Chemical Diversity and Potential Target Network of Woody Peony Flower Essential Oil from Eleven Representative Cultivars (Paeonia × suffruticosa Andr.)
Abstract
:1. Introduction
2. Results
2.1. Hydro-Distillation Yields of the Essential Oils of Woody Peony Flowers
2.2. Comparative Chemical Composition of the Flower Essential Oils
2.3. Multivariate Analysis Results
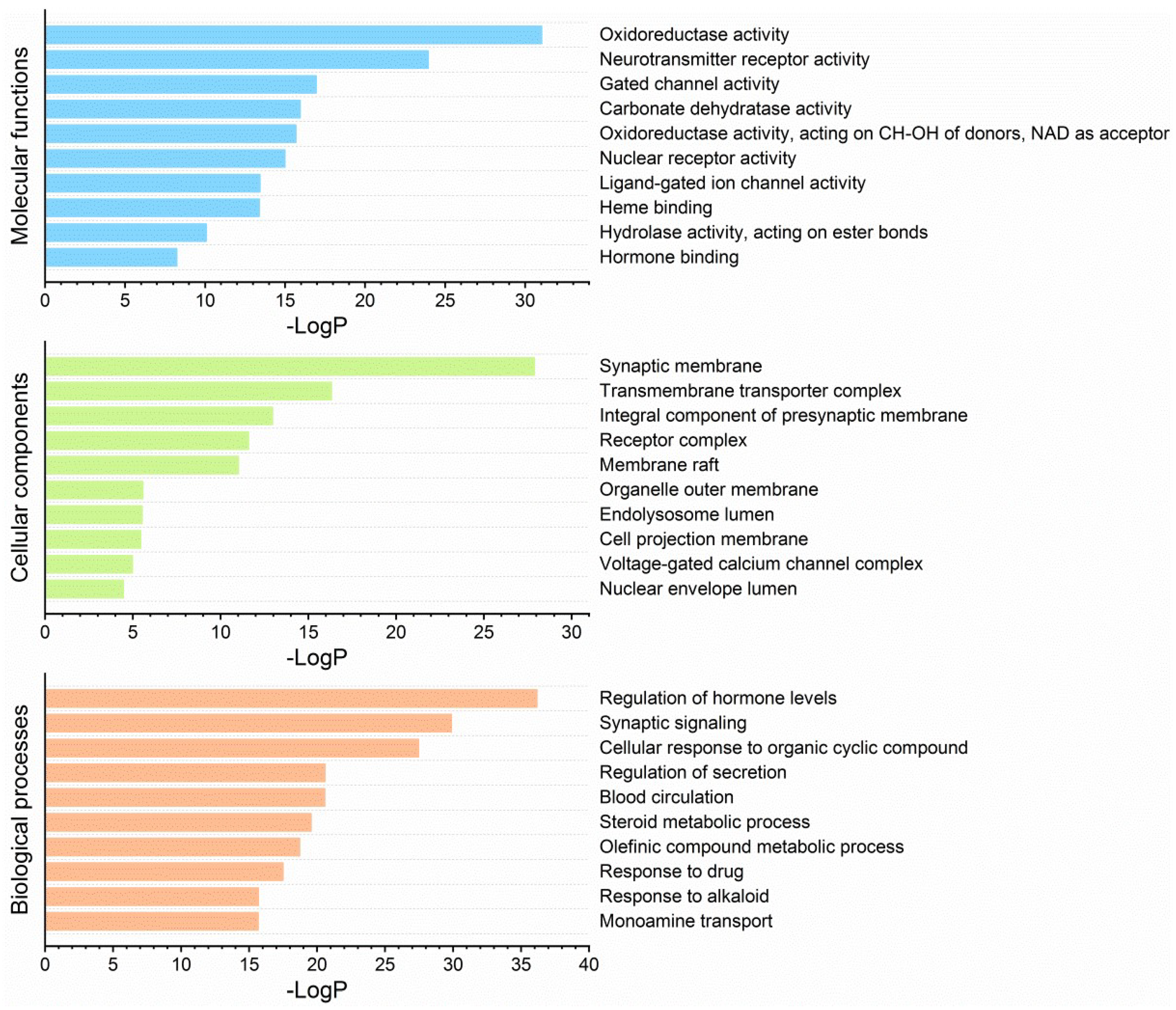
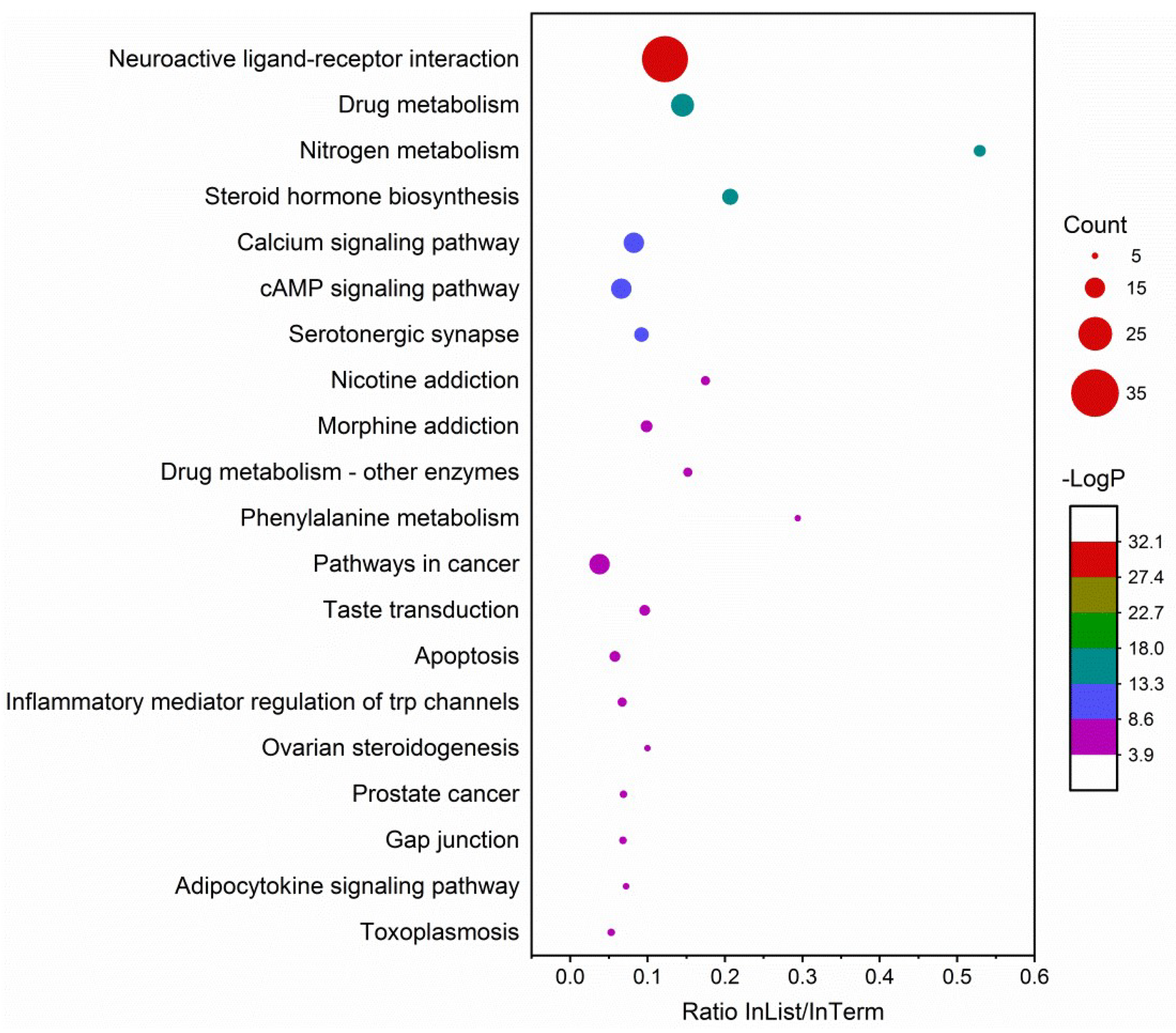
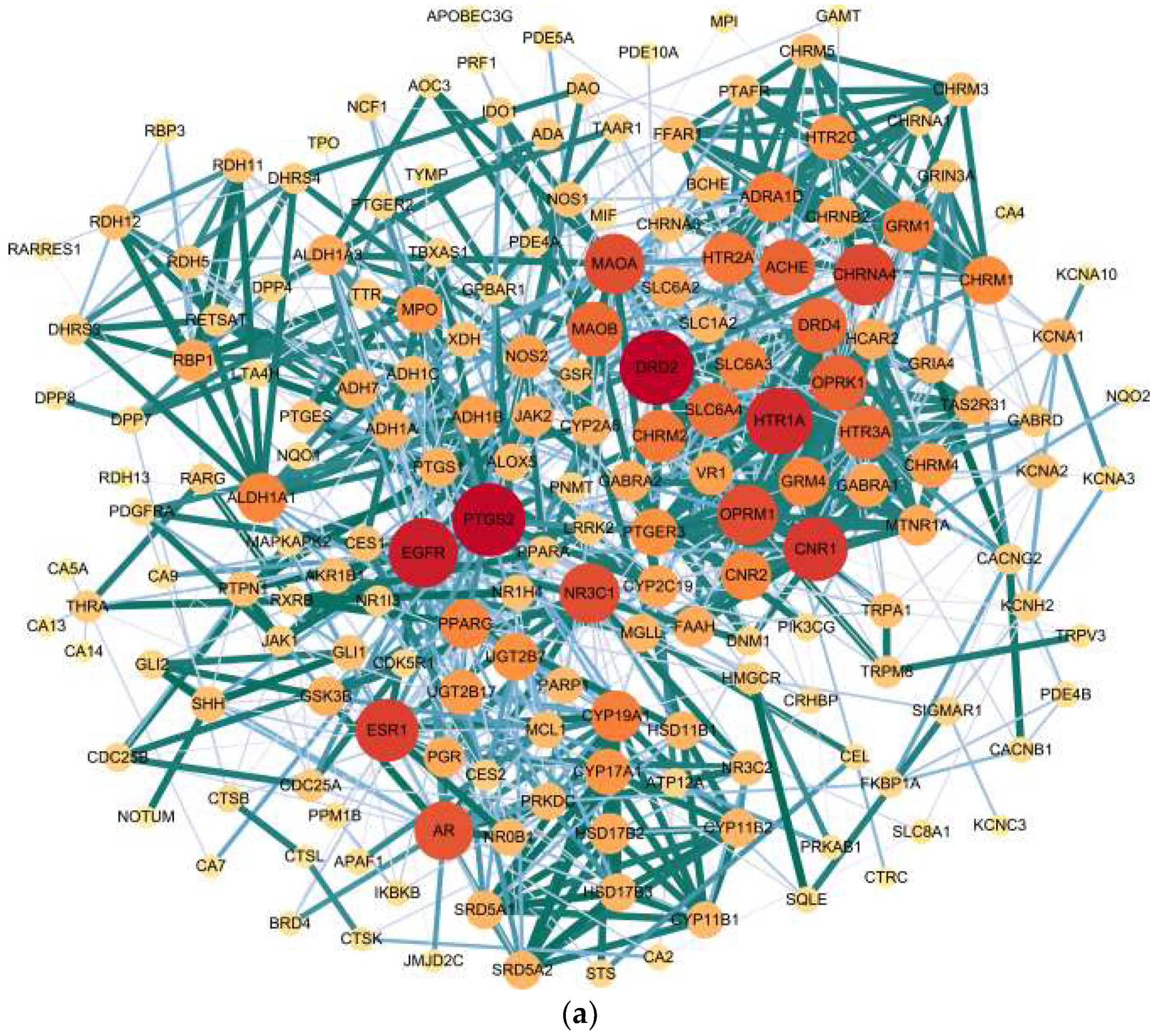
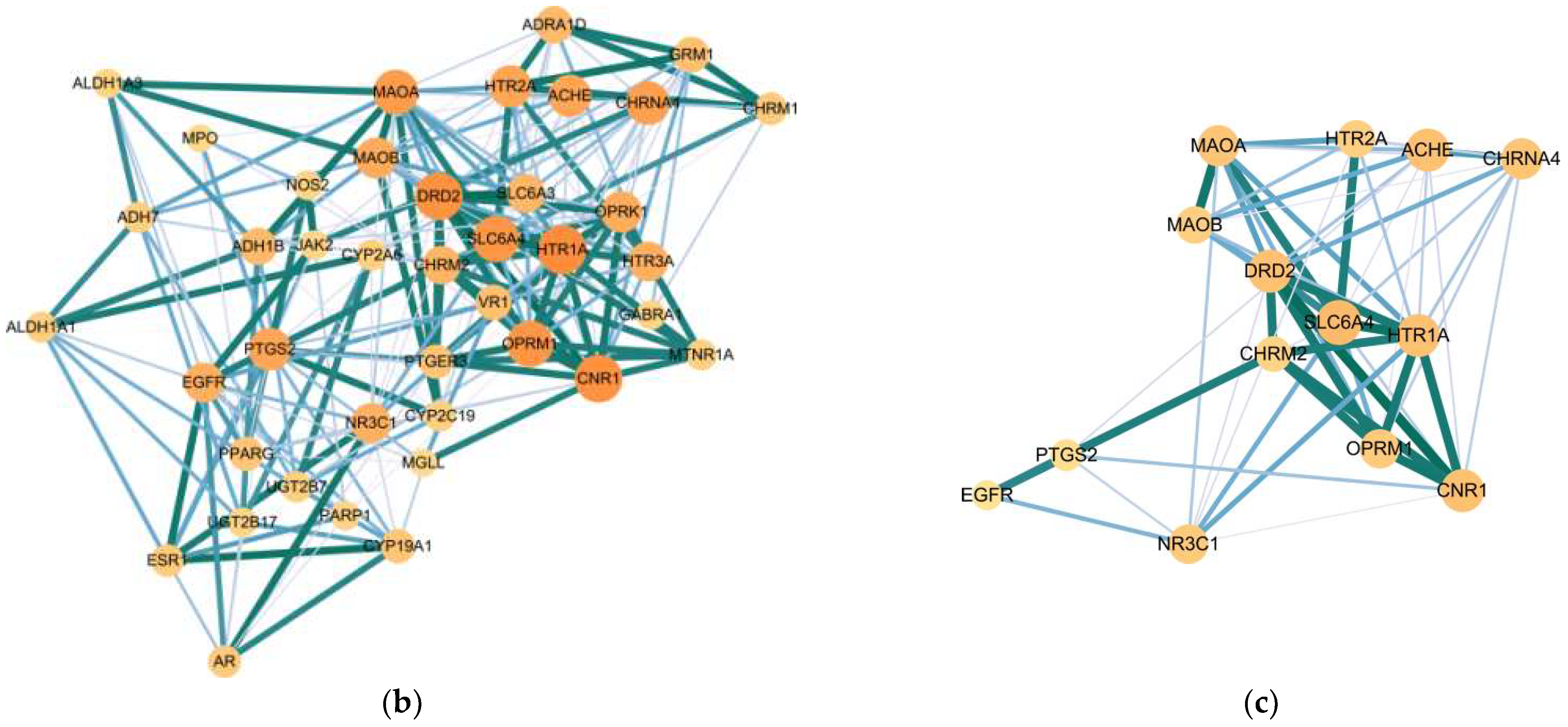
2.4. Target Network Characteristics
3. Discussion
4. Materials and Methods
4.1. Materials and Regents
4.2. Hydro-Distillation
4.3. GC-MS and GC-FID Analysis
4.4. Multivariate Analysis
4.5. Target Network Analysis
4.6. Other Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hong, D.; Pan, K.; Turland, N.J.; Paeonia, L. Flora of China; Wu, Z., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; Volume 6, pp. 127–132. [Google Scholar]
- Li, J.; Zhang, X.; Zhao, X. Tree Peony of China; Encyclopedia of China Publishing House: Beijing, China, 2011; Volume 1, pp. 2–67. [Google Scholar]
- Nanjing University of Chinese Medicine. Great Dictionary of Traditional Chinese Medicine, 2nd ed.; Zhao, G., Dai, S., Chen, R., Eds.; Shanghai Scientific & Technical Publishers: Shanghai, China, 2006; Volume 1, pp. 1576–1579. [Google Scholar]
- Zhang, Q.; Huo, R.; Ma, Y.; Yan, S.; Yang, L.; Chen, F. A novel microwave-assisted steam distillation approach for separation of essential oil from tree peony (Paeonia suffruticosa Andrews) petals: Optimization, kinetic, chemical composition and antioxidant activity. Ind. Crops Prod. 2020, 154, 112669. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Yuan, W.; Hu, J.; Lv, Z. Application of GC × GC coupled with TOF–MS for the trace analysis of chemical components and exploration the characteristic aroma profile of essential oils obtained from two tree peony species (Paeonia rockii and Paeonia ostii). Eur. Food Res. Technol. 2021, 247, 2591–2608. [Google Scholar] [CrossRef]
- Han, C.V.; Bhat, R. In vitro control of food-borne pathogenic bacteria by essential oils and solvent extracts of underutilized flower buds of Paeonia suffruticosa (Andr.). Ind. Crops Prod. 2014, 54, 203–208. [Google Scholar] [CrossRef]
- Zhang, X.X.; Sun, J.Y.; Niu, L.X.; Zhang, Y.L. Chemical compositions and antioxidant activities of essential oils extracted from the petals of three wild tree peony species and eleven cultivars. Chem. Biodivers. 2017, 14, e1700282. [Google Scholar] [CrossRef]
- Lei, G.; Li, J.; Zheng, T.; Yao, J.; Chen, J.; Duan, L. Comparative chemical profiles of essential oils and hydrolate extracts from fresh flowers of eight Paeonia suffruticosa Andr. cultivars from Central China. Molecules 2018, 23, 3268. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.; Song, C.; Luo, Y. Chemical composition of hydrosol volatiles of flowers from ten Paeonia × suffruticosa Andr. cultivars from Luoyang, China. Nat. Prod. Res. 2021, 35, 3509–3513. [Google Scholar] [CrossRef]
- Nezhadasad Aghbash, B.; Dehghan, G.; Movafeghi, A.; Talebpour, A.H.; Pouresmaeil, M.; Maggi, F.; Sabzi Nojadeh, M. Chemical compositions and biological activity of essential oils from four populations of Satureja macrantha C.A. Mey. J. Essent. Oil Res. 2021, 33, 133–142. [Google Scholar] [CrossRef]
- Najar, B.; Pieracci, Y.; Cervelli, C.; Flamini, G.; Pistelli, L. Volatolomics of three south African Helichrysum species grown in pot under protected environment. Molecules 2021, 26, 7283. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Sachett, F.H.; Agostini, F.; Boettcher, G.N.; Sulzbach, M.; Gonzatto, M.P.; Schwarz, S.F.; Pauletti, G.F. Chemical composition of petitgrain (leaf) essential oil of different Citrus rootstocks and scion cultivars. J. Essent. Oil Res. 2020, 32, 394–406. [Google Scholar] [CrossRef]
- Sulzbach, M.; da Silva, M.A.S.; Gonzatto, M.P.; Marques, M.M.O.; Böettcher, G.N.; Silvestre, W.P.; Silva, J.C.R.L.; Pauletti, G.F.; Schwarz, S.F. Effect of distillation methods on the leaf essential oil of some Citrus cultivars. J. Essent. Oil Res. 2021, 33, 452–463. [Google Scholar] [CrossRef]
- Kessler, J.C.; Vieira, V.A.; Martins, I.M.; Manrique, Y.A.; Afonso, A.; Ferreira, P.; Mandim, F.; Ferreira, I.C.F.R.; Barros, L.; Rodrigues, A.E.; et al. Obtaining aromatic extracts from portuguese Thymus mastichina L. by hydrodistillation and supercritical fluid extraction with CO2 as potential flavouring additives for food applications. Molecules 2022, 27, 694. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Lv, R.; Qian, H.; Chen, X.; Yang, C.F. Study on the multitarget mechanism and key active ingredients of Herba Siegesbeckiae and volatile oil against rheumatoid arthritis based on network pharmacology. Evid. Based Complement. Altern. Med. 2019, 2019, 8957245. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, N.; Hu, J.; Wang, H.; Duan, D.; Ma, L.; Xiao, J.; Wang, X. Analysis of the main active ingredients and bioactivities of essential oil from Osmanthus fragrans Var. thunbergii using a complex network approach. BMC Syst. Biol. 2017, 11, 144. [Google Scholar] [CrossRef]
- Khan, R.A.; Hossain, R.; Siyadatpanah, A.; Al-Khafaji, K.; Khalipha, A.B.R.; Dey, D.; Asha, U.H.; Biswas, P.; Saikat, A.S.M.; Chenari, H.A.; et al. Diterpenes/diterpenoids and their derivatives as potential bioactive leads against dengue virus: A computational and network pharmacology study. Molecules 2021, 26, 6821. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology. NIST Chemistry WebBook, NIST Standard Reference Database Number 69. 2021. Available online: https://webbook.nist.gov/chemistry/ (accessed on 20 March 2021).
- Royal Society of Chemistry. ChemSpider Online Database. 2021. Available online: https://www.chemspider.com/ (accessed on 21 March 2021).
- Joshi, R.K. Volatile constituents of leaf, stem and flower of the traditional shrub Pogostemon plectranthoides Desf. from the Western Ghats, India. Nat. Prod. Res. 2022, 36, 411–413. [Google Scholar] [CrossRef]
- Setzer, W.N. Germacrene D cyclization: An ab initio investigation. Int. J. Mol. Sci. 2008, 9, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Han, X.; Yuan, W.; Wang, X.; Meng, D.; Hu, J.; Lv, Z. Salt intervention for the diversities of essential oil composition, aroma and antioxidant activities of Kushui rose (R. setate × R. rugosa). Ind. Crops Prod. 2020, 150, 112417. [Google Scholar] [CrossRef]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. C. R. Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Wang, Y.; Gao, S.; Du, D.; Fu, J.; Dong, L. Anthocyanin biosynthesis and accumulation in developing flowers of tree peony (Paeonia suffruticosa) ‘Luoyang Hong’. Postharvest Biol. Technol. 2014, 97, 11–22. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R.; Adinarayana, G.; Kumar, A.N.; Rajput, D.K.; Syamasundar, K.V. Chemical-profile variations in essential oils isolated from lemongrass (Cymbopogon flexuosus) biomass and condensate wastewater by re-distillation and solvent extraction techniques. J. Essent. Oil Res. 2016, 28, 557–564. [Google Scholar] [CrossRef]
- Marques, T.H.C.; Marques, M.L.B.G.C.B.; dos Santos Lima, D.; Siqueira, H.D.S.; Neto, J.D.N.; Branco, M.S.B.G.C.; de Souza, A.A.; de Sousa, D.P.; de Freitas, R.M. Evaluation of the neuropharmacological properties of nerol in mice. World J. Neurosci. 2013, 3, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Farokhcheh, M.; Hejazian, L.; Akbarnejad, Z.; Pourabdolhossein, F.; Hosseini, S.M.; Mehraei, T.M.; Soltanpour, N. Geraniol improved memory impairment and neurotoxicity induced by zinc oxide nanoparticles in male wistar rats through its antioxidant effect. Life Sci. 2021, 282, 119823. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.L.; Matos, J.P.S.C.F.; Picot, L.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. Citronellol, a monoterpene alcohol with promising pharmacological activities—A systematic review. Food Chem. Toxicol. 2019, 123, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Sanekata, A.; Tanigawa, A.; Takoi, K.; Nakayama, Y.; Tsuchiya, Y. Identification and characterization of geranic acid as a unique flavor compound of hops (Humulus lupulus L.) variety Sorachi Ace. J. Agric. Food Chem. 2018, 66, 12285–12295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lü, J.; He, Z.; Zhang, F.; Zhang, S.; Zhang, H. Investigations into the production of volatile compounds in Korla fragrant pears (Pyrus sinkiangensis Yu). Food Chem. 2020, 302, 125337. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, Y.; Miyata, S.; Nakashima, T. Mixture of cis-3-hexenol and trans-2-hexenal attenuates behavioral and stress responses induced by 2,5-dihydro-2,4,5-trimethylthiazoline and electric footshock stress in rats. Physiol. Behav. 2011, 103, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Criswell, H.E.; Ming, Z.; Pleasant, N.; Griffith, B.L.; Mueller, R.A.; Breese, G.R. Macrokinetic analysis of blockade of NMDA-gated currents by substituted alcohols, alkanes and ethers. Brain Res. 2004, 1015, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Reguilon, M.D.; Mińarro, J.; De Feo, V.; Rodriguez-Arias, M. Lavandula angustifolia essential oil and linalool counteract social aversion induced by social defeat. Molecules 2018, 23, 2694. [Google Scholar] [CrossRef] [Green Version]
- Souto-Maior, F.N.; de Carvalho, F.L.; de Morais, L.C.S.L.; Netto, S.M.; de Sousa, D.P.; de Almeida, R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Rios, E.R.V.; Rocha, N.F.M.; Carvalho, A.M.R.; Vasconcelos, L.F.; Dias, M.L.; de Sousa, D.P.; de Sousa, F.C.F.; de França Fonteles, M.M. TRP and ASIC channels mediate the antinociceptive effect of citronellyl acetate. Chem. Biol. Interact. 2013, 203, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Maia, W.M.N.; Andrade, F.D.C.P.D.; Filgueiras, L.A.; Mendes, A.N.; Assunção, A.F.C.; Rodrigues, N.D.S.; Marques, R.B.; Filho, A.L.M.M.; de Sousa, D.P.; Lopes, L.D.S. Antidepressant activity of rose oxide essential oil: Possible involvement of serotonergic transmission. Heliyon 2021, 7, e06620. [Google Scholar] [CrossRef] [PubMed]
- do Vale, T.G.; Furtado, E.C.; Santos, J.G.; Viana, G.S.B. Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill.) N.E. Brown. Phytomedicine 2002, 9, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Hacke, A.C.M.; Miyoshi, E.; Marques, J.A.; Pereira, R.P. Cymbopogon citratus (DC.) Stapf, citral and geraniol exhibit anticonvulsant and neuroprotective effects in pentylenetetrazole-induced seizures in zebrafish. J. Ethnopharmacol. 2021, 275, 114142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, C.; Cheng, B.; Wan, H.; Luo, L.; Pan, H.; Zhang, Q. Volatile compound analysis and aroma evaluation of tea-scented roses in China. Ind. Crops Prod. 2020, 155, 112735. [Google Scholar] [CrossRef]
- Oshima, T.; Ito, M. Sedative effects of l-menthol, d-camphor, phenylethyl alcohol, and geraniol. J. Nat. Med. 2021, 75, 319–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J.; Zeng, F. Volatile composition analysis of tree peony (Paeonia section Moutan DC.) seed oil and the effect of oxidation during storage. J. Food Sci. 2021, 86, 3467–3479. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2007, 36, D901–D906. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2017, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohtani, K.; Imajo, A.; Izu, H.; Nakamura, H.; Shiraishi, K. Comparison of the neurotoxicities between volatile organic compounds and fragrant organic compounds on human neuroblastoma SK-N-SH cells and primary cultured rat neurons. Toxicol. Rep. 2015, 2, 729–736. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2020, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Cai, C.; Liu, X.; Ku, X.; Jiang, H.; Gao, D.; Li, H. ChemMapper: A versatile web server for exploring pharmacology and chemical structure association based on molecular 3D similarity method. Bioinformatics 2013, 29, 1827–1829. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, H.; Li, H. SHAFTS: A Hybrid Approach for 3D Molecular Similarity Calculation. 1. Method and Assessment of Virtual Screening. J. Chem. Inf. Model. 2011, 51, 2372–2385. [Google Scholar] [CrossRef]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2015, 44, D1045–D1053. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, F.; Wang, Y.; Li, C.; Zhang, X.; Li, H.; Diao, L.; Gu, J.; Wang, W.; Li, D.; et al. BATMAN-TCM: A Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Consortium, T.G.O. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2020, 49, D325–D334. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2016, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2019, 48, D845–D855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2020, 49, D605–D612. [Google Scholar] [CrossRef]

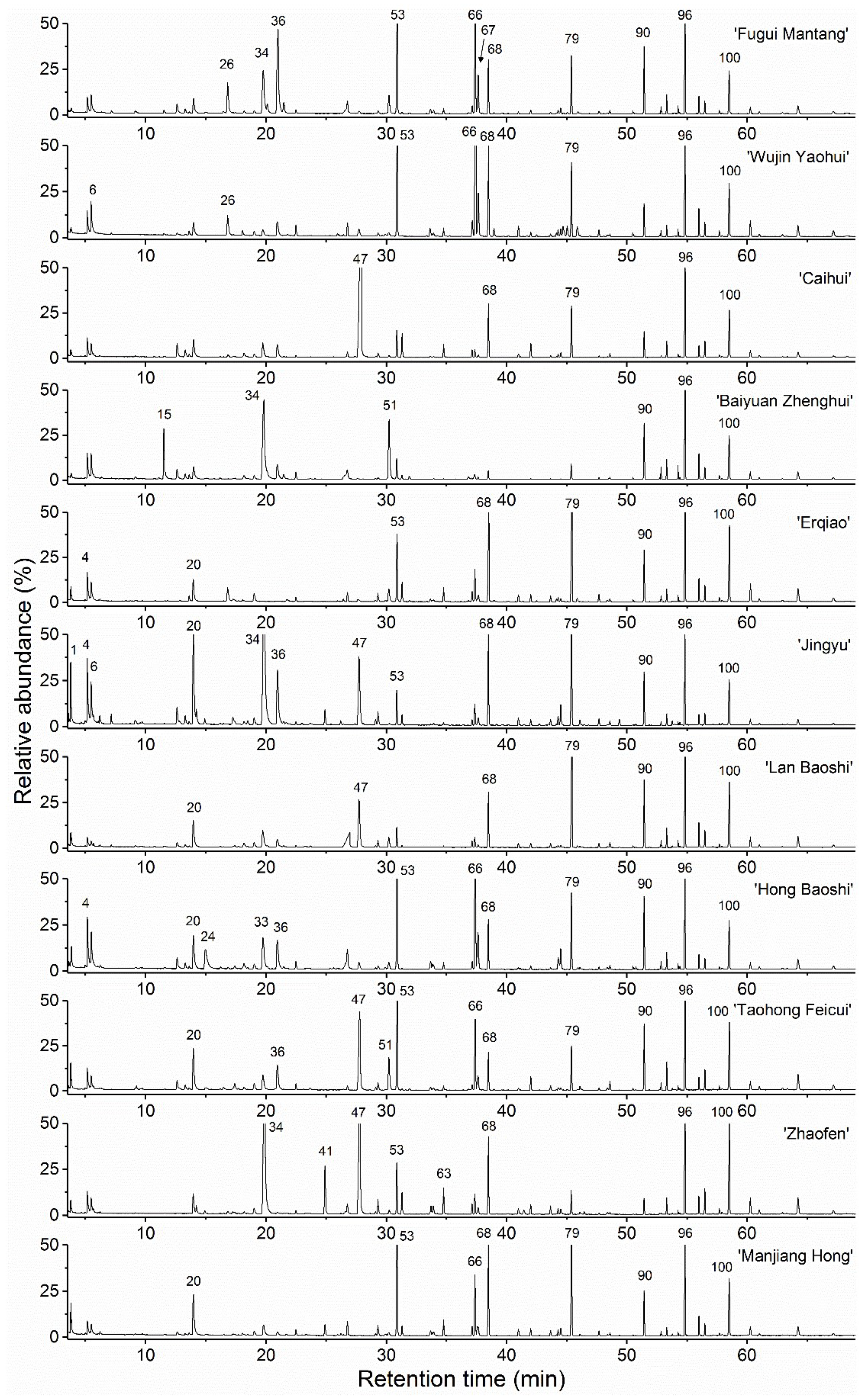





| Cultivar Name | Cultivar Name Translated | Code | Specimen No. | EO Yield (w/w%) a |
|---|---|---|---|---|
| ‘Fugui Mantang’ | Full of wealth and rank | FGMT | PS190412-01 | 0.14 ± 0.05 B |
| ‘Wujin Yaohui’ | Dark gold that glitters | WJYH | PS190412-02 | 0.13 ± 0.02 B |
| ‘Caihui’ | Colorful painting | CH | PS190412-03 | 0.17 ± 0.03 A,B |
| ‘Baiyuan Zhenghui’ | Competing for shining in the great flower garden | BYZH | PS190412-04 | 0.17 ± 0.04 A,B |
| ‘Erqiao’ | Two distinct beauties | EQ | PS190412-05 | 0.18 ± 0.05 A,B |
| ‘Jingyu’ | In honor of Horticulturist Jingyu Sun | JY | PS190412-06 | 0.11 ± 0.01 B |
| ‘Lan Baoshi’ | Blue gems | LBS | PS190412-07 | 0.19 ± 0.06 A,B |
| ‘Hong Baoshi’ | Red gems | HBS | PS190412-08 | 0.16 ± 0.02 A,B |
| ‘Taohong Feicui’ | Peach red with flying emerald green | THFC | PS190412-09 | 0.25 ± 0.04 A |
| ‘Zhaofen’ | Pink flowers from Zhao (horticulturist in Qing Dynasty) | ZF | PS190412-10 | 0.13 ± 0.02 B |
| ‘Manjiang Hong’ | Red floating plants all over the river | MJH | PS190412-11 | 0.16 ± 0.03 A,B |
| No. | RIdet a | RIref b | Compound c | FGMT d | WJYH | CH | BYZH | EQ | JY | LBS | HBS | THFC | ZF | MJH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 800 | Octane | 0.1 | 0.1 | 0.7 | 0.1 | 0.6 | 2.3 | 0.6 | 0.4 | 0.9 | 0.3 | 1.3 |
| 2 | 802 | 801 | Hexanal | 0.2 | 0.3 | - | 0.3 | 0.4 | - | 1.0 | 1.0 | 0.9 | 0.3 | 1.2 |
| 3 | 852 | 854 | trans-2-Hexenal | - | - | - | - | - | 0.2 | - | 0.2 | - | - | - |
| 4 | 860 | 858 | cis-3-Hexen-1-ol | 1.4 | 2.1 | 2.3 | 2.9 | 3.2 | 4.2 | 1.2 | 4.0 | 1.9 | 1.7 | 1.5 |
| 5 | 870 | 868 | 1,4-Dimethylbenzene | 0.1 | - | - | 0.2 | 0.2 | - | - | 0.3 | tr | 0.1 | 0.1 |
| 6 | 873 | 871 | 1-Hexanol | 1.7 | 3.7 | 2.1 | 2.9 | 2.5 | 2.9 | 0.7 | 3.6 | 1.0 | 1.2 | 0.9 |
| 7 | 882 | 882 | 2,6-Dimethyl-1,5-heptadiene | 0.3 | - | - | 0.4 | - | 0.8 | 0.3 | - | - | 0.3 | - |
| 8 | 899 | 900 | Nonane | - | - | - | - | - | 0.2 | - | - | - | - | - |
| 9 | 903 | 902 | Heptanal | - | - | 0.2 | - | 0.1 | 0.5 | 0.2 | 0.2 | - | - | 0.3 |
| 10 | 931 | 932 | α-Pinene | 0.2 | - | - | - | - | 0.5 | 0.2 | - | - | - | - |
| 11 | 989 | 989 | Prenylacetone | 0.3 | - | - | 0.3 | - | 0.4 | - | - | - | - | - |
| 12 | 992 | 993 | 2-Pentylfuran | - | - | - | - | - | - | - | - | 0.3 | - | - |
| 13 | 1005 | 1004 | Octanal | - | - | - | - | - | 0.2 | - | - | - | - | - |
| 14 | 1038 | 1037 | cis-β-Ocimene | - | - | - | tr | - | - | - | - | - | - | - |
| 15 | 1049 | 1048 | trans-β-Ocimene | 0.3 | - | - | 6.7 | - | - | - | - | - | - | - |
| 16 | 1050 | 1046 | Benzeneacetaldehyde | - | - | - | - | - | - | - | tr | - | - | - |
| 17 | 1075 | 1074 | cis-Linalool oxide furan | 1.2 | 0.2 | 2.0 | 1.5 | - | 1.6 | 0.7 | 1.1 | 1.2 | - | 0.4 |
| 18 | 1091 | 1088 | trans-Linalool oxide furan | 0.5 | - | 1.0 | 0.7 | - | 0.7 | 0.3 | 0.6 | 0.5 | - | - |
| 19 | 1099 | 1100 | Undecane | - | 0.3 | 0.4 | 0.5 | 0.7 | 0.2 | - | 0.2 | - | - | 0.2 |
| 20 | 1107 | 1104 | Nonanal | - | - | - | - | 3.2 | 7.2 | 4.8 | 3.8 | 5.4 | 1.8 | 6.4 |
| 21 | 1108 | 1106 | Linalool | 1.9 | 1.6 | 3.1 | 1.8 | - | - | - | - | - | - | - |
| 22 | 1113 | 1111 | cis-Rose oxide | - | - | - | - | - | 1.3 | - | - | - | 0.7 | - |
| 23 | 1128 | 1127 | trans-Rose oxide | - | - | - | - | - | 0.4 | - | - | - | 0.3 | - |
| 24 | 1130 | 1127 | Benzeneethanol | - | - | - | - | - | - | - | 4.0 | 0.4 | - | - |
| 25 | 1164 | 1160 | cis-3-Nonen-1-ol | - | - | - | - | - | - | - | - | 0.4 | - | - |
| 26 | 1171 | 1170 | Hydroquinone dimethyl ether | 4.1 | 2.7 | 0.4 | - | 2.5 | - | - | - | - | 0.2 | - |
| 27 | 1181 | 1176 | 1-Nonanol | - | - | - | - | 0.5 | 0.9 | - | - | - | - | - |
| 28 | 1184 | 1180 | Myrtanal | - | - | - | - | - | - | - | 0.3 | 0.8 | - | - |
| 29 | 1199 | 1200 | Dodecane | - | 0.5 | - | - | 0.2 | - | - | - | - | - | - |
| 30 | 1202 | 1201 | α-Terpineol | 0.3 | - | 0.8 | 0.7 | - | 0.3 | 0.7 | 0.6 | 0.3 | - | - |
| 31 | 1208 | 1208 | Decanal | - | - | - | - | - | 0.3 | - | - | - | - | - |
| 32 | 1221 | 1220 | 4,7-Dimethylbenzofuran | 0.4 | 0.6 | 0.5 | 0.6 | 1.5 | 0.7 | 0.9 | 0.6 | 1.0 | 0.6 | 0.7 |
| 33 | 1237 | 1230 | Nerol | - | 0.9 | 2.5 | - | - | - | 3.6 | 4.2 | 2.5 | - | - |
| 34 | 1239 | 1231 | Citronellol | 7.9 | - | - | 21.6 | - | 23.1 | - | - | - | 25.8 | 2.2 |
| 35 | 1246 | 1240 | Neral | 1.2 | - | - | - | - | - | - | - | - | - | - |
| 36 | 1264 | 1256 | Geraniol | 13.0 | 2.2 | 2.4 | 3.0 | - | 5.6 | 1.7 | 3.9 | 4.2 | - | 0.7 |
| 37 | 1276 | 1270 | Geranial | 1.4 | - | - | 0.8 | - | 0.2 | - | - | - | - | - |
| 38 | 1283 | 1275 | 1-Decanol | - | - | - | - | 0.4 | - | - | - | - | - | - |
| 39 | 1299 | 1300 | Tridecane | 0.3 | 0.9 | 0.2 | 0.8 | 0.5 | 0.2 | 0.2 | 0.6 | 0.6 | 0.3 | 0.6 |
| 40 | 1327 | 1326 | Geranic acid methyl ester | - | - | - | - | - | 0.2 | - | - | - | - | - |
| 41 | 1355 | 1354 | Citronellyl acetate | - | - | - | - | - | 1.0 | - | - | - | 3.9 | 1.4 |
| 42 | 1386 | 1383 | Geranyl acetate | - | - | - | - | - | 0.3 | - | - | - | - | - |
| 43 | 1391 | 1385 | trans-3-Tetradecene | - | - | - | - | 0.2 | - | - | - | - | - | - |
| 44 | 1396 | 1393 | Geranic acid | 0.8 | - | - | 1.4 | - | - | 3.5 | 1.0 | - | 0.3 | - |
| 45 | 1399 | 1400 | Tetradecane | 1.3 | 1.1 | 0.7 | 1.6 | 0.9 | - | 2.2 | 2.0 | 0.4 | 0.8 | 1.4 |
| 46 | 1414 | 1407 | Eugenol methyl ether | - | - | 0.2 | - | - | - | - | - | - | - | - |
| 47 | 1423 | 1418 | Phloroglucinol trimethyl ether | 0.4 | 1.2 | 36.8 | - | 0.3 | 7.3 | 9.9 | 0.8 | 12.9 | 15.6 | - |
| 48 | 1457 | 1455 | Geranyl acetone | - | - | 0.1 | - | 0.2 | 0.4 | 0.2 | - | 0.3 | 0.2 | 0.3 |
| 49 | 1461 | 1462 | 2,6,10-Trimethyltridecane | 0.3 | 0.4 | 0.5 | 0.2 | 1.0 | 1.0 | 0.9 | 0.3 | 0.8 | 1.1 | 1.2 |
| 50 | 1476 | 1475 | 1,14-Pentadecadiene * | - | 0.2 | - | - | - | - | - | - | - | - | - |
| 51 | 1483 | 1481 | Germacrene D | 2.2 | 0.6 | 0.3 | 10.5 | 2.1 | - | 1.6 | 0.9 | 4.1 | - | 0.4 |
| 52 | 1484 | 1485 | Citronellyl isobutyrate | - | - | - | - | - | - | - | - | - | 0.4 | - |
| 53 | 1500 | 1500 | Pentadecane | 10.1 | 11.1 | 2.7 | 2.5 | 7.1 | 1.9 | 2.3 | 13.2 | 9.0 | 3.6 | 12.8 |
| 54 | 1510 | 1508 | α-Farnesene | - | - | 2.4 | 0.6 | 2.1 | 0.6 | 0.2 | - | - | 1.6 | 1.0 |
| 55 | 1526 | 1524 | δ-Cadinene | - | - | - | 0.4 | - | - | - | - | - | - | - |
| 56 | 1570 | 1570 | 3-Methylpentadecane | - | 0.8 | - | - | - | - | - | - | - | - | - |
| 57 | 1571 | 1570 | cis-9-Tridecen-1-ol * | 0.4 | - | - | - | 0.3 | - | - | 0.7 | 0.3 | - | 0.6 |
| 58 | 1572 | 1572 | Citronellyl 2-methylbutanoate | - | - | - | - | - | - | - | - | - | 0.7 | - |
| 59 | 1575 | 1577 | 1,15-Hexadecadiene * | - | 0.2 | - | - | 0.2 | - | - | 0.4 | - | - | 0.3 |
| 60 | 1577 | 1577 | Citronellyl 3-methylbutanoate | - | - | - | - | - | - | - | - | - | 0.8 | - |
| 61 | 1578 | 1579 | cis-3-Hexadecene | 0.2 | 0.3 | - | - | 0.2 | - | - | 0.3 | - | - | - |
| 62 | 1578 | 1580 | cis-3-Hexenyl benzoate | - | - | - | - | - | 0.1 | - | - | - | - | 0.5 |
| 63 | 1599 | 1600 | Hexadecane | 0.4 | 0.6 | 1.2 | - | 1.3 | 0.1 | 0.2 | 0.5 | 0.3 | 1.8 | 1.5 |
| 64 | 1654 | 1651 | τ-Muurolol | - | - | - | 0.6 | - | - | - | - | - | - | - |
| 65 | 1663 | 1665 | 2-Methylhexadecane | 0.6 | 1.6 | 0.7 | - | 1.0 | 0.3 | 0.7 | 0.6 | 0.4 | 0.7 | 0.9 |
| 66 | 1669 | 1667 | 6,9-Heptadecadiene | 9.0 | 15.5 | 0.8 | - | 3.2 | 1.2 | 1.1 | 12.4 | 6.4 | 1.4 | 6.1 |
| 67 | 1676 | 1676 | trans-8-Heptadecene | 3.8 | 4.9 | 0.4 | 0.3 | 1.1 | 0.6 | 0.4 | 4.0 | 2.0 | 0.7 | 1.9 |
| 68 | 1700 | 1700 | Heptadecane | 4.2 | 7.3 | 5.2 | 0.9 | 10.1 | 5.1 | 6.2 | 3.3 | 3.2 | 5.2 | 9.3 |
| 69 | 1713 | 1714 | 2-Pentadecanol | - | 0.8 | - | - | 0.2 | - | - | - | - | - | - |
| 70 | 1770 | 1770 | 3-Methylheptadecane | 0.1 | 0.8 | 0.2 | - | 0.7 | 0.4 | 0.4 | - | - | 0.3 | 0.6 |
| 71 | 1799 | 1800 | Octadecane | 0.3 | 0.3 | 1.4 | - | 0.7 | 0.3 | 0.5 | 0.3 | 1.1 | 0.7 | 0.7 |
| 72 | 1814 | 1812 | 2-Hexadecanol | - | 0.2 | - | - | - | - | - | - | - | - | - |
| 73 | 1820 | 1819 | Hexadecanal | - | - | - | - | - | 0.1 | - | - | - | - | - |
| 74 | 1848 | 1845 | Hexahydrofarnesyl acetone | - | 0.3 | 0.2 | - | 0.6 | 0.4 | 0.5 | - | 0.5 | 0.6 | 0.7 |
| 75 | 1863 | 1864 | 2-Methyloctadecane | - | 0.3 | - | - | 0.3 | 0.1 | - | - | - | - | - |
| 76 | 1873 | 1874 | 9-Nonadecene | 0.4 | 0.7 | 0.5 | - | 0.3 | 1.2 | 0.2 | 1.7 | 0.3 | 0.4 | 0.8 |
| 77 | 1879 | 1879 | 1,18-Nonadecadiene * | - | 1.4 | - | - | - | - | - | - | - | - | - |
| 78 | 1889 | 1892 | 1-Nonadecene | - | 1.2 | - | - | - | - | - | - | - | - | - |
| 79 | 1900 | 1900 | Nonadecane | 4.5 | 5.4 | 5.2 | 1.6 | 12.4 | 7.4 | 13.3 | 5.2 | 3.8 | 1.5 | 12.6 |
| 80 | 1915 | 1909 | 2-Heptadecanol | - | 1.2 | - | - | 0.5 | - | 0.2 | - | - | - | - |
| 81 | 1917 | 1920 | Heptadecanal | 0.2 | - | - | - | - | - | - | 0.2 | - | - | - |
| 82 | 1922 | 1919 | Farnesyl acetone | - | - | - | - | - | 0.3 | - | 0.2 | 0.3 | - | - |
| 83 | 1971 | 1972 | 3-Methylnonadecane | 0.3 | 0.6 | 0.2 | 0.2 | 0.7 | 0.4 | 0.5 | 0.2 | 0.2 | - | 0.5 |
| 84 | 1992 | 1984 | Hexadecanoic acid | - | - | - | - | 0.3 | - | - | 0.2 | 0.3 | - | - |
| 85 | 1999 | 2000 | Eicosane | 0.2 | 0.2 | 0.4 | 0.3 | 0.4 | 0.3 | 0.5 | 0.3 | 0.8 | 0.2 | 0.2 |
| 86 | 2027 | 2024 | Octadecanal | - | - | - | - | - | 0.4 | - | - | - | - | - |
| 87 | 2066 | 2069 | Linoleyl alcohol | - | 0.4 | - | - | - | - | - | - | - | - | - |
| 88 | 2067 | 2064 | 2-Methyleicosane | 0.2 | - | - | 0.2 | 0.2 | - | - | 0.2 | 0.2 | - | - |
| 89 | 2075 | 2080 | 1-Octadecanol | - | - | - | - | - | - | - | 0.2 | - | - | - |
| 90 | 2100 | 2100 | Heneicosane | 3.9 | 2.1 | 1.9 | 4.6 | 3.9 | 2.2 | 5.5 | 3.9 | 4.3 | 0.8 | 3.3 |
| 91 | 2174 | 2172 | 3-Methylheneicosane | 0.4 | 0.3 | 0.1 | 0.8 | 0.4 | 0.2 | 0.5 | 0.4 | 0.4 | - | 0.4 |
| 92 | 2199 | 2200 | Docosane | 0.9 | 0.5 | 1.0 | 1.3 | 0.7 | 0.4 | 1.3 | 0.7 | 1.5 | 0.7 | 0.5 |
| 93 | 2230 | 2229 | Eicosanal | - | - | - | - | - | 0.2 | - | - | - | - | - |
| 94 | 2262 | 2264 | 2-Methyldocosane | 0.4 | 0.3 | 0.2 | 0.8 | 0.4 | 0.1 | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 |
| 95 | 2272 | 2273 | 1-Eicosanol | - | - | 0.1 | 0.2 | - | 0.1 | - | 0.2 | - | - | - |
| 96 | 2300 | 2300 | Tricosane | 9.4 | 9.1 | 8.6 | 13.1 | 12.3 | 4.8 | 14.3 | 7.5 | 10.8 | 8.6 | 11.7 |
| 97 | 2370 | 2372 | 3-Methyltricosane | 1.1 | 1.6 | 0.9 | 2.0 | 1.8 | 0.4 | 1.9 | 0.8 | 0.8 | 0.9 | 1.4 |
| 98 | 2399 | 2400 | Tetracosane | 0.8 | 0.8 | 1.2 | 1.0 | 1.2 | 0.5 | 1.4 | 0.6 | 1.3 | 1.3 | 0.9 |
| 99 | 2459 | 2462 | 2-Methyltetracosane | 0.2 | 0.4 | 0.2 | 0.4 | 0.4 | 0.1 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 |
| 100 | 2500 | 2500 | Pentacosane | 3.5 | 4.1 | 4.6 | 4.8 | 7.9 | 2.6 | 7.3 | 3.3 | 5.7 | 6.1 | 5.7 |
| 101 | 2570 | 2572 | 3-Methylpentacosane | 0.6 | 1.6 | 0.8 | 0.9 | 2.2 | 0.4 | 1.4 | 0.5 | 0.8 | 1.3 | 1.1 |
| 102 | 2599 | 2600 | Hexacosane | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.1 | 0.4 | 0.2 | 0.4 | 0.4 | - |
| 103 | 2659 | 2663 | 2-Methylhexacosane | - | 0.2 | - | - | - | - | - | - | 0.3 | - | - |
| 104 | 2699 | 2700 | Heptacosane | 1.1 | 1.4 | 0.8 | 1.3 | 2.0 | 0.5 | 1.9 | 1.2 | 2.0 | 1.8 | 1.4 |
| 105 | 2770 | 2772 | 3-Methylheptacosane | 0.4 | 0.9 | 0.2 | 0.4 | 0.7 | - | 0.5 | 0.4 | 0.8 | 0.5 | 0.4 |
| Sum | 99.6 | 99.2 | 99.5 | 98.9 | 99.4 | 98.9 | 99.7 | 98.7 | 99.5 | 99.0 | 99.4 |
| No. a | Compound Name | Degree Value b |
|---|---|---|
| 33 | Nerol | 29 |
| 34 | Citronellol | 29 |
| 36 | Geraniol | 28 |
| 44 | Geranic acid | 28 |
| 4 | cis-3-Hexen-1-ol | 27 |
| 6 | 1-Hexanol | 27 |
| 15 | trans-β-Ocimene | 21 |
| 35 | Neral | 21 |
| 47 | Phloroglucinol trimethyl ether | 21 |
| 17 | cis-Linalool oxide furan | 20 |
| 18 | trans-Linalool oxide furan | 20 |
| 26 | Hydroquinone dimethyl ether | 20 |
| 20 | Nonanal | 19 |
| 21 | Linalool | 19 |
| 37 | Geranial | 19 |
| 41 | Citronellyl acetate | 18 |
| 2 | Hexanal | 17 |
| 32 | 4,7-Dimethylbenzofuran | 15 |
| 22 | cis-rose oxide | 13 |
| 24 | Benzeneethanol | 9 |
| Target a | Full Name of the Target | Degree Value b |
|---|---|---|
| SLC6A4 | Sodium-dependent serotonin transporter | 11 |
| CNR1 | Cannabinoid receptor 1 | 10 |
| ACHE | Acetylcholinesterase | 10 |
| HTR1A | 5-Hydroxytryptamine receptor 1A | 10 |
| DRD2 | Dopamine D2 receptor | 10 |
| MAOA | Monoamine oxidase type A | 9 |
| CHRNA4 | Neuronal acetylcholine receptor subunit alpha-4 | 9 |
| NR3C1 | Glucocorticoid receptor | 8 |
| OPRM1 | Mu-type opioid receptor | 8 |
| HTR2A | 5-Hydroxytryptamine receptor 2A | 7 |
| MAOB | Monoamine oxidase type B | 7 |
| CHRM2 | Muscarinic acetylcholine receptor M2 | 6 |
| PTGS2 | Prostaglandin G/H synthase 2 | 4 |
| EGFR | Epidermal growth factor receptor | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, G.; Song, C.; Wen, X.; Gao, G.; Qi, Y. Chemical Diversity and Potential Target Network of Woody Peony Flower Essential Oil from Eleven Representative Cultivars (Paeonia × suffruticosa Andr.). Molecules 2022, 27, 2829. https://doi.org/10.3390/molecules27092829
Lei G, Song C, Wen X, Gao G, Qi Y. Chemical Diversity and Potential Target Network of Woody Peony Flower Essential Oil from Eleven Representative Cultivars (Paeonia × suffruticosa Andr.). Molecules. 2022; 27(9):2829. https://doi.org/10.3390/molecules27092829
Chicago/Turabian StyleLei, Gaoming, Chaoying Song, Xinyue Wen, Guoyu Gao, and Yanjie Qi. 2022. "Chemical Diversity and Potential Target Network of Woody Peony Flower Essential Oil from Eleven Representative Cultivars (Paeonia × suffruticosa Andr.)" Molecules 27, no. 9: 2829. https://doi.org/10.3390/molecules27092829
APA StyleLei, G., Song, C., Wen, X., Gao, G., & Qi, Y. (2022). Chemical Diversity and Potential Target Network of Woody Peony Flower Essential Oil from Eleven Representative Cultivars (Paeonia × suffruticosa Andr.). Molecules, 27(9), 2829. https://doi.org/10.3390/molecules27092829





