Microstructure, Electromagnetic Properties, and Microwave Absorption Mechanism of SiO2-MnO-Al2O3 Based Manganese Ore Powder for Electromagnetic Protection
Abstract
:1. Introduction
2. Experiment
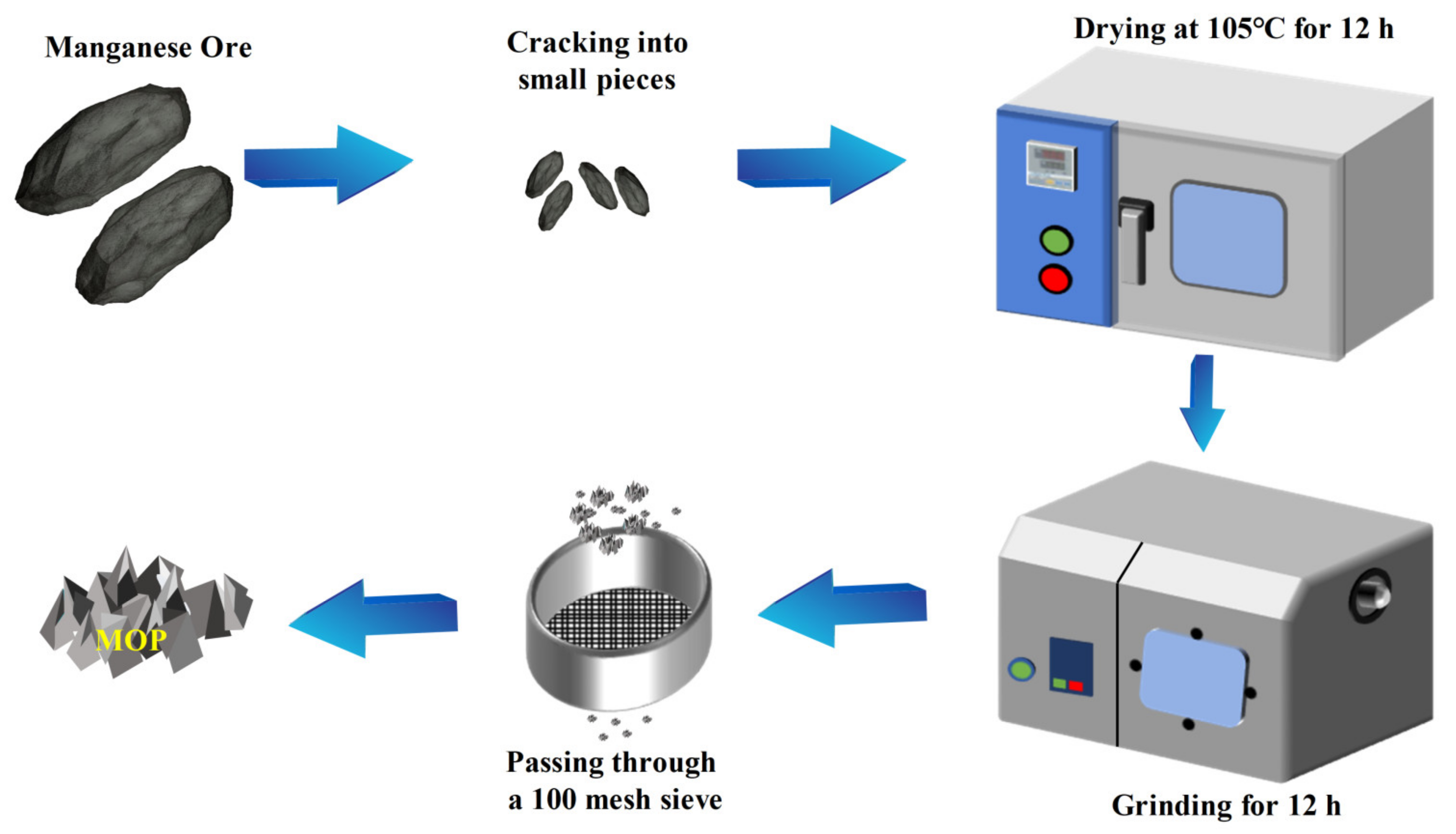
2.1. Materials
2.2. Characterization
3. Results and Discussion
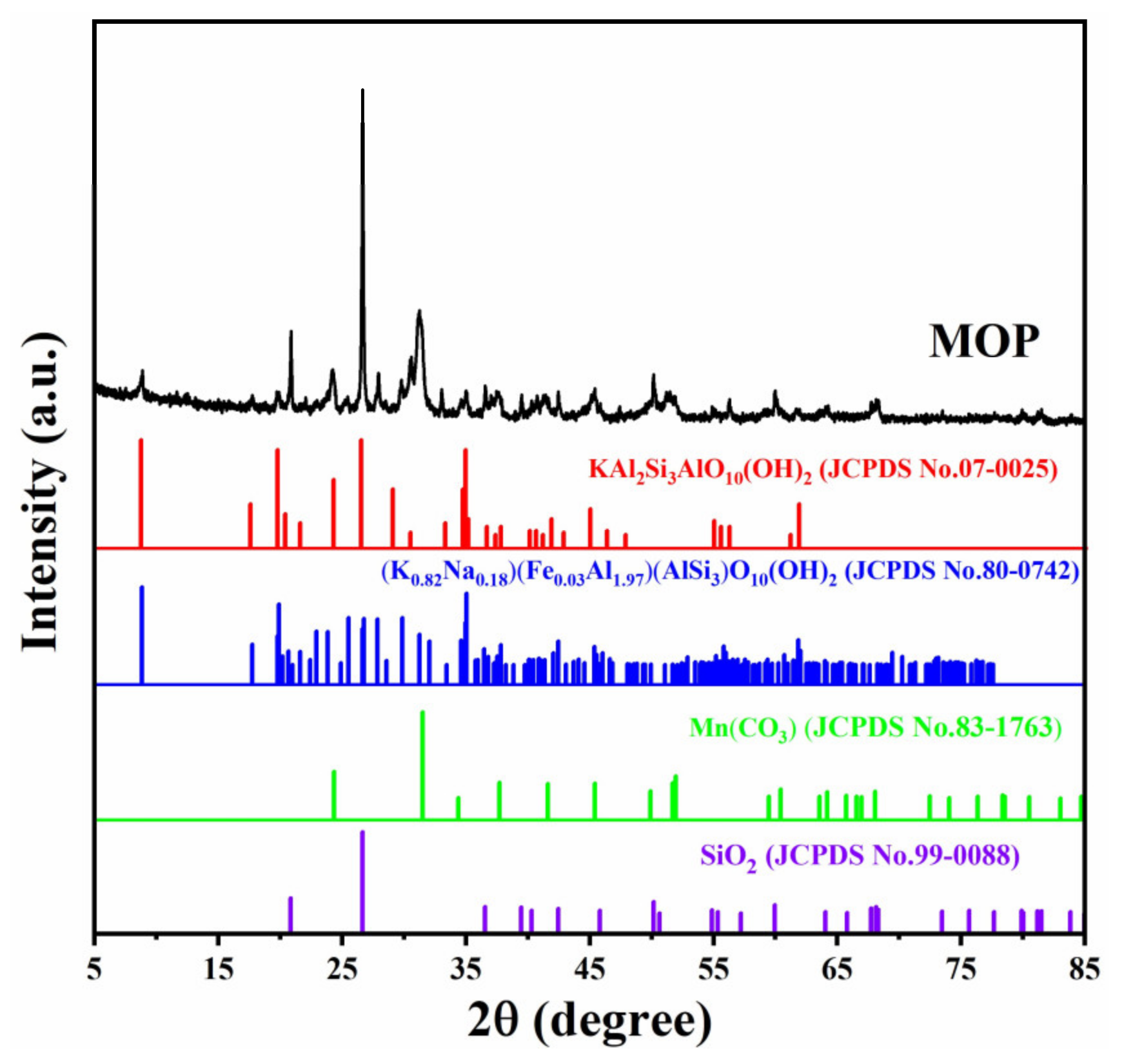
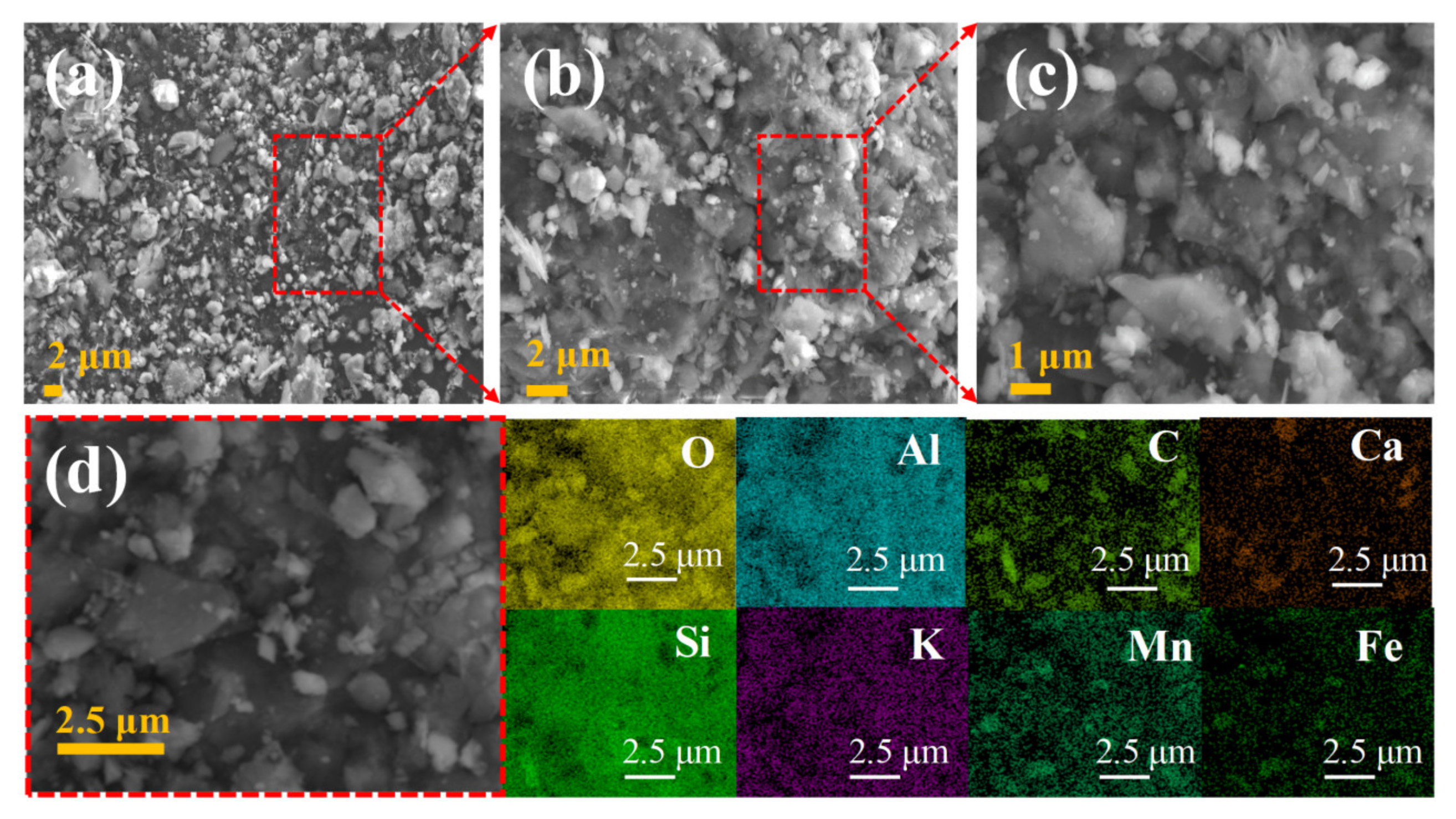
3.1. Compositional and Microstructures of MOP
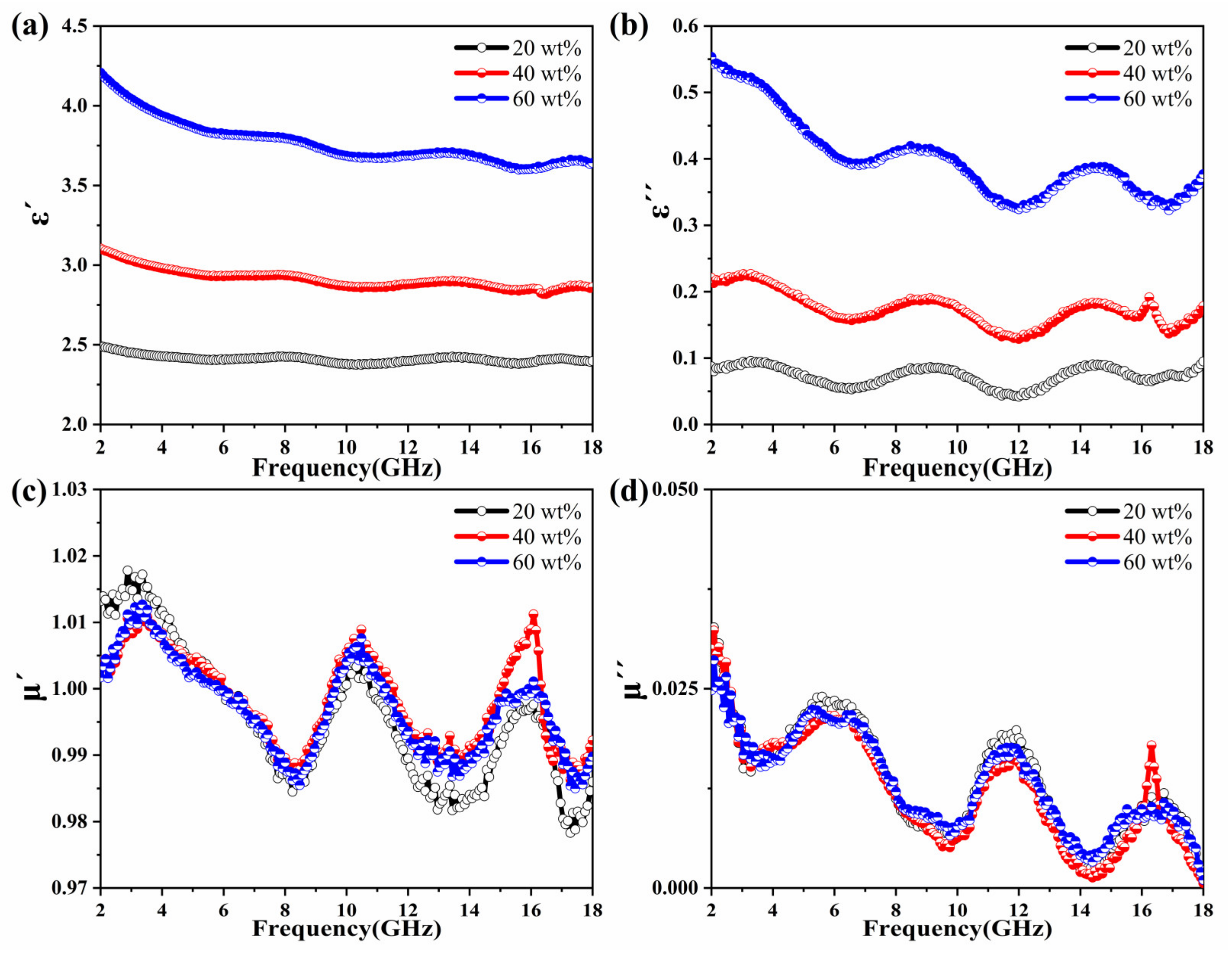
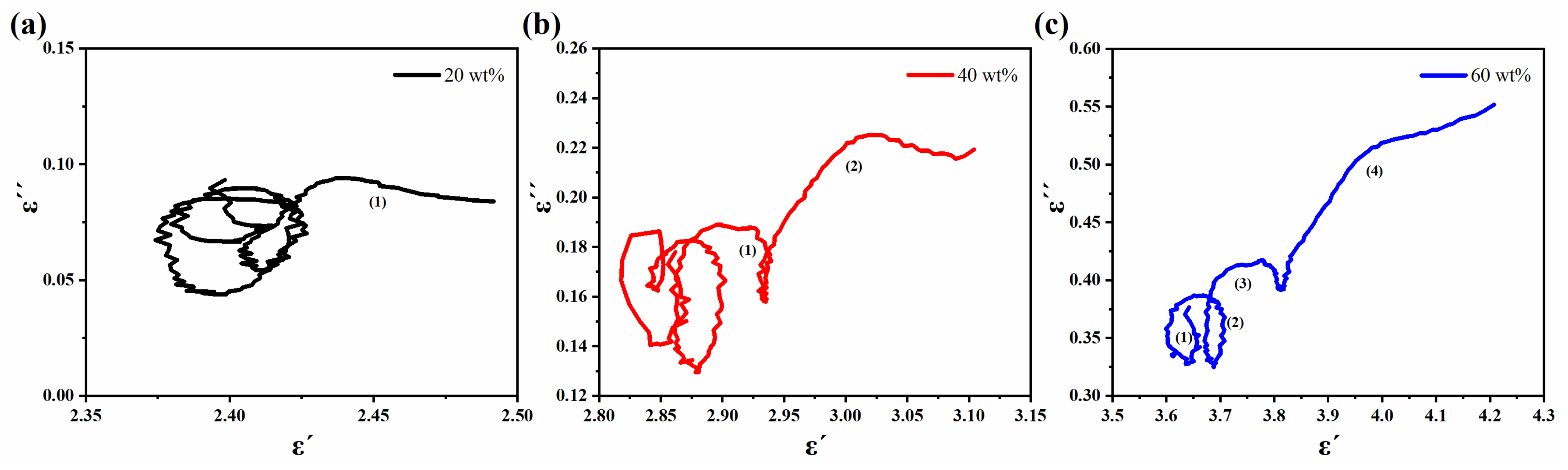
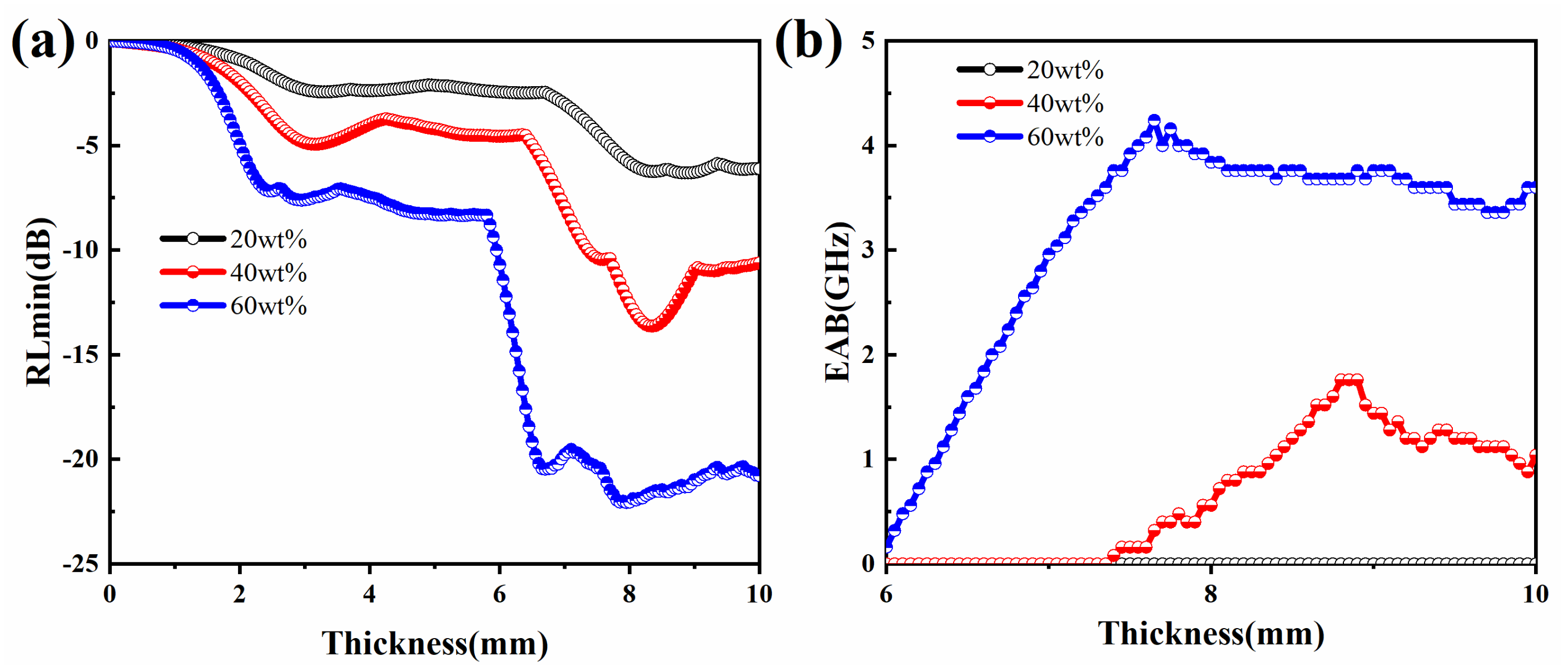
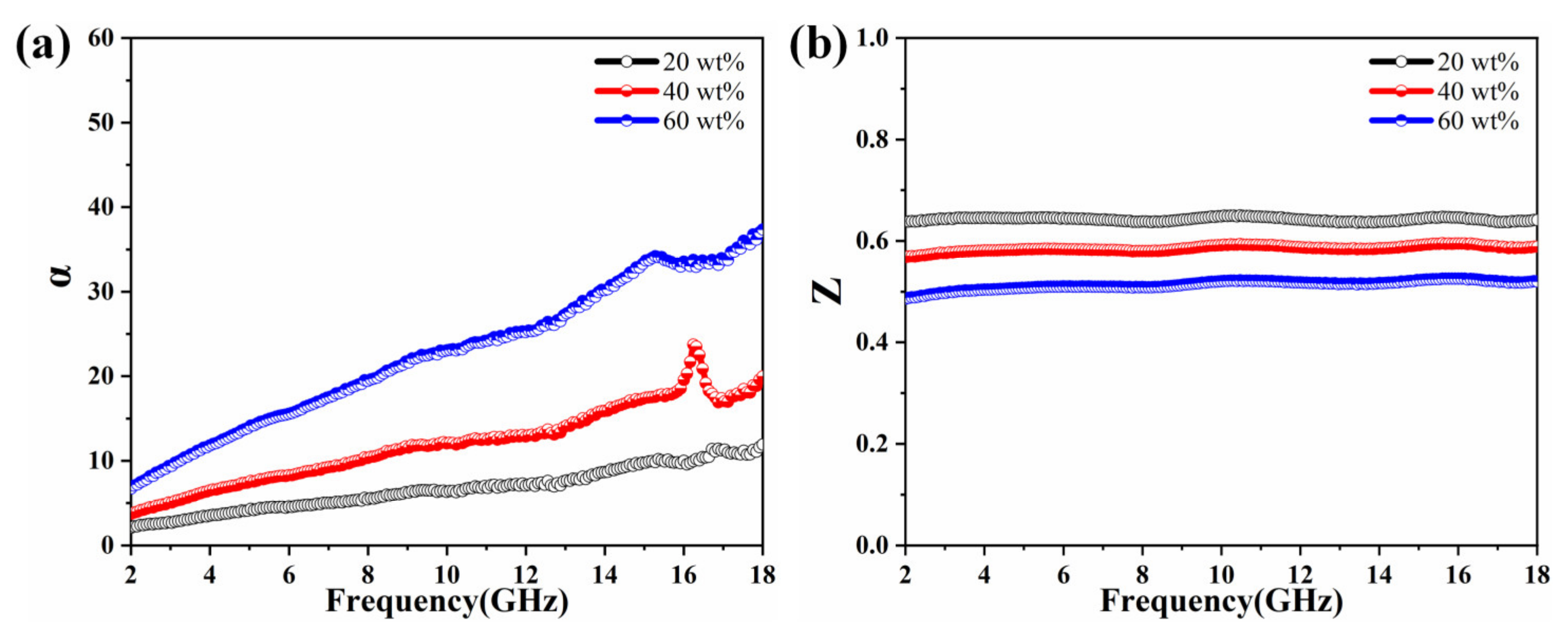
3.2. Electromagnetic Characteristics and Microwave Absorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Camargo, F.P.; do Prado, P.F.; Tonello, P.S.; Dos Santos, A.C.A.; Duarte, I.C.S. Bioleaching of Toxic Metals from Sewage Sludge by Co-Inoculation of Acidithiobacillus and the Biosurfactant-Producing Yeast Meyerozyma Guilliermondii. J. Environ. Manag. 2018, 211, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, K.; Zeng, L. Removal of Phosphorus by a Composite Metal Oxide Adsorbent Derived from Manganese Ore Tailings. J. Hazard. Mater. 2012, 217–218, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic Life and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef]
- Song, J.; Yang, X.; Chen, P.; Liu, R.; Luo, D.; Li, J.; Liu, J. Sintering Technology and Properties of Permeable Bricks Prepared Using Manganese Tailings. J. Ceram. Processing Res. 2021, 22, 283–288. [Google Scholar] [CrossRef]
- He, D.; Shu, J.; Wang, R.; Chen, M.; Wang, R.; Gao, Y.; Liu, R.; Liu, Z.; Xu, Z.; Tan, D.; et al. A Critical Review on Approaches for Electrolytic Manganese Residue Treatment and Disposal Technology: Reduction, Pretreatment, and Reuse. J. Hazard. Mater. 2021, 418, 126235. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.Q.; Qin, F.; Jiang, J.B. Road Performance of Concrete Incorporating Manganese Slag. Adv. Mater. Res. 2011, 402, 457–462. [Google Scholar] [CrossRef]
- Miao, X.; Bai, Z.; Qiu, G.; Tang, S.; Guo, M.; Cheng, F.; Zhang, M. Preparation of Transparent Mn-Doped CaF2 Glass-Ceramics from Silicon-Manganese Slag: Dependence of Colour-Controllable Change on Slag Addition and Crystallization Behavior. J. Eur. Ceram. Soc. 2020, 40, 3249–3261. [Google Scholar] [CrossRef]
- Navarro, R.; Alcocel, E.G.; Sánchez, I.; Garcés, P.; Zornoza, E. Mechanical Properties of Alkali Activated Ground SiMn Slag Mortars with Different Types of Aggregates. Constr. Build. Mater. 2018, 186, 79–89. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D. Preparation of Baking-Free Brick from Manganese Residue and Its Mechanical Properties. J. Nanomater. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Liu, X.; Tang, B.; Mukiza, E.; Zhang, N. Preparation of Non-Sintered Permeable Bricks Using Electrolytic Manganese Residue: Environmental and NH3-N Recovery Benefits. J. Hazard. Mater. 2019, 378, 120768. [Google Scholar] [CrossRef]
- Tang, B.; Gao, S.; Wang, Y.; Liu, X.; Zhang, N. Pore Structure Analysis of Electrolytic Manganese Residue Based Permeable Brick by Using Industrial CT. Constr. Build. Mater. 2019, 208, 697–709. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Jiao, X.; Sun, P.; Li, J.; Wuri, L.; Zhang, T.C. Electrolytic Manganese Residue Based Autoclaved Bricks with Ca(OH)2 and Thermal-Mechanical Activated K-Feldspar Additions. Constr. Build. Mater. 2020, 230, 116848. [Google Scholar] [CrossRef]
- Yang, P.-A.; Huang, Y.; Li, R.; Huang, X.; Ruan, H.; Shou, M.; Li, W.; Zhang, Y.; Li, N.; Dong, L. Optimization of Fe@Ag Core–Shell Nanowires with Improved Impedance Matching and Microwave Absorption Properties. Chem. Eng. J. 2022, 430, 132878. [Google Scholar] [CrossRef]
- Ma, L.; Li, S.; Liu, F.; Ma, S.; Han, E.-H.; Zhang, Z. Metal-Organic Framework-Derived Co/C Composite with High Magnetization as Broadband Electromagnetic Wave Absorber. J. Alloys Compd. 2022, 906, 164257. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevic, J.; Svirskas, S.; Banys, J.; Plausinaitiene, V.; Bychanok, D.; Maksimenko, S.A.; Selskis, A.; Sokal, A.; Lapko, K.N.; et al. Silicon Carbide/Phosphate Ceramics Composite for Electromagnetic Shielding Applications: Whiskers vs Particles. Appl. Phys. Lett. 2019, 114, 183105. [Google Scholar] [CrossRef]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Kotsilkova, R.; Ivanov, E.; Menseidov, D.; Naddeo, C.; Romano, V.; Guadagno, L.; Adami, R.; et al. Nanocarbon/Poly(Lactic) Acid for 3D Printing: Effect of Fillers Content on Electromagnetic and Thermal Properties. Materials 2019, 12, 2369. [Google Scholar] [CrossRef]
- Jiao, Y.; Song, Q.; Yin, X.; Han, L.; Li, W.; Li, H. Grow Defect-Rich Bamboo-like Carbon Nanotubes on Carbon Black for Enhanced Microwave Absorption Properties in X Band. J. Mater. Sci. Technol. 2022, 119, 200–208. [Google Scholar] [CrossRef]
- Qian, J.; Shui, A.; Du, B.; Cai, M.; He, C.; Zeng, S.; Zhong, X.; Lou, J. Synthesis and Tunable Electromagnetic Shielding and Absorption Performance of the Three-Dimensional SiC Nanowires/Carbon Fiber Composites. J. Eur. Ceram. Soc. 2022, 42, 4154–4161. [Google Scholar] [CrossRef]
- Meisak, D.; Gurnevich, E.; Plyushch, A.; Bychanok, D.; Georgiev, V.; Kotsilkova, R.; Kuzhir, P. Robust Design of Compact Microwave Absorbers and Waveguide Matched Loads Based on DC-Conductive 3D-Printable Filament. J. Phys. D Appl. Phys. 2020, 53, 305301. [Google Scholar] [CrossRef]
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, 1906769. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Zheng, Q.; Cao, M.-S. Graphene-Wrapped Multiloculated Nickel Ferrite: A Highly Efficient Electromagnetic Attenuation Material for Microwave Absorbing and Green Shielding. Nano Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Wen, G.; Wu, G.L.; Lei, T.Q.; Zhou, Y.; Guo, Z.X. Co-Enhanced SiO2-BN Ceramics for High-Temperature Dielectric Applications. J. Eur. Ceram. Soc. 2000, 20, 1923–1928. [Google Scholar] [CrossRef]
- Xu, D.; Qiao, J.; Wu, N.; Liu, W.; Wang, F.; Lv, L.; Pan, J.; Dong, Y.; Liu, J. Facile Synthesis of Three-Dimensional Porous Co/MnO Composites Derived from Bimetal Oxides for Highly Efficient Electromagnetic Wave Absorption. ACS Sustain. Chem. Eng. 2019, 33, 8687–8695. [Google Scholar] [CrossRef]
- Mei, H.; Yang, D.; Yang, W.; Yao, L.; Yao, Y.; Cheng, L.; Zhang, L. 3D-Printed Impedance Gradient Al2O3 Ceramic with in-Situ Growing Needle-like SiC Nanowires for Electromagnetic Wave Absorption. Ceram. Int. 2021, 47, 31990–31999. [Google Scholar] [CrossRef]
- Lu, Y.; You, W.; Cai, C.; Yu, X.; Zhao, Y.; Liu, X.; Guo, J.; Zhang, X.; Zeng, W.; Che, R. Insights into the Micro Magnetic Loss Mechanism of Microwave Absorption by Off-Axis Electron Holography. J. Magn. Magn. Mater. 2019, 475, 24–29. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Lv, H.; Ji, G.; Du, Y. A Novel Hierarchically Porous Magnetic Carbon Derived from Biomass for Strong Lightweight Microwave Absorption. Carbon 2019, 142, 245–253. [Google Scholar] [CrossRef]
- Lü, Y.; Wang, Y.; Li, H.; Lin, Y.; Jiang, Z.; Xie, Z.; Kuang, Q.; Zheng, L. MOF-Derived Porous Co/C Nanocomposites with Excellent Electromagnetic Wave Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 13604–13611. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Duan, W.; Ren, S.; Zhang, L.; Cheng, L. Hierarchical Graphene/SiC Nanowire Networks in Polymer-Derived Ceramics with Enhanced Electromagnetic Wave Absorbing Capability. J. Eur. Ceram. Soc. 2016, 36, 2695–2703. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Wei, X.; Yu, R.; Shui, J. Porous CNTs/Co Composite Derived from Zeolitic Imidazolate Framework: A Lightweight, Ultrathin, and Highly Efficient Electromagnetic Wave Absorber. ACS Appl. Mater. Interfaces 2016, 8, 34686–34698. [Google Scholar] [CrossRef]
- Song, C.; Yin, X.; Han, M.; Li, X.; Hou, Z.; Zhang, L.; Cheng, L. Three-Dimensional Reduced Graphene Oxide Foam Modified with ZnO Nanowires for Enhanced Microwave Absorption Properties. Carbon 2017, 116, 50–58. [Google Scholar] [CrossRef]
- Lai, Y.; Lv, L.; Fu, H. Preparation and Study of Al2O3@PPy@rGO Composites with Microwave Absorption Properties. J. Alloys Compd. 2020, 832, 152957. [Google Scholar] [CrossRef]
- He, J.; Gao, S.; Zhang, Y.; Zhang, X.; Li, H. N-Doped Residual Carbon from Coal Gasification Fine Slag Decorated with Fe3O4 Nanoparticles for Electromagnetic Wave Absorption. J. Mater. Sci. Technol. 2022, 104, 98–108. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell Thickness-Dependent Microwave Absorption of Core–Shell Fe3O4@C Composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef]
- Xiong, J.; Xiang, Z.; Zhao, J.; Yu, L.; Cui, E.; Deng, B.; Liu, Z.; Liu, R.; Lu, W. Layered NiCo Alloy Nanoparticles/Nanoporous Carbon Composites Derived from Bimetallic MOFs with Enhanced Electromagnetic Wave Absorption Performance. Carbon 2019, 154, 391–401. [Google Scholar] [CrossRef]
- Yan, L.; Liu, J.; Zhao, S.; Zhang, B.; Gao, Z.; Ge, H.; Chen, Y.; Cao, M.; Qin, Y. Coaxial Multi-Interface Hollow Ni-Al2O3-ZnO Nanowires Tailored by Atomic Layer Deposition for Selective-Frequency Absorptions. Nano Res. 2017, 10, 1595–1607. [Google Scholar] [CrossRef]
- Sun, G.; Dong, B.; Cao, M.; Wei, B.; Hu, C. Hierarchical Dendrite-Like Magnetic Materials of Fe3O4, γ-Fe2O3, and Fe with High Performance of Microwave Absorption. Chem. Mater. 2011, 23, 1587–1593. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhang, T.; Chang, H.; Xiao, P.; Chen, H.; Huang, Z.; Chen, Y. Broadband and Tunable High-Performance Microwave Absorption of an Ultralight and Highly Compressible Graphene Foam. Adv. Mater. 2015, 27, 2049–2053. [Google Scholar] [CrossRef]
- Chen, C.; Bao, S.; Zhang, B.; Chen, Y.; Chen, W.; Wang, C. Coupling Fe@Fe3O4 Nanoparticles with Multiple-Walled Carbon Nanotubes with Width Band Electromagnetic Absorption Performance. Appl. Surf. Sci. 2019, 467–468, 836–843. [Google Scholar] [CrossRef]
- Bai, B.; Zhu, Y.; Miao, J.; Wang, X.; Bi, S.; Kong, L.; Liu, W.; Zhang, L. Electromagnetic Wave Absorption Performance and Mechanisms of Geoploymer-Based Composites Containing Core-Shell SiO2@Fe3O4 Nanoparticles. Ceram. Int. 2022, 48, 2755–2762. [Google Scholar] [CrossRef]
- Wang, Y.; Di, X.; Lu, Z.; Cheng, R.; Wu, X.; Gao, P. Controllable Heterogeneous Interfaces of Cobalt/Carbon Nanosheets/RGO Composite Derived from Metal-Organic Frameworks for High-Efficiency Microwave Attenuation. Carbon 2022, 187, 404–414. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, X.; Wu, G.; Kara, U.I.; Wang, X. Engineering Defects in 2D G-C3N4 for Wideband, Efficient Electromagnetic Absorption at Elevated Temperature. J. Mater. Chem. A 2021, 9, 19710–19718. [Google Scholar] [CrossRef]
- Wu, F.; Li, Q.; Liu, Z.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Fabrication of Binary MOF-Derived Hybrid Nanoflowers via Selective Assembly and Their Microwave Absorbing Properties. Carbon 2021, 182, 484–496. [Google Scholar] [CrossRef]
- Ge, J.; Liu, L.; Cui, Y.; Li, R.; Meng, F.; Wang, F. Optimizing the Electromagnetic Wave Absorption Performances of Designed Fe3O4@SiO2@MnO2 Hybrids. Ceram. Int. 2020, 46, 15325–15332. [Google Scholar] [CrossRef]
- Yang, J.; Shen, Z.; Hao, Z. Microwave Characteristics of Sandwich Composites with Mesophase Pitch Carbon Foams as Core. Carbon 2004, 42, 1882–1885. [Google Scholar] [CrossRef]
- Guo, X.; Lu, J.; Liu, J.; Liu, C.; Tong, Y.; Li, J.; Sun, H.; Peng, H.; Wu, S.; Feng, Y.; et al. Enhanced Electromagnetic Wave Absorption Properties of PDCs-SiCN(Ni) Fibers by in-Situ Formed CNTs and Ni2Si. Ceram. Int. 2022, 48, 20495–20505. [Google Scholar] [CrossRef]
- Wu, Z.; Qian, X.; Pei, K.; You, W.; Li, X.; Jin, C.; Che, R. Drawing Advanced Electromagnetic Functional Composites with Ultra-Low Filler Loading. Chem. Eng. J. 2020, 399, 125720. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, X.; Guo, Y.; Huang, H.; Zhang, H.; Wang, T.; Wen, N.; Chen, H.; Cong, T.; Muhammad, J.; et al. Structural Engineering of Hierarchical Aerogels Comprised of Multi-Dimensional Gradient Carbon Nanoarchitectures for Highly Efficient Microwave Absorption. Nano-Micro Lett. 2021, 13, 144. [Google Scholar] [CrossRef]
- Lu, B.; Dong, X.L.; Huang, H.; Zhang, X.F.; Zhu, X.G.; Lei, J.P.; Sun, J.P. Microwave Absorption Properties of the Core/Shell-Type Iron and Nickel Nanoparticles. J. Magn. Magn. Mater. 2008, 320, 1106–1111. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.D.; Hui, S.; Xiao, T.D.; Ge, S.; Hines, W.A.; Budnick, J.I.; Taylor, G.W. Microwave Magnetic Properties of Co50/(SiO2)50 Nanoparticles. Appl. Phys. Lett. 2002, 80, 4404–4406. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Tian, R.; Li, H.; Zhou, Y.; Duan, H.; Liu, H. Porous Co–C Core–Shell Nanocomposites Derived from Co-MOF-74 with Enhanced Electromagnetic Wave Absorption Performance. ACS Appl. Mater. Interfaces 2018, 10, 11333–11342. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Y.; Di, X.; Cheng, R.; Yang, L.; Gao, P. Design of Hierarchical Core-Shell ZnFe2O4@MnO2@RGO Composite with Heterogeneous Interfaces for Enhanced Microwave Absorption. Ceram. Int. 2022, 48, 5217–5228. [Google Scholar] [CrossRef]
- Fu, L.-S.; Jiang, J.-T.; Xu, C.-Y.; Zhen, L. Synthesis of Hexagonal Fe Microflakes with Excellent Microwave Absorption Performance. CrystEngComm 2012, 14, 6827. [Google Scholar] [CrossRef]
- Wang, H.; Dai, Y.; Gong, W.; Geng, D.; Ma, S.; Li, D.; Liu, W.; Zhang, Z. Broadband Microwave Absorption of CoNi@C Nanocapsules Enhanced by Dual Dielectric Relaxation and Multiple Magnetic Resonances. Appl. Phys. Lett. 2013, 102, 223113. [Google Scholar] [CrossRef]
- Cao, M.-S.; Cai, Y.-Z.; He, P.; Shu, J.-C.; Cao, W.-Q.; Yuan, J. 2D MXenes: Electromagnetic Property for Microwave Absorption and Electromagnetic Interference Shielding. Chem. Eng. J. 2019, 359, 1265–1302. [Google Scholar] [CrossRef]
- Chen, D.; Wang, G.-S.; He, S.; Liu, J.; Guo, L.; Cao, M.-S. Controllable Fabrication of Mono-Dispersed RGO–Hematite Nanocomposites and Their Enhanced Wave Absorption Properties. J. Mater. Chem. A 2013, 1, 5996. [Google Scholar] [CrossRef]
- Huang, B.; Yue, J.; Wei, Y.; Huang, X.; Tang, X.; Du, Z. Enhanced Microwave Absorption Properties of Carbon Nanofibers Functionalized by FeCo Coatings. Appl. Surf. Sci. 2019, 483, 98–105. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Xing, H.; Li, H. Controlled Reduction Synthesis of Yolk-Shell Magnetic@void@C for Electromagnetic Wave Absorption. Chem. Eng. J. 2020, 387, 124149. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and Leaching Behavior of Mine Tailings-Based Geopolymer Bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Du, B.; Zhou, C.; Duan, N. Recycling of Electrolytic Manganese Solid Waste in Autoclaved Bricks Preparation in China. J. Mater. Cycles Waste Manag. 2014, 16, 258–269. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Du, Y.; Bai, J.; Fan, H.; Zhang, H.; Peng, Y.; Li, F. One-Pot Synthesis of CoFe2O4/Graphene Oxide Hybrids and Their Conversion into FeCo/Graphene Hybrids for Lightweight and Highly Efficient Microwave Absorber. J. Mater. Chem. A 2015, 3, 5535–5546. [Google Scholar] [CrossRef]
- Wang, C.; Han, X.; Xu, P.; Zhang, X.; Du, Y.; Hu, S.; Wang, J.; Wang, X. The Electromagnetic Property of Chemically Reduced Graphene Oxide and Its Application as Microwave Absorbing Material. Appl. Phys. Lett. 2011, 98, 072906. [Google Scholar] [CrossRef]
- Wang, C.; Murugadoss, V.; Kong, J.; He, Z.; Mai, X.; Shao, Q.; Chen, Y.; Guo, L.; Liu, C.; Angaiah, S.; et al. Overview of Carbon Nanostructures and Nanocomposites for Electromagnetic Wave Shielding. Carbon 2018, 140, 696–733. [Google Scholar] [CrossRef]
- Kuang, J.; Jiang, P.; Ran, F.; Cao, W. Conductivity-Dependent Dielectric Properties and Microwave Absorption of Al-Doped SiC Whiskers. J. Alloys Compd. 2016, 687, 227–231. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; He, H.; Ye, T.; Deng, Q.; Wei, H.; Zhao, F.; Wang, Q. Nano-SiC-Decorated Y2Si2O7 Ceramic for Enhancing Electromagnetic Waves Absorption Performance. Ceram. Int. 2022, 48, 20168–20175. [Google Scholar] [CrossRef]
- Cao, M.; Wang, X.; Cao, W.; Fang, X.; Wen, B.; Yuan, J. Thermally Driven Transport and Relaxation Switching Self-Powered Electromagnetic Energy Conversion. Small 2018, 14, 1800987. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, L.; Zhang, Y.; Zhang, L. Flexible SiC/Si3N4 Composite Nanofibers with in Situ Embedded Graphite for Highly Efficient Electromagnetic Wave Absorption. ACS Appl. Mater. Interfaces 2017, 9, 28844–28858. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Xiao, G.; Wang, T.-S.; Ouyang, Q.-Y.; Qi, L.-H.; Ma, Y.; Gao, P.; Zhu, C.-L.; Cao, M.-S.; Jin, H.-B. Porous Fe3O4/Carbon Core/Shell Nanorods: Synthesis and Electromagnetic Properties. J. Phys. Chem. C 2011, 115, 13603–13608. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, X.; Zhao, W.; Deng, J.; Fan, B.; Shao, G.; Bai, Z.; Zhang, R. Facile Synthesis of Yolk–Shell Ni@void@SnO2(Ni3Sn2) Ternary Composites via Galvanic Replacement/Kirkendall Effect and Their Enhanced Microwave Absorption Properties. Nano Res. 2017, 10, 331–343. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, R.; Zheng, W.; Yang, P.; Rao, J.; Huang, X.; Wang, D.; Du, Z.; Yao, K.; Zhang, Y. Microstructure, Electromagnetic Properties, and Microwave Absorption Mechanism of SiO2-MnO-Al2O3 Based Manganese Ore Powder for Electromagnetic Protection. Molecules 2022, 27, 3758. https://doi.org/10.3390/molecules27123758
Cai R, Zheng W, Yang P, Rao J, Huang X, Wang D, Du Z, Yao K, Zhang Y. Microstructure, Electromagnetic Properties, and Microwave Absorption Mechanism of SiO2-MnO-Al2O3 Based Manganese Ore Powder for Electromagnetic Protection. Molecules. 2022; 27(12):3758. https://doi.org/10.3390/molecules27123758
Chicago/Turabian StyleCai, Rui, Wei Zheng, Pingan Yang, Jinsong Rao, Xin Huang, Dashuang Wang, Zhilan Du, Kexin Yao, and Yuxin Zhang. 2022. "Microstructure, Electromagnetic Properties, and Microwave Absorption Mechanism of SiO2-MnO-Al2O3 Based Manganese Ore Powder for Electromagnetic Protection" Molecules 27, no. 12: 3758. https://doi.org/10.3390/molecules27123758
APA StyleCai, R., Zheng, W., Yang, P., Rao, J., Huang, X., Wang, D., Du, Z., Yao, K., & Zhang, Y. (2022). Microstructure, Electromagnetic Properties, and Microwave Absorption Mechanism of SiO2-MnO-Al2O3 Based Manganese Ore Powder for Electromagnetic Protection. Molecules, 27(12), 3758. https://doi.org/10.3390/molecules27123758






