Neuropeltis acuminata (P. Beauv.): Investigation of the Chemical Variability and In Vitro Anti-inflammatory Activity of the Leaf Essential Oil from the Ivorian Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Detailed Analysis of Essential Oil Samples S3, S6, S13, S20, and S24
2.1.1. Isolation and Structural Elucidation of δ-Cadinen-11-ol
2.1.2. Chemical Composition of Leaf Essential Oil Samples S3, S6, S13, S20 and S24
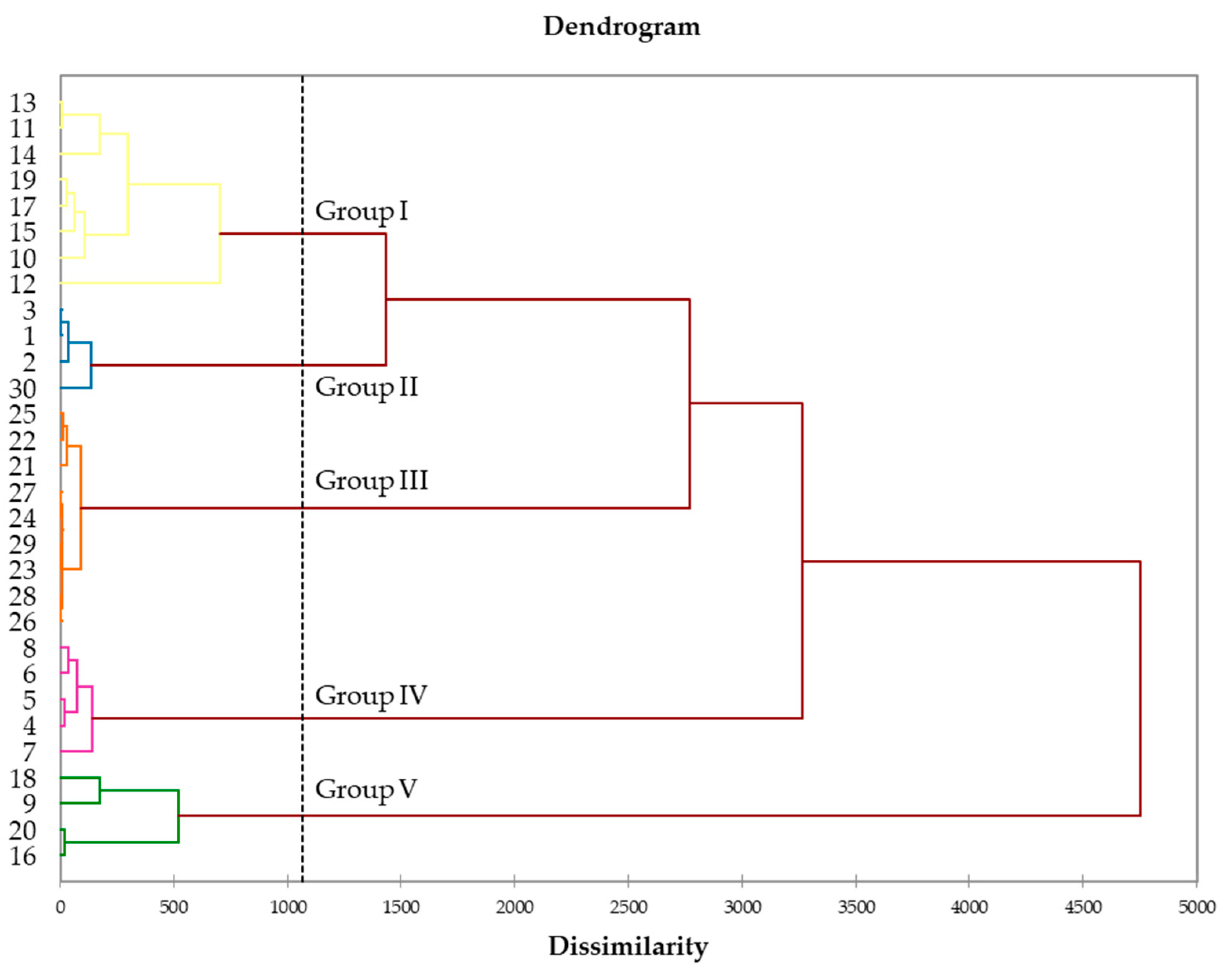
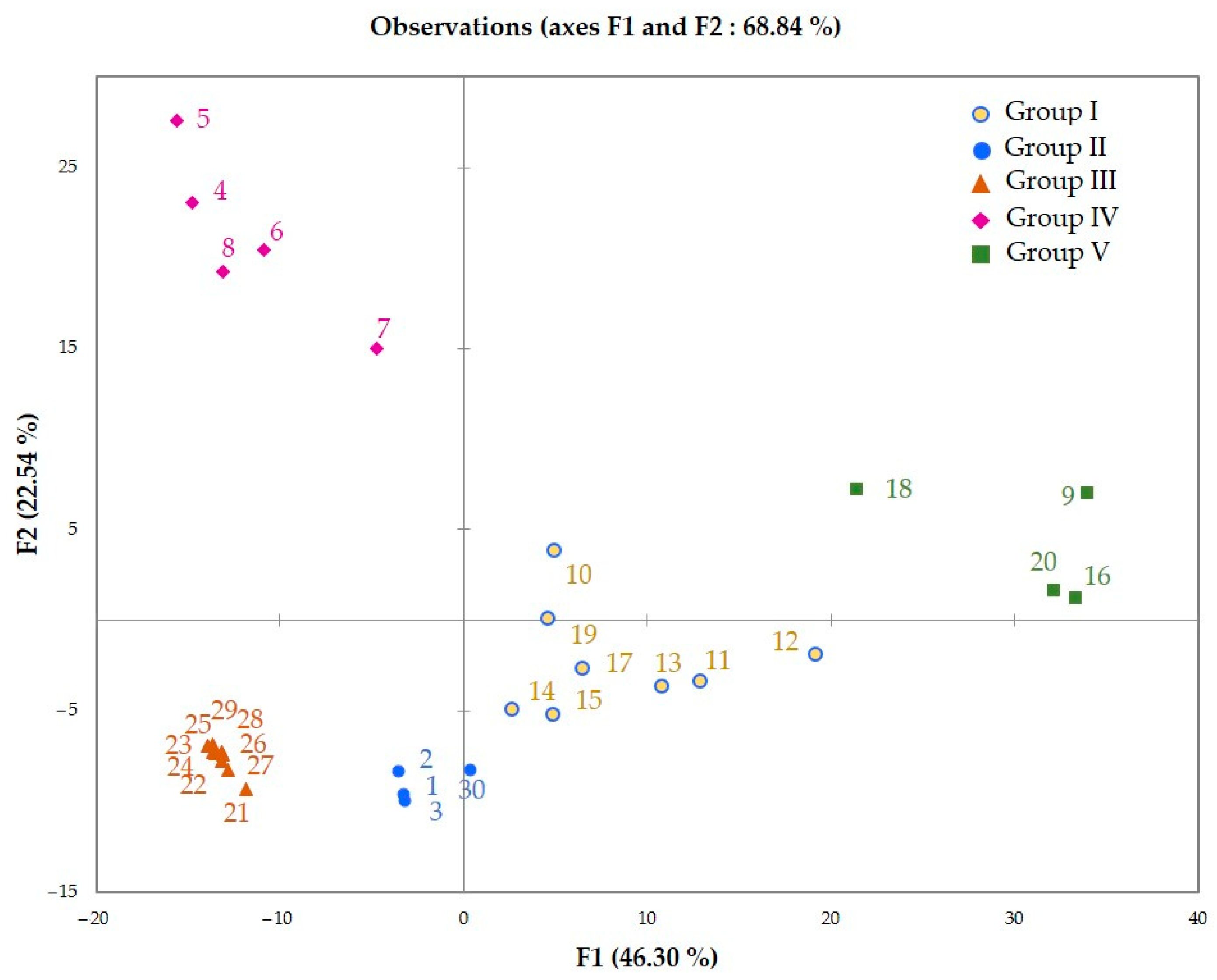
2.2. Chemical Variability of Leaf Essential Oil from N. acuminata
2.3. Evaluation of In Vitro Anti-Inflammatory Activity
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oil Isolation and Fractionation
3.3. Gas Chromatography
3.4. Gas Chromatography-Mass Spectrometry in Electron Impact Mode
3.5. Gas Chromatography-High Resolution Mass Spectrometry
3.6. Nuclear Magnetic Resonance
3.7. Identification of Individual Components
3.8. Statistical Analysis
3.9. In Vitro Anti-Inflammatory Capacity of Neuropeltis acuminata Leaf Essential Oil
3.10. Spectral Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lejoly, J.; Lisowski, S. Le genre Neuropeltis Wall. (Convolvulaceae) au Zaïre, au Gabon et au Cameroun. Bull. Jard. Bot. Natl. Belg. 1984, 54, 471–482. Available online: http://www.jstor.org/stable/3667854 (accessed on 18 March 2022). [CrossRef]
- Breteler, F.J. Description of a new species of Neuropeltis (Convolvulaceae) with a synopsis and a key to all African species. Plant Ecol. Evol. 2010, 143, 176–180. [Google Scholar] [CrossRef]
- Burkill, H.M. The Useful Plants of West Tropical Africa, Volume 1, Families A–D, 2nd ed.; Royal Botanic Gardens: Richmond, UK, 1985; p. 960. [Google Scholar]
- Tra Bi, F.H.; Kouamé, F.N.; Traoré, D. Utilisation of climbers in two forest reserves in West Côte d’Ivoire. In Forest Climbing Plants of West Africa, Diversity, Ecology and Management; Bongers, F., Parren, M.P.E., Traoré, D., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 167–181. [Google Scholar]
- Irvine, F.R. Woody Plants of Ghana, with Special Reference to Their Uses; Oxford University Press: London, UK, 1961; p. 868. [Google Scholar]
- Ebanda, F.B.; Evoung, Y.S.N.; Ateba, J.A.; Tchinda, J.B.S.; Yona, A.M.T.; Laynde, T. Evaluation of micromechanical properties of Neuropeltis acuminatas (NA) fibers. Int. J. Acad. Res. Reflect. 2019, 7, 23–36. [Google Scholar]
- Betene, A.D.O.; Betene, F.E.; Martoïa, F.; Dumont, P.J.J.; Atangana, A.; Noah, P.M.A. Physico-Chemical and Thermal Characterization of Some Lignocellulosic Fibres: Ananas comosus (AC), Neuropeltis acuminatas (NA) and Rhecktophyllum camerunense (RC). J. Miner. Mater. Charact. Eng. 2020, 8, 205–222. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Boti, J.B.; Kablan, A.C.L.; Ballo, D.; Paoli, M.; Brunini, V.; Tomi, F. Chemical Variability and in vitro Anti-Inflammatory Activity of Leaf Essential Oil from Ivorian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels. Molecules 2021, 26, 6228. [Google Scholar] [CrossRef] [PubMed]
- Kambiré, D.A.; Boti, J.B.; Ouattara, Z.A.; Thierry, A.Y.; Barat, N.; Bighelli, A.; Tomi, F. Chemical composition of root and stem bark essential oils from Ivorian Isolona dewevrei: Structural elucidation of a new natural germacrone. Nat. Prod. Res. 2021, 36, 2105–2111. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Boti, J.B.; Ouattara, Z.A.; Yapi, T.A.; Bighelli, A.; Tomi, F.; Casanova, J. Leaf essential oil from Ivoirian Isolona dewevrei (Annonaceae): Chemical composition and structure elucidation of four new natural sesquiterpenes. Flavour Fragr. J. 2021, 36, 22–33. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Boti, J.B.; Yapi, T.A.; Ouattara, Z.A.; Bighelli, A.; Casanova, J.; Tomi, F. New Natural Oxygenated Sesquiterpenes and Chemical Composition of Leaf Essential Oil from Ivoirian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels. Molecules 2020, 25, 5613. [Google Scholar] [CrossRef]
- Nea, F.; Kambiré, D.A.; Genva, M.; Tanoh, E.A.; Wognin, E.L.; Martin, H.; Brostaux, Y.; Tomi, F.; Lognay, G.C.; Tonzibo, Z.F.; et al. Composition, Seasonal Variation, and Biological Activities of Lantana camara Essential Oils from Côte d’Ivoire. Molecules 2020, 25, 2400. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Boti, J.B.; Yapi, A.T.; Ouattara, Z.A.; Paoli, M.; Bighelli, A.; Tomi, F.; Casanova, J. Composition and intraspecific chemical variability of leaf essential oil of Laggera pterodonta from Côte d’Ivoire. Chem. Biodivers. 2020, 17, e1900504. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Yapi, A.T.; Boti, J.B.; Ouattara, Z.A.; Tonzibo, Z.F.; Filippi, J.J.; Bighelli, A.; Tomi, F. Two new eudesman-4α-ol epoxides from the stem essential oil of Laggera pterodonta from Côte d’Ivoire. Nat. Prod. Res. 2020, 34, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Kambiré, D.A.; Boti, J.B.; Filippi, J.-J.; Tonzibo, Z.F.; Tomi, F. Characterization of a new epoxy-hydroxycarvotanacetone derivative from the leaf essential oil of Laggera pterodonta from Côte d’Ivoire. Nat. Prod. Res. 2019, 33, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Kambiré, D.A.; Yapi, T.A.; Boti, J.B.; Garcia, G.; Tomi, P.; Bighelli, A.; Tomi, F. Chemical composition of leaf essential oil of Piper umbellatum and aerial part essential oil of Piper guineense from Côte d’Ivoire. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Tomi, F.; Bradesi, P.; Bighelli, A.; Casanova, J. Computer-aided identification of individual components of essential oils using carbon-13 NMR spectroscopy. J. Magn. Reson. Anal. 1995, 1, 25–34. [Google Scholar]
- Palu, D.; Bighelli, A.; Casanova, J.; Paoli, M. Identification and Quantitation of Ursolic and Oleanolic Acids in Ilex aquifolium L. Leaf Extracts Using 13C and 1H-NMR Spectroscopy. Molecules 2019, 24, 4413. [Google Scholar] [CrossRef] [PubMed]
- Baldovini, N.; Tomi, F.; Casanova, J. Identification and quantitative determination of furanodiene, a heat-sensitive compound, in essential oil by 13C-NMR. Phytochem. Anal. 2001, 12, 58–63. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall-Weyerstahl, H.; Christiansen, C. Constituents of Ayou Essential Oil (Aydendron barbeyana Mez). Flavour Fragr. J. 1989, 4, 93–98. [Google Scholar] [CrossRef]
- Kim, H.-K.; Yun, Y.-K.; Ahn, Y.-J. Toxicity of Atractylon and Atractylenolide III Identified in Atractylodes ovata Rhizome to Dermatophagoides farinae and Dermatophagoides pteronyssinus. J. Agric. Food Chem. 2007, 55, 6027–6031. [Google Scholar] [CrossRef]
- Sciarrone, D.; Pantò, S.; Rotondo, A.; Tedone, L.; Tranchida, P.Q.; Dugo, P.; Mondello, L. Rapid collection and identification of a novel component from Clausena lansium Skeels leaves by means of three-dimensional preparative gas chromatography and nuclear magnetic resonance/infrared/mass spectrometric analysis. Anal. Chim. Acta 2013, 785, 119–125. [Google Scholar] [CrossRef]
- Look, S.A.; Buchholz, K.; Fenical, W. 12-Hydroxy-E-γ-bisabolene, a new sesquiterpene alcohol from a Caribbean sea whip of the genus Pseudopterogorgia (Gorgonacea, Cnidaria). Experientia 1984, 40, 931–933. [Google Scholar] [CrossRef]
- Terpenoids Library Website. Available online: https://massfinder.com/wiki/Terpenoids_Library_List (accessed on 14 April 2022).
- Maridass, M. Inter-Generic Relationship of Ocimum and Origanum Based on GC-MS Volatile Oils Data using Software NTSPSpc Version 2.0. Ethnobot. Leafl. 2009, 13, 83–88. Available online: https://ethnoleaflets.com/leaflets/gcms.htm (accessed on 1 February 2022).
- Cavalli, J.-F.; Tomi, F.; Bernadini, A.-F.; Casanova, J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Boti, J.B.; Yao, P.A.; Koukoua, G.; N’Guessan, T.Y.; Casanova, J. Components and chemical variability of Isolona campanulata Engler & Diels leaf oil. Flavour Fragr. J. 2006, 21, 166–170. [Google Scholar] [CrossRef]
- Vellutini, M.; Baldovini, N.; De Rocca Serra, D.; Tomi, T.; Casanova, J. β-Cyclolavandulyl and β-isocyclolavandulyl esters from Peucedanum paniculatum L., an endemic species to Corsica. Phytochemistry 2005, 66, 1956–1962. [Google Scholar] [CrossRef]
- Albano, S.M.; Lima, A.S.; Miguel, M.G.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C. Antioxidant, Anti-5-lipoxygenase and Antiacetylcholinesterase Activities of Essential Oils and Decoction Waters of Some Aromatic Plants. Rec. Nat. Prod. 2012, 6, 35–48. [Google Scholar]
- Melo, R.M.; Corrêa, V.F.S.; Amorim, A.C.L.; Miranda, A.L.P.; Rezende, C.M. Identification of Impact Aroma Compounds in Eugenia uniflora L. (Brazilian Pitanga) Leaf Essential Oil. J. Braz. Chem. Soc. 2007, 18, 179–183. [Google Scholar] [CrossRef]
- Paolini, J.; Tomi, P.; Bernardini, A.-F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed analysis of the essential oil from Cistus albidus L. by combination of GC/RI, GC/MS and 13C-NMR spectroscopy. Nat. Prod. Res. 2008, 22, 1270–1278. [Google Scholar] [CrossRef]
- Carrasco, A.; Ortiz-Ruiz, V.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula stoechas essential oil from Spain: Aromatic profile determined by gas chromatography-mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Ind. Crops Prod. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thyme essential oils from Spain: Aromatic profile ascertained by GC-MS and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug Anal. 2018, 26, 529–544. [Google Scholar] [CrossRef]
- Whitman, S.; Gezginci, M.; Timmermann, B.N.; Holman, T.R. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J. Med. Chem. 2002, 45, 2659–2661. [Google Scholar] [CrossRef]
- Jazet Dongmo, P.M.; Kuate, J.; Ngouana, V.; Damesse, F.; Tchinda Sonwa, E.; Amvam Zollo, P.H.; Menut, C. Comparaison des propriétés anti-radicalaires et anti-inflammatoires des huiles essentielles de Citrus reticulata var. Madagascar et Citrus sinensis var. Casagrande du Cameroun. Fruits 2008, 63, 201–208. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Gono-Bwalya, A.B.; van Zyl, R.L.; van Vuuren, S.F.; Lourens, A.C.U.; Başer, K.H.C.; Demirci, B.; Lindsey, K.L.; van Staden, J.; et al. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J. Ethnopharmacol. 2005, 102, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Cachet, T.; Brevard, H.; Chaintreau, A.; Demyttenaere, J.; French, L.; Gassenmeier, K.; Joulain, D.; Koenig, T.; Leijs, H.; Liddle, P.; et al. IOFI recommended practice for the use of predicted relative-response factors for the rapid quantification of volatile flavouring compounds by GC-FID. Flavour Fragr. J. 2016, 31, 191–194. [Google Scholar] [CrossRef]
- König, W.A.; Hochmuth, D.H.; Joulain, D. Terpenoids and Related Constituents of Essential Oils. Library of MassFinder 2.1; Institute of Organic Chemistry: Hamburg, Germany, 2001. [Google Scholar]
- National Institute of Standards and Technology. PC Version of the Mass Spectral Library; Norwalk: Connecticut, CT, USA, 2014.
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured: Carol Stream, IL, USA, 2007; p. 455. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 1998; p. 853. [Google Scholar]
- Eshwarappa, R.S.B.; Ramachandra, Y.L.; Subaramaihha, S.R.; Subbaiah, S.G.P.; Austin, R.S.; Dhananjaya, B.L. Anti-Lipoxygenase activity of leaf gall extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae). Pharmacogn. Res. 2016, 8, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Vyas, V.K.; Variya, B.; Patel, P.; Qureshi, G.; Ghate, M. Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives. Bioorg. Chem. 2016, 67, 130–138. [Google Scholar] [CrossRef]
- Malti, C.E.W.; Baccati, C.; Mariani, M.; Hassani, F.; Babali, B.; Atik-Bekkara, F.; Paoli, M.; Maury, J.; Tomi, F.; Bekhechi, C. Biological Activities and Chemical Composition of Santolina africana Jord. et Fourr. Aerial Part Essential Oil from Algeria: Occurrence of Polyacetylene Derivatives. Molecules 2019, 24, 204. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef]
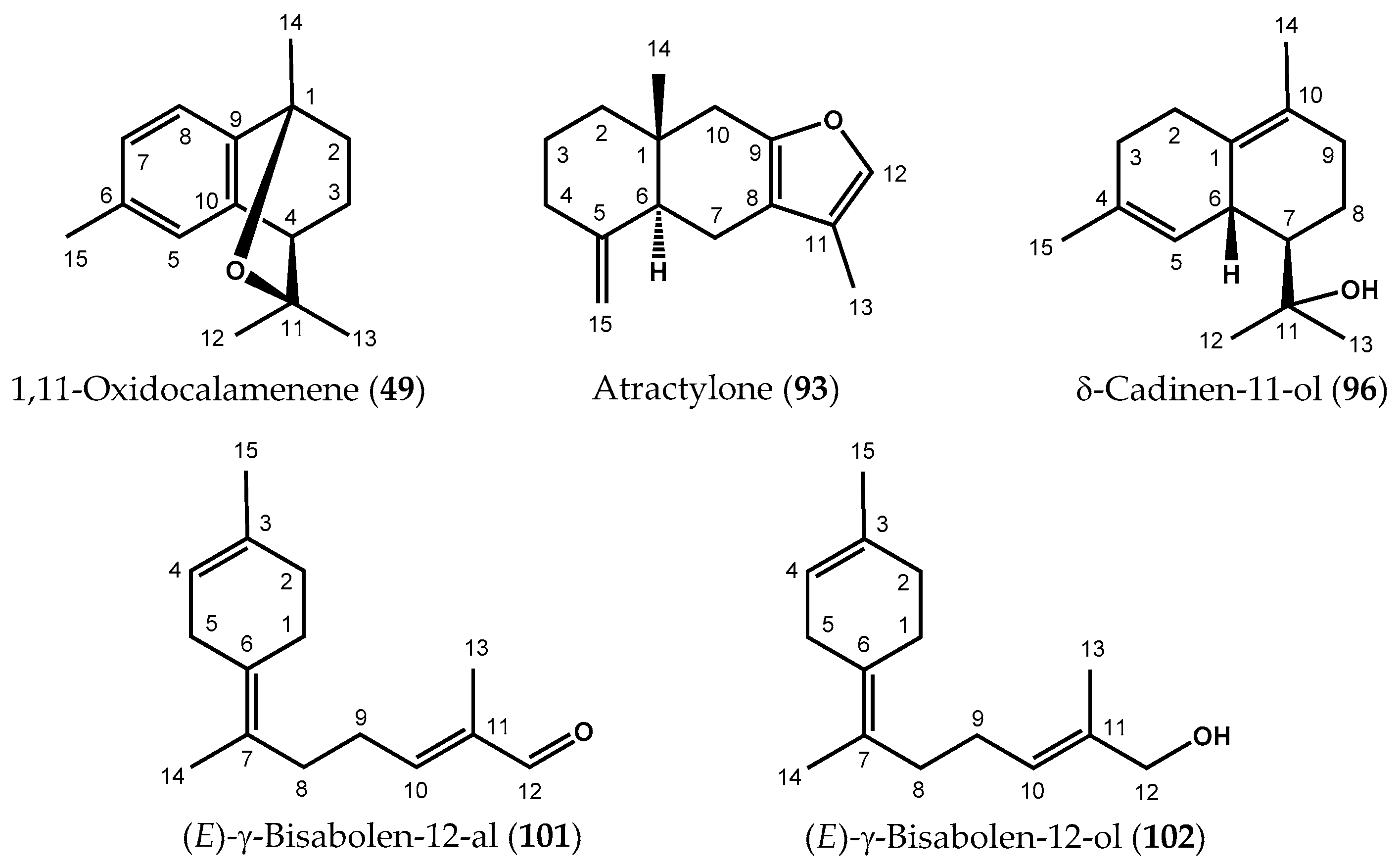
| N °C | 49 | 93 | 101 | 102 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exp | [20] | Exp | [21] | Exp | [22] | Exp | Exp * | [23] * | |
| 1 | 71.90 | 71.9 | 37.35 | 37.5 | 26.64 | 26.5 | 26.87 | 27.24 | 27.2 |
| 2 | 21.09 | 21.1 | 39.31 | 39.4 | 31.72 | 31.6 | 31.79 | 32.05 | 31.9 |
| 3 | 32.03 | 32.0 | 23.59 | 23.5 | 134.09 | 134.1 | 134.82 | 135.39 | 135.2 |
| 4 | 43.52 | 43.5 | 36.65 | 36.8 | 120.53 | 120.4 | 120.73 | 121.18 | 121.0 |
| 5 | 120.25 | 120.2 | 149.41 | 149.5 | 29.74 | 29.6 | 29.71 | 27.24 | 29.9 |
| 6 | 136.50 | 136.5 | 45.75 | 45.9 | 129.94 | 129.8 | 128.67 | 128.93 | 128.7 |
| 7 | 125.29 | 125.3 | 20.90 | 21.0 | 124.29 | 124.3 | 125.56 | 125.63 | 125.4 |
| 8 | 126.48 | 126.5 | 116.15 | 116.3 | 32.44 | 32.3 | 33.77 | 34.23 | 34.1 |
| 9 | 138.90 | 138.9 | 149.83 | 150.0 | 28.12 | 28.1 | 26.55 | 26.95 | 26.8 |
| 10 | 142.15 | 142.1 | 41.96 | 42.1 | 154.39 | 154.9 | 126.11 | 125.45 | 125.4 |
| 11 | 73.72 | 73.7 | 119.55 | 119.7 | 139.40 | 139.2 | 134.16 | 133.91 | 133.8 |
| 12 | 29.36 | 29.4 | 136.96 | 137.1 | 195.24 | 195.6 | 68.96 | 68.63 | 68.3 |
| 13 | 27.78 | 27.8 | 8.15 | 8.3 | 9.14 | 9.2 | 13.60 | 13.62 | 13.6 |
| 14 | 22.12 | 22.1 | 17.56 | 17.7 | 18.24 | 18.2 | 18.36 | 18.49 | 18.4 |
| 15 | 21.37 | 21.4 | 107.28 | 107.4 | 23.34 | 23.4 | 23.40 | 23.56 | 23.5 |
| N°C | δ 13C (ppm) | DEPT | δ 1H (ppm) | Multiplicity (J, Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|---|---|
| 1 | 130.26 | C | – | – | – | – | – |
| 2 | 27.45 | CH2 | α2.70 | ddd (12.0, 4.5, 2.6) | 2β,3 | 5,7,10,3,6,4,1 | 2β,3,9,14 |
| β1.98 | m | 2α,3 | 1,3,4,6,10 | 2α,3,6 | |||
| 3 | 32.32 | CH2 | 2.04 | m | 3,2α | 2,1,4 | 2α,2β,15 |
| 4 | 134.13 | C | – | – | – | – | – |
| 5 | 128.74 | CH | 5.71 | sept (1.5) | 6 | 15,3,6,7,1 | 6,15 |
| 6 | 38.58 | CH | 2.79 | br s | 5,7 | – | 2β,5,9,8β,12,13 |
| 7 | 49.85 | CH | 1.41 | ddd (11.0, 8.1, 3.1) | 6,8 | 1,5,6,8,9,11,12,13 | 9 |
| 8 | 24.53 | CH2 | α1.69 | m | 7,9,8β | 9,6,7,11,10 | 8β,9 |
| β1.35 | m | 7,9,8α | 6,7,10,11,12,13 | 6,8α,9 | |||
| 9 | 31.40 | CH2 | 1.95 | m | 9, 8b, 8a | 1,10,7,8,14 | 2α,6,7,8α,8β,14 |
| 10 | 124.12 | C | – | – | – | – | – |
| 11 | 74.57 | C | – | – | – | – | – |
| 12 | 26.58 | CH3 | 1.23 | s | – | 8,13,7,11 | 6 |
| 13 | 29.97 | CH3 | 1.29 | s | – | 8,12,6,7,11 | 6 |
| 14 | 18.55 | CH3 | 1.67 | br s | – | 1,10,9 | 2α,9 |
| 15 | 23.41 | CH3 | 1.64 | br s | – | 3,5,4 | 3,5 |
| N° | Compounds a | RIl b | RIa | RIp | RRF | S3 (%) | S6 (%) | S13 (%) | S20 (%) | S24 (%) | Identification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (Z)-Hex-3-en-1-ol | 837 | 840 | 1388 | 0.826 | 0.1 | 0.1 | 0.1 | tr | – | RI, MS |
| 2 | Hexanol | 851 | 854 | 1355 | 0.826 | 0.1 | – | 0.1 | 0.1 | tr | RI, MS |
| 3 | α-Thujene | 932 | 923 | 1020 | 0.765 | 0.2 | 0.2 | 0.1 | – | 0.1 | RI, MS, 13C-NMR |
| 4 | α-Pinene | 936 | 931 | 1016 | 0.765 | 0.1 | tr | tr | tr | 0.2 | RI, MS, 13C-NMR |
| 5 | Sabinene | 973 | 966 | 1127 | 0.765 | 0.6 | 0.3 | 0.5 | 0.1 | 0.6 | RI, MS, 13C-NMR |
| 6 | β-Pinene | 978 | 971 | 1116 | 0.765 | 0.4 | tr | tr | tr | 0.2 | RI, MS, 13C-NMR |
| 7 | Myrcene | 987 | 981 | 1166 | 0.765 | 0.1 | 0.1 | – | tr | tr | RI, MS |
| 8 | α-Terpinene | 1013 | 1010 | 1186 | 0.765 | 0.1 | – | 0.1 | – | 0.1 | RI, MS |
| 9 | β-Phellandrene * | 1023 | 1022 | 1215 | 0.698 | – | tr | 0.1 | – | 0.1 | RI, MS |
| 10 | Limonene * | 1025 | 1022 | 1205 | 0.765 | 0.1 | 0.1 | – | tr | 0.1 | RI, MS |
| 11 | (Z)-β-Ocimene | 1029 | 1026 | 1237 | 0.765 | tr | 0.1 | 0.1 | – | 0.3 | RI, MS, 13C-NMR |
| 12 | (E)-β-Ocimene | 1041 | 1037 | 1255 | 0.765 | 1.2 | 0.8 | 1.5 | 1.7 | 1.2 | RI, MS, 13C-NMR |
| 13 | γ-Terpinene | 1051 | 1049 | 1250 | 0.765 | tr | 0.2 | 0.1 | – | 0.2 | RI, MS, 13C-NMR |
| 14 | Terpinolene | 1082 | 1079 | 1288 | 0.765 | 0.2 | 0.1 | 0.1 | – | 0.2 | RI, MS, 13C-NMR |
| 15 | Linalool | 1086 | 1085 | 1550 | 0.869 | tr | 0.1 | tr | 0.1 | tr | RI, MS |
| 16 | Terpinen-4-ol | 1164 | 1163 | 1604 | 0.869 | 0.1 | 0.1 | tr | – | tr | RI, MS |
| 17 | Neral | 1215 | 1217 | 1680 | 0.887 | 0.2 | 0.1 | 0.1 | – | 0.2 | RI, MS, 13C-NMR |
| 18 | Geraniol | 1235 | 1236 | 1837 | 0.869 | 0.1 | 0.2 | 0.2 | – | 0.1 | RI, MS, 13C-NMR |
| 19 | Geranial | 1244 | 1244 | 1732 | 0.887 | 0.1 | 0.1 | 0.1 | – | 0.1 | RI, MS |
| 20 | Thymol | 1267 | 1268 | 2190 | 0.808 | 1.3 | tr | tr | tr | tr | RI, MS, 13C-NMR |
| 21 | Carvacrol | 1278 | 1277 | 2228 | 0.808 | 0.1 | 0.1 | tr | – | – | RI, MS |
| 22 | Cogeijerene | 1285 c | 1282 | 1540 | 0.808 | 0.1 | 0.1 | 0.2 | tr | tr | RI, MS, 13C-NMR |
| 23 | Bicycloelemene | 1338 | 1332 | 1483 | 0.751 | 0.1 | tr | 0.1 | – | 0.1 | RI, MS |
| 24 | δ-Elemene | 1340 | 1335 | 1472 | 0.751 | 0.4 | 0.2 | 2.2 | 0.2 | 2.6 | RI, MS, 13C-NMR |
| 25 | α-Cubebene | 1355 | 1348 | 1459 | 0.751 | 0.1 | 0.2 | tr | – | 0.1 | RI, MS, 13C-NMR |
| 26 | Cyclosativene | 1378 | 1369 | 1483 | 0.751 | 0.1 | 0.1 | 0.1 | – | 0.1 | RI, MS |
| 27 | α-Ylangene | 1376 | 1371 | 1468 | 0.751 | 0.1 | 0.1 | 0.1 | tr | – | RI, MS |
| 28 | α-Copaene | 1379 | 1375 | 1493 | 0.751 | 0.1 | 0.1 | 0.1 | tr | tr | RI, MS |
| 29 | β-Bourbonene | 1378 | 1383 | 1520 | 0.751 | 0.1 | 0.1 | tr | – | 0.1 | RI, MS |
| 30 | β-Cubebene * | 1390 | 1387 | 1539 | 0.751 | 0.2 | 0.3 | 0.4 | 0.1 | 1.0 | RI, MS, 13C-NMR |
| 31 | β-Elemene * | 1389 | 1387 | 1591 | 0.751 | 4.4 | 0.6 | 1.6 | 4.6 | 1.2 | RI, MS, 13C-NMR |
| 32 | Cyperene | 1402 | 1399 | 1528 | 0.751 | 0.2 | 0.1 | 0.2 | – | tr | RI, MS, 13C-NMR |
| 33 | α-Gurjunene | 1413 | 1409 | 1531 | 0.751 | 0.7 | tr | 0.2 | 0.1 | tr | RI, MS, 13C-NMR |
| 34 | (E)-β-Caryophyllene | 1421 | 1417 | 1597 | 0.751 | 1.8 | 3.4 | 20.0 | 34.4 | 1.0 | RI, MS, 13C-NMR |
| 35 | β-Copaene | 1430 | 1426 | 1591 | 0.751 | 0.5 | 0.1 | 0.7 | 0.1 | 3.1 | RI, MS, 13C-NMR |
| 36 | γ-Elemene # | 1429 | 1427 | 1640 | 0.751 | 5.5 | 0.3 | 1.3 | 0.3 | 1.1 | RI, MS, 13C-NMR |
| 37 | trans-α-Bergamotene | 1434 | 1432 | 1586 | 0.751 | 0.1 | tr | tr | tr | 0.4 | RI, MS, 13C-NMR |
| 38 | α-Guaiene | 1440 | 1435 | 1591 | 0.751 | tr | 0.1 | – | tr | 0.2 | RI, MS, 13C-NMR |
| 39 | Sesquisabinene A | 1435 | 1436 | 1647 | 0.751 | tr | tr | tr | tr | 0.6 | RI, MS, 13C-NMR |
| 40 | Guaia-6,9-diene | 1443 | 1437 | 1606 | 0.751 | tr | tr | 0.1 | 0.1 | 0.3 | RI, MS, 13C-NMR |
| 41 | β-Gurjunene (Calarene) | 1437 | 1444 | 1591 | 0.751 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 42 | (E)-β-Farnesene | 1446 | 1446 | 1661 | 0.751 | 0.1 | 0.4 | 0.1 | tr | 0.6 | RI, MS, 13C-NMR |
| 43 | α-Humulene | 1455 | 1450 | 1670 | 0.751 | 5.8 | 1.6 | 2.7 | 27.1 | 1.7 | RI, MS, 13C-NMR |
| 44 | cis-β-Bergamotene | 1435 d | 1452 | 1671 | 0.751 | 0.3 | 0.2 | 0.2 | 1.1 | tr | RI, MS, 13C-NMR |
| 45 | allo-Aromadendrene | 1462 | 1456 | 1640 | 0.751 | 0.2 | tr | 0.1 | 0.2 | 0.1 | RI, MS, 13C-NMR |
| 46 | Ishwarane | 1468 | 1461 | 1645 | 0.751 | 0.1 | tr | 0.3 | – | – | RI, MS, 13C-NMR |
| 47 | γ-Muurolene | 1474 | 1469 | 1688 | 0.751 | 0.1 | tr | 0.2 | 0.1 | 0.1 | RI, MS, 13C-NMR |
| 48 | 4,5-diepi-Aristolochene | 1470 | 1471 | 1705 | 0.751 | 0.3 | – | tr | – | – | RI, MS, 13C-NMR |
| 49 | 1,11-Oxidocalamenene | 1474 | 1472 | 1883 | 0.830 | 1.0 | 0.4 | 0.5 | 2.7 | 0.1 | RI, MS, 13C-NMR |
| 50 | Germacrene D | 1479 | 1475 | 1709 | 0.751 | 3.0 | 1.1 | 5.2 | 1.0 | 10.2 | RI, MS, 13C-NMR |
| 51 | trans-β-Bergamotene | 1480 | 1478 | 1684 | 0.751 | 0.1 | tr | tr | – | 0.1 | RI, MS |
| 52 | β-Selinene | 1486 | 1481 | 1718 | 0.751 | 0.5 | 0.3 | 0.2 | 0.4 | 0.3 | RI, MS, 13C-NMR |
| 53 | Furanodiene # | 1485 | 1482 | 1873 | 0.853 | 0.4 | 0.1 | 0.4 | 0.3 | 3.9 | RI, MS, 13C-NMR |
| 54 | Furano-elemene (Curzerene) # | 1485 | 1484 | 1873 | 0.853 | 0.1 | tr | 0.1 | tr | 0.1 | RI, MS |
| 55 | 4-epi-Cubebol | 1490 | 1487 | 1871 | 0.819 | 0.2 | 0.4 | – | – | – | RI, MS, 13C-NMR |
| 56 | Bicyclogermacrene | 1494 | 1490 | 1732 | 0.751 | 0.9 | 0.7 | 0.7 | 0.3 | 0.1 | RI, MS, 13C-NMR |
| 57 | α-Selinene | 1494 | 1491 | 1723 | 0.751 | 0.7 | 0.2 | 0.4 | 0.2 | 2.2 | RI, MS, 13C-NMR |
| 58 | α-Muurolene | 1496 | 1494 | 1705 | 0.751 | 2.8 | 2.2 | 1.0 | 6.6 | 0.3 | RI, MS, 13C-NMR |
| 59 | β-Bisabolene | 1503 | 1500 | 1727 | 0.751 | 0.8 | 0.9 | 0.4 | 0.2 | 1.5 | RI, MS, 13C-NMR |
| 60 | Cubebol | 1514 | 1505 | 1885 | 0.819 | tr | tr | 0.7 | tr | 0.2 | RI, MS, 13C-NMR |
| 61 | γ-Cadinene | 1507 | 1507 | 1758 | 0.751 | 0.1 | – | 0.1 | – | 0.1 | RI, MS |
| 62 | (Z)-γ-Bisabolene | 1505 | 1510 | 1732 | 0.751 | 0.1 | tr | tr | 0.1 | 0.1 | RI, MS |
| 63 | δ-Cadinene | 1520 | 1514 | 1758 | 0.751 | 2.4 | 0.8 | 8.4 | 0.9 | 7.0 | RI, MS, 13C-NMR |
| 64 | Kessane | 1533 | 1521 | 1761 | 0.751 | 5.2 | 2.3 | 11.5 | 2.7 | – | RI, MS, 13C-NMR |
| 65 | (E)-γ-Bisabolene | 1521 | 1522 | 1758 | 0.751 | 1.1 | 0.4 | tr | tr | 3.4 | RI, MS, 13C-NMR |
| 66 | Selina-4(15),7(11)-diene | 1534 | 1528 | 1778 | 0.751 | 0.5 | 8.3 | 0.1 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 67 | β-Elemol | 1541 | 1534 | 2079 | 0.819 | 0.6 | 4.4 | 1.2 | 0.9 | 1.8 | RI, MS, 13C-NMR |
| 68 | Selina-3,7(11)-diene | 1542 | 1537 | 1778 | 0.751 | 0.2 | tr | tr | tr | 0.2 | RI, MS, 13C-NMR |
| 69 | cis-Cadinene ether | 1551 | 1545 | 1860 | 0.830 | – | – | 0.4 | – | tr | RI, MS, 13C-NMR |
| 70 | (E)-Nerolidol | 1553 | 1548 | 2042 | 0.819 | 0.5 | 23.3 | 1.6 | 0.7 | 0.2 | RI, MS, 13C-NMR |
| 71 | Germacrene B # | 1552 | 1551 | 1829 | 0.751 | 15.5 | 0.9 | 3.2 | 0.7 | 3.5 | RI, MS, 13C-NMR |
| 72 | Palustrol | 1569 | 1561 | 1924 | 0.819 | 1.5 | 1.2 | 0.2 | 0.1 | tr | RI, MS, 13C-NMR |
| 73 | cis-Sesquisabinene hydrate | 1565e | 1564 | 2081 | 0.819 | 0.1 | tr | 0.6 | 0.1 | tr | RI, MS, 13C-NMR |
| 74 | Caryophyllene oxide | 1578 | 1570 | 1978 | 0.830 | 0.1 | tr | 0.3 | 2.5 | tr | RI, MS, 13C-NMR |
| 75 | Curzerenone | 1588 | 1575 | 2025 | 0.841 | – | – | – | 0.1 | 0.3 | RI, MS, 13C-NMR |
| 76 | 7-epi-cis-Sesquisabinene hydrate | 1579 e | 1576 | 2099 | 0.819 | 0.3 | tr | 0.7 | tr | 0.1 | RI, MS, 13C-NMR |
| 77 | Viridiflorol | 1592 | 1581 | 2081 | 0.819 | 0.9 | tr | 0.1 | 0.2 | 0.2 | RI, MS, 13C-NMR |
| 78 | Guaiol | 1593 | 1584 | 2088 | 0.819 | 0.6 | 12.2 | 2.3 | 1.8 | 11.6 | RI, MS, 13C-NMR |
| 79 | Ledol * | 1600 | 1593 | 2025 | 0.819 | 12.5 | 0.3 | 1.3 | 1.0 | 0.2 | RI, MS, 13C-NMR |
| 80 | Copaborneol * | 1595 | 1593 | 2183 | 0.819 | 1.4 | tr | 0.1 | – | 0.2 | RI, MS, 13C-NMR |
| 81 | Eudesm-5-en-11-ol | 1600 f | 1595 | 2132 | 0.819 | tr | 0.2 | 0.3 | tr | 0.8 | RI, MS, 13C-NMR |
| 82 | neo-Intermedeol | 1601 g | 1599 | 2146 | 0.819 | 0.1 | tr | tr | tr | 0.2 | RI, MS, 13C-NMR |
| 83 | epi-Cubenol | 1602 | 1606 | 2048 | 0.819 | tr | 0.2 | 0.2 | 0.4 | 0.3 | RI, MS, 13C-NMR |
| 84 | Alismol | 1619 | 1610 | 2248 | 0.830 | tr | – | tr | 0.5 | 0.1 | RI, MS, 13C-NMR |
| 85 | Eremoligenol | 1614 | 1614 | 2196 | 0.819 | 0.1 | – | – | tr | 0.2 | RI, MS, 13C-NMR |
| 86 | 10-epi-γ-Eudesmol | 1609 | 1617 | 2096 | 0.819 | 1.4 | 0.3 | 1.3 | tr | 0.3 | RI, MS, 13C-NMR |
| 87 | τ-Cadinol | 1633 | 1625 | 2175 | 0.819 | 0.3 | – | 0.2 | tr | 0.4 | RI, MS, 13C-NMR |
| 88 | τ-Muurolol | 1633 | 1628 | 2184 | 0.819 | 0.7 | 0.2 | 0.8 | tr | 0.7 | RI, MS, 13C-NMR |
| 89 | α-Muurolol | 1618 h | 1630 | 2212 | 0.819 | 0.2 | tr | 0.6 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 90 | β-Eudesmol | 1641 | 1634 | 2225 | 0.819 | 0.2 | 6.3 | 1.9 | 0.7 | 0.5 | RI, MS, 13C-NMR |
| 91 | α-Cadinol | 1643 | 1637 | 2228 | 0.819 | 1.2 | 0.7 | 0.5 | tr | tr | RI, MS, 13C-NMR |
| 92 | α-Eudesmol | 1653 | 1638 | 2216 | 0.819 | tr | 2.1 | 0.6 | tr | tr | RI, MS, 13C-NMR |
| 93 | Atractylone | 1652 i | 1639 | 2121 | 0.841 | 1.0 | 3.3 | 2.7 | 1.5 | 10.0 | RI, MS, 13C-NMR |
| 94 | Intermedeol | 1626 h | 1641 | 2249 | 0.819 | 0.1 | 0.8 | 0.2 | 0.2 | tr | RI, MS, 13C-NMR |
| 95 | Bulnesol * | 1665 | 1651 | 2207 | 0.819 | 0.4 | 5.2 | 1.5 | 1.0 | 6.2 | RI, MS, 13C-NMR |
| 96 | δ-Cadinen-11-ol * | j | 1651 | 2271 | 0.819 | 0.8 | 2.9 | 0.2 | 0.1 | 1.8 | RI, MS, 13C-NMR |
| 97 | α-Bisabolol | 1673 | 1666 | 2208 | 0.819 | 0.3 | tr | 0.5 | 0.1 | 0.6 | RI, MS, 13C-NMR |
| 98 | epi-α-Bisabolol | 1667 k | 1668 | 2214 | 0.819 | 0.2 | – | 0.2 | – | 0.1 | RI, MS, 13C-NMR |
| 99 | Cadina-1(10),4-dien-8α-ol | 1682 | 1671 | 2306 | 0.819 | – | 0.4 | 8.1 | 0.4 | 0.3 | RI, MS, 13C-NMR |
| 100 | Germacrone | 1684 | 1673 | 2221 | 0.841 | 0.8 | 0.2 | tr | 0.1 | 0.1 | RI, MS, 13C-NMR |
| 101 | (E)-γ-Bisabolen-12-al | 1790 l | 1761 | 2348 | 0.841 | 0.7 | 1.1 | tr | tr | 1.9 | RI, MS, 13C-NMR |
| 102 | (E)-γ-Bisabolen-12-ol | j | 1776 | 2549 | 0.819 | 6.1 | 2.5 | 1.0 | 0.3 | 7.1 | RI, MS, 13C-NMR |
| 103 | (E)-Phytol | 2114 | 2098 | 2609 | 0.974 | 0.4 | 0.3 | 0.1 | 0.1 | 0.1 | RI, MS, 13C-NMR |
| Monoterpene hydrocarbons | 3.0 | 1.9 | 2.6 | 1.8 | 3.3 | ||||||
| Oxygenated monoterpenes | 1.9 | 0.7 | 0.4 | 0.1 | 0.4 | ||||||
| Sesquiterpene hydrocarbons | 55.4 | 26.2 | 62.2 | 81.7 | 43.8 | ||||||
| Oxygenated sesquiterpenes | 34.8 | 68.7 | 31.3 | 15.8 | 50.7 | ||||||
| Other compounds | 0.6 | 0.4 | 0.3 | 0.2 | 0.1 | ||||||
| Total | 95.7 | 97.9 | 96.8 | 99.6 | 98.3 |
| Component [a] | RIa [b] | RIp [b] | Group I | Group II | Group III | Group IV | Group V | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M% ± SD | Min | Max | M% ± SD | Min | Max | M% ± SD | Min | Max | M% ± SD | Min | Max | M% ± SD | Min | Max | |||
| β-Elemene | 1387 | 1591 | 2.2 ± 0.9 | 1.1 | 3.7 | 4.6 ± 0.5 | 4.1 | 5.2 | 1.4 ± 0.2 | 1.1 | 1.7 | 0.8 ± 0.3 | 0.5 | 1.1 | 3.1 ± 1.1 | 2.1 | 4.6 |
| (E)-β-Caryophyllene | 1417 | 1597 | 17.6 ± 4.2 | 12.5 | 25.0 | 2.6 ± 1.4 | 1.6 | 4.7 | 1.1 ± 0.1 | 1.0 | 1.2 | 3.3 ± 3.6 | 0.9 | 9.4 | 36.6 ± 5.9 | 32.5 | 45.4 |
| γ-Elemene # | 1427 | 1640 | 1.5 ± 0.7 | 0.9 | 3.2 | 5.0 ± 0.8 | 4.1 | 5.8 | 1.2 ± 0.4 | 0.8 | 2.0 | 0.4 ± 0.2 | 0.2 | 0.7 | 0.5 ± 0.3 | 0.3 | 0.9 |
| α-Humulene | 1450 | 1670 | 2.5 ± 1.3 | 1.1 | 5.2 | 6.6 ± 2.1 | 5.0 | 9.7 | 1.3 ± 0.3 | 0.5 | 1.7 | 1.0 ± 0.6 | 0.5 | 1.8 | 18.9 ± 12.1 | 6.3 | 31.2 |
| Germacrene D | 1475 | 1709 | 4.3 ± 1.1 | 3.0 | 5.8 | 3.5 ± 1.4 | 2.4 | 5.6 | 10.6 ± 1.6 | 9.0 | 14.4 | 1.2 ± 0.5 | 0.5 | 2.0 | 1.7 ± 0.8 | 1.0 | 2.4 |
| Furanodiene # | 1482 | 1873 | 0.2 ± 0.2 | tr | 0.4 | 0.6 ± 0.7 | 0.1 | 1.6 | 4.0 ± 1.2 | 2.2 | 5.9 | 0.3 ± 0.2 | 0.1 | 0.6 | 0.4 ± 0.2 | 0.2 | 0.6 |
| α-Muurolene | 1494 | 1705 | 1.2 ± 0.8 | 0.2 | 2.5 | 2.0 ± 1.3 | 0.1 | 2.9 | 0.1 ± 0.1 | tr | 0.3 | 2.7 ± 0.5 | 2.2 | 3.4 | 6.2 ± 3.5 | 2.8 | 10.9 |
| δ-Cadinene | 1514 | 1758 | 6.2 ± 3.4 | 1.8 | 12.0 | 2.2 ± 0.6 | 1.3 | 2.8 | 7.3 ± 0.4 | 6.5 | 7.8 | 0.8 ± 0.3 | 0.5 | 1.2 | 0.9 ± 0.4 | 0.4 | 1.4 |
| Kessane | 1521 | 1761 | 10.3 ± 9.9 | 1.4 | 32.5 | 4.8 ± 0.6 | 4.2 | 5.5 | – | – | – | 1.6 ± 1.3 | 0.1 | 2.7 | 1.2 ± 1.1 | 0.4 | 2.7 |
| Selina-4(15),7(11)-diene | 1528 | 1778 | 1.0 ± 1.5 | tr | 4.3 | 0.5 ± 0.1 | 0.3 | 0.5 | 0.2 ± 0.1 | tr | 0.3 | 9.5 ± 1.7 | 7.4 | 11.2 | 1.1 ± 1.1 | 0.1 | 2.2 |
| β-Elemol | 1534 | 2079 | 2.2 ± 1.1 | 1.0 | 4.0 | 0.5 ± 0.3 | tr | 0.8 | 1.8 ± 0.3 | 1.5 | 2.5 | 4.5 ± 0.5 | 3.9 | 5.0 | 1.6 ± 0.9 | 0.9 | 2.9 |
| (E)-Nerolidol | 1548 | 2042 | 1.9 ± 2.1 | tr | 6.6 | 0.6 ± 0.1 | 0.4 | 0.7 | 0.2 ± 0.0 | 0.2 | 0.2 | 23.8 ± 5.4 | 15.8 | 30.8 | 3.0 ± 3.4 | 0.3 | 7.8 |
| Germacrene B # | 1551 | 1829 | 3.4 ± 0.8 | 2.2 | 4.8 | 13.4 ± 3.1 | 10.2 | 16.6 | 3.4 ± 1.0 | 2.3 | 5.1 | 0.4 ± 0.5 | tr | 0.9 | 1.3 ± 0.7 | 0.7 | 2.2 |
| Guaiol | 1584 | 2088 | 4.2 ± 2.5 | 2.0 | 7.8 | 0.4 ± 0.3 | tr | 0.7 | 11.4 ± 2.0 | 6.9 | 13.2 | 12.4 ± 1.3 | 10.9 | 13.8 | 2.5 ± 0.8 | 1.8 | 3.5 |
| Ledol | 1593 | 2025 | 3.4 ± 3.1 | 0.4 | 8.2 | 10.6 ± 2.7 | 7.3 | 13.2 | 0.2 ± 0.2 | tr | 0.6 | 0.6 ± 0.8 | tr | 1.9 | 1.8 ± 1.1 | 0.7 | 2.9 |
| Atractylone | 1639 | 2121 | 1.9 ± 1.2 | tr | 3.3 | 1.2 ± 1.4 | tr | 3.2 | 9.6 ± 2.3 | 4.8 | 12.1 | 3.0 ± 0.3 | 2.6 | 3.3 | 1.5 ± 0.5 | 0.8 | 2.1 |
| Bulnesol | 1651 | 2207 | 2.5 ± 1.2 | 1.4 | 4.2 | 0.4 ± 0.1 | 0.2 | 0.5 | 5.9 ± 1.2 | 3.2 | 7.2 | 6.5 ± 1.1 | 5.2 | 7.6 | 1.7 ± 0.9 | 1.0 | 3.0 |
| Cadina-1(10),4-dien-8α-ol | 1671 | 2306 | 3.6 ± 4.8 | tr | 12.1 | – | – | – | 0.3 ± 0.1 | 0.1 | 0.6 | 0.1 ± 0.2 | tr | 0.4 | 0.6 ± 0.7 | tr | 1.6 |
| (E)-γ-Bisabolen-12-ol | 1776 | 2549 | 1.0 ± 0.4 | 0.4 | 1.6 | 5.8 ± 0.4 | 5.2 | 6.1 | 7.3 ± 1.3 | 5.3 | 9.4 | 4.4 ± 2.6 | 2.5 | 8.8 | 0.8 ± 0.5 | 0.3 | 1.4 |
| Anti-Inflammatory Activity (Percentage Inhibition of LOX) | IC50 (mg mL−1) | ||
|---|---|---|---|
| Oil concentration (mg mL−1) | Inhibition (%) | Essential oil | 0.059 ± 0.001 |
| 0.0125 | 15.20 ± 0.30 | *NDGA | 0.013 ± 0.003 |
| 0.0250 | 24.59 ± 1.42 | ||
| 0.0500 | 47.35 ± 2.09 | ||
| 0.0800 | 64.92 ± 2.87 | ||
| 0.1000 | 81.87 ± 0.33 | ||
| Fraction Sample | F1 P 100% | F2 P 100% | F3 P/DE 98/2 | F4 P/DE 95/5 | F5 P/DE 90/10 | F6 P/DE 80/20 | F7 DE 100% |
|---|---|---|---|---|---|---|---|
| S3 (2.605 g) | 0.928 | 0.612 | 0.115 | 0.159 | 0.456 | 0.219 | 0.024 |
| S6 (3.280 g) | 0.698 | 0.224 | 0.174 | 0.049 | 1.542 | 0.515 | 0.011 |
| S13 (2.318 g) | 1.109 | 0.349 | 0.107 | 0.095 | 0.232 | 0.290 | 0.019 |
| S20 (2.148 g) | 1.311 | 0.423 | 0.155 | 0.019 | 0.131 | 0.037 | 0.014 |
| S24 (4.110 g) | 1.411 | 0.536 | 0.686 | 0.058 | 0.929 | 0.399 | 0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kambiré, D.A.; Kablan, A.C.L.; Yapi, T.A.; Vincenti, S.; Maury, J.; Baldovini, N.; Tomi, P.; Paoli, M.; Boti, J.B.; Tomi, F. Neuropeltis acuminata (P. Beauv.): Investigation of the Chemical Variability and In Vitro Anti-inflammatory Activity of the Leaf Essential Oil from the Ivorian Species. Molecules 2022, 27, 3759. https://doi.org/10.3390/molecules27123759
Kambiré DA, Kablan ACL, Yapi TA, Vincenti S, Maury J, Baldovini N, Tomi P, Paoli M, Boti JB, Tomi F. Neuropeltis acuminata (P. Beauv.): Investigation of the Chemical Variability and In Vitro Anti-inflammatory Activity of the Leaf Essential Oil from the Ivorian Species. Molecules. 2022; 27(12):3759. https://doi.org/10.3390/molecules27123759
Chicago/Turabian StyleKambiré, Didjour Albert, Ahmont Claude Landry Kablan, Thierry Acafou Yapi, Sophie Vincenti, Jacques Maury, Nicolas Baldovini, Pierre Tomi, Mathieu Paoli, Jean Brice Boti, and Félix Tomi. 2022. "Neuropeltis acuminata (P. Beauv.): Investigation of the Chemical Variability and In Vitro Anti-inflammatory Activity of the Leaf Essential Oil from the Ivorian Species" Molecules 27, no. 12: 3759. https://doi.org/10.3390/molecules27123759
APA StyleKambiré, D. A., Kablan, A. C. L., Yapi, T. A., Vincenti, S., Maury, J., Baldovini, N., Tomi, P., Paoli, M., Boti, J. B., & Tomi, F. (2022). Neuropeltis acuminata (P. Beauv.): Investigation of the Chemical Variability and In Vitro Anti-inflammatory Activity of the Leaf Essential Oil from the Ivorian Species. Molecules, 27(12), 3759. https://doi.org/10.3390/molecules27123759








