1,5-Benzothiazepine Derivatives: Green Synthesis, In Silico and In Vitro Evaluation as Anticancer Agents
Abstract
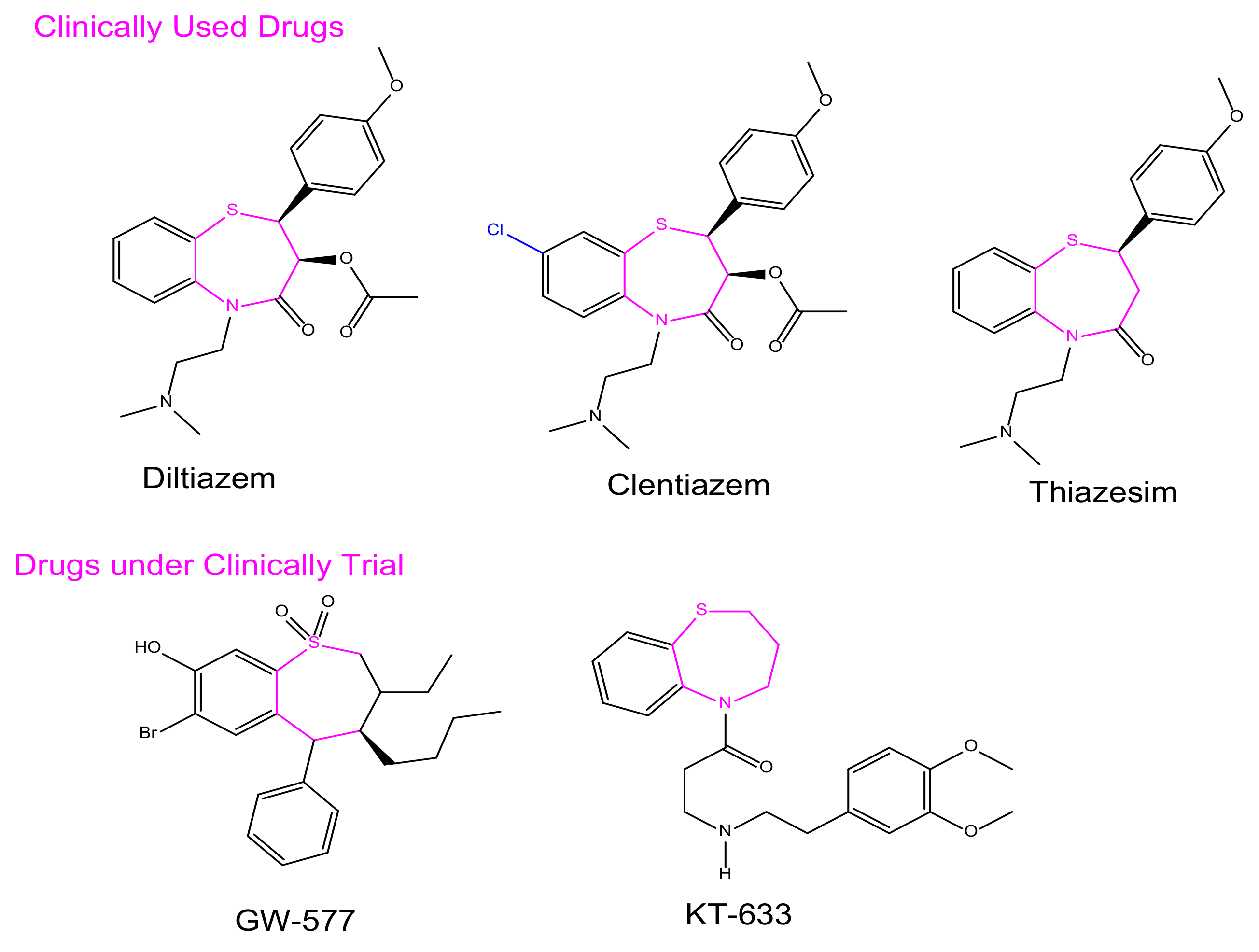
1. Introduction
2. Results and Discussion
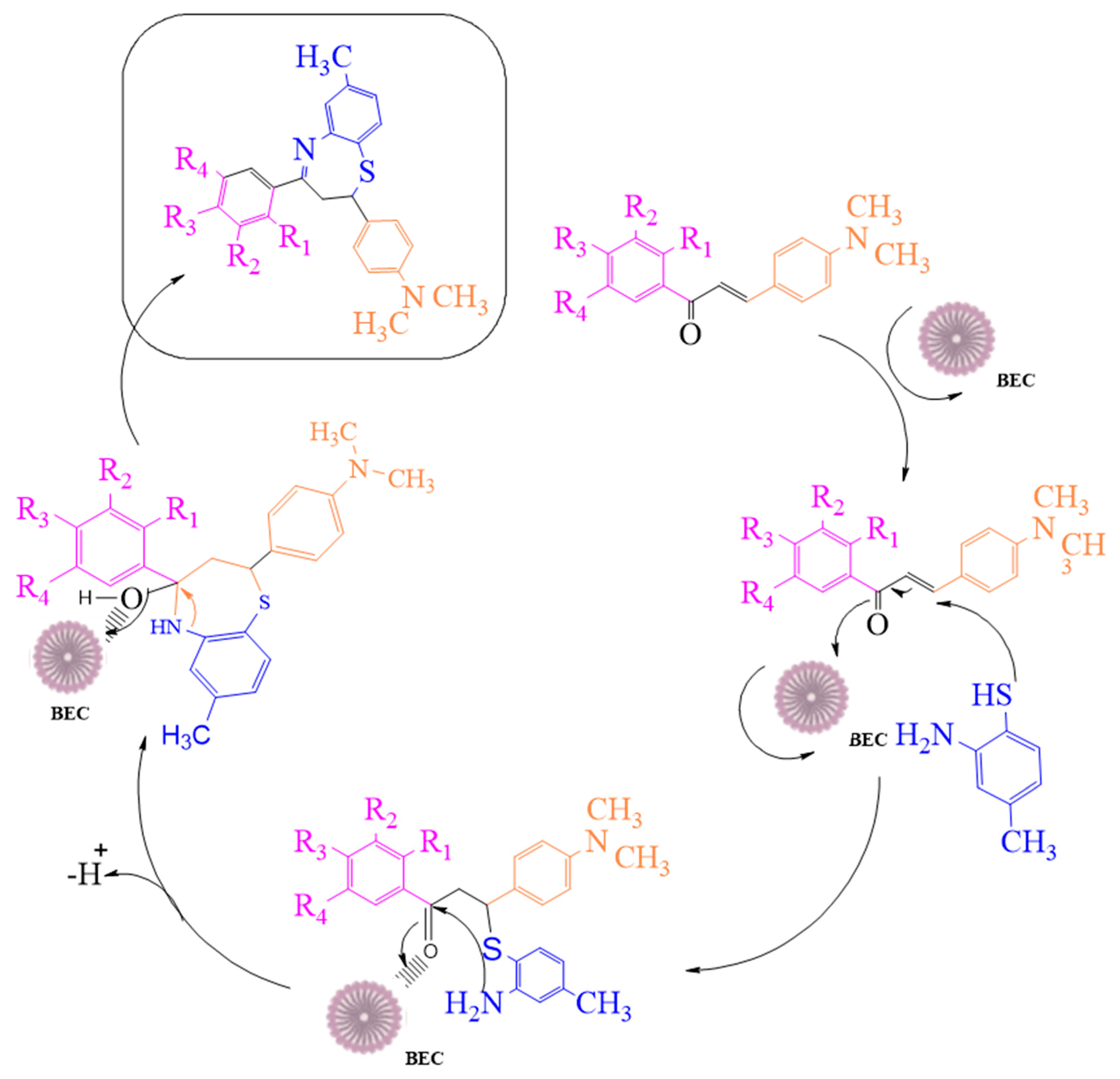
2.1. Synthesis of 2,3-Dihydro-1,5-benzothiazepines
2.2. Biological Evaluation
Anti-Proliferative Activity
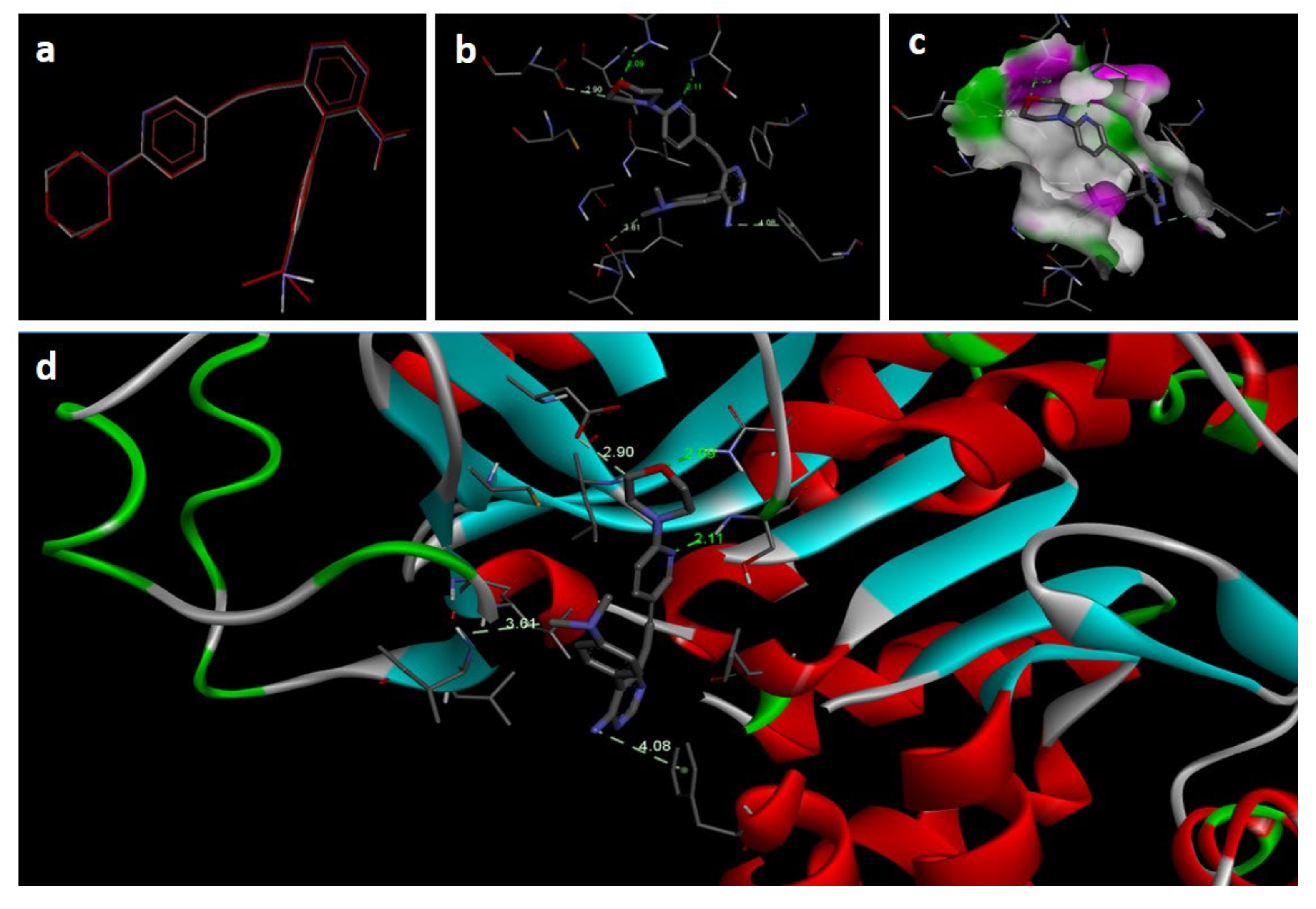
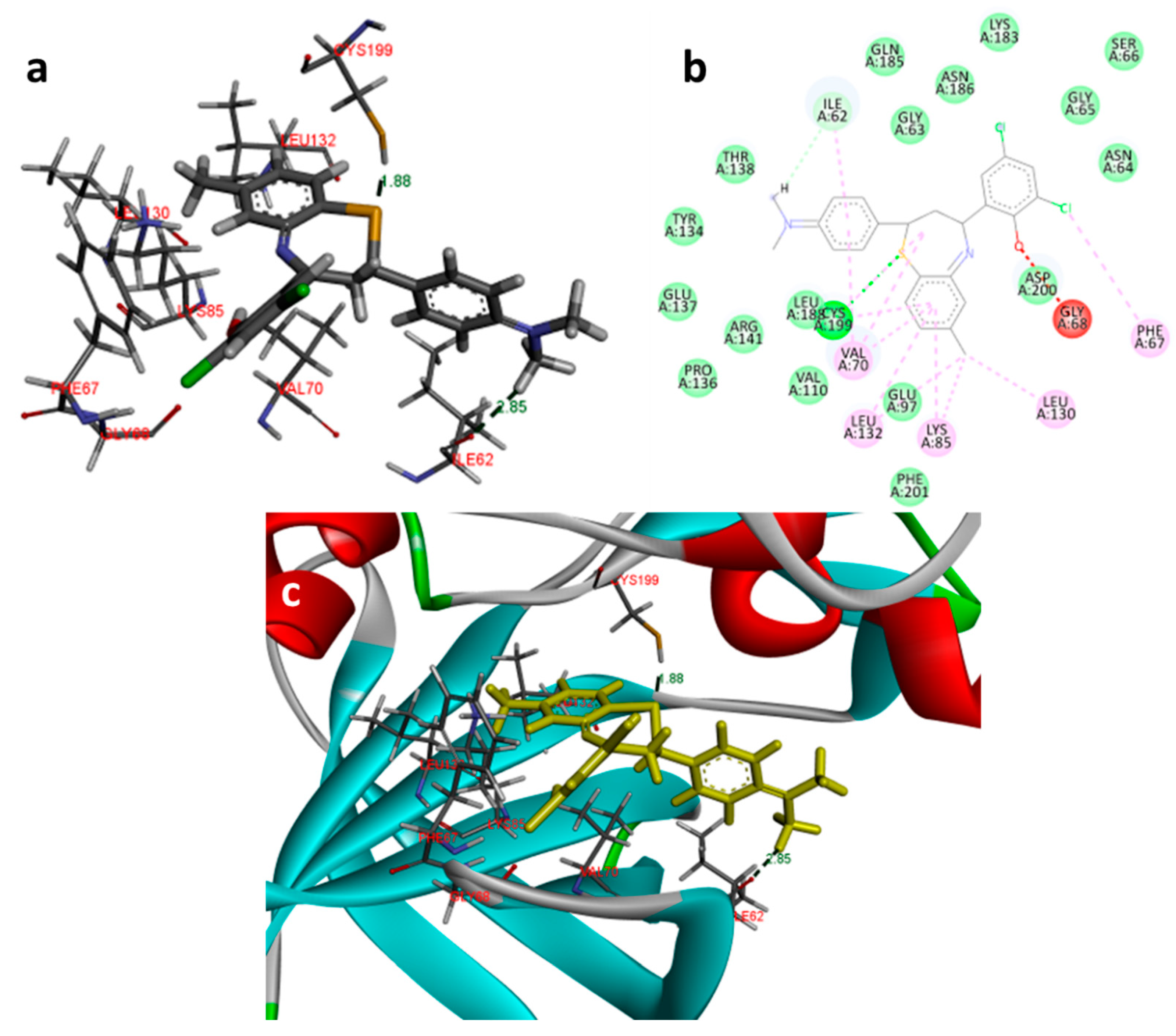
2.3. Molecular Docking
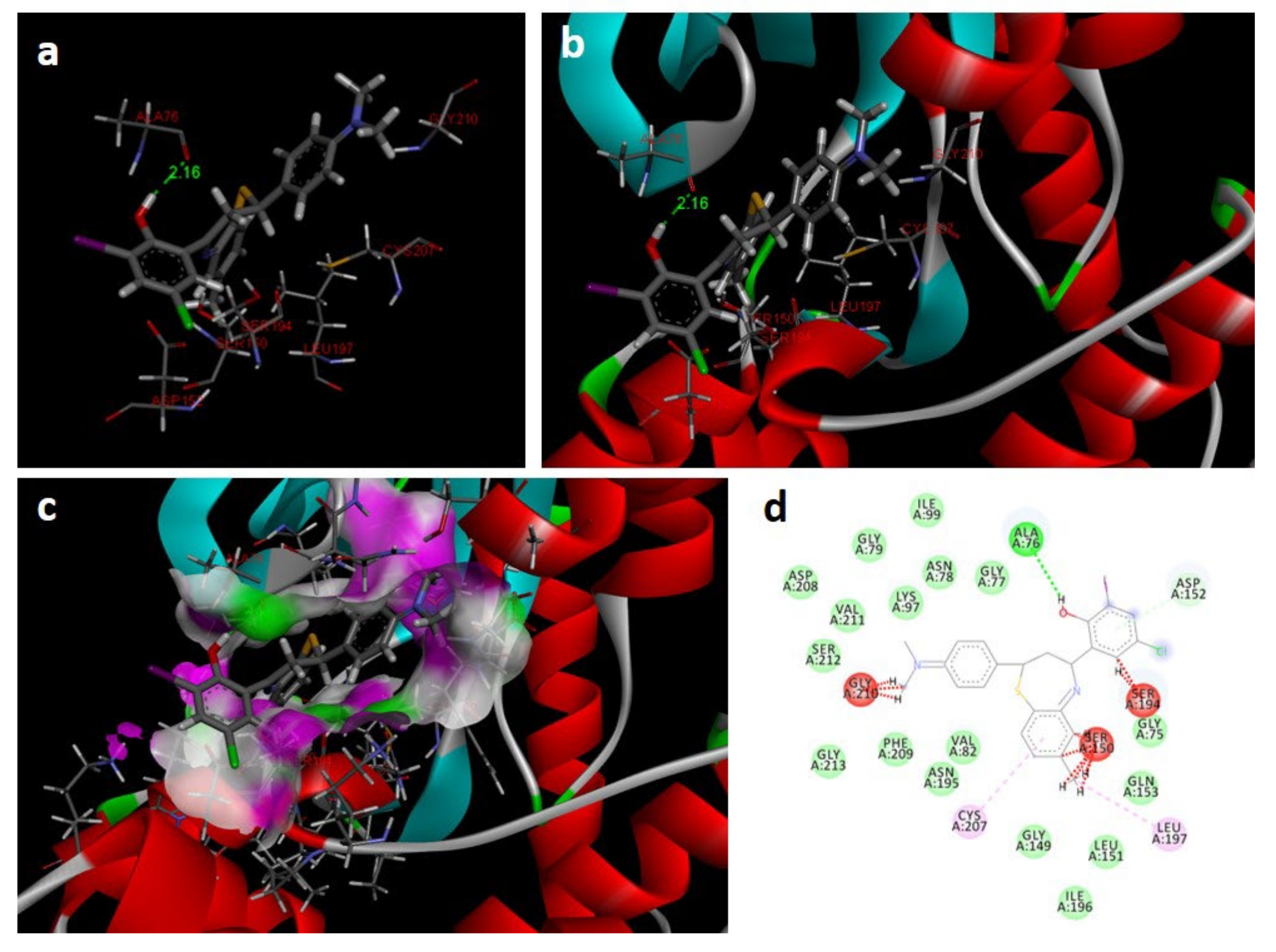
2.4. Potency of BTZ Derivatives to Inhibit GSK-3β
3. Materials and Methods
3.1. Synthesis of the 2,3-Dihydro-1,5-benzothiazepines
The General Method Used for the Synthesis of 2,4-(Substituted-aryl)-2,3-dihydro-1,5-benzothiazepines
3.2. Biological Evaluation
Anti-Proliferative Activity
3.3. Molecular Docking
3.3.1. Small Molecule Preparation
3.3.2. Protein Preparation
3.3.3. Docking Methodology
3.4. GSK-3β Inhibitory Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Saha, D.; Jain, G.; Sharma, A. Benzothiazepines: Chemistry of a privileged scaffold. RSC Adv. 2015, 5, 70619–70639. [Google Scholar] [CrossRef]
- Bariwal, J.B.; Upadhyay, K.D.; Manvar, A.T.; Trivedi, J.C.; Singh, J.S.; Jain, K.S.; Shah, A.K. 1,5-Benzothiazepine, a versatile pharmacophore: A review. Eur. J. Med. Chem. 2008, 43, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Arya, K.; Prabhakar, B. Ionic liquid confined zeolite system: An approach towards water mediated room temperature synthesis of spiro[pyrazolo[3,4-e]benzothiazepines]. Green Chem. 2013, 15, 2885. [Google Scholar] [CrossRef]
- Van der Poorten, O.; van den Hauwe, R.; Hollanders, K.; Maes, B.U.W.; Tourwé, D.; Jida, M.; Ballet, S. Rapid construction of substituted 3-amino-1,5-benzothiazepin-4(5 H)-one dipeptide scaffolds through an Ugi-4CR—Ullmann cross-coupling sequence. Org. Biomol. Chem. 2018, 16, 1242–1246. [Google Scholar] [CrossRef]
- Khan, A.J.; Baseer, M.A. Lanthanum nitrate-catalyzed synthesis of new 2, 3-dihydro-1, 5-benzothiazepines. Orient. J. Chem. 2011, 27, 1759–1762. [Google Scholar]
- Chaffman, M.; Brogden, R.N. Diltiazem: A review of its pharmacological properties and therapeutic efficacy. Drugs 1985, 29, 387–454. [Google Scholar] [CrossRef]
- Passalacqua, T.G.; Dutra, L.A.; de Almeida, L.; Velásquez, A.M.A.; Torres, F.A.E.; Yamasaki, P.R.; dos Santos, M.B.; Regasini, L.O.; Michels, P.A.M.; Bolzani, V.d.S.; et al. Synthesis and evaluation of novel prenylated chalcone derivatives as anti-leishmanial and anti-trypanosomal compounds. Bioorg. Med. Chem. Lett. 2015, 25, 3342–3345. [Google Scholar] [CrossRef]
- Nikalje, A.P.; Ghodke, M.; Tiwari, S. Exploring potential of 1, 5-benzothiazepines: A brief review. Asian J. Res. Chem. 2013, 6, 182–186. [Google Scholar]
- Smith, L.; Wong, W.C.; Kiselyov, A.S.; Burdzovic-Wizemann, S.; Mao, Y.; Xu, Y.; Duncton, M.A.J.; Kim, K.; Piatnitski, E.L.; Doody, J.F.; et al. Novel tricyclic azepine derivatives: Biological evaluation of pyrimido[4,5-b]-1,4-benzoxazepines, thiazepines, and diazepines as inhibitors of the epidermal growth factor receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 2006, 16, 5102–5106. [Google Scholar] [CrossRef]
- Wang, H.; Gu, S.; Yan, Q.; Ding, L.; Chen, F.-E. Asymmetric catalysis in synthetic strategies for chiral benzothiazepines. Green Synth. Catal. 2020, 1, 12–25. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, L.; Kramer, H.F.; Tomberlin, G.H.; Townsend, C.; Meissner, G. Stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex by the 1,4-benzothiazepine derivative S107. PLoS ONE 2013, 8, e54208. [Google Scholar] [CrossRef]
- Dawane, B.S.; Shaikh, B.M.; Khandare, N.T.; Kamble, V.T.; Chobe, S.S.; Konda, S.G. Eco-friendly polyethylene glycol-400: A rapid and efficient recyclable reaction medium for the synthesis of thiazole derivatives. Green Chem. Lett. Rev. 2010, 3, 205–208. [Google Scholar] [CrossRef]
- Dawane, B.S.; Konda, S.G.; Shaikh, B.M.; Chobe, S.S.; Khandare, N.T.; Kamble, V.T.; Bhosale, R.B. Synthesis and in vitro antimicrobial activity of some new 1-thiazolyl-2-pyrazoline derivatives. Synthesis 2010, 1, 009. [Google Scholar]
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Succinic acid: A new platform chemical for biobased polymers from renewable resources. Chem. Eng. Technol. 2008, 31, 647–654. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Ragauskas, A.J. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Sarda, S.R.; Kale, J.D.; Wasmatkar, S.K.; Kadam, V.S.; Ingole, P.G.; Jadhav, W.N.; Pawar, R.P. An efficient protocol for the synthesis of 2-amino-4,6-diphenylpyridine-3-carbonitrile using ionic liquid ethylammonium nitrate. Mol. Divers. 2009, 13, 545–549. [Google Scholar] [CrossRef]
- Chennu, M.M.P.R.; Abdul, R.S.; Yejella, R.P. Molecular docking based screening of G6PS with 1, 5 Benzothiazepine derivates for a potential inhibitor. Bioinformation 2015, 11, 525–528. [Google Scholar] [CrossRef][Green Version]
- Morton, G.C.; Salvino, J.M.; Labaudinière, R.F.; Herpin, T.F. Novel solid-phase synthesis of 1,5-benzothiazepine-4-one derivatives. Tetrahedron Lett. 2000, 41, 3029–3033. [Google Scholar] [CrossRef]
- Prasad, D.C.; Rao, D.A.V.; Rao, M.V. 1, 5-benzothiazepines: An update. J. Global Trends Pharm. Sci. 2014, 5, 1769–1786. [Google Scholar]
- Chobe, S.S.; Dawane, B.S.; Tumbi, K.M.; Nandekar, P.P.; Sangamwar, A.T. An ecofriendly synthesis and DNA binding interaction study of some pyrazolo [1,5-a]pyrimidines derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7566–7572. [Google Scholar] [CrossRef]
- Chobe, S.S.; Kamble, R.D.; Patil, S.D.; Acharya, A.P.; Hese, S.V.; Yemul, O.S.; Dawane, B.S. Green approach towards synthesis of substituted pyrazole-1,4-dihydro,9-oxa,1,2,6,8-tetrazacyclopentano[b]naphthalene-5-one derivatives as antimycobacterial agents. Med. Chem. Res. 2013, 22, 5197–5203. [Google Scholar] [CrossRef]
- Dickerson, T.J.; Reed, N.N.; Janda, K.D. Soluble polymers as scaffolds for recoverable catalysts and reagents. Chem. Rev. 2002, 102, 3325–3344. [Google Scholar] [CrossRef]
- Kumar, R.R.; Perumal, S. A facile synthesis and highly atom economic 1,3-dipolar cycloaddition of hexahydropyrido[3,4-c][1,5]benzothiazepines with nitrile oxide: Stereoselective formation of hexahydro[1,2,4]oxadiazolo[5,4-d]pyrido[3,4-c][1,5]benzothiazepines. Tetrahedron 2007, 63, 7850–7857. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, R.; Chakraborti, A.K. ‘On water’ synthesis of 2,4-diaryl-2,3-dihydro-1,5-benzothiazepines catalysed by sodium dodecyl sulfate (SDS). Tetrahedron Lett. 2008, 49, 4269–4271. [Google Scholar] [CrossRef]
- Tanaka, H.; Kawakami, T.; Yang, Z.B.; Komiyama, K.; Omura, S. Potentiation of cytotoxicity and antitumor activity of adenosine analogs by the adenosine deaminase inhibitor adecypenol. J. Antibiot. 1989, 42, 1722–1724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.G.; Ma, G.Y.; Kou, F.; Liu, W.J.; Sun, Q.Y.; Guo, G.J.; Ma, X.D.; Guo, S.J.; Jian-Ning, Z. Reynoutria japonica from traditional Chinese medicine: A source of competitive adenosine deaminase inhibitors for anticancer. Comb. Chem. High Throughput Screen. 2019, 22, 113–122. [Google Scholar] [CrossRef]
- Ni, H.; Li, Y.H.; Hao, R.L.; Li, H.; Hu, S.Q.; Li, H.H. Identification of adenosine deaminase inhibitors from Tofu wastewater and litchi peel and their synergistic anticancer and antibacterial activities with cordycepin. Int. J. Food Sci. Techol. 2016, 51, 1168–1176. [Google Scholar] [CrossRef]
- Kawazoe, H.; Bilim, V.N.; Ugolkov, A.V.; Yuuki, K.; Naito, S.; Nagaoka, A.; Kato, T.; Tomita, Y. GSK-3 inhibition in vitro and in vivo enhances antitumor effect of sorafenib in renal cell carcinoma (RCC). Biochem. Biophys. Res. Commun. 2012, 423, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Augello, G.; Emma, M.R.; Cusimano, A.; Azzolina, A.; Montalto, G.; Mc Cubrey, J.A.; Cervello, M. The role of GSK-3 in cancer immunotherapy: GSK-3 inhibitors as a new frontier in cancer treatment. Cells 2020, 9, 1427. [Google Scholar] [CrossRef]
- Winfield, H.J.; Cahill, M.M.; O’Shea, K.D.; Pierce, L.T.; Robert, T.; Ruchaud, S.; Bach, S.; Marchand, P.; Mc Carthy, F.O. Synthesis and anticancer activity of novel bisindolylhydroxymaleimide derivatives with potent GSK-3 kinase inhibition. Bioorg. Med. Chem. 2018, 26, 4209–4224. [Google Scholar] [CrossRef]
- Singh, V.J.; Sharma, B.; Chawla, P.A. Recent developments in mitogen activated protein kinase inhibitors as potential anticancer agents. Bioorg. Chem. 2021, 114, 105161. [Google Scholar] [CrossRef]
- Caffa, I.; d’Agostino, V.; Damonte, P.; Soncini, D.; Cea, M.; Monacelli, F.; Odetti, P.; Ballestrero, A.; Provenzani, A.; Longo, V.D.; et al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget 2015, 6, 11820–11832. [Google Scholar] [CrossRef]
- Kamal, A.; Srikanth, Y.V.V.; Khan, M.N.A.; Shaik, T.B.; Ashraf, M. Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl3 and their in vitro evaluation for anticancer activity. Bioorg. Med. Chem. Lett. 2010, 20, 5229–5231. [Google Scholar] [CrossRef]
- Lakshmi, N.V.; Thirumurugan, P.; Noorulla, K.M.; Perumal, P.T. InCl3 mediated one-pot multicomponent synthesis, anti-microbial, antioxidant and anticancer evaluation of 3-pyranyl indole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 5054–5061. [Google Scholar] [CrossRef]
- Sandra, C.M.; Cortes Eduardo, C.; Simon, H.O.; Apan Teresa, R.; Camacho Antonio, N.; Lijanova, I.V.; Marcos, M.G. Anticancer activity and anti-inflammatory studies of 5-aryl-1, 4-benzodiazepine derivatives. Anti Cancer Agents Med. Chem. 2012, 12, 611. [Google Scholar] [CrossRef]
- Hunt, J.T.; Ding, C.Z.; Batorsky, R.; Bednarz, M.; Bhide, R.; Cho, Y.; Chong, S.; Chao, S.; Gullo-Brown, J.; Guo, P.; et al. Discovery of (R)-7-cyano-2, 3, 4, 5-tetrahydro-1-(1 H-imidazol-4-ylmethyl)-3-(phenylmethyl)-4-(2-thienylsulfonyl)-1 H-1, 4-benzodiazepine (BMS-214662), a farnesyltransferase inhibitor with potent preclinical antitumor activity. J. Med. Chem. 2000, 43, 3587–3595. [Google Scholar] [CrossRef]
- Kamal, A.; Reddy, K.S.; Khan, M.N.A.; Shetti, R.V.; Ramaiah, M.J.; Pushpavalli, S.N.C.V.L.; Srinivas, C.; Pal-Bhadra, M.; Chourasia, M.; Sastry, G.N.; et al. Synthesis, DNA-binding ability and anticancer activity of benzothiazole/benzoxazole–pyrrolo [2,1-c][1,4] benzodiazepine conjugates. Bioorg. Med. Chem. 2010, 18, 4747–4761. [Google Scholar] [CrossRef]
- Muchmore, S.W.; Smith, R.A.; Stewart, A.O.; Cowart, M.D.; Gomtsyan, A.; Matulenko, M.A.; Yu, H.; Severin, J.M.; Bhagwat, S.S.; Lee, C.-H.; et al. Crystal structures of human adenosine kinase inhibitor complexes reveal two distinct binding modes. J. Med. Chem. 2006, 49, 6726–6731. [Google Scholar] [CrossRef]
- Dan, N.T.; Quang, H.D.; van Truong, V.; Do Nghi, H.; Cuong, N.M.; Cuong, T.D.; Toan, T.Q.; Bach, L.G.; Anh, N.H.T.; Mai, N.T.; et al. Design, synthesis, structure, in vitro cytotoxic activity evaluation and docking studies on target enzyme GSK-3β of new indirubin-3′-oxime derivatives. Sci. Rep. 2020, 10, 11429. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Kitamura, M.; Fujiwara, D.; Sawa, M.; Shirai, T.; Fujii, I.; Tada, T. Structure of mitogen-activated protein kinase kinase 1 in the DFG-out conformation. Acta Cryst. 2021, F77, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Baki, A.; Bielik, A.; Molnár, L.; Szendrei, G.; Keserü, G.M. A high throughput luminescent assay for glycogen synthase kinase-3 inhibitors. Assay Drug. Dev. Technol. 2007, 5, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hulcová, D.; Breiterová, K.; Siatka, T.; Klímová, K.; Davani, L.; Šafratová, M.; Hošťálková, A.; de Simone, A.; Andrisano, V.; Cahlíková, L. Amaryllidaceae alkaloids as potential glycogen synthase kinase-3β inhibitors. Molecules 2018, 23, 719. [Google Scholar] [CrossRef]
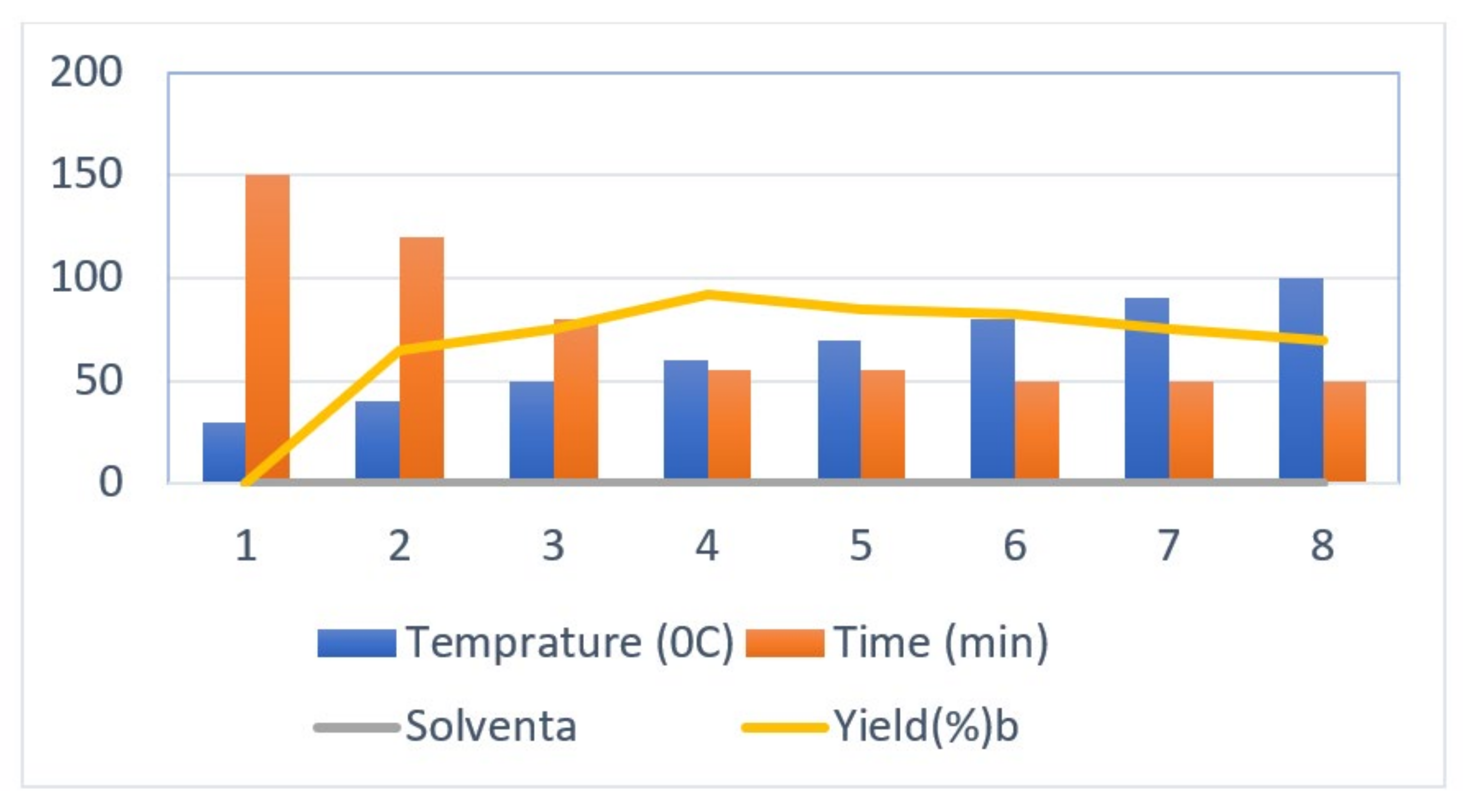


| Entry No. | Temperatures (°C) | Period (min) | Solvent a | Yield (%) b |
|---|---|---|---|---|
| 1 | 30 | 150 | PEG-400 | -- |
| 2 | 40 | 120 | PEG-400 | 35 |
| 3 | 50 | 80 | PEG-400 | 68 |
| 4 | 60 | 55 | PEG-400 | 92 |
| 5 | 70 | 55 | PEG-400 | 85 |
| 6 | 80 | 50 | PEG-400 | 82 |
| 7 | 90 | 50 | PEG-400 | 75 |
| 8 | 100 | 50 | PEG-400 | 70 |
| Entry | Solvent | Period (min) | Yield (%) |
|---|---|---|---|
| 1 | EtOH | 255 | 62 |
| 2 | DCM | 220 | 68 |
| 3 | CH3CN | 175 | 70 |
| 4 | PEG-400 | 55 | 92 |
| Compound | Substitutions | IC50 (µM) | % Inhibition (100 µM) | ||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | Hep G-2 | DU-145 | L02 | |
| 2a | OH | I | H | I | 7.52 ± 0.13 | 32.74 ± 0.13 | 28.23 ± 1.68 |
| 2b | OH | I | H | CH3 | 8.26 ± 0.26 | 41.34 ± 0.12 | 19.65 ± 0.97 |
| 2c | OH | Cl | H | Cl | 3.29 ± 0.15 | 20.45 ± 0.19 | 19.29 ± 1.25 |
| 2d | OH | I | H | Cl | 7.89 ± 0.22 | 36.17 ± 0.14 | 31.42 ± 1.14 |
| 2e | OH | Br | H | CH3 | 6.87 ± 0.15 | 37.52 ± 0.27 | 52.16 ± 1.42 |
| 2f | OH | Br | H | Cl | 4.38 ± 0.11 | 24.58 ± 0.13 | 28.41 ± 1.18 |
| 2g | OH | Br | H | Br | 8.32 ± 0.16 | 28.53 ± 0.14 | 15.56 ± 1.29 |
| 2h | OH | I | H | Br | 8.56 ± 0.14 | 36.80 ± 0.18 | 46.12 ± 1.05 |
| 2i | OH | H | CH3 | Cl | 7.74 ± 0.08 | 40.64 ± 0.09 | 27.47 ± 1.32 |
| 2j | OH | H | H | Br | 4.77 ± 0.21 | 15.42 ± 0.16 | 21.65 ± 1.03 |
| Methotrexate | - | 4.68 ± 0.17 | 21.96 ± 0.15 | 78.23 ± 1.86 | |||
| S. No | Ligand No. | AK | GSK-3 β | MAPK | |||
|---|---|---|---|---|---|---|---|
| Bond Energy | Ki (µM) | Bond Energy | Ki (µM) | Bond Energy | Ki (µM) | ||
| 1 | Native ligand | −7.88 | 1.66 | −8.01 | 9.03 | −14.78 | 21.23 |
| 2 | 2a | −5.22 | 11.87 | −5.05 | 12.52 | −2.37 | 69.17 |
| 3 | 2b | −6.39 | 7.36 | −1.04 | 36.05 | −0.35 | 76.97 |
| 4 | 2c | −7.65 | 2.49 | −4.32 | 17.13 | −6.82 | 51.98 |
| 5 | 2d | −7.41 | 3.42 | −4.78 | 13.58 | −3.97 | 62.99 |
| 6 | 2e | −2.68 | 21.68 | −0.54 | 29.93 | −3.26 | 65.73 |
| 7 | 2f | −4.58 | 14.35 | −5.27 | 11.67 | −2.86 | 67.27 |
| 8 | 2g | −5.36 | 11.33 | −0.04 | 32.20 | −0.95 | 74.65 |
| 9 | 2h | −4.85 | 13.30 | −0.74 | 29.18 | −0.40 | 76.78 |
| 10 | 2i | −4.08 | 16.28 | −1.78 | 25.14 | −4.06 | 62.64 |
| 11 | 2j | −3.99 | 16.63 | −1.76 | 25.25 | −11.71 | 33.09 |
| Compound | R1 | R2 | R3 | R4 | % of Inhibition at 50 µM * |
|---|---|---|---|---|---|
| 2a | OH | I | H | I | 62.35 ± 2.21 |
| 2b | OH | I | H | CH3 | 60.74 ± 2.85 |
| 2c | OH | Cl | H | Cl | 95.21 ± 3.64 |
| 2d | OH | I | H | Cl | 61.14 ± 1.98 |
| 2e | OH | Br | H | CH3 | 66.63 ± 2.51 |
| 2f | OH | Br | H | Cl | 89.70 ± 1.65 |
| 2g | OH | Br | H | Br | 87.62 ± 2.54 |
| 2h | OH | I | H | Br | 61.93 ± 3.47 |
| 2i | OH | H | CH3 | Cl | 61.52 ± 3.26 |
| 2j | OH | H | H | Br | 92.65 ± 1.89 |
| SB-415286 | 98.62 ± 1.63 |
| PDB ID | Co-Crystallized Ligand or Inhibitor | Resolution | Reference |
|---|---|---|---|
| 2I6B | 5-[4-(Dimethylamino)Phenyl]-6-[(6-Morpholin-4-yl pyridin-3-yl)Ethynyl]Pyrimidin-4-Amine (89I) | 2.30 Å | [40] |
| 1Q41 | (Z)-1h,1’h-[2,3’]Biindolylidene-3,2’-Dione-3-Oxime (IXM) | 2.10 Å | [41] |
| 3w8q | Phosphothiophosphoric Acid-Adenylate Ester (AGS) | 2.20 Å | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haroun, M.; Chobe, S.S.; Alavala, R.R.; Mathure, S.M.; Jamullamudi, R.N.; Nerkar, C.K.; Gugulothu, V.K.; Tratrat, C.; Islam, M.M.; Venugopala, K.N.; et al. 1,5-Benzothiazepine Derivatives: Green Synthesis, In Silico and In Vitro Evaluation as Anticancer Agents. Molecules 2022, 27, 3757. https://doi.org/10.3390/molecules27123757
Haroun M, Chobe SS, Alavala RR, Mathure SM, Jamullamudi RN, Nerkar CK, Gugulothu VK, Tratrat C, Islam MM, Venugopala KN, et al. 1,5-Benzothiazepine Derivatives: Green Synthesis, In Silico and In Vitro Evaluation as Anticancer Agents. Molecules. 2022; 27(12):3757. https://doi.org/10.3390/molecules27123757
Chicago/Turabian StyleHaroun, Michelyne, Santosh S. Chobe, Rajasekhar Reddy Alavala, Savita M. Mathure, Risy Namratha Jamullamudi, Charushila K. Nerkar, Vijay Kumar Gugulothu, Christophe Tratrat, Mohammed Monirul Islam, Katharigatta N. Venugopala, and et al. 2022. "1,5-Benzothiazepine Derivatives: Green Synthesis, In Silico and In Vitro Evaluation as Anticancer Agents" Molecules 27, no. 12: 3757. https://doi.org/10.3390/molecules27123757
APA StyleHaroun, M., Chobe, S. S., Alavala, R. R., Mathure, S. M., Jamullamudi, R. N., Nerkar, C. K., Gugulothu, V. K., Tratrat, C., Islam, M. M., Venugopala, K. N., Habeebuddin, M., Telsang, M., Sreeharsha, N., & Anwer, M. K. (2022). 1,5-Benzothiazepine Derivatives: Green Synthesis, In Silico and In Vitro Evaluation as Anticancer Agents. Molecules, 27(12), 3757. https://doi.org/10.3390/molecules27123757









