Monoterpenoid Glycosides from the Leaves of Ligustrum robustum and Their Bioactivities
Abstract
:1. Introduction
2. Material and Methods
2.1. General Experimental Procedure
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Acid Hydrolysis of Compounds 1–8
2.5. Enzymatic Hydrolysis of Compounds 1–2
2.6. Determination of Bioactivities
2.7. Statistical Analyses
3. Results and Discussion
3.1. Identification of Compounds 1–11
3.2. The Bioactivities of Compounds 1–11
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- He, Z.D.; Lau, K.M.; But, P.P.-H.; Jiang, R.W.; Dong, H.; Ma, S.C.; Fung, K.P.; Ye, W.C.; Sun, H.D. Antioxidative glycosides from the leaves of Ligustrum robustum. J. Nat. Prod. 2003, 66, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.M.; Liu, F.; He, Z.D.; Ji, M.; Chu, X.X.; Kang, Z.Y.; Cai, D.Y.; Gao, N.N. Anti-obesity effect of total phenylpropanoid glycosides from Ligustrum robustum Blume in fatty diet-fed mice via up-regulating leptin. J. Ethnopharmacol. 2015, 169, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Ke, J.X.; Lu, D.Y.; Corke, H. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum. J. Agric. Food Chem. 2009, 57, 6082–6089. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.M.; He, Z.D.; Dong, H.; Fung, K.P.; But, P.P.-H. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J. Ethnopharmacol. 2002, 83, 63–71. [Google Scholar] [CrossRef]
- Xie, Z.M.; Zhou, T.; Liao, H.Y.; Ye, Q.; Liu, S.; Qi, L.; Huang, J.; Zuo, H.J.; Pei, X.F. Effects of Ligustrum robustum on gut microbes and obesity in rats. World J. Gastroenterol. 2015, 21, 13042–13054. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Xu, L.J.; Wu-Lan, T.N.; Shi, R.B.; Xiao, P.G. Chemical constituents from Ligustrum robustum Bl. Biochem. Syst. Ecol. 2010, 38, 398–401. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Liu, Y.; Xu, L.J.; Guo, N.; Shi, R.B.; Xiao, P.G. Two new phenethanol glycosides from Ligustrum robustum. Chin. Chem. Lett. 2011, 22, 326–329. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, H.J.; Sun, H.D.; Pan, L.T.; Yao, P.; Chen, D.Y. Monoterpenoid glycosides from Ligustrum robustum. Phytochemistry 1998, 48, 1013–1018. [Google Scholar] [CrossRef]
- Tian, J.; Sun, H.D. New monoterpenoid glycosides from Ligustrum robustum. Chin. J. Appl. Environ. Biol. 1999, 5, 501–506. [Google Scholar]
- Yu, Z.L.; Zeng, W.C. Antioxidant, antibrowning, and cytoprotective activities of Ligustrum robustum (Roxb.) Blume extract. J. Food Sci. 2013, 78, 1354–1362. [Google Scholar]
- Yu, Z.L.; Gao, H.X.; Zhang, Z.; He, Z.; He, Q.; Jia, L.R.; Zeng, W.C. Inhibitory effects of Ligustrum robustum (Roxb.) Blume extract on α-amylase and α-glucosidase. J. Funct. Foods 2015, 19, 204–213. [Google Scholar] [CrossRef]
- Lu, S.-H.; Zuo, H.-J.; Shi, J.-X.; Li, C.-R.; Li, Y.-H.; Wang, X.; Li, L.-R.; Huang, J. Two new glycosides from the leaves of Ligustrum robustum and their antioxidant activities and inhibitory effects on α-glucosidase and α-amylase. S. Afr. J. Bot. 2019, 125, 521–526. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, J.E.; Han, J.S. Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur. J. Pharmacol. 2018, 834, 152–156. [Google Scholar] [CrossRef]
- Sirdah, M.M. Protective and therapeutic effectiveness of taurine in diabetes mellitus: A rationale for antioxidant supplementation. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Spadiene, A.; Savickiene, N.; Ivanauskas, L.; Jakstas, V.; Skesters, A.; Silova, A.; Rodovicius, H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J. Food Drug Anal. 2014, 22, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Zemestani, M.; Rafraf, M.; Asghari-Jafarabadi, M. Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrition 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Buckley, D.; Duke, G.; Heuer, T.S.; O’Farrell, M.; Wagman, A.S.; McCulloch, W.; Kemble, G. Fatty acid synthase—Modern tumor cell biology insights into a classical oncology target. Pharmacol. Therapeut. 2017, 177, 23–31. [Google Scholar] [CrossRef]
- Fan, H.J.; Wu, D.; Tian, W.X.; Ma, X.F. Inhibitory effects of tannic acid on fatty acid synthase and 3T3-L1 preadipocyte. Biochim. Biophys. Acta 2013, 1831, 1260–1266. [Google Scholar] [CrossRef]
- Lu, T.B.; Schubert, C.; Cummings, M.D.; Bignan, G.; Connolly, P.J.; Smans, K.; Ludovici, D.; Parker, M.H.; Meyer, C.; Rocaboy, C.; et al. Design and synthesis of a series of bioavailable fatty acid synthase (FASN) KR domain inhibitors for cancer therapy. Bioorg. Med. Chem. Lett. 2018, 28, 2159–2164. [Google Scholar] [CrossRef]
- Wu, D.; Ma, X.F.; Tian, W.X. Pomegranate husk extract, punicalagin and ellagic acid inhibit fatty acid synthase and adipogenesis of 3T3-L1 adipocyte. J. Func. Foods 2013, 5, 633–641. [Google Scholar] [CrossRef]
- He, Z.D.; Ueda, S.; Akaji, M.; Fujita, T.; Inoue, K.; Yang, C.R. Monoterpenoid and phenylethanoid glycosides from Ligustrum Pedunculare. Phytochemistry 1994, 36, 709–716. [Google Scholar] [CrossRef]
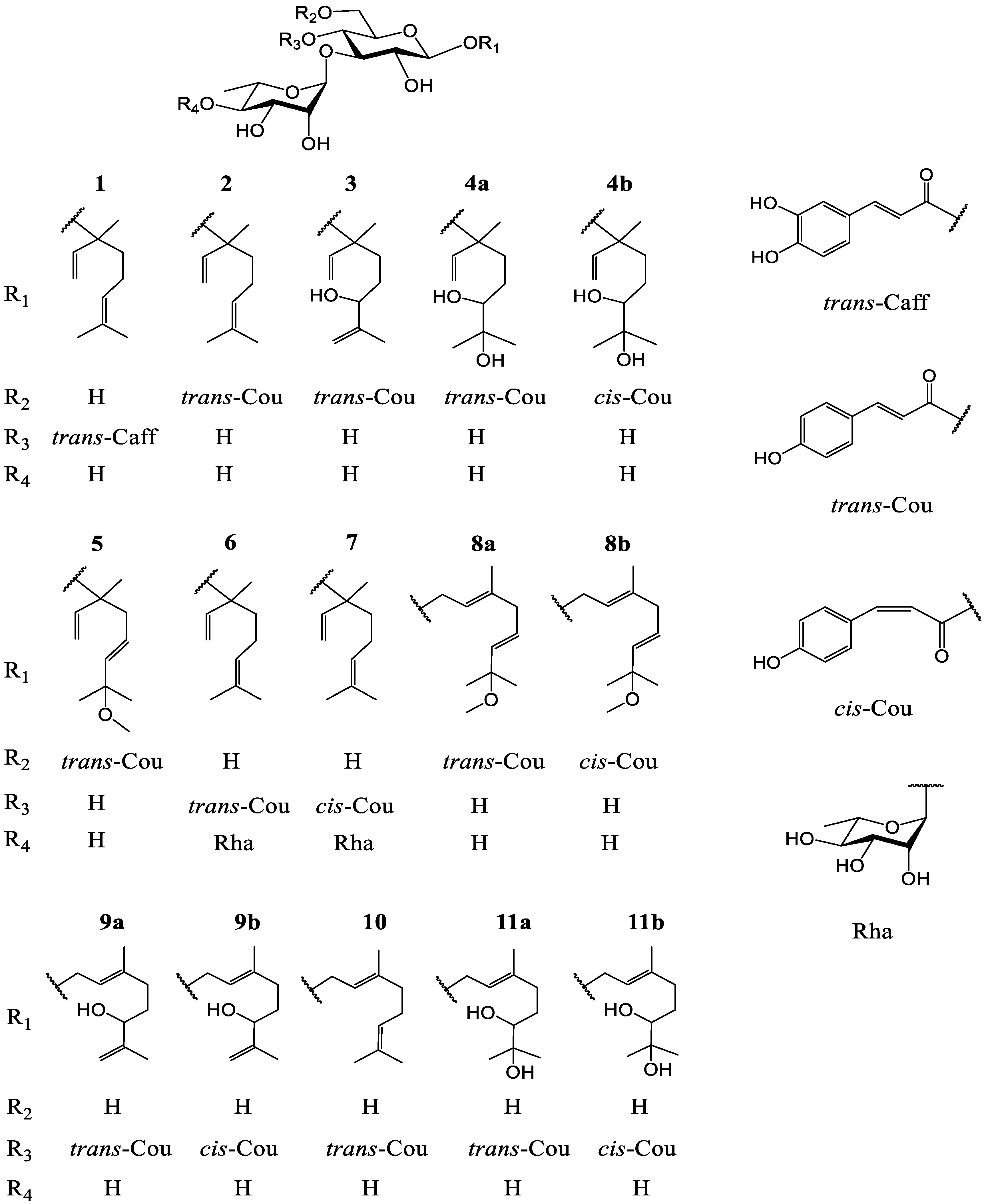

| No | 1 b | 2 b | 3 c | 4a b | 4b b |
| 1 | 5.22 dd (10.8, 1.2) | 5.19 dd (10.8, 1.2) | 5.19 br. d (10.8) | 5.19 dd (10.8, 2.0) | 5.19 dd (10.8, 2.0) |
| 5.26 dd (17.6, 1.2) | 5.23 dd (18.0, 1.2) | 5.24 br. d (18.0) | 5.24 dd (18.0, 2.0) | 5.24 dd (18.0, 2.0) | |
| 2 | 5.93 dd (17.6, 10.8) | 5.90 dd (18.0, 10.8) | 5.90 dd (18.0, 10.8) | 5.92 dd (18.0, 10.8) | 5.92 dd (18.0, 10.8) |
| 4 | 1.58 m | 1.56 m | 1.22 dd (10.8, 2.4) | 1.57 m | 1.57 m |
| 1.62 m | 1.60 m | 1.90 m | 1.90 m | ||
| 5 | 2.04 m | 2.02 m | 1.60 m | 1.32 m | 1.32 m |
| 1.70 m | 1.70 m | ||||
| 6 | 5.10 tt (7.2, 1.6) | 5.07 tt (7.2, 1.2) | 3.95 m | 3.21 dd (10.4, 2.0) | 3.21 dd (10.4, 2.0) |
| 8 | 1.67 s | 1.62 br. s | 4.78 br. s | 1.11 s | 1.11 s |
| 4.88 br. s | |||||
| 9 | 1.60 s | 1.56 br. s | 1.67 s | 1.14 s | 1.14 s |
| 10 | 1.39 s | 1.34 s | 1.35 s | 1.36 s | 1.36 s |
| 7-OCH3 | |||||
| Glc | |||||
| 1′ | 4.43 d (8.0) | 4.39 d (8.0) | 4.38 d (8.4) | 4.41 d (8.0) | 4.36 d (8.0) |
| 2′ | 3.36 m | 3.29 m | 3.29 m | 3.29 m | 3.27 m |
| 3′ | 3.77 t (9.2) | 3.49 m | 3.48 m | 3.50 t (8.8) | 3.46 t (8.8) |
| 4′ | 4.89 m | 3.34 m | 3.32 m | 3.33 m | 3.29 m |
| 5′ | 3.45 m | 3.49 m | 3.48 m | 3.47 m | 3.42 m |
| 6′ | 3.49 m | 4.30 dd (12.0, 6.8) | 4.30 dd (12.0, 6.6) | 4.30 dd (12.0, 6.0) | 4.25 dd (12.0, 6.0) |
| 3.57 m | 4.45 dd (12.0, 2.4) | 4.45 dd (12.0, 2.4) | 4.45 dd (12.0, 2.4) | 4.40 dd (12.0, 2.4) | |
| inner- Rha | |||||
| 1″ | 5.18 d (1.6) | 5.17 d (2.0) | 5.17 d (1.8) | 5.18 d (2.0) | 5.15 d (2.0) |
| 2″ | 3.91 dd (3.2, 1.6) | 3.94 dd (3.2, 2.0) | 3.94 m | 3.94 dd (3.2, 2.0) | 3.94 dd (3.2, 2.0) |
| 3″ | 3.58 m | 3.70 dd (9.6, 3.2) | 3.70 dd (9.6, 3.6) | 3.70 dd (9.6, 3.2) | 3.70 dd (9.6, 3.2) |
| 4″ | 3.29 t (9.6) | 3.40 t (9.6) | 3.39 t (9.6) | 3.39 t (9.6) | 3.39 t (9.6) |
| 5″ | 3.56 m | 4.00 dd (9.6, 6.4) | 4.00 m | 3.99 dd (9.6, 6.4) | 3.99 dd (9.6, 6.4) |
| 6″ | 1.08 d (6.4) | 1.25 d (6.4) | 1.24 d (6.6) | 1.25 d (6.4) | 1.24 d (6.4) |
| outer- Rha | |||||
| 1′′′ | |||||
| 2′′′ | |||||
| 3′′′ | |||||
| 4′′′ | |||||
| 5′′′ | |||||
| 6′′′ | |||||
| Ester | |||||
| 2′′′′ | 7.05 d (2.0) | 7.45 d (8.8) | 7.46 d (8.4) | 7.46 d (8.8) | 7.64 d (8.8) |
| 3′′′′ | 6.81 d (8.8) | 6.80 d (8.4) | 6.81 d (8.8) | 6.76 d (8.8) | |
| 5′′′′ | 6.77 d (8.0) | 6.81 d (8.8) | 6.80 d (8.4) | 6.81 d (8.8) | 6.76 d (8.8) |
| 6′′′′ | 6.95 dd (8.0, 2.0) | 7.45 d (8.8) | 7.46 d (8.4) | 7.46 d (8.8) | 7.64 d (8.8) |
| 7′′′′ | 7.58 d (16.0) | 7.64 d (16.0) | 7.64 d (16.2) | 7.64 d (16.0) | 6.87 d (12.8) |
| 8′′′′ | 6.27 d (16.0) | 6.33 d (16.0) | 6.33 d (16.2) | 6.34 d (16.0) | 5.78 d (12.8) |
| No | 5 b | 6 b | 7 b | 8a b | 8b b |
| 1 | 5.19 dd (10.8, 1.2) | 5.23 dd (10.8, 1.6) | 5.22 dd (10.8, 1.6) | 4.27 d (8.0) | 4.27 d (8.0) |
| 5.22 dd (17.6, 1.2) | 5.25 dd (17.6, 1.6) | 5.24 dd (17.6, 1.6) | |||
| 2 | 5.90 dd (17.6, 10.8) | 5.93 dd (17.6, 10.8) | 5.92 dd (17.6, 10.8) | 5.41 t (8.0) | 5.41 t (8.0) |
| 4 | 2.36 d (7.2) | 1.58 m | 1.58 m | 2.76 d (10.2) | 2.76 d (10.2) |
| 1.62 m | 1.62 m | ||||
| 5 | 5.64 dt (16.0, 7.2) | 2.05 m | 2.04 m | 5.55 m | 5.55 m |
| 6 | 5.40 d (16.0) | 5.10 m | 5.10 m | 5.44 d (15.6) | 5.44 d (15.6) |
| 8 | 1.20 s | 1.67 s | 1.67 s | 1.23 s | 1.23 s |
| 9 | 1.20 s | 1.60 s | 1.60 s | 1.23 s | 1.23 s |
| 10 | 1.33 s | 1.39 s | 1.38 s | 1.65 s | 1.65 s |
| 7-OCH3 | 3.09 s | 3.12 s | 3.12 s | ||
| Glc | |||||
| 1′ | 4.41 d (8.0) | 4.44 d (7.6) | 4.41 d (8.0) | 4.31 d (8.0) | 4.27 d (8.0) |
| 2′ | 3.31 m | 3.37 m | 3.37 m | 3.30 m | 3.28 m |
| 3′ | 3.50 t (8.8) | 3.77 t (9.6) | 3.77 t (9.6) | 3.51 m | 3.46 m |
| 4′ | 3.35 m | 4.91 t (9.6) | 4.86 t (9.6) | 3.37 m | 3.33 m |
| 5′ | 3.49 m | 3.46 m | 3.46 m | 3.51 m | 3.47 m |
| 6′ | 4.32 dd (12.0, 7.2) | 3.50 m | 3.50 m | 4.35 dd (12.0, 6.0) | 4.31 dd (12.0, 6.0) |
| 4.45 dd (12.0, 2.0) | 3.57 m | 3.57 m | 4.50 dd (12.0, 2.0) | 4.48 dd (12.0, 2.0) | |
| inner- Rha | |||||
| 1″ | 5.17 d (2.0) | 5.19 d (2.0) | 5.29 d (2.0) | 5.17 d (2.0) | 5.16 d (2.0) |
| 2″ | 3.94 dd (3.6, 2.0) | 3.86 dd (3.2, 2.0) | 3.82 dd (3.2, 2.0) | 3.94 m | 3.92 m |
| 3″ | 3.70 dd (9.6, 3.6) | 3.68 dd (9.6, 3.2) | 3.68 dd (9.6, 3.2) | 3.70 dd (9.6, 3.2) | 3.68 dd (9.6, 3.2) |
| 4″ | 3.40 t (9.6) | 3.39 m | 3.45 m | 3.40 m | 3.40 m |
| 5″ | 4.00 dd (9.6, 6.4) | 3.59 m | 3.60 m | 4.00 dd (9.6, 6.4) | 4.00 dd (9.6, 6.4) |
| 6″ | 1.25 d (6.4) | 1.08 d (6.0) | 1.21 d (6.4) | 1.24 d (6.4) | 1.23 d (6.4) |
| outer- Rha | |||||
| 1′′′ | 5.04 d (2.0) | 5.13 d (2.0) | |||
| 2′′′ | 3.90 dd (3.2, 2.0) | 3.82 dd (3.2, 2.0) | |||
| 3′′′ | 3.51 m | 3.51 m | |||
| 4′′′ | 3.32 m | 3.34 m | |||
| 5′′′ | 3.46 m | 3.46 m | |||
| 6′′′ | 1.04 d (6.0) | 1.21 d (6.4) | |||
| Ester | |||||
| 2′′′′ | 7.45 d (8.4) | 7.48 d (8.4) | 7.72 d (8.4) | 7.45 d (8.4) | 7.65 d (8.4) |
| 3′′′′ | 6.81 d (8.4) | 6.82 d (8.4) | 6.77 d (8.4) | 6.80 d (8.4) | 6.75 d (8.4) |
| 5′′′′ | 6.81 d (8.4) | 6.82 d (8.4) | 6.77 d (8.4) | 6.80 d (8.4) | 6.75 d (8.4) |
| 6′′′′ | 7.45 d (8.4) | 7.48 d (8.4) | 7.72 d (8.4) | 7.45 d (8.4) | 7.65 d (8.4) |
| 7′′′′ | 7.63 d (16.0) | 7.66 d (16.0) | 6.98 d (12.8) | 7.64 d (16.0) | 6.87 d (12.8) |
| 8′′′′ | 6.32 d (16.0) | 6.33 d (16.0) | 5.76 d (12.8) | 6.35 d (16.0) | 5.79 d (12.8) |
| No | 1 a | 2 a | 3 a | 4a a | 4b a |
| 1 | 115.9 | 115.7 | 115.8 | 115.9 | 115.9 |
| 2 | 144.3 | 144.3 | 144.3 | 144.3 | 144.3 |
| 3 | 81.6 | 81.5 | 81.4 | 81.5 | 81.5 |
| 4 | 42.6 | 42.5 | 30.2 | 39.9 | 39.9 |
| 5 | 23.6 | 23.6 | 30.1 | 26.4 | 26.4 |
| 6 | 125.7 | 125.7 | 76.9 | 80.1 | 80.1 |
| 7 | 132.2 | 132.1 | 148.7 | 73.9 | 73.9 |
| 8 | 25.9 | 25.8 | 111.4 | 24.9 | 24.9 |
| 9 | 17.7 | 17.7 | 17.7 | 25.8 | 25.8 |
| 10 | 23.2 | 23.5 | 23.5 | 23.9 | 23.9 |
| 7-OCH3 | |||||
| Glc | |||||
| 1′ | 99.4 | 99.3 | 99.4 | 99.4 | 99.3 |
| 2′ | 76.3 | 75.7 | 75.8 | 75.8 | 75.8 |
| 3′ | 82.0 | 84.4 | 84.4 | 84.2 | 84.2 |
| 4′ | 70.7 | 70.8 | 70.8 | 70.7 | 70.7 |
| 5′ | 75.7 | 75.0 | 75.1 | 75.1 | 75.0 |
| 6′ | 62.5 | 65.0 | 64.9 | 64.9 | 62.7 |
| inner-Rha | |||||
| 1″ | 103.1 | 102.8 | 102.8 | 102.7 | 102.4 |
| 2″ | 72.4 | 72.4 | 72.4 | 72.4 | 72.4 |
| 3″ | 72.0 | 72.3 | 72.3 | 72.3 | 72.3 |
| 4″ | 73.8 | 74.0 | 74.0 | 74.0 | 74.0 |
| 5″ | 70.4 | 70.0 | 70.0 | 70.0 | 70.0 |
| 6″ | 18.5 | 17.9 | 17.9 | 17.9 | 17.9 |
| outer-Rha | |||||
| 1′′′ | |||||
| 2′′′ | |||||
| 3′′′ | |||||
| 4′′′ | |||||
| 5′′′ | |||||
| 6′′′ | |||||
| Ester | |||||
| 1′′′′ | 127.6 | 127.1 | 126.9 | 127.1 | 127.6 |
| 2′′′′ | 115.2 | 131.1 | 131.2 | 131.2 | 133.8 |
| 3′′′′ | 146.8 | 116.9 | 117.0 | 116.9 | 115.9 |
| 4′′′′ | 149.8 | 161.5 | 161.9 | 161.4 | 160.2 |
| 5′′′′ | 116.5 | 116.9 | 117.0 | 116.9 | 115.9 |
| 6′′′′ | 123.2 | 131.1 | 131.2 | 131.2 | 133.8 |
| 7′′′′ | 148.0 | 146.7 | 148.7 | 146.8 | 145.3 |
| 8′′′′ | 114.7 | 115.0 | 114.8 | 115.0 | 116.2 |
| CO | 168.3 | 169.0 | 169.0 | 169.0 | 168.1 |
| No | 5 a | 6 b | 7 b | 8a a | 8b a |
| 1 | 116.0 | 115.9 | 115.9 | 66.3 | 66.3 |
| 2 | 144.0 | 144.3 | 144.3 | 122.3 | 122.3 |
| 3 | 81.2 | 81.6 | 81.6 | 140.9 | 140.9 |
| 4 | 45.5 | 42.6 | 42.6 | 43.5 | 43.5 |
| 5 | 127.4 | 23.6 | 23.7 | 129.3 | 129.3 |
| 6 | 139.2 | 125.7 | 125.7 | 138.1 | 138.1 |
| 7 | 76.5 | 132.2 | 132.2 | 76.4 | 76.4 |
| 8 | 26.1 | 25.9 | 25.9 | 26.2 | 26.2 |
| 9 | 26.1 | 17.7 | 17.7 | 26.2 | 26.2 |
| 10 | 23.5 | 23.2 | 23.1 | 16.6 | 16.6 |
| 7-OCH3 | 50.7 | 50.6 | 50.6 | ||
| Glc | |||||
| 1′ | 99.3 | 99.4 | 99.4 | 102.6 | 102.6 |
| 2′ | 75.8 | 76.3 | 76.5 | 75.6 | 75.6 |
| 3′ | 84.2 | 81.9 | 79.8 | 84.0 | 84.0 |
| 4′ | 70.8 | 70.6 | 70.4 | 70.5 | 70.4 |
| 5′ | 75.0 | 75.7 | 75.6 | 75.5 | 75.4 |
| 6′ | 64.9 | 62.4 | 62.5 | 64.7 | 64.5 |
| inner-Rha | |||||
| 1″ | 102.8 | 102.7 | 101.9 | 102.7 | 102.8 |
| 2″ | 72.4 | 72.7 | 72.9 | 72.4 | 72.4 |
| 3″ | 72.3 | 72.9 | 73.0 | 72.2 | 72.2 |
| 4″ | 74.0 | 81.7 | 80.6 | 74.0 | 74.0 |
| 5″ | 70.0 | 68.9 | 68.6 | 70.0 | 70.3 |
| 6″ | 17.9 | 19.2 | 18.9 | 17.9 | 17.9 |
| outer-Rha | |||||
| 1′′′ | 103.5 | 103.2 | |||
| 2′′′ | 72.3 | 72.3 | |||
| 3′′′ | 72.3 | 72.3 | |||
| 4′′′ | 73.8 | 73.9 | |||
| 5′′′ | 70.3 | 70.3 | |||
| 6′′′ | 17.7 | 17.8 | |||
| Ester | |||||
| 1′′′′ | 127.1 | 127.0 | 127.5 | 126.9 | 127.5 |
| 2′′′′ | 131.2 | 131.4 | 134.3 | 131.2 | 133.8 |
| 3′′′′ | 116.9 | 117.0 | 116.0 | 117.0 | 116.0 |
| 4′′′′ | 161.4 | 161.5 | 160.3 | 161.7 | 160.3 |
| 5′′′′ | 116.9 | 117.0 | 116.0 | 117.0 | 116.0 |
| 6′′′′ | 131.2 | 131.4 | 134.3 | 131.2 | 133.8 |
| 7′′′′ | 146.7 | 147.5 | 147.4 | 146.9 | 145.3 |
| 8′′′′ | 115.0 | 114.8 | 115.8 | 114.8 | 116.2 |
| CO | 168.9 | 168.2 | 166.9 | 169.1 | 168.1 |
| Compounds | FAS IC50 (μM) b | α-Glucosidase Inhibition at 0.1 mM (%) | α-Amylase Inhibition at 0.1 mM (%) | DPPH IC50 (μM) b | ABTS•+ IC50 (μM) b |
|---|---|---|---|---|---|
| 1 | NA c | NA | NA | 19.74 ± 0.23 b | 6.91 ± 0.10 a |
| 2 | 2.36 ± 0.10 a | 48.1 ± 4.3 b | 31.5 ± 0.5 b | >250 | 9.41 ± 0.22 c |
| 3 | 21.77 ± 0.38 c | 27.3 ± 0.3 c | 32.5 ± 6.3 b | NA | 16.00 ± 0.69 g |
| 4 | >100 | NA | 28.2 ± 3.9 b | NA | 9.66 ± 0.17 cd |
| 5 | 23.71 ± 0.45 d | 13.8 ± 2.0 d | 35.6 ± 2.0 b | NA | 6.93 ± 0.01 a |
| 6 | 4.78 ± 0.14 b | 12.0 ± 1.7 d | 26.1 ± 3.0 b | NA | 11.30 ± 0.16 e |
| 7 | >100 | NA | NA | NA | 20.21 ± 0.33 j |
| 8 | 25.83 ± 0.47 e | 24.7 ± 3.5 c | 31.4 ± 1.9 b | NA | 19.50 ± 0.46 i |
| 9 | 21.67 ± 0.46 c | 12.4 ± 5.6 d | 29.2 ± 8.4 b | NA | 18.66 ± 0.47 h |
| 10 | 4.68 ± 0.16 b | 28.7 ± 2.1 c | NA | NA | 15.10 ± 0.10 f |
| 11 | 61.74 ± 0.45 f | NA | 31.3 ± 1.3 b | NA | 7.92 ± 0.23 b |
| Orlistat d | 4.46 ± 0.13 b | ||||
| Acarbose d | 93.2 ± 0.1 a | 51.8 ± 2.5 a | |||
| L-(+)-ascorbic acid d | 13.66 ± 0.13 a | 10.06 ± 0.19 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.-H.; Huang, J.; Zuo, H.-J.; Zhou, Z.-B.; Yang, C.-Y.; Huang, Z.-L. Monoterpenoid Glycosides from the Leaves of Ligustrum robustum and Their Bioactivities. Molecules 2022, 27, 3709. https://doi.org/10.3390/molecules27123709
Lu S-H, Huang J, Zuo H-J, Zhou Z-B, Yang C-Y, Huang Z-L. Monoterpenoid Glycosides from the Leaves of Ligustrum robustum and Their Bioactivities. Molecules. 2022; 27(12):3709. https://doi.org/10.3390/molecules27123709
Chicago/Turabian StyleLu, Shi-Hui, Jing Huang, Hao-Jiang Zuo, Zhong-Bo Zhou, Cai-Yan Yang, and Zu-Liang Huang. 2022. "Monoterpenoid Glycosides from the Leaves of Ligustrum robustum and Their Bioactivities" Molecules 27, no. 12: 3709. https://doi.org/10.3390/molecules27123709
APA StyleLu, S.-H., Huang, J., Zuo, H.-J., Zhou, Z.-B., Yang, C.-Y., & Huang, Z.-L. (2022). Monoterpenoid Glycosides from the Leaves of Ligustrum robustum and Their Bioactivities. Molecules, 27(12), 3709. https://doi.org/10.3390/molecules27123709








