Noncovalent Interactions and Crystal Structure Prediction of Energetic Materials
Abstract
:1. Introduction
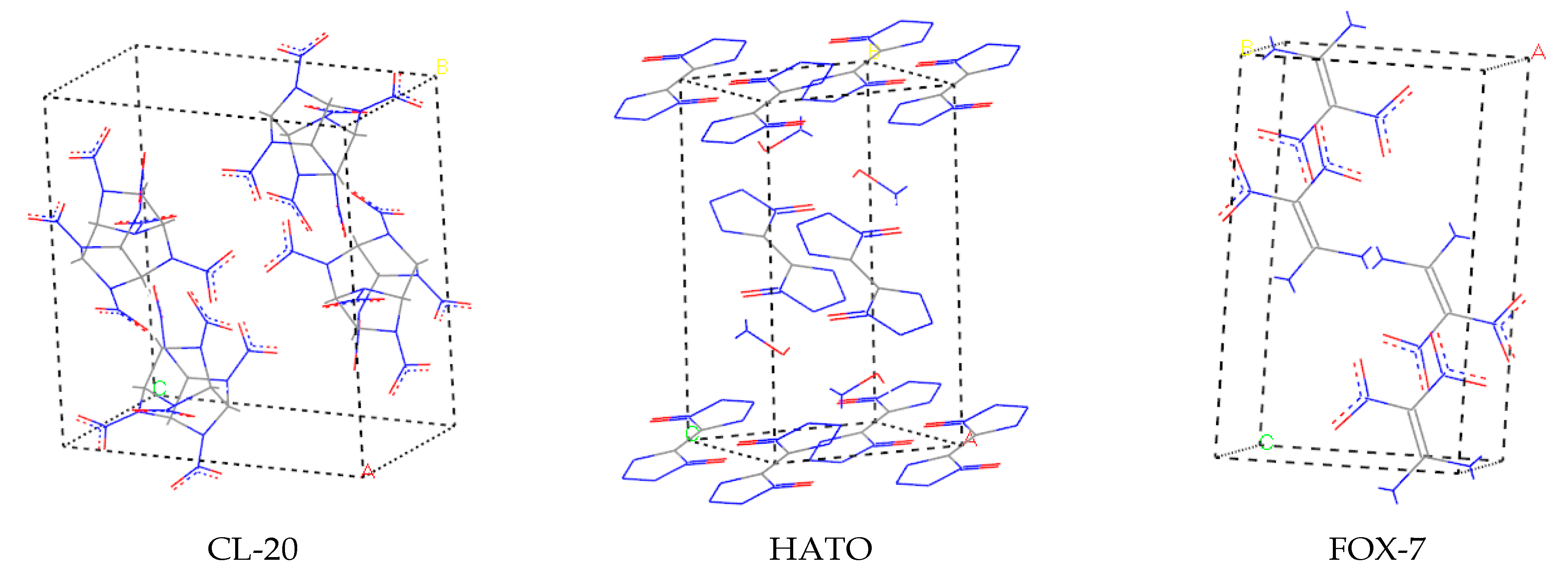
2. Results and Discussion
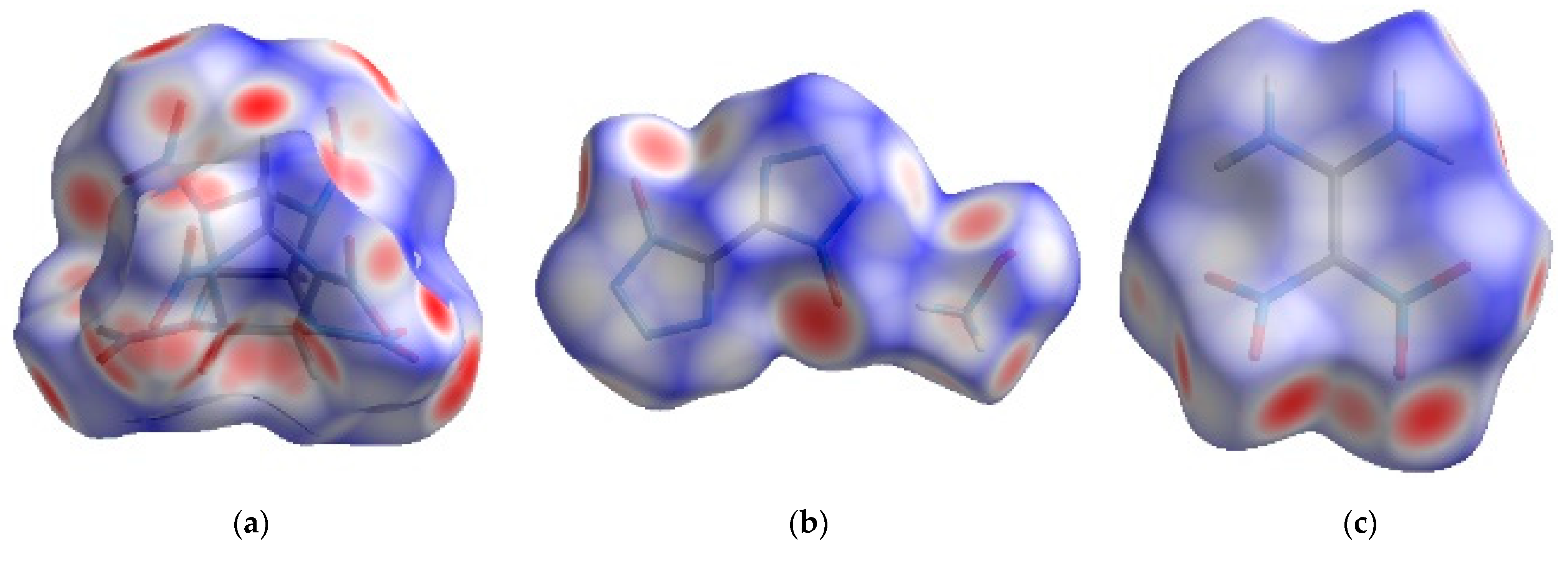
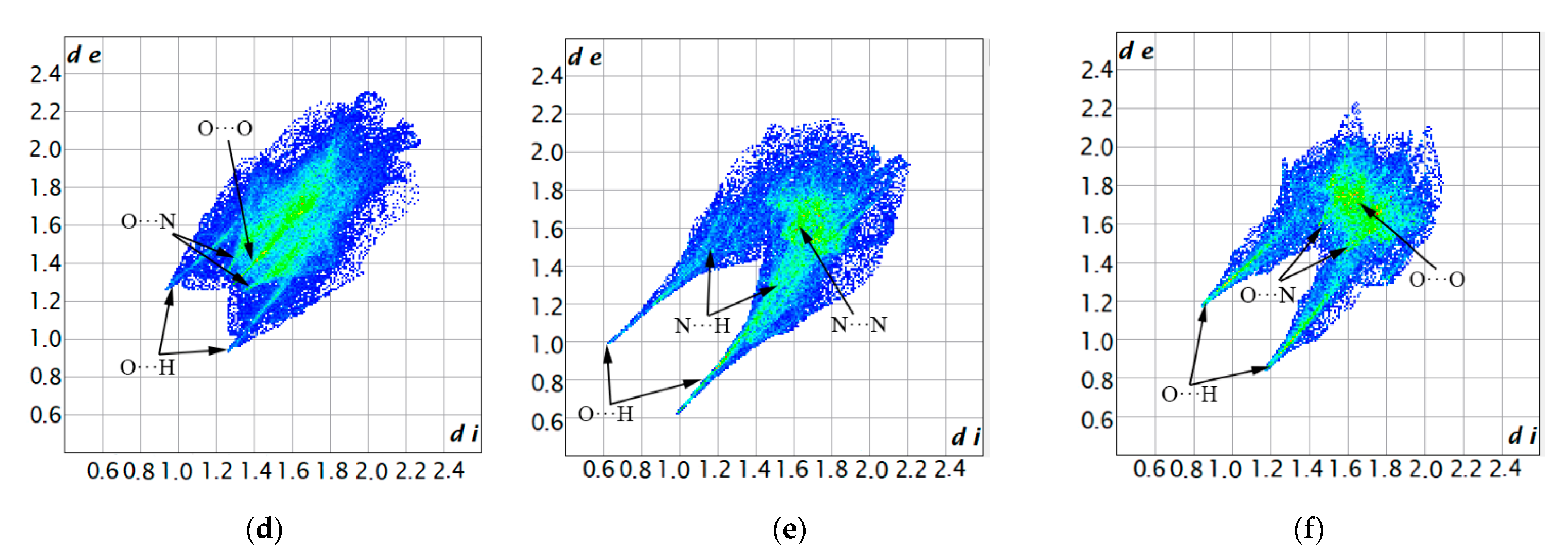
2.1. Hirshfeld Surface Analysis
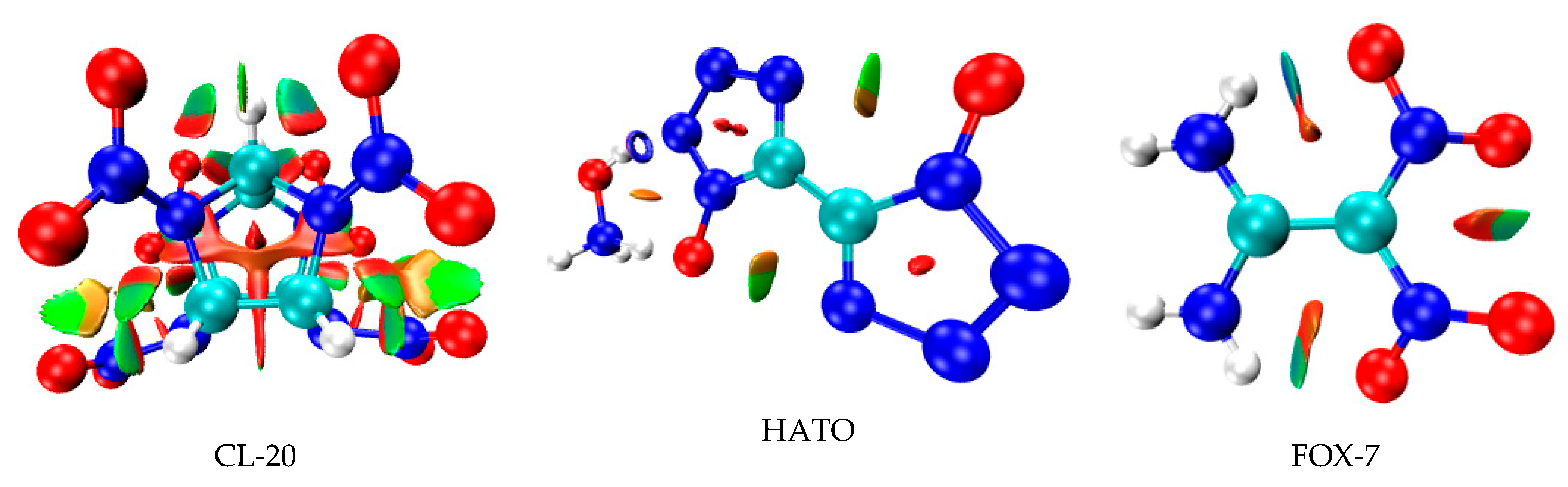
2.2. Reduced Density Gradient Analysis
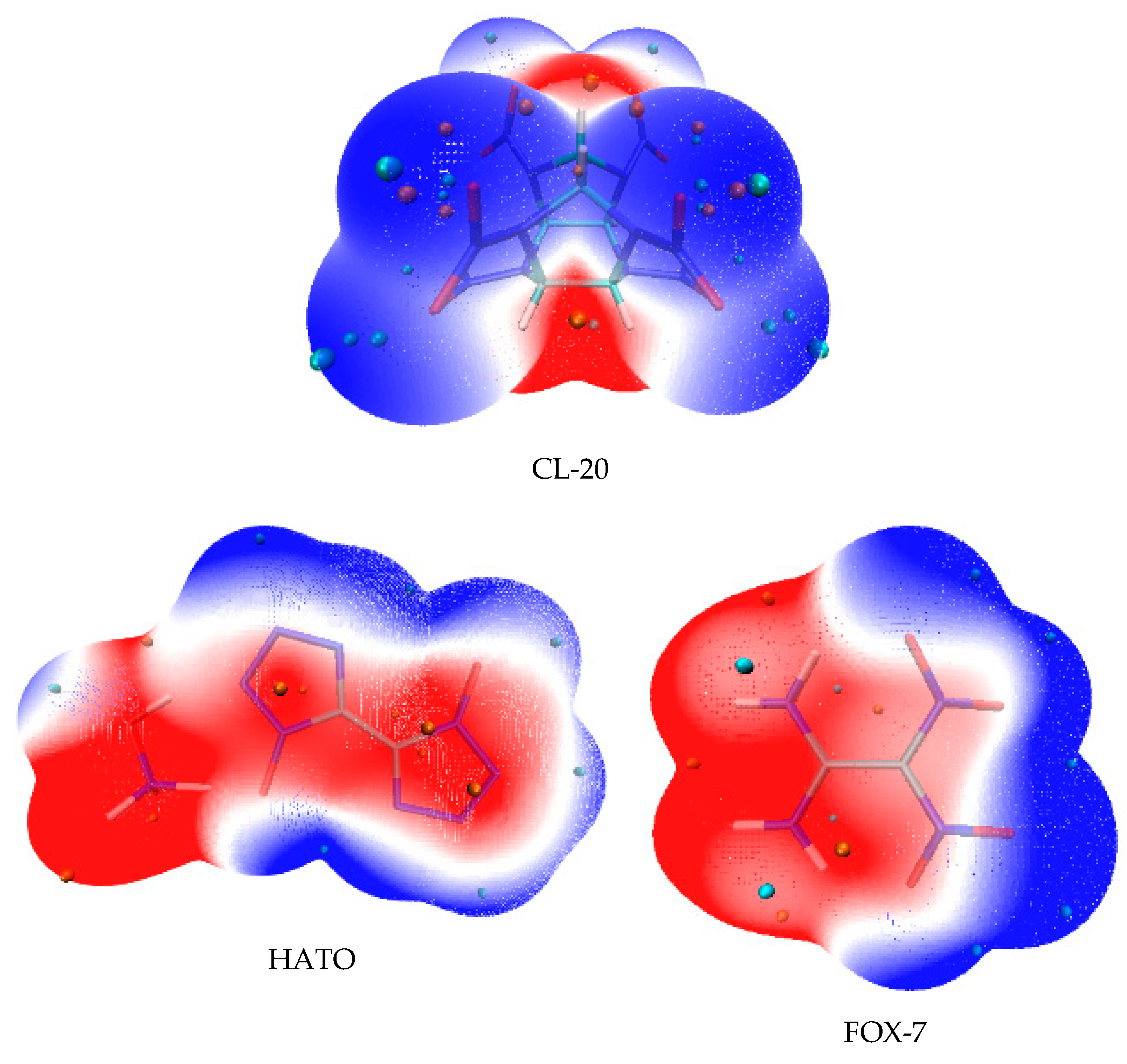
2.3. Electrostatic Potential Surface Analysis
2.4. Crystal Morphology in Vacuum and Dominant Crystal Facets Analysis
2.5. Effect of Ethyl Acetate, Acetonitrile, Acetone, DMF, DMSO, and NMP
3. Experimental Computational Details
3.1. Hirshfeld Surface
3.2. Reduced Density Gradient
3.3. Electrostatic Potential Surface
3.4. Model and Computational Details
3.4.1. Construction of Models
3.4.2. Choice of Force Field
3.4.3. Structure Optimization and Morphology in Vacuum
3.4.4. Construction of Interface Models
3.4.5. Molecular Dynamic Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Duan, B.-H.; Shu, Y.-J.; Liu, N.; Lu, Y.-Y.; Wang, B.-Z.; Lu, X.-M.; Zhang, J.-Q. Comparative studies on structure, sensitivity and mechanical properties of CL-20/DNDAP cocrystal and composite by molecular dynamics simulation. RSC Adv. 2018, 8, 34690–34698. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-B.; Gong, J.; Hu, X.-Y.; Ju, X. Comparative investigation on the thermostability, sensitivity, and mechanical performance of RDX/HMX energetic cocrystal and its mixture. J. Mol. Model. 2020, 26, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xiao, J.-J.; Liu, Q.; Zhao, F.; Xiao, H.-M. Comparative study on structure, energetic and mechanical properties of a 3-CL-20/HMX cocrystal and its composite with molecular dynamics simulation. J. Mater. Chem. A 2014, 2, 13898–13904. [Google Scholar] [CrossRef]
- Gamekkanda, J.-C.; Sinha, A.-S.; Aakeroy, C.-B. Cocrystals and salts of tetrazole-based energetic materials. Cryst. Growth Des. 2020, 20, 2432–2439. [Google Scholar] [CrossRef]
- Zhang, C. Origins of the energy and safety of energetic materials and of the energy & safety contradiction. Propellants Explos. Pyrotech. 2018, 43, 855–856. [Google Scholar]
- Bolton, O.; Matzger, A.-J. Improved stability and smart-material functionality realized in an energetic cocrystal. Angew. Chem. Int. Ed. 2011, 50, 8960–8963. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.-Q.; Wang, K.; Zhang, W.; Luca, L.-T.-D.; Fan, X.-Z.; Li, J.-Q. CL-20-based cocrystal energetic materials: Simulation, preparation and performance. Molecules 2020, 25, 4311–4330. [Google Scholar] [CrossRef]
- Hang, G.-Y.; Yu, W.-L.; Wang, W.-T.; Wang, J.-T. Theoretical investigations into effects of adulteration crystal defect on properties of CL-20/TNT cocrystal explosive. Comput. Mater. Sci. 2019, 156, 77–83. [Google Scholar] [CrossRef]
- Fischer, N.; Fischer, D.; Klapotke, T.-M.; Piercey, D.-G.; Stierstorfer, J. Pushing the limits of energetic materials—The synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J. Mater. Chem. 2012, 22, 20418–20422. [Google Scholar] [CrossRef]
- Fischer, N.; Klapotke, T.-M.; Reymann, M.; Stierstorfer, J. Nitrogen-rich salts of 1 h,1′h-5,5′-Bitetrazole-1,1′-diol: Energetic materials with high thermal stability. Eur. J. Inorg. Chem. 2013, 12, 2167–2180. [Google Scholar] [CrossRef]
- An, Q.; Cheng, T.; Goddard, W.-A.; Zybin, S.-V. Anisotropic impact sensitivity and shock induced plasticity of TKX-50 (dihydroxylammonium 5,5′-bis(tetrazole)-1,1′-diolate) single crystals: From large-scale molecular dynamics simulations. J. Phys. Chem. C 2015, 119, 2196–2207. [Google Scholar] [CrossRef]
- Yan, C.; Qi, X.-J.; Wang, K.-C.; Jin, J.-H.; Cheng, G.-B.; Liu, T.-L.; Yang, H.-W.; Zhang, Q.-H. Revisiting the reactive chemistry of FOX-7: Cyclization of FOX-7 affords the fused-ring polynitro compounds. Chem. Commun. 2019, 19, 3497–3500. [Google Scholar] [CrossRef] [PubMed]
- Bemm, U.; Ostmark, H. 1,1-diamino-2,2-dinitroethylene:t a novel energetic material with infinite layers in two dimensions. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 1997–1999. [Google Scholar] [CrossRef]
- Evers, J.; Klapotke, T.-M.; Mayer, P.; Oehlinger, G.; Welch, J. α- and β-FOX-7, polymorphs of a high energy density material, studied by X-ray single crystal and powder investigations in the temperature range from 200 to 423 K. Inorg. Chem. 2006, 45, 4996–5007. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.-J.; Evers, J.; Gobel, M.; Klapotke, T.-M.; Mayer, P.; Oehlinger, G.; Welch, J.-M. γ-FOX-7: Structure of a high energy density material immediately prior to decomposition. Propellants Explos. Pyrotech. 2007, 32, 478–495. [Google Scholar] [CrossRef]
- Xiong, S.-L.; Chen, S.-S.; Jin, S.-H.; Zhang, C.-Y. Molecular dynamics simulations on dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate/hexanitrohexaazaisowurtzitane cocrystal. RSC Adv. 2016, 6, 4221–4226. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, F.; Li, J.; He, L.; Ren, Y.-Y.; Wang, X.-L.; Cao, D.-L. Morphology prediction of 5,5′-bistetrazole-1,1′-diolate (BTO) crystal in solvents with different models using molecular dynamics simulation. J. Cryst. Growth 2020, 548, 125843–125854. [Google Scholar] [CrossRef]
- Spackman, M.-A.; McKinnon, J.-J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- McKinnon, J.-J.; Jayatilaka, D.; Spackman, M.-A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, S.-G.; Li, H.-Z.; Peng, X.-H. Structure and detonation performance of a novel HMX/LLM-105 cocrystal explosive. J. Phys. Org. Chem. 2013, 26, 898–907. [Google Scholar] [CrossRef]
- Gao, H.-F.; Zhang, S.-H.; Ren, F.-D.; Liu, F.; Gou, R.-J.; Ding, X. Theoretical insight into the co-crystal explosive of 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane (CL-20)/1,1-diamino-2,2-dinitroethylene (FOX-7). Comput. Mater. Sci. 2015, 107, 33–41. [Google Scholar] [CrossRef]
- Xu, Y.-G.; Tian, L.-L.; Li, D.-X.; Wang, P.-C.; Lu, M. A series of energetic cyclo-pentazolate salts: Rapid synthesis, characterization, and promising performance. J. Mater. Chem. A. 2019, 7, 12468–12479. [Google Scholar] [CrossRef]
- Johnson, E.-R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.-J.; Yang, W.-T. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-R.; Li, H.-Z.; Gou, R.-J.; Zhang, C.-Y. Packing structures of CL-20-based cocrystals. Cryst. Growth Des. 2018, 18, 7065–7078. [Google Scholar] [CrossRef]
- Ren, J.-R.; Chen, D.; Liu, G.-R.; Wang, K.-C.; Fan, G.-J.; Yu, Y.-W.; Zhang, C.-Y.; Li, H.-Z. Crystal structure evolution of the high energetic compound carbonic dihydrazidinium bis[3-(5-nitroimino-1,2,4-triazolate)] induced by solvents. CrystEngComm 2020, 22, 593–601. [Google Scholar] [CrossRef]
- Ma, Y.; Meng, L.-Y.; Li, H.-Z.; Zhang, C.-Y. Enhancing intermolecular interactions and their anisotropy to build low-impact-sensitivity energetic crystals. CrystEngComm 2017, 19, 3145–3155. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Zhang, Q.-H.; Vo, T.-T.; Parrish, D.-A.; Shreeve, J.-M. Energetic salts with π-stacking and hydrogen-bonding interactions lead the way to future energetic materials. J. Am. Chem. Soc. 2015, 137, 1697–1704. [Google Scholar] [CrossRef]
- Bu, R.-P.; Liu, G.-R.; Zhong, K.; Wang, R.; Xiong, Y.; Zhang, C.-Y. Relationship between the molecular structure and stacking mode: Characteristics of the D2h and D3h molecules in planar layer-stacked crystals. Cryst. Growth Des. 2021, 21, 6847–6861. [Google Scholar] [CrossRef]
- Xie, Z.-B.; Hu, S.-Q.; Cao, X. Theoretical insight into the influence of molecular ratio on the binding energy and mechanical property of HMX/2-picoline-N-oxide cocrystal, cooperativity effect and surface electrostatic potential. Mol. Phys. 2016, 114, 2164–2176. [Google Scholar] [CrossRef]
- Chen, L.-Z.; She, C.-C.; Pan, H.-X.; Wang, J.-L.; Cao, D.-L. Habit prediction of 3,4,5-trinitro-1H-pyrazole in four solvent mediums using a molecular dynamics simulation. J. Cryst. Growth 2019, 507, 58–64. [Google Scholar] [CrossRef]
- Spackman, P.-R.; Turner, M.-J.; McKinnon, J.-J.; Wolff, S.-K.; Grimwood, D.-J.; Jayatilaka, D.; Spackman, M.-A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Khrustalev, V.-N.; Grishina, M.-M.; Matsulevich, Z.-V.; Lukiyanova, J.-M.; Borisova, G.-N.; Osmanov, V.-K.; Novikov, A.-S.; Kirichuk, A.-A.; Borisov, A.-V.; Solari, E.; et al. Novel cationic 1,2,4-selenadiazoles: Synthesis via addition of 2-pyridylselenyl halides to unactivated nitriles, structures and four-center Se⋯N contacts. Dalton Trans. 2021, 50, 10689–10691. [Google Scholar] [CrossRef] [PubMed]
- Grudova, M.-V.; Kubasov, A.-S.; Khrustalev, V.-N.; Novikov, A.-S.; Kritchenkov, A.-S.; Nenajdenko, V.-G.; Borisov, A.-V.; Tskhovrebov, A.-G. Exploring supramolecular assembly space of cationic 1,2,4-selenodiazoles: Effect of the substituent at the carbon atom and anions. Molecules 2022, 27, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Artemjev, A.-A.; Astafiev, A.-A.; Vologzhanina, A.-V.; Kubasov, A.-S.; Burkin, G.-M.; Novikov, A.-S.; Kritchenkov, A.-S.; Kirichuk, A.-A.; Tskhovrebov, A.-G. Triarylazoimidazole-ZnII, CdII, and HgII complexes: Structures, photophysics, and antibacterial properties. Crystals 2022, 12, 680–693. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.-W. Multiwfn: A Multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Zhang, J.; Kim, E.-C.; Lam, K.; Chung, H.-J.; Tajkhorshid, E. PIP2-dependent coupling of voltage sensor and pore domains in Kv7.2 channel. Commun. Biol. 2021, 4, 1189. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.-F.; Shi, C.-Y.; Zhao, S.-X.; Mei, Z.-S.; Pan, Y.-G. Molecular dynamics simulations of a cyclotetramethylene tetra-nitramine/hydrazine 5,5’-bitetrazole-1,1’-diolate cocrystal. RSC Adv. 2019, 9, 19390–19396. [Google Scholar] [CrossRef] [PubMed]
- Bunte, S.-W.; Sun, H. Molecular modeling of energetic materials: The parameterization and validation of nitrate esters in the COMPASS force field. J. Phys. Chem. B 2000, 104, 2477–2489. [Google Scholar] [CrossRef]
- Wang, F. Study on the Interaction and Thermal Stability of TKX-50/Additives System; Beijing Institute of Technology: Beijing, China, 2017. [Google Scholar]
- Liu, Y. Research on Design and Controlled Assembly of Nitroamine Explosive Cocrystal; North University of China: Taiyuan, China, 2020. [Google Scholar]

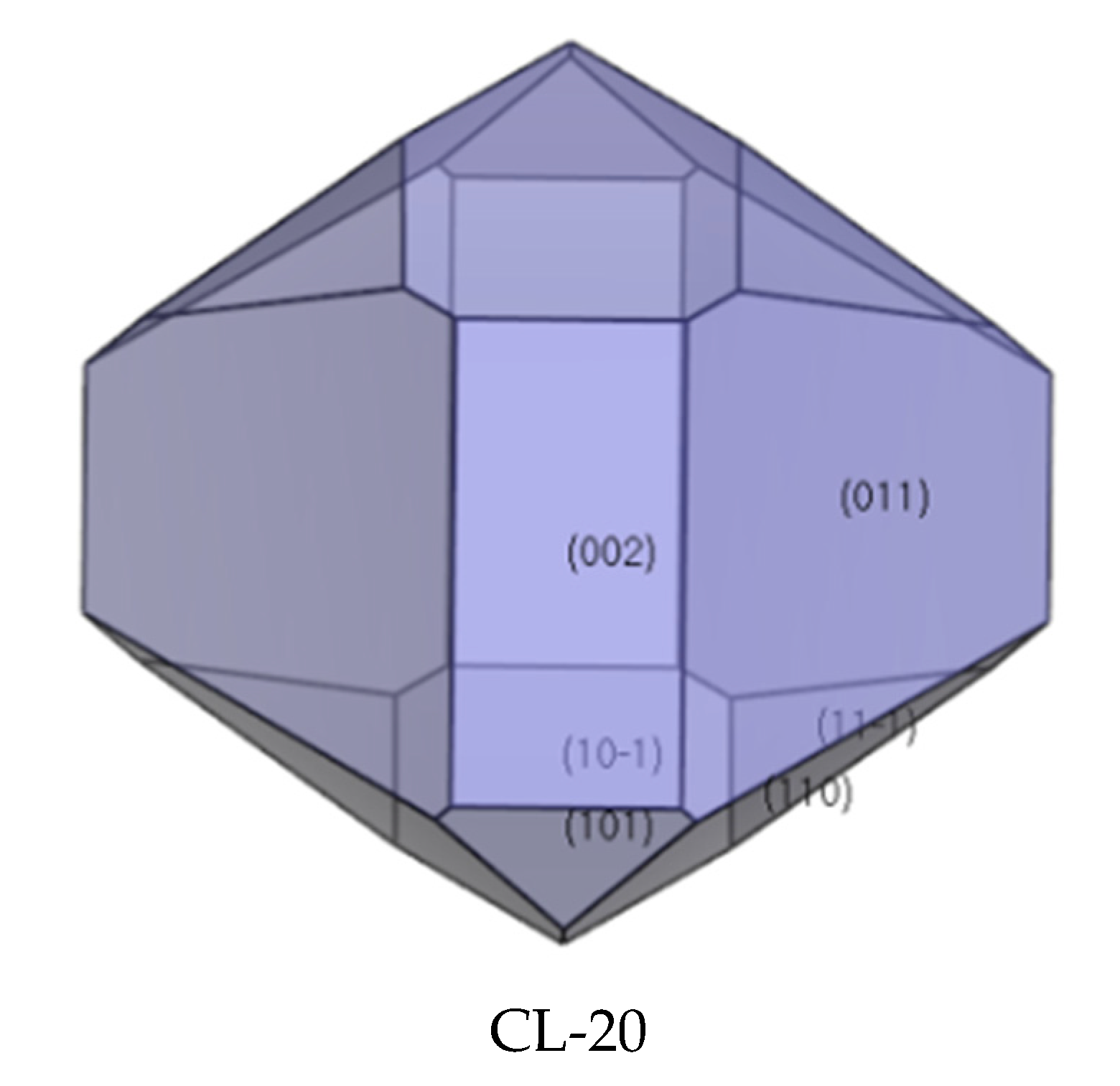
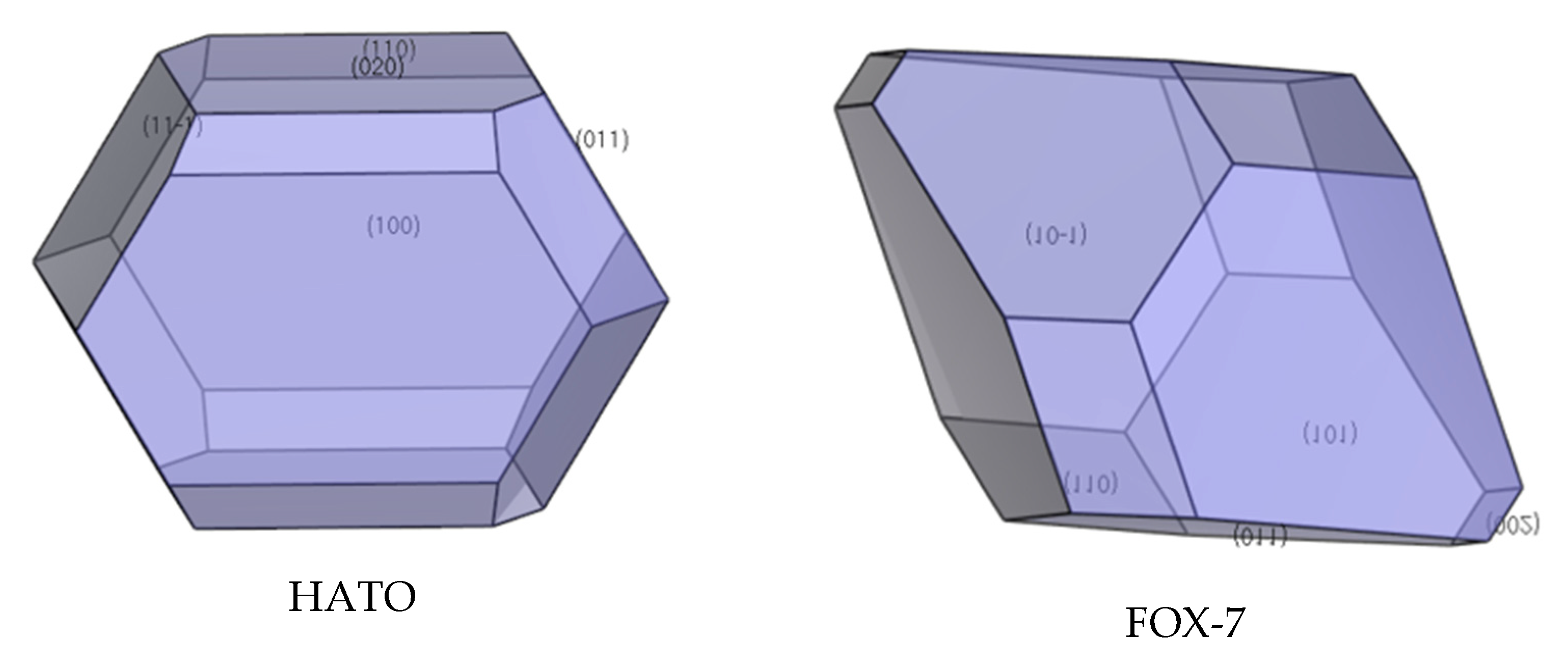
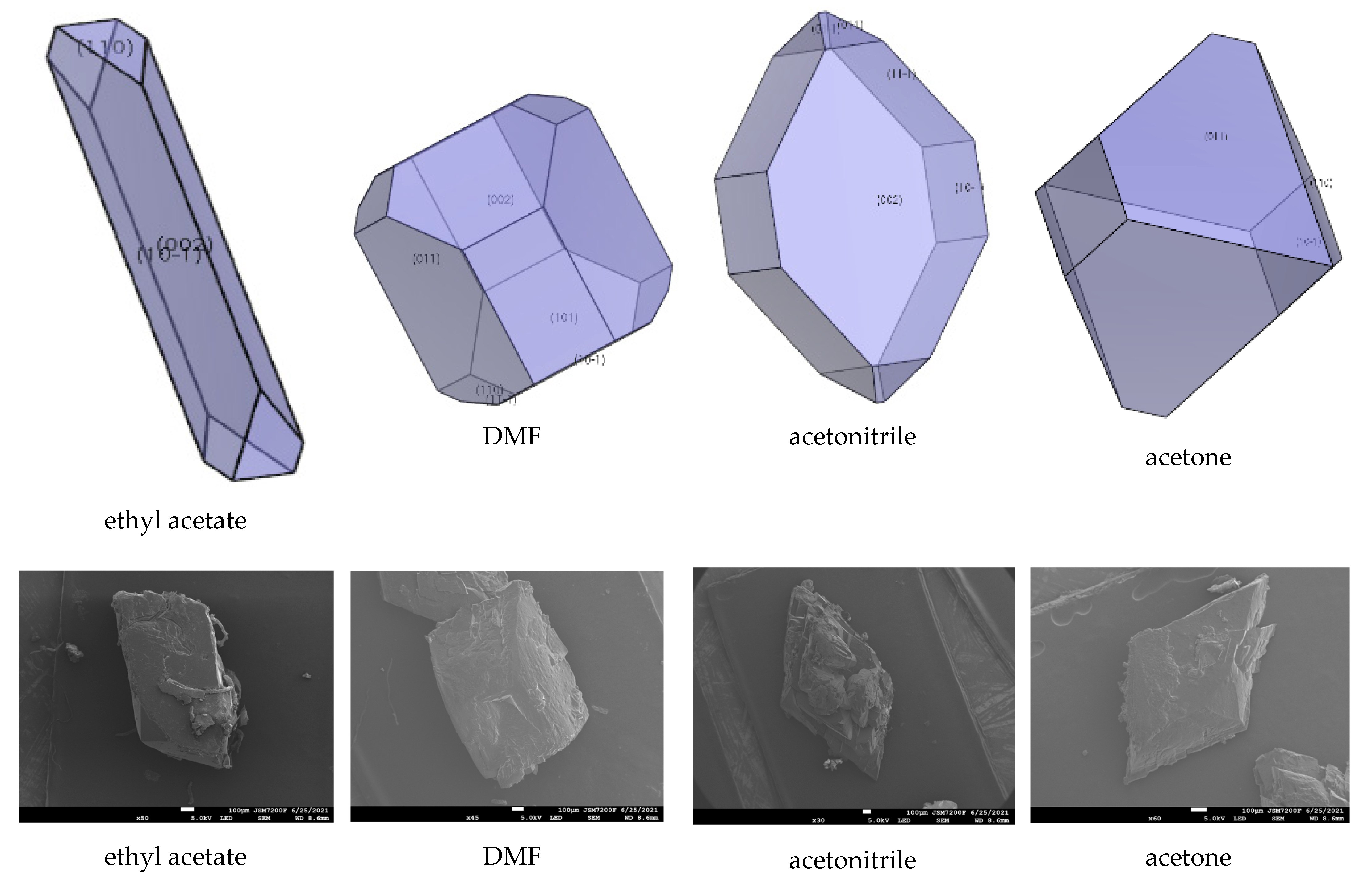
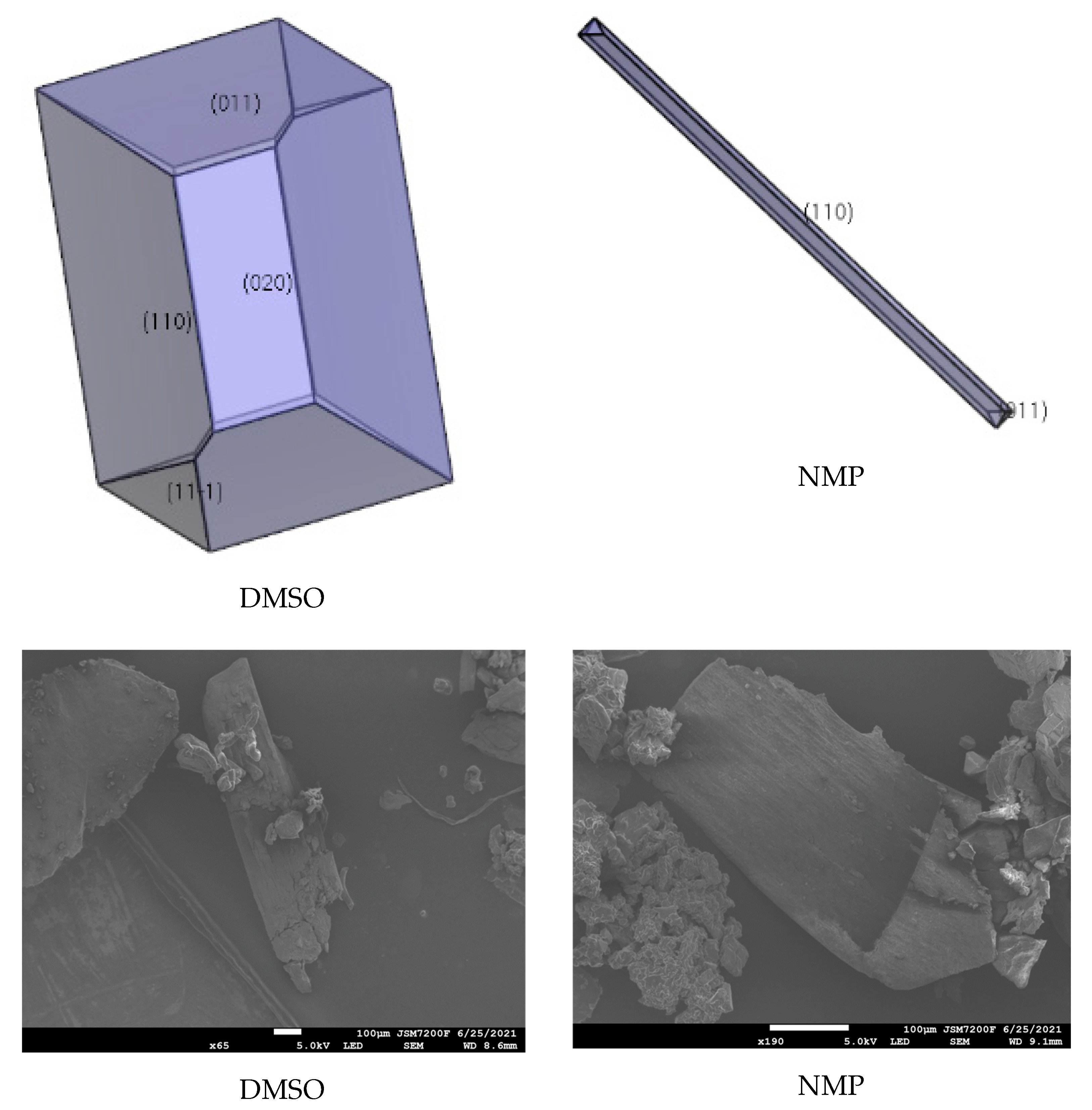
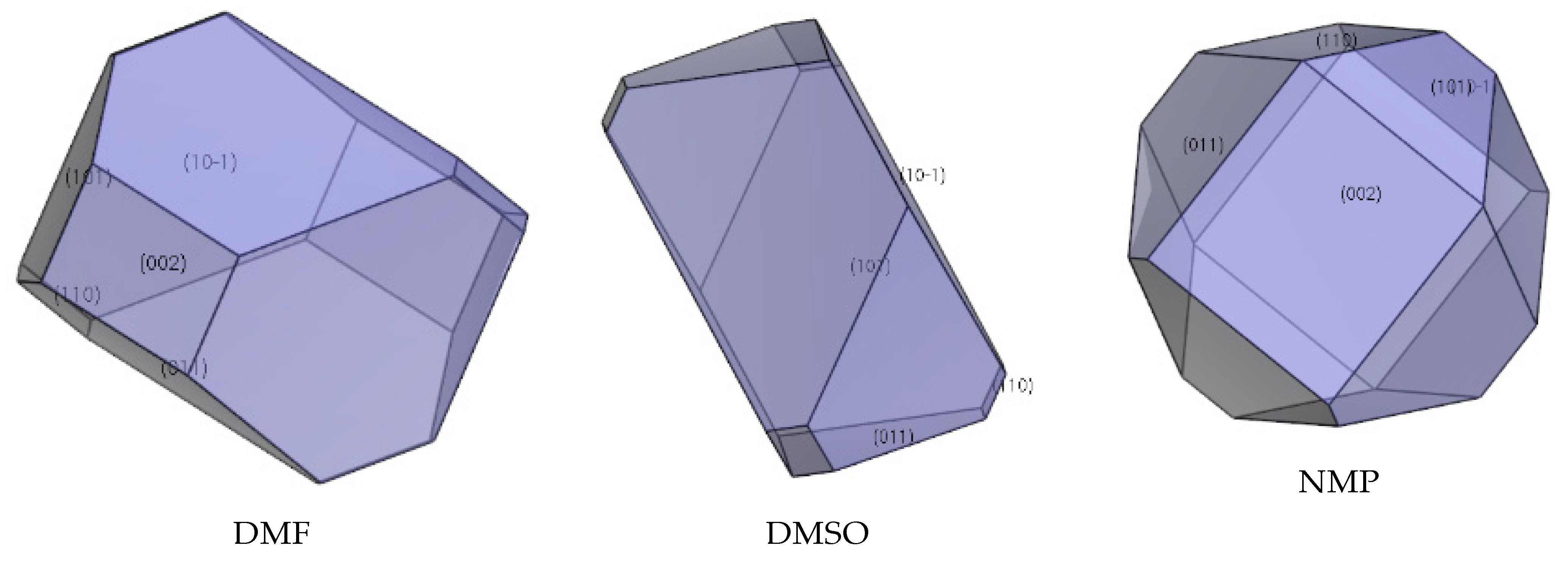
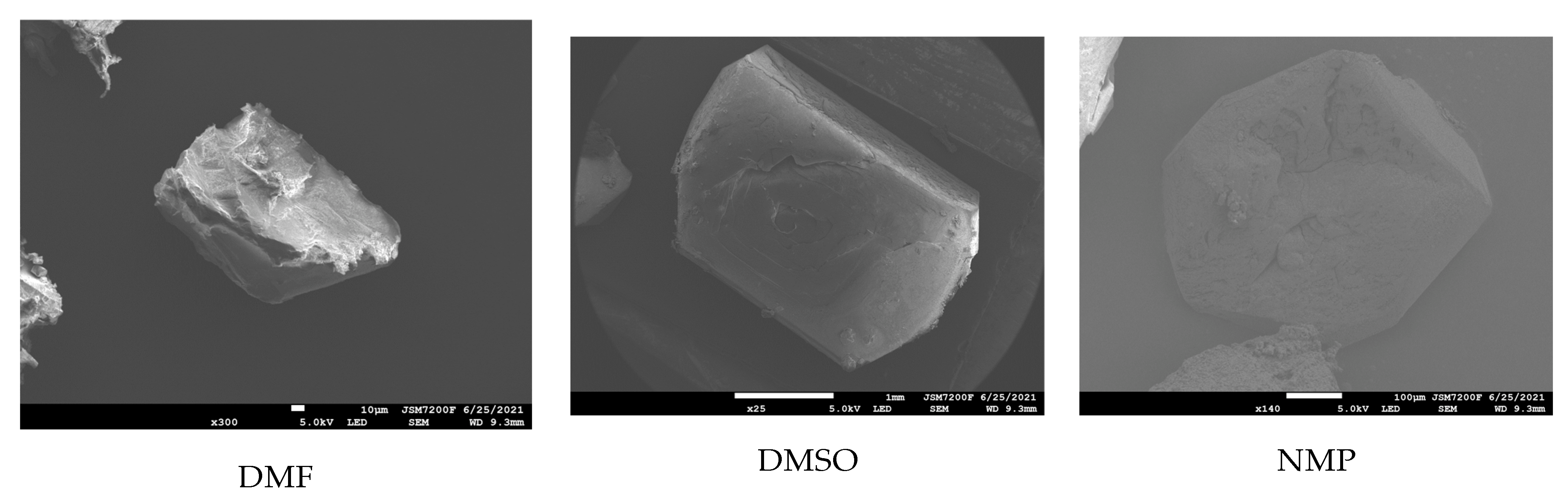
| Facet | Multiplicity | dhkl/Å | Surface Area | Eatt(Total) /(kcal/mol) | Eatt(vdW) /(kcal/mol) | Eatt(Electrostatic) /(kcal/mol) | Distance/Å | Total Facet Area (%) |
|---|---|---|---|---|---|---|---|---|
| {0 1 1} | 4 | 8.81 | 153.78 | −84.78 | −33.51 | −51.27 | 84.78 | 39.67 |
| {1 0 −1} | 2 | 8.06 | 168.01 | −87.92 | −34.69 | −53.23 | 87.92 | 13.14 |
| {1 1 0} | 4 | 6.94 | 195.31 | −91.04 | −38.95 | −52.09 | 91.04 | 26.12 |
| {1 1 −1} | 4 | 6.73 | 201.15 | −95.97 | −38.15 | −57.81 | 95.97 | 8.10 |
| {0 0 2} | 2 | 6.33 | 106.85 | −92.86 | −37.69 | −55.17 | 92.86 | 10.27 |
| {1 0 1} | 2 | 2.29 | 215.46 | −110.24 | −47.12 | −63.12 | 110.24 | 2.71 |
| Facet | Multiplicity | dhkl/Å | Surface Area | Eatt(Total) /(kcal/mol) | Eatt(vdW) /(kcal/mol) | Eatt(Electrostatic) /(kcal/mol) | Distance/Å | Total Facet Area (%) |
|---|---|---|---|---|---|---|---|---|
| {1 0 0} | 2 | 6.59 | 70.49 | −20.11 | −10.33 | −9.78 | 20.11 | 29.59 |
| {1 1 0} | 4 | 5.66 | 82.11 | −26.62 | −14.76 | −11.86 | 26.62 | 8.60 |
| {0 2 0} | 2 | 5.52 | 42.11 | −24.39 | −13.57 | −10.82 | 24.39 | 21.84 |
| {0 1 1} | 4 | 5.50 | 84.57 | −29.70 | −11.56 | −18.14 | 29.70 | 27.67 |
| {1 1 −1} | 4 | 4.47 | 104.06 | −30.39 | −18.29 | −12.10 | 30.39 | 12.30 |
| Facet | Multiplicity | dhkl/Å | Surface Area | Eatt(Total) /(kcal/mol) | Eatt(vdW) /(kcal/mol) | Eatt(Electrostatic) /(kcal/mol) | Distance/Å | Total Facet Area (%) |
|---|---|---|---|---|---|---|---|---|
| {0 1 1} | 4 | 5.79 | 86.64 | −37.04 | −22.26 | −14.78 | 37.04 | 50.25 |
| {1 0 −1} | 2 | 5.75 | 87.11 | −36.61 | −21.04 | −15.57 | 36.61 | 24.61 |
| {0 0 2} | 2 | 5.68 | 44.15 | −63.85 | −22.94 | −40.91 | 63.85 | 1.75 |
| {1 0 1} | 2 | 5.61 | 89.29 | −49.43 | −17.38 | −32.05 | 49.43 | 12.85 |
| {1 1 0} | 4 | 4.70 | 106.72 | −46.51 | −25.10 | −21.41 | 46.51 | 10.53 |
| {h l k} | Eint/(kcal/mol) | Es/(kcal/mol) | Eatt */(kcal/mol) | CED/(kcal/mol) | Total Facet Area (%) | |
|---|---|---|---|---|---|---|
| ethyl acetate | {0 1 1} | −8454.70 | −6825.84 | 6741.06 | −6205.59 | − |
| {1 0 −1} | −178.58 | −148.39 | 60.47 | −6029.59 | 36.38 | |
| {1 1 0} | −560.59 | −326.85 | 235.81 | −6049.49 | 11.97 | |
| {1 1 −1} | −724.11 | −449.72 | 353.76 | −5684.58 | − | |
| {0 0 2} | −44.07 | −45.73 | −47.13 | −3886.14 | 51.66 | |
| {1 0 1} | −671.64 | −375.71 | 265.38 | −5984.99 | − | |
| DMF | {0 1 1} | −799.08 | −334.68 | 249.90 | −8076.94 | 48.38 |
| {1 0 −1} | −778.27 | −425.29 | 337.37 | −6253.02 | 14.74 | |
| {1 1 0} | −896.18 | −486.36 | 395.32 | −6422.75 | 4.99 | |
| {1 1 −1} | −975.45 | −502.34 | 406.37 | −5620.82 | 1.18 | |
| {0 0 2} | −553.03 | −328.25 | 235.40 | −3980.93 | 14.17 | |
| {1 0 1} | −864.72 | −388.23 | 277.99 | −5809.20 | 16.54 | |
| acetonitrile | {0 1 1} | −881.48 | −456.95 | 372.18 | −6475.99 | 4.11 |
| {1 0 −1} | −758.41 | −335.41 | 267.48 | −6367.03 | 8.86 | |
| {1 1 0} | −1354.59 | −509.52 | 418.48 | −6424.70 | − | |
| {1 1 −1} | −930.51 | −389.32 | 293.35 | −6151.77 | 28.51 | |
| {0 0 2} | −340.98 | −193.02 | 100.17 | −4189.15 | 58.53 | |
| {1 0 1} | −1147.79 | −426.61 | 316.38 | −6287.97 | − | |
| acetone | {0 1 1} | −8454.70 | −6825.84 | 6741.06 | −5979.91 | 48.96 |
| {1 0 −1} | −178.58 | −148.39 | 60.47 | −5807.82 | 6.38 | |
| {1 1 0} | −560.59 | −326.85 | 235.81 | −5854.99 | 44.66 | |
| {1 1 −1} | −724.11 | −449.72 | 353.76 | −5391.30 | − | |
| {0 0 2} | −44.07 | −45.73 | −47.13 | −3798.27 | − | |
| {1 0 1} | −671.64 | −375.71 | 265.48 | −5671.363 | − |
| {h l k } | Eint/(kcal/mol) | Es/(kcal/mol) | Eatt */(kcal/mol) | CED/(kcal/mol) | Total Facet Area (%) | |
|---|---|---|---|---|---|---|
| DMSO | {1 0 0} | −258.23 | −461.19 | 441.08 | −2648.26 | − |
| {1 1 0} | −222.28 | −347.89 | 321.27 | −2633.03 | 53.62 | |
| {0 2 0} | −224.44 | −463.73 | 439.34 | −2025.01 | 8.95 | |
| {0 1 1} | −312.43 | −486.03 | 456.32 | −2665.47 | 29.59 | |
| {1 1 −1} | −340.49 | −510.72 | 480.34 | −2633.51 | 7.84 | |
| NMP | {1 0 0} | −163.59 | −357.24 | 337.13 | −2996.00 | − |
| {1 1 0} | −13.64 | −14.69 | −11.94 | −3031.07 | 97.97 | |
| {0 2 0} | −90.73 | −278.65 | 254.26 | −2249.33 | − | |
| {0 1 1} | −188.83 | −322.94 | 293.24 | −3104.75 | 2.03 | |
| {1 1 −1} | −246.51 | −339.18 | 308.79 | −3017.11 | − |
| {h l k} | Eint/(kcal/mol) | Es/(kcal/mol) | Eatt */(kcal/mol) | CED/(kcal/mol) | Total Facet Area (%) | |
|---|---|---|---|---|---|---|
| DMF | {0 1 1} | −256.06 | −182.86 | 145.83 | −4643.31 | 46.35 |
| {1 0 −1} | −225.41 | −162.57 | 125.96 | −4609.75 | 31.50 | |
| {0 0 2} | −253.23 | −244.83 | 180.99 | −3238.98 | 9.19 | |
| {1 0 1} | −332.22 | −243.21 | 193.77 | −4551.62 | 10.93 | |
| {1 1 0} | −355.10 | −254.96 | 208.45 | −4537.00 | 2.04 | |
| DMSO | {0 1 1} | −243.04 | −252.54 | 215.50 | −3915.75 | 29.29 |
| {1 0 −1} | −158.55 | −161.89 | 125.28 | −3842.51 | 36.76 | |
| {0 0 2} | −222.09 | −402.84 | 338.99 | −2642.95 | − | |
| {1 0 1} | −202.64 | −190.50 | 141.07 | −3834.91 | 31.15 | |
| {1 1 0} | −336.46 | −289.11 | 242.60 | −3635.65 | 2.81 | |
| NMP | {0 1 1} | −231.32 | −204.53 | 167.49 | −4817.41 | 26.78 |
| {1 0 −1} | −227.52 | −200.47 | 163.86 | −4750.23 | 14.81 | |
| {0 0 2} | −133.69 | −216.62 | 152.78 | −3403.93 | 21.33 | |
| {1 0 1} | −251.54 | −236.91 | 187.48 | −4556.55 | 8.65 | |
| {1 1 0} | −311.19 | −212.39 | 165.88 | −4630.11 | 28.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; An, C.; Liu, N.; Wang, M.; Ye, B.; Liao, D. Noncovalent Interactions and Crystal Structure Prediction of Energetic Materials. Molecules 2022, 27, 3755. https://doi.org/10.3390/molecules27123755
Liu Y, An C, Liu N, Wang M, Ye B, Liao D. Noncovalent Interactions and Crystal Structure Prediction of Energetic Materials. Molecules. 2022; 27(12):3755. https://doi.org/10.3390/molecules27123755
Chicago/Turabian StyleLiu, Yan, Chongwei An, Ning Liu, Minchang Wang, Baoyun Ye, and Dongjie Liao. 2022. "Noncovalent Interactions and Crystal Structure Prediction of Energetic Materials" Molecules 27, no. 12: 3755. https://doi.org/10.3390/molecules27123755
APA StyleLiu, Y., An, C., Liu, N., Wang, M., Ye, B., & Liao, D. (2022). Noncovalent Interactions and Crystal Structure Prediction of Energetic Materials. Molecules, 27(12), 3755. https://doi.org/10.3390/molecules27123755







