Mass Balance and Compositional Analysis of Biomass Outputs from Cacao Fruits
Abstract
1. Introduction
2. Results and Discussion
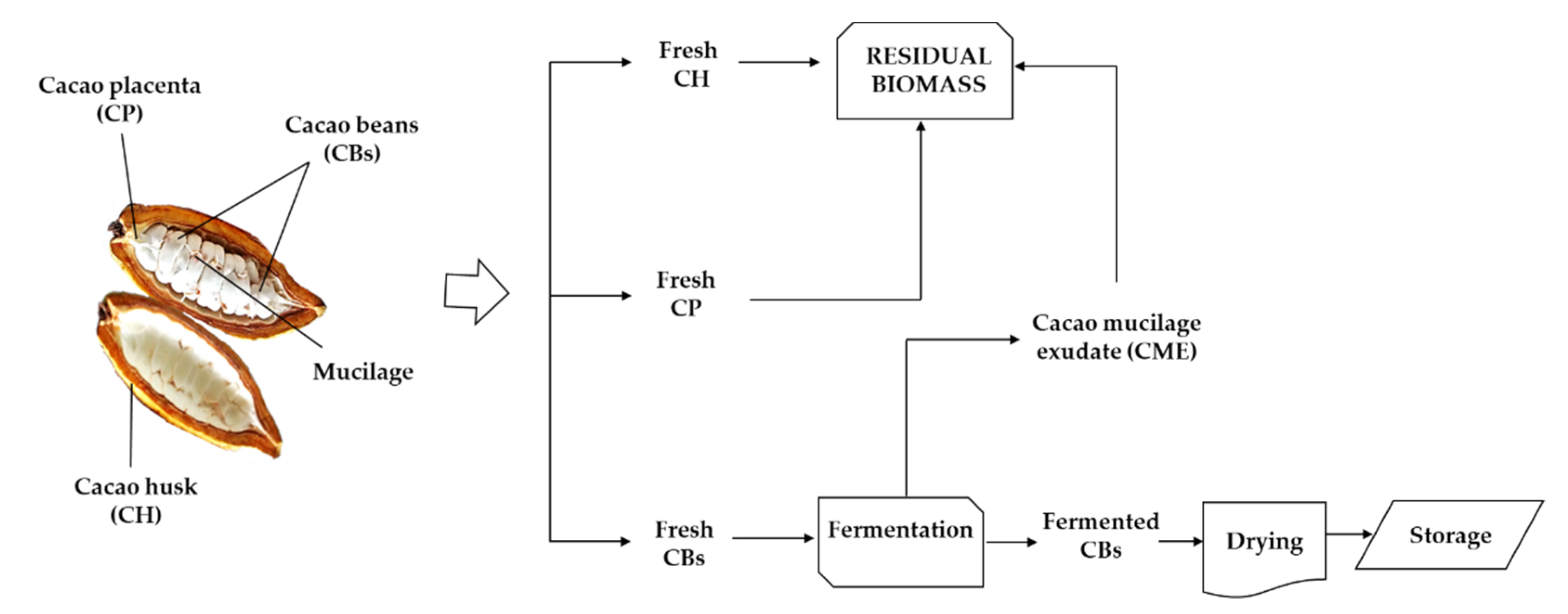
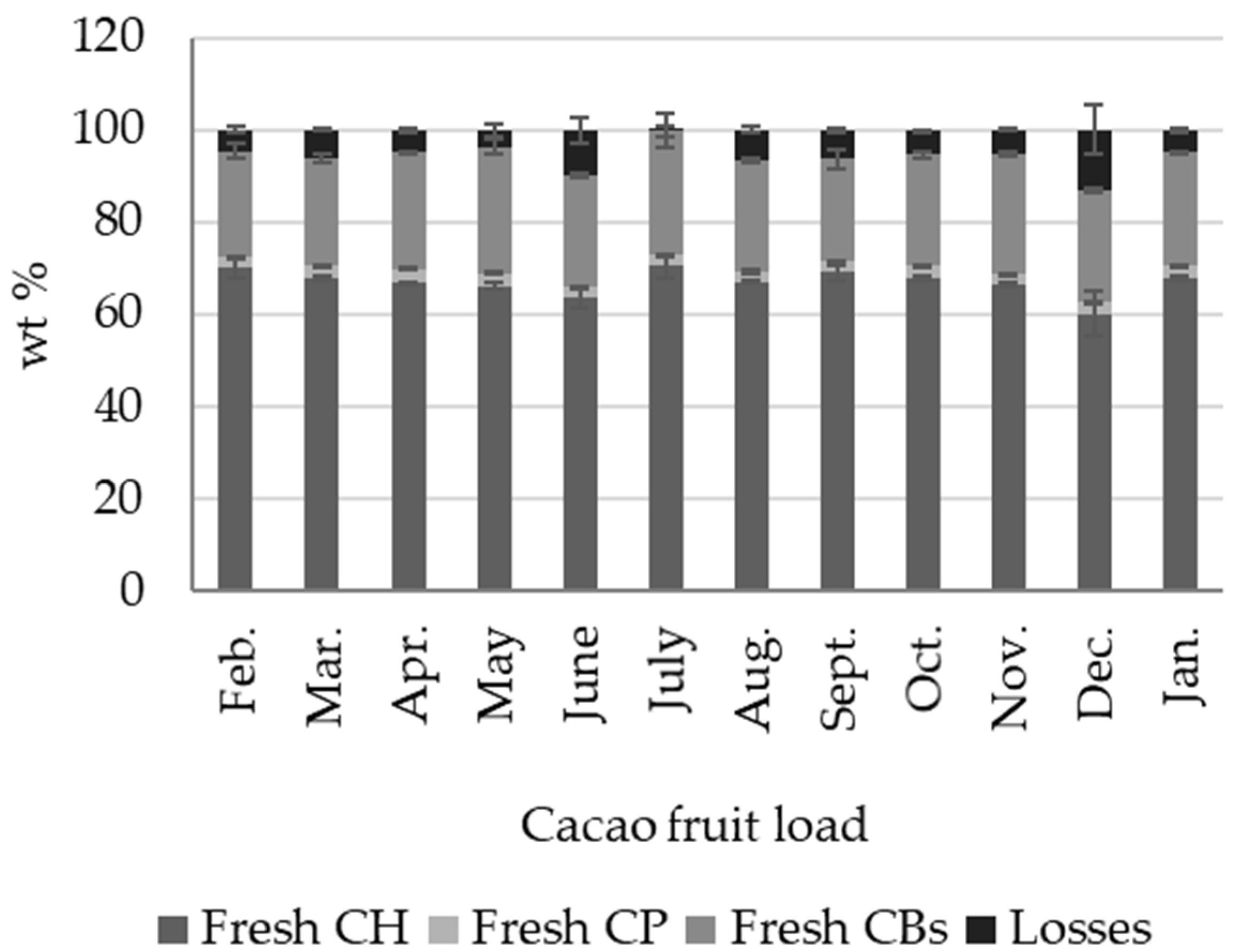
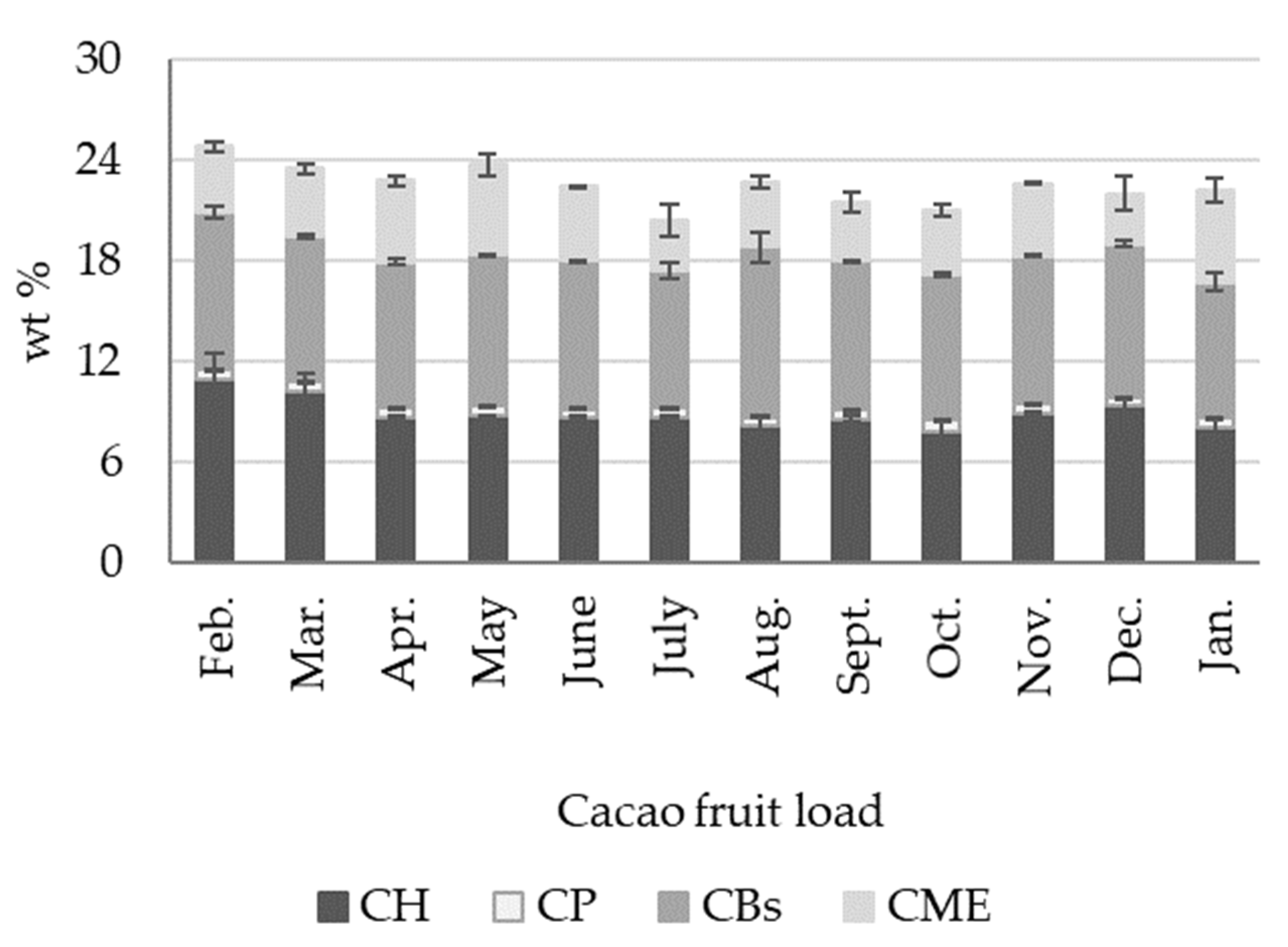
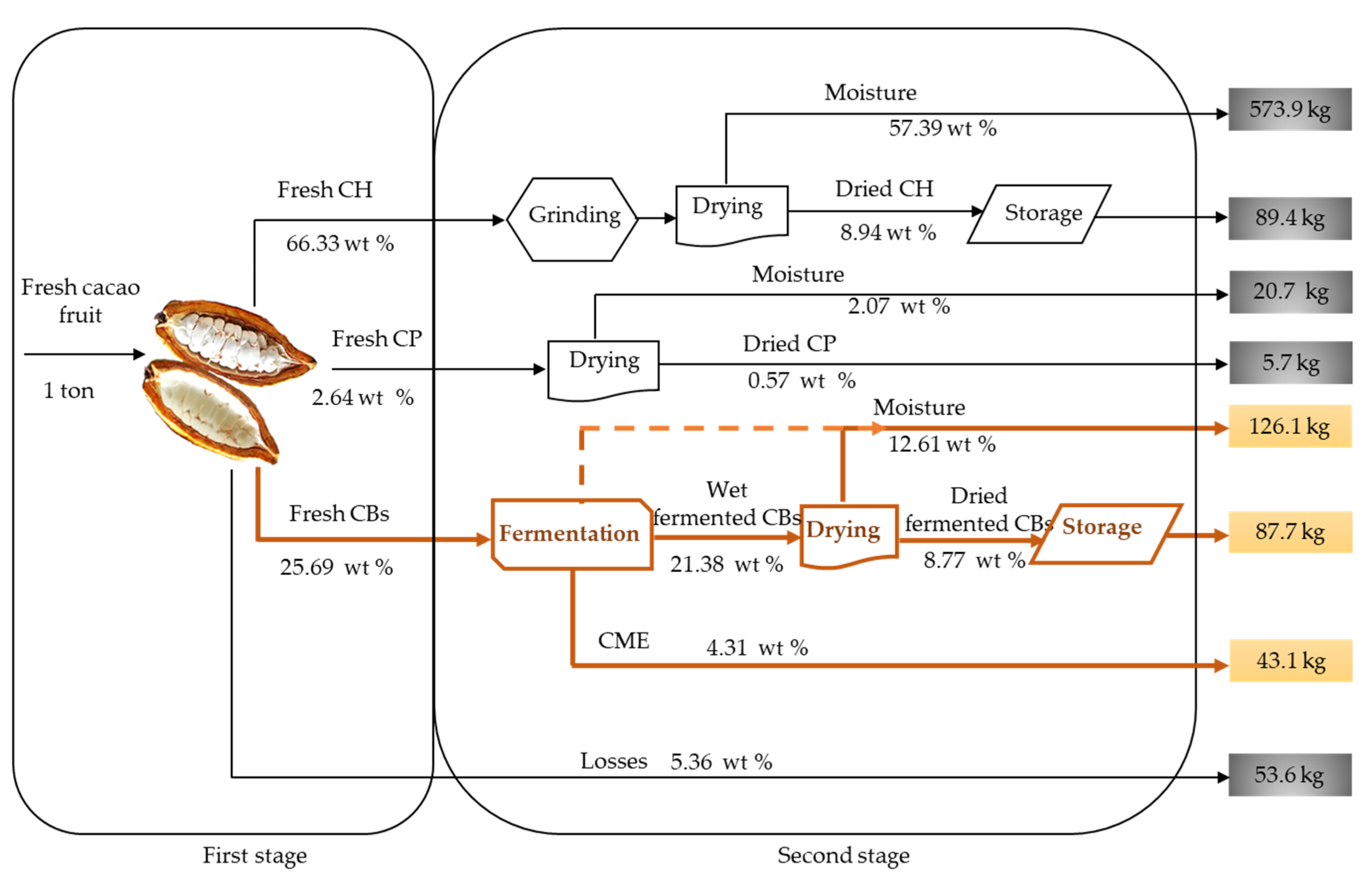
2.1. Cacao Fruit Mass Balance
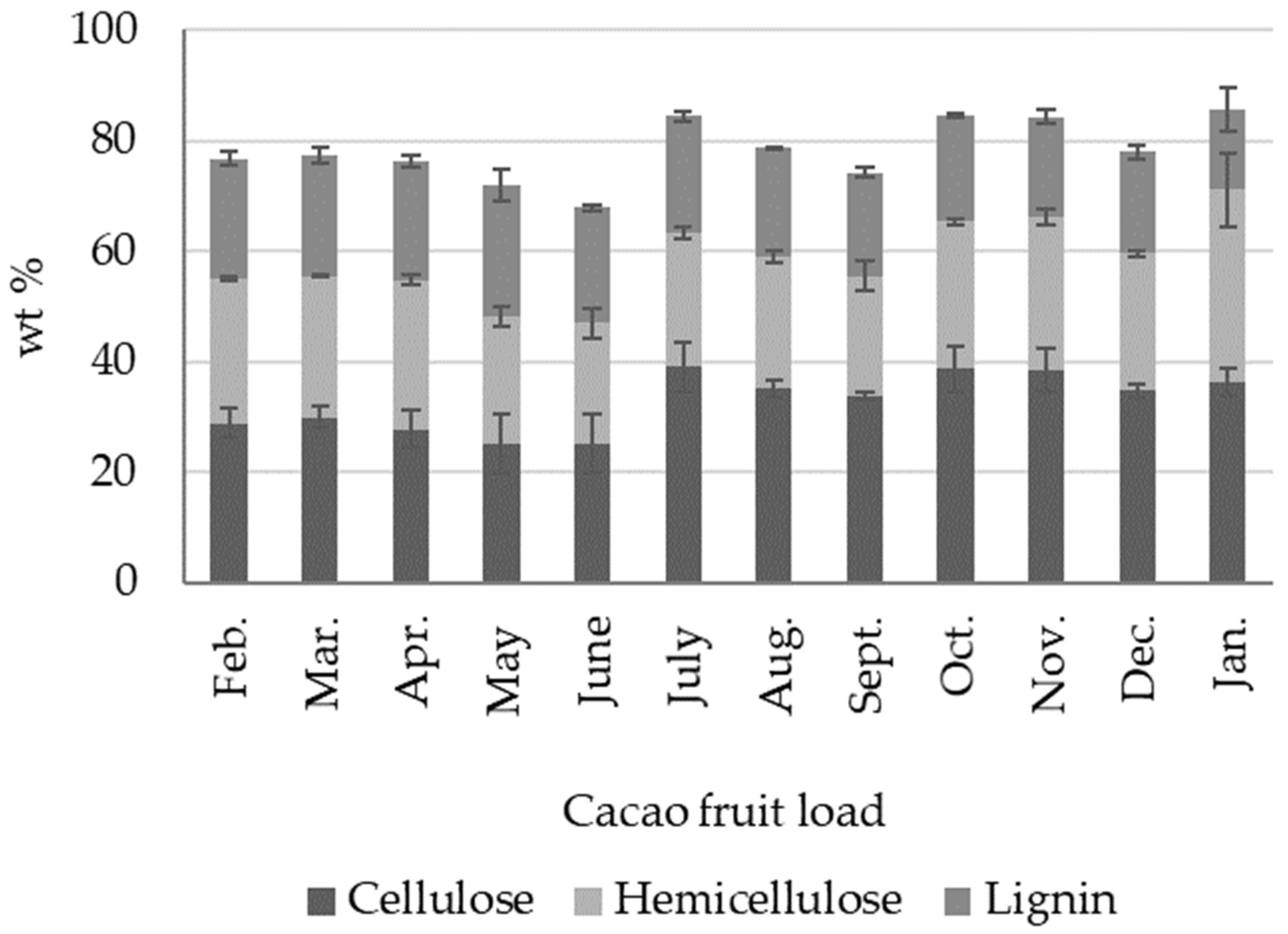
2.2. Compositional Analysis
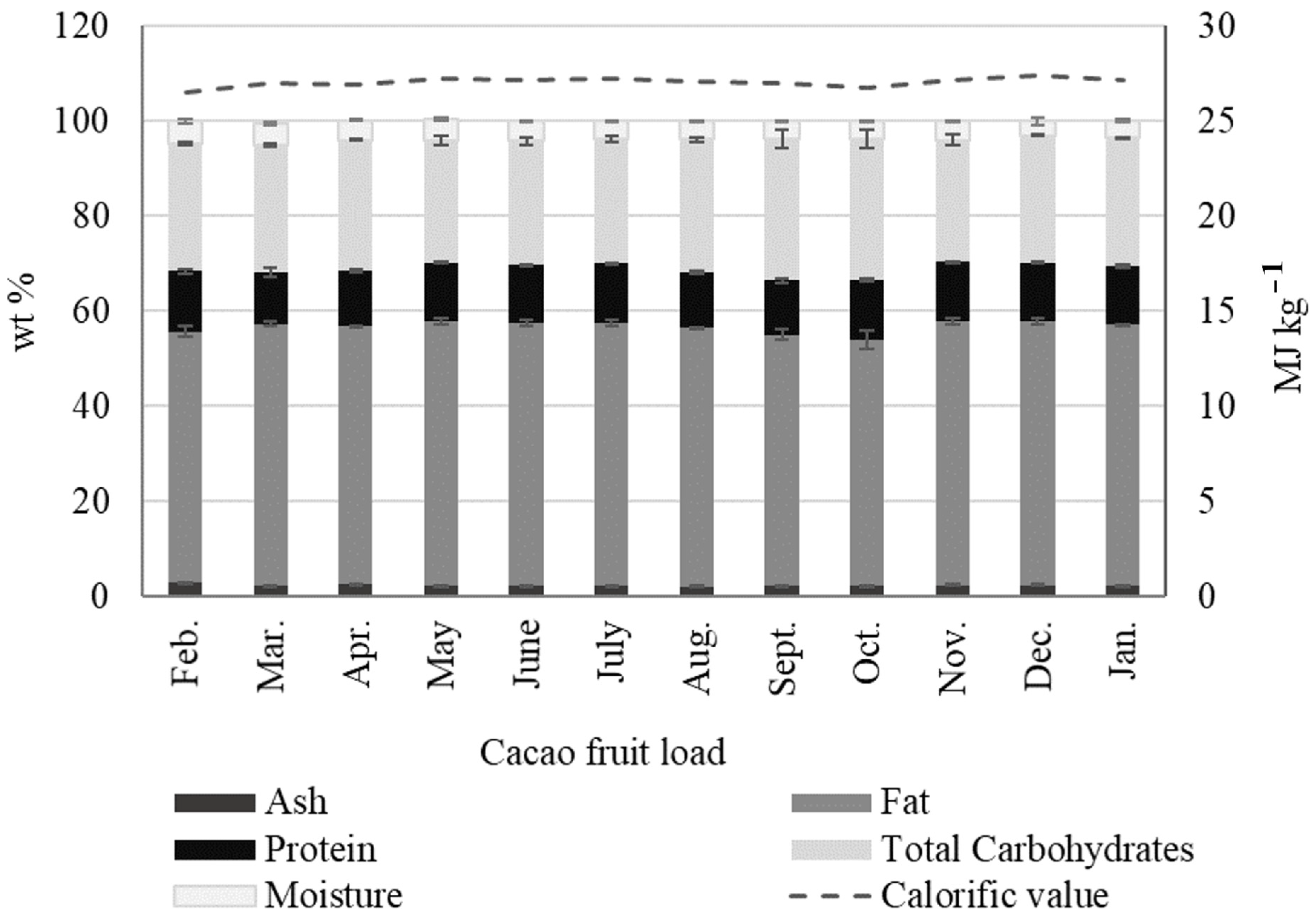
2.2.1. Cacao Fruit Husk (CH)
2.2.2. Cacao Beans (CBs)
2.2.3. Cacao Placenta (CP)
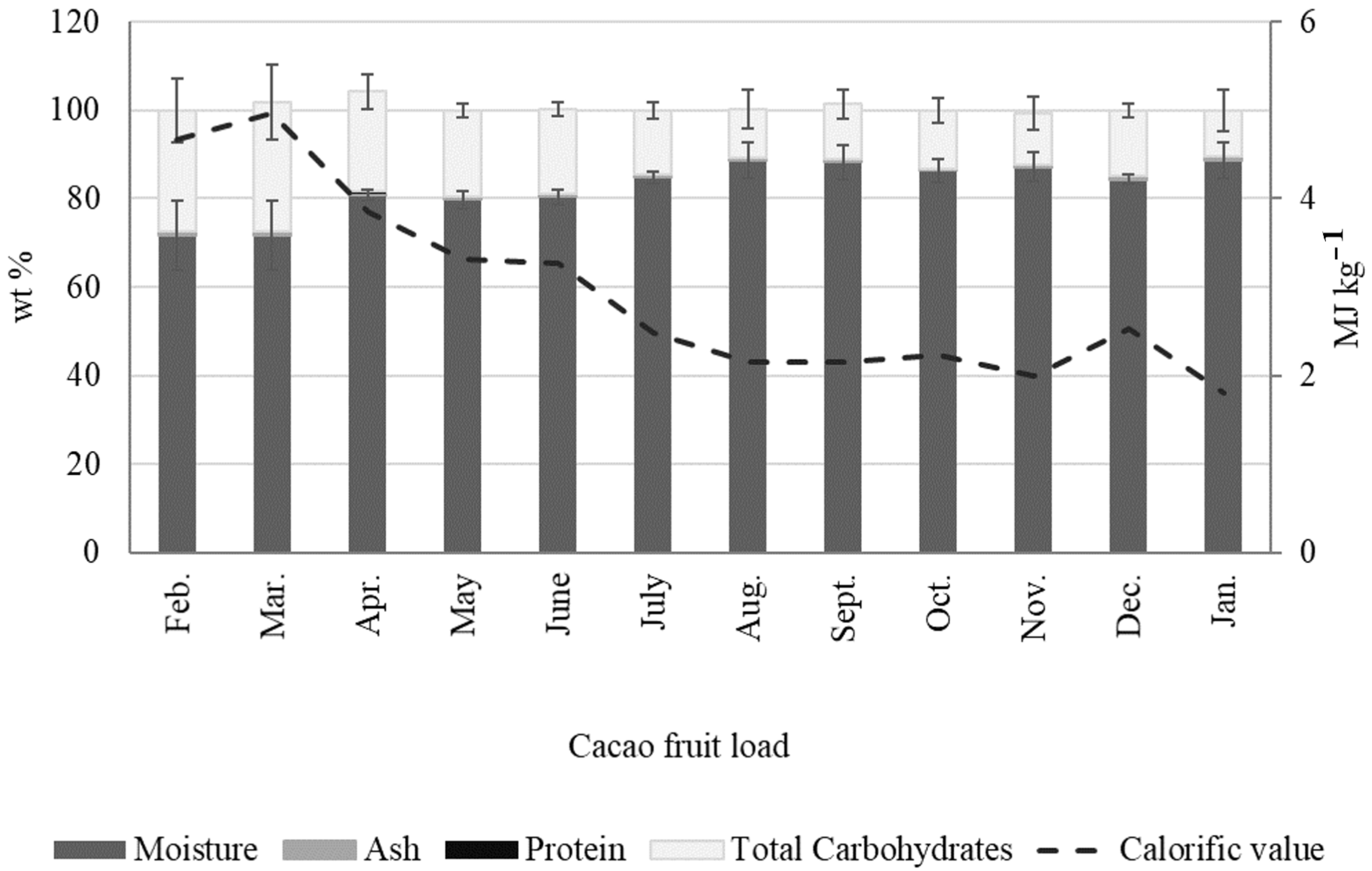
2.2.4. Cacao Mucilage Exudate (CME)
3. Materials and Experimental Methods
3.1. Area of Study
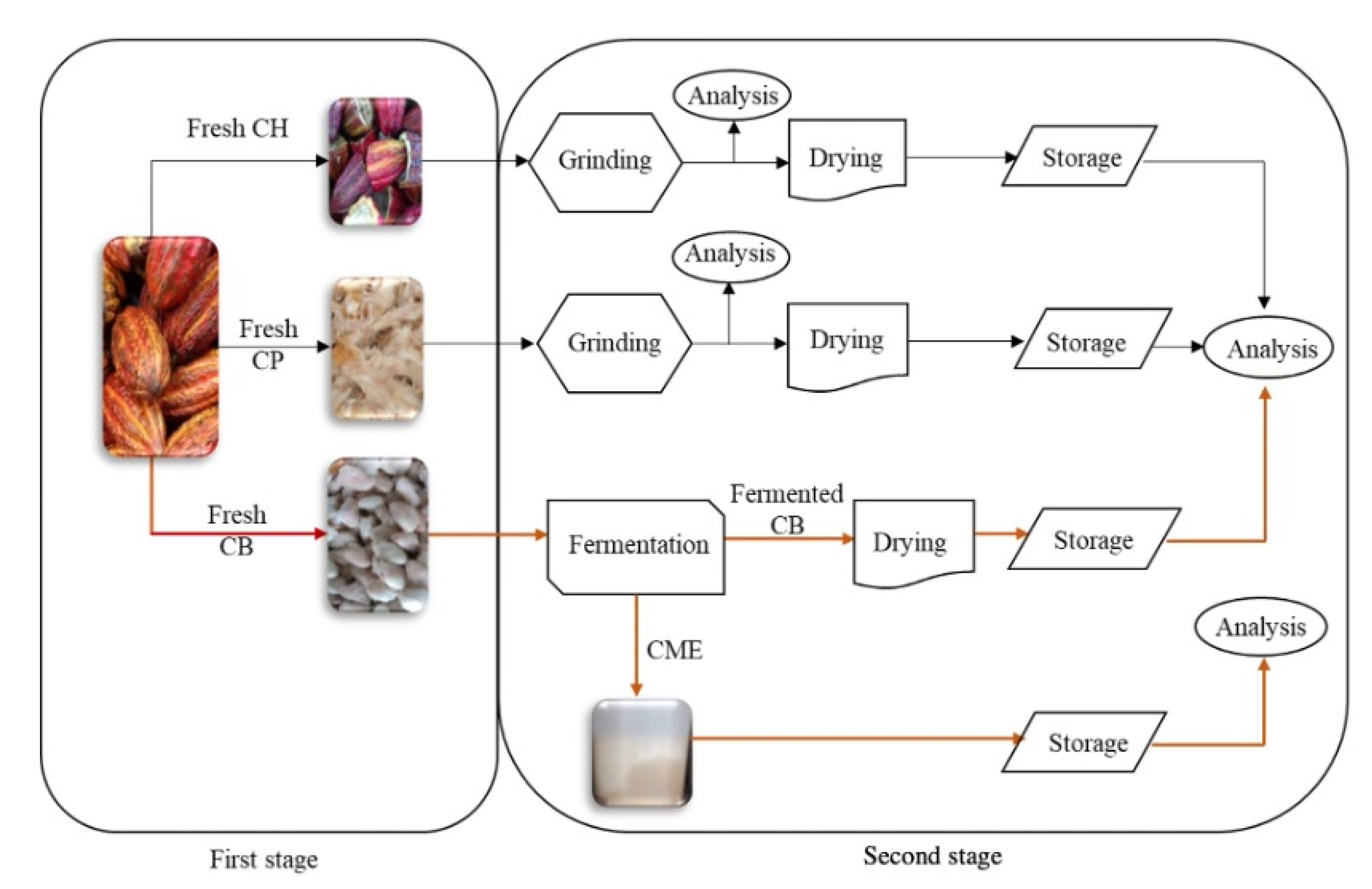
3.2. Cacao Fruit Collection and Treatment
3.3. Compositional Analyses
4. Conclusions
- We identified five distinctive biomass outputs from cacao fruit: cacao husk (CH), cacao beans (CBs), cacao placenta (CP), and cacao mucilage exudate (CME). CH, CBs, and CP are solid lignocellulosic outputs that comprise, in terms of dry weight, 8.92 ± 0.90 wt %, 8.87 ± 0.52 wt %, and 0.57 ± 0.05 wt % of the cacao fruit weight, respectively. Moisture, on the other hand, constitutes most of the biomass weight (71.6 ± 2.29 wt %). Cellulose and lignin contents in CH are time-dependent, reaching maximum values during the crop’s primary harvest season (October–January).
- Dried CH is mostly used as an energy source in the cacao-producing regions of the world. We found no significant changes in CH calorific values during the crops’ yearly cycle, with an average of 13.69 ± 0.43 MJ kg−1. This value is similar to the calorific value content in other residual biomass outputs, such as rice straw/husk, soybean cake, potato peels, rapeseed, sugarcane bagasse, and cotton cake.
- As a lignocellulosic output, CH can potentially be processed via physical, chemical, or biological methods to produce added-value byproducts, such as simple sugars, ethanol, hydrogen, biobutanol, and volatile organic acids, among others. Likewise, the structural biopolymers in CH, such as cellulose, can be the precursors of high-performance materials, such as nanocrystals and nanofibers. The hemicelluloses in CH, which are rich in heteropolymers like xylan, glucuronoxylan, arabinoxylan, xyloglucans, and galactomannans, could become a potential source of probiotics such as xylo-oligosaccharides.
- CB contains carbohydrates, fats, protein, ash, and phenolic compounds. The contents of these materials in Colombian CCN51 CBs do not change significantly during the yearly crop cycle and are within the range of CBs from different geographical sources. Interestingly, the total polyphenol content in CBs is time-dependent, reaching maxima values during the harvest seasons. For instance, from January to April, CBs exhibit 49.97 to 61.68 mg GAE/g, and, from November to December, 52.12 to 57.58 mg GAE/g. The total polyphenols in CCN51 CBs reach minimum values of 36.21 to 42.52 mg GAE/g from May to September.
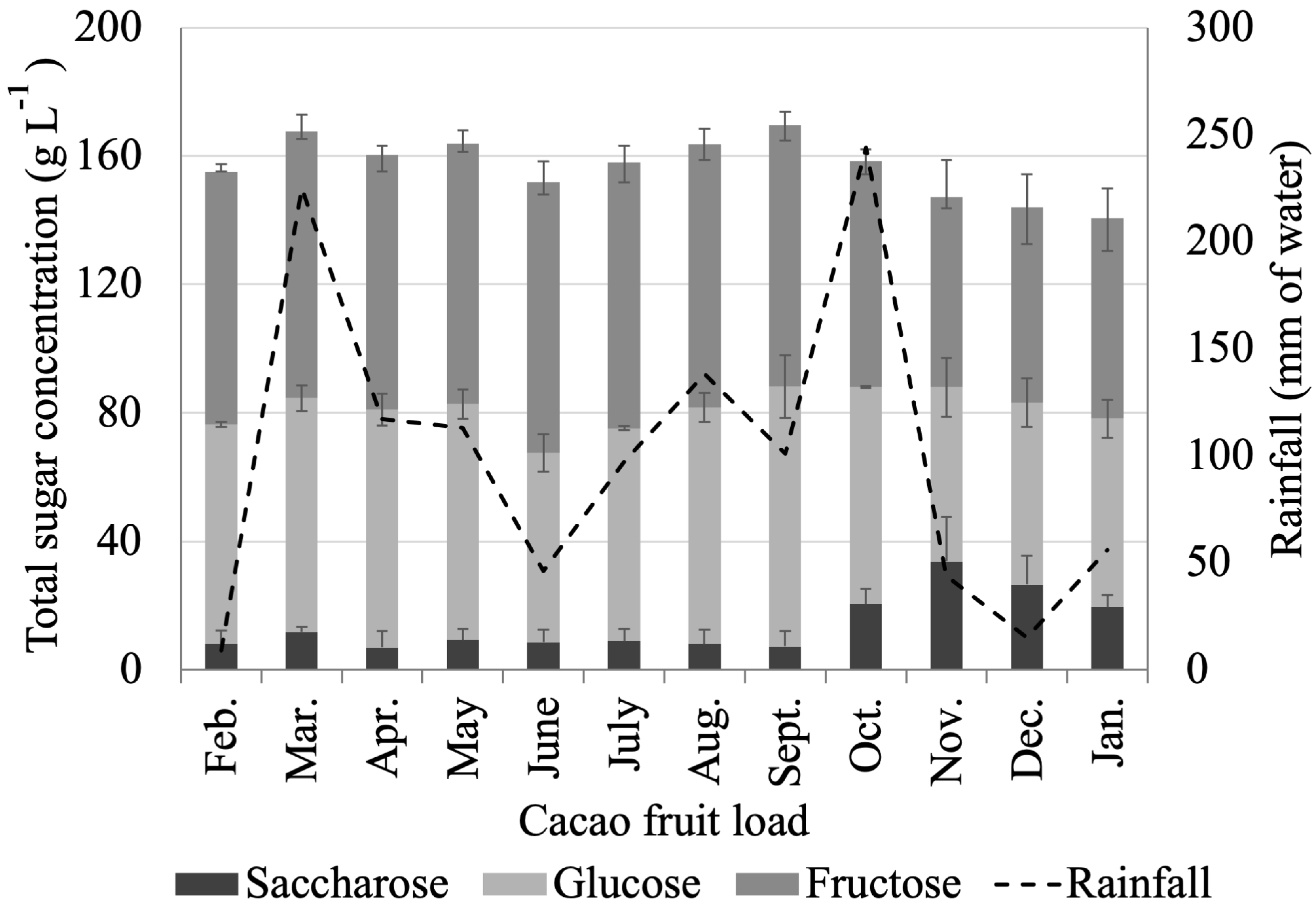
- Cacao mucilage exudate (CME) is a liquid biomass output that is equivalent to 4.13 ± 0.80 wt % of the cacao fruit. CME is rich in simple sugars (glucose, fructose, and saccharose) and minerals (K), with an average of 20 wt % of total carbohydrates. Interestingly, the total carbohydrate content in CME changes dramatically during the year, with a minimum of 10 wt % from August to January and a maximum of 29 wt % in March. Likewise, we observed a positive correlation between sugar content in CME and rainfall, with the highest sugar content in CME being measured during April when rainfall was at its highest.
- CME uses include fermentation to produce alcohol and concentration to produce syrups and jams. However, the high nutrient content of CME makes it an ideal culture media for biotechnological applications, particularly biopolymer production, as demonstrated recently by our group.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- ICCO International Cocoa Organization. Quarterly Bulletin of Cacao Statistics, Issue No. 2—Volume XLVI. 2020. Available online: https://www.icco.org/icco-documentation/quarterly-bulletin-of-cocoa-statistics/ (accessed on 5 March 2021).
- Arvelo, M.; González, D.; Delgado, T.; Maroto, S.; Montoya, P. Estado Actual Sobre la Producción, el Comercio y Cultivo del Cacao en América; Instituto Interamericano de Cooperación para la Agricultura, Fundación Colegio de Postgraduados en Ciencias Agrícolas. C.R., IICA: San José, CA, USA, 2017. [Google Scholar]
- Fedecacao-Federación Nacional de Cacaoteros. Boletín de Prensa. Así Quedó el Ranking de Producción de Cacao en Colombia. Publicado 18/021/2020. 2020. Available online: http://www.fedecacao.com.co/portal/index.php/es/2015-04-23-20-00-33/1193-boletin-de-prensa-asi-quedo-el-ranking-de-produccion-de-cacao-en-colombia/ (accessed on 12 May 2021).
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, L.Y.; Kouassi, E.K.A.; Soro, D.; Soro, Y.; Yao, K.B.; Adouby, K.; Drogui, A.P.; Tyagi, D.R.; Aina, P.M. Cocoa pod husks as potential sources of renewable high-value-added products: A review of current valorizations and future prospects. BioResources 2021, 16, 1988–2020. [Google Scholar] [CrossRef]
- Mendoza-Meneses, C.J.; Feregrino-Pérez, A.A.; Gutiérrez-Antonio, C. Potential Use of Industrial Cocoa Waste in Biofuel Production. J. Chem. 2021, 2021, 3388067. [Google Scholar] [CrossRef]
- Indiarto, R.; Raihani, Z.; Dewi, M.; Zsahra, A. A Review of Innovation in Cocoa Bean Processing By-Products. Int. J. 2021, 9, 1162–1169. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Osueke, C.O.; Olayanju, T.M.A.; Lawal, A. Co-digestion of Theobroma cacao (Cocoa) pod husk and poultry manure for energy generation: Effects of pretreatment methods. Bioresour. Technol. 2019, 283, 229–241. [Google Scholar] [CrossRef]
- Antwi, E.; Engler, N.; Nelles, M.; Schuch, A. Anaerobic digestion and the effect of hydrothermal pretreatment on the biogas yield of cocoa pods residues. Waste Manag. 2019, 88, 131–140. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Adesulu-Dahunsi, A.T.; Izebere, J.O. Cleaner energy through liquefaction of Cocoa (Theobroma cacao) pod husk: Pretreatment and process optimization. J. Clean. Prod. 2019, 226, 578–588. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; ViudaMatos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Kilama, G.; Lating, P.O.; Byaruhanga, J.; Biira, S. Quantification and characterization of cocoa pod husks for electricity generation in Uganda. Energy Sustain. Soc. 2019, 9, 22. [Google Scholar] [CrossRef]
- Villota, S.M.; Lei, H.W.; Villota, E.; Qian, M.; Lavarias, J.; Taylan, V.; Agulto, I.; Mateo, W.; Valentin, M.; Denson, M. Microwave-assisted activation of waste cocoa pod husk by H3PO4 and KOH-comparative insight into textural properties and pore development. ACS Omega 2019, 44, 7088–7095. [Google Scholar] [CrossRef]
- Tsai, W.T.; Jiang, T.J.; Lin, Y.Q. Conversion of de-ashed cocoa pod husk into high-surface-area microporous carbon materials by CO2 physical activation. J. Mater. Cycles Waste Manag. 2019, 21, 308–314. [Google Scholar] [CrossRef]
- Olakunle, M.O.; Inyinbor, A.A.; Dada, A.O.; Bello, O.S. Combating dye pollution using cocoa pod husks: A sustainable approach. Int. J. Sustain. Eng. 2018, 11, 4–15. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Murniati Budianta, W.; Rivera, K.K.P.; Arazo, R.O. Removal of sodium diclofenac from aqueous solution by adsorbents derived from cocoa pod husks. J. Environ. Chem. Eng. 2017, 2, 1465–1474. [Google Scholar] [CrossRef]
- Betiku, E.; Etim, A.O.; Pereao, O.; Ojumu, T.V. Two-step conversion of neem (Azadirachta indica) seed oil into fatty methyl esters using a heterogeneous biomass-based catalyst: An example of cocoa pod husk. Energy Fuels 2017, 31, 6182–6193. [Google Scholar] [CrossRef]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ojo, S.A.; Gueguim-Kana, E.B.; Beukes, L.S. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: Its antimicrobial, antioxidant and larvicidal activities. J. Nanostruct. Chem. 2016, 6, 159–216. [Google Scholar] [CrossRef]
- Adi-Dako, O.; Ofori-Kwakye, K.; El Boakye-Gyasi, M.; Bekoe, S.O.; Okyem, S. In Vitro Evaluation of Cocoa Pod Husk Pectin as a Carrier for Chronodelivery of Hydrocortisone Intended for Adrenal Insufficiency. J. Drug Deliv. 2018, 2017, 828405. [Google Scholar] [CrossRef]
- Lubis, M.; Harahap, M.B.; Ginting, M.H.S.; Maysarah, S. The effect of ethylene glycol as plasticizer against mechanical properties of bioplastic originated from jackfruit seed starch and cocoa pod husk. Nusant. Biosci. 2018, 10, 76–80. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Yeng, C.M. Effect of green coupling agent from waste oil fatty acid on the properties of polypropylene/cacao pod husk composites. Polym. Bull. 2016, 73, 3465–3484. [Google Scholar] [CrossRef]
- El-Shekeil, Y.A.; Sapuan, S.M.; Algrafi, M.W. Effect of fiber loading on mechanical and morphological properties of cocoa pod husk fibers reinforced thermoplastic polyurethane composites. Mater. Des. 2014, 64, 330–333. [Google Scholar] [CrossRef]
- Esong, R.N.; Etchu, K.A.; Bayemi, P.H.; Tan, P.V. Effects of the dietary replacement of maize with sun-dried cacao pods on the performance of growing rabbits. Trop. Anim. Health Prod. 2015, 47, 1411–1416. [Google Scholar] [CrossRef]
- Oddoye, E.O.K.; Rhule, S.W.A.; Agyente-Badu, K.; Anchirinah, V.; Owusu Ansah, F. Fresh cocoa pod husk as an ingredient in the diets of growing pigs. Sci. Res. Essays 2010, 5, 1141–1144. [Google Scholar]
- Anvoh, K.Y.B.; Zoro, B.A.; Gnakr, D. Production and characterization of juice from mucilage of cacao beans and its transformation into marmalade. Pak. J. Nutr. 2009, 8, 129–133. [Google Scholar] [CrossRef]
- Badrie, N.; Escalante, M.; Bekele, F.L. Production and quality characterization of pulp from cacao beans from Trinidad: Effects of varying levels of pulp on value-added carbonated cacao beverages. In Proceedings of the CFCS Meeting, 49th Annual Meeting, Caribbean Food Crops Society 49™ Annual Meeting, 30 June–6 July 2013. [Google Scholar] [CrossRef]
- Toth, J.; Lopata, J.; Schweizer, C.; Pachard, S. Method for Production and Use of Syrup Derived from the Fruit Pulp of the Cacao Pod. WO 2017/044610 A1, 16 March 2017. [Google Scholar]
- Dias, D.R.; Schwan, R.F.; Freire, E.S.; Serôdio, R.D.S. Elaboration of a fruit wine from cocoa (Theobroma cacao L.) pulp. Int. J. Food Sci. Technol. 2007, 42, 319–329. [Google Scholar] [CrossRef]
- Puerari, C.; Teixeira-Magalhães, K.; Freitas-Schwan, R. New cocoa pulp-based kefir beverages: Microbiological, chemical composition and sensory analysis. Food Res. Int. 2012, 48, 634–640. [Google Scholar] [CrossRef]
- Quimbita, F.; Rodriguez, P.; Vera, E. Uso del exudado y placenta del cacao para la obtención de subproductos. Rev. Tecnol. ESPOL—RTE 2013, 26, 8–15. [Google Scholar]
- Segura, J.; García, M.I.; Tigre, A.; Dominguez, V.; Barragán, U.; Guamán, J.; Ramón, R.; Bayas-Morejón, F. Elaboration of an alcoholic drink from the aerobic fermentation of mucilage of cacao (Theobroma cacao L.). Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 1375–1380. [Google Scholar]
- Nguyen, T.T.; Nguyen, T.A.; Ho, T.T.; Nguyen, T.T. A study of wine fermentation from mucilage of cacao beans (Theobroma cacao L.). Dalat Univ. J. Sci. 2016, 6, 387–397. [Google Scholar]
- Romero-Cortés, T.; Cuervo-Parra, J.A.; Robles-Olvera, V.J.; Rangel-Cortes, E.; López-Pérez, P.A. Experimental and kinetic production of ethanol using mucilage juice residues from cacao processing. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
- Saavedra-Sanabria, O.L.; Durán, D.; Cabezas, J.; Hernández, I.; Blanco-Tirado Combariza, M.Y. Cellulose biosynthesis using simple sugars available in residual cacao mucilage exudate. Carbohydr. Polym. 2021, 274, 118645. [Google Scholar] [CrossRef]
- Morejón-Lucio, R.; Vera-Chang, J.; Vallejo-Torres, C.; Morales-Rodríguez, W.; Díaz-Ocampo, R.; Alvarez-Aspiazu, A. Valor nutricional de la placenta deshidratada de cacao (Theobroma cacao L.) nacional, para la elaboración de barras nutricionales. Revista 2018, 5, 57–62. [Google Scholar]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and Its By-Products: Identification and Utilization. In Chocolate in Health and Nutrition; Watson, R., Preedy, V., Zibadi, S., Eds.; (Nutrition and Health); Humana Press: Totowa, NJ, USA, 2013; Volume 7. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Modified Cocoa Pod Husk-Filled Polypropylene Composites by Using Methacrylic Acid. BioResources 2013, 8, 3260–3275. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Karen, H.; Nieto-Figueroa, K.H.; Oomah, D. Cacao (Theobroma cacao L.) pod husk: Renewable source of bioactive Compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, N.H. Proximate composition, extraction, and purification of theobromine from Cacao pod husk (Theobroma Cacao L.). Technologies 2017, 5, 14. [Google Scholar] [CrossRef]
- Laconi, E.B.; Jayanegara, A. Improving nutritional quality of cocoa pod (Theobroma cacao) through chemical and biological treatments for ruminant feeding: In vitro and in vivo evaluation. Asian Australas. J. Anim. Sci. 2015, 28, 343–350. [Google Scholar] [CrossRef]
- Ashade, O.O.; Osineye, O.M. Effect of replacing maize with cocoa pod husk in the nutrition of Oreochromis niloticus. In Proceedings of the 25th Annual Conference of the Fisheries Society of Nigeria (FISON), Lagos, Nigeria, 25–29 October 2010; pp. 504–511. [Google Scholar] [CrossRef][Green Version]
- Daud, Z.; Mohd Kassim, A.S.; Aripin, A.S.; Awang, H.; Mohd Hatta, M.Z. Chemical composition and morphological of cocoa pod husks and cassava peels for pulp and paper production. Aust. J. Basic Appl. Sci. 2013, 7, 406–411. [Google Scholar]
- Kalita, E.; Nath, B.K.; Deb, P.; Agan, F.; Islam, M.R.; Saikia, K. High quality fluorescent cellulose nanofibers from endemic rice husk: Isolation and characterization. Carbohydr. Polym. 2015, 122, 308–313. [Google Scholar] [CrossRef]
- Karp, S.G.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. Pretreatment Strategies for Delignification of Sugarcane Bagasse: A Review. Braz. Arch. Biol. Technol. 2013, 56, 679–689. [Google Scholar] [CrossRef]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Titiloye, J.O.; Bakar, M.S.A.; Odetoye, T.E. Thermochemical characterization of agricultural wastes from West Africa. Ind. Crops Prod. 2013, 47, 199–203. [Google Scholar] [CrossRef]
- Santana, N.B.; Dias, J.C.T.; Rezende, R.P.; Franco, M.; Oliveira, L.K.S.; Souza, L.O. Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE 2018, 13, e0195206. [Google Scholar] [CrossRef] [PubMed]
- Ward-Doria, M.; Arzuaga-Garrido, J.; Ojeda, K.A.; Sánchez, E. Production of biogas from acid and alkaline pretreated cocoa pod husk (Theobroma cacao L.). Int. J. ChemTech Res. IJCRGG 2016, 9, 252–260. [Google Scholar]
- Mansur, D.; Tago, T.; Masuda, T.; Abimanyu, H. Conversion of cacao pod husks by pyrolysis and catalytic reaction to produce useful chemicals. Biomass Bioenergy 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Adeyi, O. Proximate composition of some agricultural wastes in Nigeria and their potential use in activated carbon production. J. Appl. Sci. Environ. Manag. 2010, 14, 55–58. [Google Scholar] [CrossRef]
- Alemawor, F.; Dzogbefia, V.P.; Oddoy, E.O.K.; Oldham, J.H. Enzyme cocktail for enhancing poultry utilization of cocoa pod husk. Sci. Res. Essay 2009, 4, 555–559. Available online: http://www.academicjournals.org/SRE (accessed on 12 May 2021).
- Vriesmann, L.C.; de Mello Castanho Amboni, R.D.; de Oliveira Petkowicz, C.L. Cacao pod husks (Theobroma cacao L.): Composition and hot-water-soluble pectins. Ind. Crops Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S. Agrowaste-based composites from cocoa pod husk and polypropylene: Effect of filler content and chemical treatment. J. Thermoplast. Compos. Mater. 2016, 29, 1332–1351. [Google Scholar] [CrossRef]
- Cruz, G.; Pirilä, M.; Huuhtanen, M.; Carrión, L.; Alvarenga, E.; Keiski, R.L. Production of activated carbon from cacao (Theobroma cacao) pod husk. J. Civ. Environ. Eng. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Tambunan, B.H.; Pambudi, N.A. A preliminary study on use of cacao pod husk as a renewable source of energy in Indonesia. Energy Sustain. Dev. 2012, 16, 74–77. [Google Scholar] [CrossRef]
- Erol, M.; Haykiri-Acma, H.; Küçükbayrak, S. Calorific value estimation of biomass from their proximate analyses data. Renew. Energy 2010, 35, 170–173. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second-generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Serra, B.J.; Aragay, B.M. Composition of dietary fibre in cocoa husk. Z. Für Lebensm. Und—Forsch. A 1988, 207, 105–109. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; Mason, S.M.; Gomez, L.; et al. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Sandoval, A.J.; Barreiro, J.A.; De Sousa, A.; Valera, D.; López, J.V.; Müller, J.A. Composition and thermogravimetric characterization of components of Venezuelan fermented and dry Trinitario cocoa beans (Theobroma cacao L.): Whole beans, peeled beans and shells. Rev. Téc. Ing. Univ. Zulia 2019, 42, 39–47. [Google Scholar] [CrossRef]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.; Sand Saalia, F.K. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. [Google Scholar] [CrossRef]
- Bertazzo, A.; Comai, S.; Brunato, I.; Zancato, M.; Costa, C.V. The content of protein and non-protein (free and protein-bound) tryptophan in Theobroma cacao beans. Food Chem. 2011, 124, 93–96. [Google Scholar] [CrossRef]
- Valussi, M.; Minto, C. Cacao as a Globalised Functional Food: Review on Cardiovascular Effects of Chocolate Consumption. Open Agric. J. 2016, 10, 36–51. [Google Scholar] [CrossRef]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The Content of Polyphenolic Compounds in Cocoa Beans (Theobroma cacao L.), Depending on Variety, Growing Region, and Processing Operations: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cacao powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- Goude, K.A.; Adingra, K.M.D.; Gbotognon, O.J.; Kouadio, E.J.P. Biochemical characterization, nutritional and antioxidant potentials of cocoa placenta (Theobroma Cacao L.). Ann. Food Sci. Technol. 2019, 20, 603–613. [Google Scholar]
- Delgado-Gutiérrez, N.A. Plan de Manejo Integral de Residuos Derivados de la Extracción de la Pulpa de Cacao en la Hacienda Bellavista, Luz de América, Provincia de Azuay-Ecuador. Ph.D. Thesis, Universidad de Cuenca, Cuenca, Ecuador, 2018. [Google Scholar]
- Sánchez-Prieto, V.; Ahmed-El, S.; Yépez-Anchundia, M.; Mosquera, C.; Arizaga, R.; Cadena, N. Elaboración de alimento balanceado para pollo broiler a base de subproductos de cacao (cáscara, cascarilla y placenta) Elaboration of balanced feed for chicken broiler based on cocoa by-products (shell, husk and placenta). Espirales Rev. Multidiscip. De Investig. 2018, 2, 1–13. Available online: https://www.researchgate.net/publication/323583608 (accessed on 12 May 2021).
- Damsere, J.D.; Boateng, M.; Amoah, K.O.; Frimpong, Y.O.; Okai, D.B. The response of pigs to diets containing varying levels of cocoa placenta meal (CPM) supplemented with an exogenous enzyme complex. Afr. J. Agric. Res. 2020, 15, 538–545. [Google Scholar] [CrossRef][Green Version]
- Leite, P.B.; Machado, W.M.; Guimarães, A.G.; Mafra de Carvalho, G.B.; Magalhães-Guedes, K.T.; Druzian, J.I. Cocoa’s residual honey: Physicochemical characterization and potential as a fermentative substrate by Saccharomyces cerevisiae. Sci. World J. 2019, 7, 5698089. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Torres, C.A.; Díaz-Ocampo, R.; Morales-Rodríguez, W.; Soria-Velasco, R.; Vera-Chang, J.F.; Baren-Cedeño, C. Utilización del mucílago de cacao, tipo nacional y trinitario, en la obtención de jalea. Espamciencia 2016, 7, 51–58. [Google Scholar]
- Arteaga-Estrella, Y. Estudio del desperdicio del mucilago de cacao en el cantón naranjal (provincia del Guayas). ECA Sinergia 2013, 4, 49–59. [Google Scholar]
- Cubillos-Bojacá, A.F.; García-Muñoz, M.C.; Calvo-Salamanca, A.M.; Carvajal-Rojas, G.H.; Tarazona-Díaz, M.P. Study of the physical and chemical changes during the maturation of three cocoa clones, EET8, CCN51, and ICS60. J. Sci. Food Agric. 2019, 99, 5910–5917. [Google Scholar] [CrossRef]
- Bertoldi, D.; Barbero, A.; Camin, F.; Caligiani, A.; Larcher, R. Multielemental fingerprinting and geographic traceability of Theobroma cacao beans and cocoa products. Food Control 2016, 65, 46–53. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Kruszewski, B.; Obiedziński, M.W. Multivariate analysis of essential elements in raw cocoa and processed T chocolate mass materials from three different manufacturers. LWT—Food Sci. Technol. 2018, 98, 113–123. [Google Scholar] [CrossRef]
- Ganda-Putra, G.P.; Wartini, N.M.; Trisna Darmayanti, L.P. Characteristics of Cocoa Vinegar from Pulp Liquids Fermentation by Various Methods. In Proceedings of the of the 2nd International Conference on Biosciences and Medical Engineering (ICBME2019) AIP Conf. Proc. Bali, Indonesia, 11–12 April 2019. [Google Scholar] [CrossRef]
- Ministerio de Agricultura y Desarrollo. 2017. Available online: https://www.agronet.gov.co/Documents/SANTANDER_2017.pdf/ (accessed on 26 June 2019).
- AOAC 931.04-1931; Loss on Drying (Moisture) in Cacao Products. AOAC International: Rockville, MD, USA, 2015.
- AOAC 925.10-1925; Solids (Total) and Loss on Drying (Moisture). AOAC International: Rockville, MD, USA, 2014.
- AOAC 972.15-1974; Ash of Cacao Products. AOAC International: Rockville, MD, USA, 2015.
- AOAC 970.22-1970; Nitrogen (Total) in Cacao Products. AOAC International: Rockville, MD, USA, 2015.
- AOAC 2001.11-2005; Protein (Crude) in Animal Feed, Forage (Plant). AOAC International: Rockville, MD, USA, 2015.
- AOAC 930.20-1930; Crude Fiber in Cacao Products. AOAC International: Rockville, MD, USA, 2015.
- AOAC 920.75-1920; Procedure for Separation of Fat in Cacao Products. AOAC International: Rockville, MD, USA, 2015.
- Standard Methods Committee of the American Public Health Association, American Water Works Association, and Water Environment Federation. 5530 phenols In Standard Methods for the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- AOAC 925.36-1925; Sugars (Reducing) in Fruits and Fruit Products. AOAC International: Rockville, MD, USA, 2020.
- AOAC 931.12-1931(1996); Calomel in Ointments. Titrimetric Method. AOAC International: Rockville, MD, USA, 2015.
- AOAC 960.19-1960; pH of Wines. AOAC International: Rockville, MD, USA, 2015.
- AOAC 985.35-1988; Minerals in Infant Formula, Enteral Products. AOAC International: Rockville, MD, USA, 2014.
- Standard Methods Committee of the American Public Health Association, American Water Works Association, and Water Environment Federation. 4500-norg nitrogen (organic) In Standard Methods for the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Standard Methods Committee of the American Public Health Association, American Water Works Association, and Water Environment Federation. 2540 solids In Standard Methods for the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Green, J.W. Methods in Carbohydrate Chemistry; Whistler, R.L., Ed.; Academic Press: New York, NY, USA, 1963; Volume 3, pp. 9–21. [Google Scholar]
- Kurschner, K.; Hoffer, A. Cellulose and cellulose derivative: Fresenius. J. Anal. Chem. 1993, 92, 145–154. [Google Scholar]
- Dence, C.W. The determination of lignin. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Heidelberg, Germany, 1992; pp. 33–61. [Google Scholar]

| Component. | Percentage by Weight (wt %) | |
|---|---|---|
| Moisture | 84.62 ± 1.97 | |
| Total solids | 15.38 ± 1.97 | |
| Crude fat * | 0.29 ± 0.08 | |
| Crude protein * | 5.44 ± 0.80 | |
| Crude fiber * | 30.93 ± 2.03 | |
| Ash * | 10.65 ± 1.62 | |
| Total nitrogen * | 0.88 ± 0.13 | |
| Nitrogen-free extract (NFE) * | 52.69 ± 2.50 | |
| Cellulose | Hemicellulose | Lignin | References |
|---|---|---|---|
| (wt %) | |||
| 31.7 ± 0.1 | 27.0 ± 0.1 | 21.7 ± 0.1 | [10] |
| 30.79 | 21.09 | 25.55 | [48] |
| 18.42 | 10.04 | 12.06 | [49] |
| 35 | 10 | 14.6 | [50] |
| 30.41 ± 0.20 | 11.97 ± 3.17 | 33.96 ± 1.9 | [47] |
| 35.4 ± 0.33 | 37 ± 0.5 | 14.7 ± 0.35 | [42] |
| 41.92 ± 0.09 | 35.26 ± 0.05 | 0.95 ± 0.04 | [51] |
| 26.15 ± 3 | 12.7 ± 56 | 21.16 ± 2.6 | [52] |
| 25.64 ± 3.49 | 19.96 ± 2.42 | 32.73 ± 5.15 | This work * |
| Moisture | Ash | Crude Fat | Crude Protein | Crude Fiber | Reference |
|---|---|---|---|---|---|
| wt % | |||||
| 12.5 | 12.3 | VNR | VNR | VNR | [12] |
| VNR | 11.44 ± 0.41 | 0.93 ± 0.34 | 2.42 ± 0.37 | VNR | [39] |
| 10.5 | 9 | 1.5 | 2.1 | VNR | [54] |
| VNR | VNR | 2.5 | 8.4 | 32.3 | [40] |
| 13 | 13 | 0.6 | 8 | 50 | [23] |
| VNR | 9.1–10.1 | VNR | 5.9–7.6 | 22.6–32.5 | [50] |
| VNR | VNR | 1.2–10 | 5.9–9.1 | 22.6–35.7 | [36] |
| 10.04 ± 0.3 | 12.67 ± 0.14 | VNR | VNR | 33.6 ± 0.15 | [55] |
| 6.72 ± 0.17 | 8.32 ± 0.7 | 2.24 ± 0.1 | 4.22 ± 0.07 | VNR | [11] |
| 8.5 ± 0.6 | 6.7 ± 0.2 | 1.5 ± 0.13 | 8.6 ± 0.9 | 36.6 ± 0.01 | [53] |
| 16.1 | 13.5 | VNR | VNR | VNR | [56] |
| VNR | 9.07 ± 0.04 | VNR | 9.1 ± 1.7 | 35.7 ± 0.9 | [52] |
| 9.86 ± 0.75 | 10.61 ± 1.39 | 1.61 ± 0.86 | 5.90 ± 0.91 | 31.91 ± 2.98 | This work |
| Moisture | Ash | Crude Fiber | Crude Protein | Crude Fat | Carbohydrates | Total Polyphenols (mg Gallic Acid/g) | Reference |
|---|---|---|---|---|---|---|---|
| wt % | |||||||
| 4.3 ± 0.09 | 2.3 ± 0.04 | VNR | 18.2 ± 0.13 | 52.2 ± 0.1 | 23.1 ± 0.54 | VNR | [63] |
| 6.22 ± 0.2 | 2.84 ± 0.04 | VNR | 12.25 ± 0.16 | 42.7 ± 0.6 | VNR | VNR | [61] |
| 5.95 ± 0.04 | 4.03 ± 0.01 | VNR | 12.79 ± 0.03 | VNR | 33.78 ± 0.02 | VNR | [62] * |
| 5.11 ± 0.01 | 3.56 ± 0.02 | VNR | 12.82 ± 0.01 | VNR | 36.58 ± 0.01 | VNR | [62] + |
| 3.96 ± 0.50 | 2.51 ± 0.17 | 3.20 ± 0.90 | 12.10 ± 0.44 | 54.41 ± 0.92 | 24.00 ± 2.24 | 47.31 ± 8.03 | This work |
| Moisture | Ash | Protein | Total Nitrogen | Reference |
|---|---|---|---|---|
| wt % | ||||
| 80.55 ± 0.42 | 9.34 ± 0.89 | 5.12 ± 0.02 | VNR | [68] |
| 80.925 ± 1.067 | 5.560 ± 0.424 | VNR | 1.005 ± 0.134 | [69] |
| VNR | 1.28 ± 0.051 | 1.38 ± 0.028 | VNR | [30] |
| 78.36 | 0.54 | 1.69 | 0.27 | This work |
| Moisture | Ash | Protein | Total Carbohydrates | Total Polyphenols (mg Gallic Acid/g) | Reference |
|---|---|---|---|---|---|
| wt % | |||||
| 85.86 ± 0.09 | 0.59 ± 0.15 | 1.20 ± 0.49 | 11.80 ± 0.09 | VNR | [72] |
| 77.34 a | 2.91 | 5.41 | VNR | VNR | [73] |
| 82.5 b | VNR | 0.87 | VNR | VNR | [73] |
| 80.5 | VNR | 0.38 | VNR | VNR | [74] |
| VNR c | 7.51 ± 0.14 | 5.47 ± 0.12 | 68.35 ± 0.16 | VNR | [11] |
| VNR d | 7.68 ± 0.18 | 5.56 ± 0.10 | 67.99 ± 0.14 | VNR | [11] |
| 82.67 ± 6.09 | 0.45 ± 0.01 | 0.31 ± 0.09 | 17.18 ± 6.44 | 0.55 ± 0.45 | This work |
| Percentages | Equation | Definitions |
|---|---|---|
| Wet weight | MFCH mass of fresh CH MFCF mass of fresh cacao fruit | |
| MFCP mass of fresh CP | ||
| MFCBs mass of fresh CBs | ||
| Dry weight | MDCH mass of dried CH | |
| MDCP mass of dried CP | ||
| MDFCBs mass of dried fermented CBs | ||
| Moisture content (MC) | MFCH mass of fresh CH MDCH mass of dried CH | |
| MFCP mass of fresh CP MDCP mass of dried CP | ||
| MFCBs mass of fresh CBs MDCP mass of dried CP MFCME mass of fresh CME MDCME mass of dried CME |
| Analysis | Reference Method | CH | CP | CME | CBs |
|---|---|---|---|---|---|
| Moisture | AOAC 931.04 [81] | X | X | X | |
| AOAC 925.10 [82] | X | ||||
| Ash | AOAC 972.15 [83] | X | X | X | |
| AOAC7,009/84-94205/90 | X | ||||
| Protein | AOAC 970.22 [84] | X | X | X | |
| AOAC 2001.11 [85] | X | ||||
| Crude fiber | AOAC 930.20a [86] | X | X | X | |
| Fat | AOAC 920.75a [87] | X | X | X | |
| Total carbohydrates | By difference: 100—(% Ash)—(% Total Fat)—(% Moisture)—(% Protein)—(% Fiber) | X | X | X | |
| Calorific value | By equation: (% T Carbohydrate × 4 Kcal/g) + (% Protein × 4 Kcal/g) + (% Total Fat × 9 Kcal/g) | X | X | X | |
| Total polyphenols | Standard Methods 5530 B, D [88] | X | X | ||
| Glucose | AOAC 925.36 [89] | X | |||
| Fructose | AOAC 925.36 | X | |||
| Sucrose | AOAC 925.36 | X | |||
| Total soluble solids °Brix | AOAC 931.12 [90] | X | |||
| pH 24.2 (°C) | AOAC 960.19 [91] | X | |||
| Calcium | AOAC 985.35 [92] | X | |||
| Potassium | AOAC 985.35 | X | |||
| Sodium | AOAC 985.35 | X | |||
| Aluminum | Emission mode | X | |||
| Total nitrogen | Standard Methods 4500 N [93] | X | X | X | |
| Total solids | Standard Methods 2540B [94] | X | |||
| Holocellulose | Jayme-Wise Method [95] | X | |||
| Cellulose | Kurschner and Hoffer Method [96] | X | |||
| Hemicellulose | By difference: % Holocellulose—% Cellulose | X | |||
| Lignin | Klason Method [97] | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara-Mendoza, M.; Martínez, G.R.; Blanco-Tirado, C.; Combariza, M.Y. Mass Balance and Compositional Analysis of Biomass Outputs from Cacao Fruits. Molecules 2022, 27, 3717. https://doi.org/10.3390/molecules27123717
Vergara-Mendoza M, Martínez GR, Blanco-Tirado C, Combariza MY. Mass Balance and Compositional Analysis of Biomass Outputs from Cacao Fruits. Molecules. 2022; 27(12):3717. https://doi.org/10.3390/molecules27123717
Chicago/Turabian StyleVergara-Mendoza, Marisol, Genny R. Martínez, Cristian Blanco-Tirado, and Marianny Y. Combariza. 2022. "Mass Balance and Compositional Analysis of Biomass Outputs from Cacao Fruits" Molecules 27, no. 12: 3717. https://doi.org/10.3390/molecules27123717
APA StyleVergara-Mendoza, M., Martínez, G. R., Blanco-Tirado, C., & Combariza, M. Y. (2022). Mass Balance and Compositional Analysis of Biomass Outputs from Cacao Fruits. Molecules, 27(12), 3717. https://doi.org/10.3390/molecules27123717






