanostructure ensembles. Coord. Chem. Rev. 2012, 256, 2453–2477. [Google Scholar] [CrossRef]
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular electronic components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310. [Google Scholar] [CrossRef] [Green Version]
- Mahy, J.G.; Paez, C.A.; Carcel, C.; Bied, C.; Tatton, A.S.; Damblon, C.; Heinrichs, B.; Man, M.W.C.; Lambert, S.D. Porphyrin-based hybrid silica-titania as a visible-light photocatalyst. J. Photochem. Photobiol. A 2019, 373, 66–76. [Google Scholar] [CrossRef]
- Ladomenoua, K.; Natali, M.; Iengo, E.; Charalampidis, G.; Scandola, F.; Coutsolelos, A.G. Photochemical hydrogen generation with porphyrin-based systems. Coord. Chem. Rev. 2015, 304–305, 38–54. [Google Scholar] [CrossRef]
- Wang, L.; Fan, H.; Bai, F. Porphyrin-based photocatalysts for hydrogen production. MRS Bull. 2020, 45, 49–56. [Google Scholar] [CrossRef]
- Mahmood, A.; Hu, J.-Y.; Xiao, B.; Tang, A.; Wang, X.; Zhou, E. Recent progress in porphyrin-based materials for organic solar cells. J. Mater. Chem. A 2018, 6, 16769–16797. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Mackeyev, Y.; Lee, G.-S.; Kim, H.-J.; Tachikawa, T.; Hong, S.; Lee, S.; Kim, J.; Wilson, L.J.; et al. Selective Oxidative Degradation of Organic Pollutants by Singlet Oxygen Photosensitizing Systems: Tin Porphyrin versus C60 Aminofullerene Systems. Environ. Sci. Technol. 2012, 46, 9606–9613. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, J.; Jo, H.J.; Kim, H.-J.; Choi, W. Visible Light Photocatalysts Based on Homogeneous and Heterogenized Tin Porphyrins. J. Phys. Chem. C 2008, 112, 491–499. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular porphyrin nanostructures based on coordination driven self-assembly and their visible light catalytic degradation of methylene blue dye. Nanomaterials 2020, 10, 2314. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Self-assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules 2021, 26, 3598. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Jo, H.J.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn(IV) porphyrins with Ag(I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Three Isomeric Zn(II)-Sn(IV)-Zn(II) Porphyrin-Triad-Based Supramolecular Nanoarchitectures for the Morphology-Dependent Photocatalytic Degradation of Methyl Orange. ACS Omega 2022, 7, 9775–9784. [Google Scholar] [CrossRef]
- Li, C.; Park, K.-M.; Kim, H.-J. Ionic assembled hybrid nanoparticle consisting of tin(IV) porphyrin cations and polyoxomolybdate anions, and photocatalytic hydrogen production by its visible light sensitization. Inorg. Chem. Comm. 2015, 60, 8–11. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Majima, T.; Li, C.; Kim, H.-J.; Choi, W. Tin-porphyrin sensitized TiO2 for the production of H2 under visible light. Energy Environ. Sci. 2010, 3, 1789–1795. [Google Scholar] [CrossRef] [Green Version]
- Bae, E.; Choi, W. Effect of the Anchoring Group (Carboxylate vs. Phosphonate) in Ru-Complex-Sensitized TiO2 on Hydrogen Production under Visible Light. J. Phys. Chem. B 2006, 110, 14792–14799. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Schneider, J.; Li, F.; Zhao, W.; Patel, U.; Castellano, F.N.; Eisenberg, R. Bi- and Terpyridyl Platinum(II) Chloro Complexes: Molecular Catalysts for the Photogeneration of Hydrogen from Water or Simply Precursors for Colloidal Platinum? J. Am. Chem. Soc. 2008, 130, 5056–5058. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, G.; Verma, S.; Jose, D.A.; Kumar, D.K.; Das, A.; Palit, D.K.; Ghosh, H.N. Interfacial Electron Transfer between the Photoexcited Porphyrin Molecule and TiO2 Nanoparticles: Effect of Catecholate Binding. J. Phys. Chem. B 2006, 110, 9012–9021. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, W. Photocatalytic Reactivities of Nafion-Coated TiO2 for the Degradation of Charged Organic Compounds under UV or Visible Light. J. Phys. Chem. B 2005, 109, 11667–11674. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, W. Visible-Light-Sensitized Production of Hydrogen Using Perfluorosulfonate Polymer-Coated TiO2 Nanoparticles: An Alternative Approach to Sensitizer Anchoring. Langmuir 2006, 22, 2906–2911. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Kim, S.H.; Kim, H.-J. Supramolecular Assembly of Tin(IV) Porphyrin Cations Stabilized by Ionic Hydrogen-Bonding Interactions. Bull. Korean Chem. Soc. 2015, 36, 2348–2351. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Kim, H.-J. Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2. Molecules 2022, 27, 3770. https://doi.org/10.3390/molecules27123770
Kim S-H, Kim H-J. Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2. Molecules. 2022; 27(12):3770. https://doi.org/10.3390/molecules27123770
Chicago/Turabian StyleKim, Sung-Hyun, and Hee-Joon Kim. 2022. "Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2" Molecules 27, no. 12: 3770. https://doi.org/10.3390/molecules27123770
APA StyleKim, S.-H., & Kim, H.-J. (2022). Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2. Molecules, 27(12), 3770. https://doi.org/10.3390/molecules27123770





