Chemical Characterization and Bioactivity of Commercial Essential Oils and Hydrolates Obtained from Portuguese Forest Logging and Thinning
Abstract
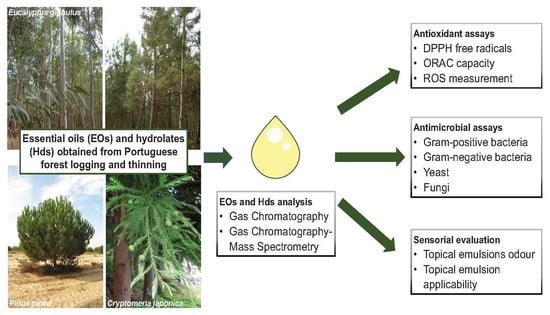
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Composition
2.2. Hydrolates Volatiles Composition
2.3. Antioxidant Activity of Essential Oils and Hydrolates
2.3.1. DPPH and ORAC Assays
2.3.2. Intracellular ROS Measurement
2.4. Antimicrobial Activity of Essential Oils and Hydrolates
2.5. Sensorial Evaluation
Questionnaire Results
3. Materials and Methods
3.1. Essential Oils and Hydrolates
3.2. Hydrolate Volatiles Extraction
3.3. Essential Oil and Hydrolate Volatiles Composition Analysis
3.3.1. Gas Chromatography (GC)-Flame Ionization Detection (FID) Analysis
3.3.2. Gas Chromatography-Mass Spectrometry (GC-MS)
3.4. Determination of Antioxidant Activity
3.4.1. DPPH Radical Scavenging Activity
3.4.2. Oxygen Radical Absorbance Capacity (ORAC)
3.4.3. In Vitro Antioxidant Activity
3.5. Evaluation of Antimicrobial Activity
3.5.1. Microbial Strains
3.5.2. Determination of Minimum Inhibitory Concentration by the Microdilution Method
3.6. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nehme, R.; Andrés, S.; Pereira, R.B.; Jenmaa, M.B.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 267–271. [Google Scholar]
- Neves, A.; Marto, J.; Duarte, A.; Gonçalves, L.M.; Pinto, P.; Figueiredo, A.C.; Ribeiro, H.M. Characterization of Portuguese Thymbra capitata, Thymus caespititius and Myrtus communis essential oils in topical formulations. Flavour Fragr. J. 2017, 32, 392–402. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.; Ribeiro, H.M.; Rodrigues, M.; Aranjo, A.R.T.S. Essential oils used in dermocosmetics: Review about its biological activities. J. Cosmet. Dermatol. 2021, 21, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.S.; Martins, A.; Faleiro, M.L.; Miguel, M.G.; Duarte, L.C.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Bioproducts from Forest Biomass: Essential oils and hydrolates from wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crops Prod. 2020, 144, 112034. [Google Scholar] [CrossRef]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential oils from residual foliage of forest tree and shrub species: Yield and antioxidant capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.S.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Hydrolates: A review on their volatiles composition, biological properties and potential uses. Phytochem. Rev. 2022. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciag, A.; Szoka, Ł.; Karna, E. Chemical Composition and Biological Activity of Abies alba and A. koreana Seed and Cone Essential Oils and Characterization of Their Seed Hydrolates. Chem. Biodivers. 2015, 15, 407–418. [Google Scholar]
- Baydar, H.; Sangun, M.K.; Erbas, S.; Kara, N. Comparison of Aroma Compounds in Distilled and Extracted Products of Sage (Salvia officinalis L.). J. Essent. Oil Bear. Plants 2013, 16, 39–44. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2015, 79, 274–282. [Google Scholar] [CrossRef]
- Hayat, U.; Jilani, M.I.; Rehman, R.; Nadeem, F. A Review on Eucalyptus globulus: A New Perspective in Therapeutics. Int. J. Chem. Biochem. Sci. 2015, 8, 85–91. [Google Scholar]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Zolfaghari, B.; Iravani, S. Essential oil constituents of the bark of Pinus pinaster from iran. J. Essent. Oil-Bear. Plants 2012, 15, 348–351. [Google Scholar] [CrossRef]
- Tümen, İ.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtcam, M. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef]
- Lugovic, L.; Mirna, S.; Ozanic-Bulic, S.; Sjerobabski-Masnec, I. Phototoxic and Photoallergic Skin Reactions. Coll. Antropol. 2011, 31, 63–67. [Google Scholar]
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungal activities of Pinus pinea essential oil. J. Pest. Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Hmamouchi, M.; Hamamouchi, J.; Zouhdi, M. Chemical and Antimicrobial Properties of Essential Oils of Five Moroccan Pinaceae. J. Essent. Oil Res. 2001, 13, 298–302. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Ates, B.; Erdogan, S.; Cenet, M.; Karaaslan, M.G. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Küpeli, A.E. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Cheng, S.S.; Lin, H.Y.; Chang, S.T. Chemical composition and antifungal activity of essential oils from different tissues of Japanese cedar (Cryptomeria japonica). J. Agric. Food Chem. 2005, 53, 614–619. [Google Scholar] [CrossRef]
- Matsunaga, T.; Hasegawa, C.; Toru, K.; Suzuki, H.; Saito, H.; Sagioka, T.; Takahashi, R.; Tsukamoto, H.; Morikawa, T.; Akiyama, T. Isolation of the Antiulcer Compound in Essential Oil from the Leaves of Cryptomeria japonica. Chem. Pharm. Bull. 2000, 23, 595–598. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Lai, W.C.; Chu, F.H.; Lin, C.T.; Shen, S.Y.; Chang, S.T. Essential oil from the leaves of Cryptomeria japonica acts as a silverfish (Lepisma saccharina) repellent and insecticide. J. Wood Sci. 2006, 52, 522–526. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Jeong, S.; Moon, S.; Kil, B.; Yun, S.; Lee, K.; Song, Y. Chemical composition and antimicrobial activity of the essential oil of Cryptomeria japonica. Phyther. Res. 2007, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, A.J.D.; Cavaleiro, J.A.S.; Delmond, B.; Filliatre, C.; Bourgeois, G. The Essential Oil of Eucalyptus globulus Labill. from Portugal. Flavour Fragr. J. 1994, 9, 51–53. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Lima, A.S.; Mendes, M.D.; Leiria, R.; Geraldes, D.A.; Figueiredo, A.C.; Trindade, H.; Pedro, L.G.; Barroso, J.G.; Sanches, J. Eucalyptus from Mata Experimental do Escaroupim (Portugal): Evaluation of the essential oil composition from sixteen species. Acta Hortic. 2011, 925, 61–66. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, M.; Bessam, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, Â.; Costa, P.M.; Belo, A.D.F. Chemical Composition, Antibacterial, Antibiofilm and Synergistic Properties of Essential Oils from Eucalyptus globulus Labill. and Seven Mediterranean Aromatic Plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.; Gago, C.; Antunes, M.; Lagoas, S.; Faleiro, M.L.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A.C. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- ISO 770:2002; Crude or rectified oils of Eucalyptus globulus (Eucalyptus globulus Labill.); International Organization for Standardization: Geneva, Switzerland, 2002.
- Carmo, M.M.; Frazão, S. The essencial oil of portuguese Pine needles. First Results. In Progress in Essential Oil Research; Brunke, E.-J., Ed.; Walter de Gruyter: New York, NY, USA, 1986; pp. 169–174. [Google Scholar]
- Rodrigues, A.M.; Mendes, M.D.; Lima, A.S.; Barbosa, P.M.; Ascensão, L.; Barroso, J.G.; Pedro, L.G.; Mota, M.M.; Figueiredo, A.C. Pinus halepensis, Pinus pinaster, Pinus pinea and Pinus sylvestris Essential Oils Chemotypes and Monoterpene Hydrocarbon Enantiomers, before and after Inoculation with the Pinewood Nematode Bursaphelenchus xylophilus. Chem. Biodivers. 2016, 14, e1600153. [Google Scholar] [CrossRef]
- Miguel, M.G.; da Silva, C.I.; Farah, L.; Braga, F.C.; Figueiredo, A.C. Effect of essential oils on the release of TNF-α and CCL2 by LPS-stimulated THP-1 cells. Plants 2021, 10, 50. [Google Scholar] [CrossRef]
- Moiteiro, C.; Esteves, T.; Ramalho, L.; Rojas, R.; Alvarez, S.; Zacchino, S.; Bragança, H. Essential Oil Characterization of Two Azorean Cryptomeria japonica Populations and Their Biological Evaluations. Nat. Prod. Commun. 2013, 8, 1785–1790. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.C.; Moiteiro, C.; Rodrigues, M.C.S.M.; Almeida, A.J.R.M. Essential Oil Composition From Cryptomeria japonica D.Don Grown in Azores: Biomass Valorization From Forest Management. Nat. Prod. Commun. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Paolini, J.; Leandri, C.; Desjobert, J.M.; Barboni, T.; Costa, J. Comparison of liquid-liquid extraction with headspace methods for the characterization of volatile fractions of commercial hydrolats from typically Mediterranean species. J. Chromatogr. A 2008, 1193, 37–49. [Google Scholar] [CrossRef]
- Inouye, S.; Takahashi, M.; Abe, S. A comparative study on the composition of forty-four hydrosols and their essential oils. Int. J. Essent. Oil Ther. 2008, 2, 89–104. [Google Scholar]
- Nakagawa, T.; Zhu, Q.; Ishikawa, H.; Ohnuki, K.; Kakino, K.; Horiuchi, N.; Shinotsuka, H.; Naito, T.; Matsumoto, T.; Minamisawa, N.; et al. Multiple uses of Essential Oil and by-products from various parts of Yakushima native cedar (Cryptomeria japonica). J. Wood Chem. Technol. 2016, 36, 42–55. [Google Scholar] [CrossRef]
- Ho, C.; Wang, E.-C.; Yu, H.-T.; Yu, H.-M.; Su, C.-Y. Compositions and antioxidant activities of essential oils of different tissues from Cryptomeria japonica D. Don. For. Res. Q. 2010, 32, 63–76. [Google Scholar]
- Souza, C.F.; Baldissera, M.D.; Silva, L.L.; Geihs, M.A.; Baldisserotto, B. Is monoterpene terpinen-4-ol the compound responsible for the anesthetic and antioxidant activity of Melaleuca alternifolia essential oil (tea tree oil) in silver catfish? Aquaculture 2018, 486, 217–223. [Google Scholar] [CrossRef]
- Cutillas, A.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Rosmarinus officinalis L. essential oils from Spain: Composition, antioxidant capacity, lipoxygenase and acetylcholinesterase inhibitory capacities, and antimicrobial activities. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 152, 1282–1292. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free. Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Sani, T.A.; Ameri, A.A.; Imani, M.; Golmakani, E.; Kamali, H. Seasonal variation in the chemical composition, antioxidant activity, and total phenolic content of Artemisia absinthium essential oils. Phcog. Res. 2015, 7, 329–334. [Google Scholar]
- Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003, 78, 570S–578S. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y. Anti-inflammatory and ECM gene expression modulations of β-eudesmol via NF-κB signaling pathway in normal human dermal fibroblasts. Biomed. Dermatol. 2018, 2, 3. [Google Scholar] [CrossRef]
- Lee, B.H.; Nam, T.G.; Park, W.J.; Kang, H.; Heo, H.J.; Chung, D.K.; Kim, G.H.; Kim, D. Antioxidative and Neuroprotective Effects of Volatile Components in Essential Oils from Chrysanthemum indicum Linné Flowers. Food Sci. Biotechnol. 2015, 24, 717–723. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Diaz-Arenas, G.L.; Agudelo, L.P.A.; Stashenko, E.; Contreras-Castillo, C.J.; da Glória, E.M. Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules 2021, 26, 2888. [Google Scholar] [CrossRef]
- Janssen, A.M.; Scheffer, J.J.C.; Svendsen, A.B. Antimicrobial activities of essential oils. A 1976–1986 literature review on possible applications. Pharm. Weekbl. Sci. Ed. 1987, 9, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Šilha, D.; Švarcová, K.; Bajer, T.; Královec, K.; Tesařová, E.; Moučková, K.; Pejchalová, M.; Bajerová, P. Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms. Molecules 2020, 25, 5654. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; Apers, S.; de Bruyne, T.; Miert, S.V.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Chemical Composition and Antifungal Activity of Essential Oils of Some Aromatic Medicinal Plants Growing in the Democratic Republic of Congo. J. Essent. Oil Res. 2002, 14, 382–387. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, A.; Burló, F.; Carbonell-Barrachina, A.A. Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Schreiner, L.; Bauer, P.; Buettner, A. Resolving the smell of wood-identification of odour-active compounds in Scots pine (Pinus sylvestris L.). Sci. Rep. 2018, 8, 8294. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Mishra, S. Plant Monoterpenoids (Prospective Pesticides). In Ecofriendly Pest Management for Food Security, 1st ed.; Omkar, Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 507–524. [Google Scholar]
- Pauwels, M.; Rogiers, V. Human health safety evaluation of cosmetics in the EU: A legally imposed challenge to science. Toxicol. Appl. Pharmacol. 2010, 243, 260–274. [Google Scholar] [CrossRef]
- ISO 7609:1985; Essential oils—Analysis by gas chromatography on capillary columns—General method; International Organization for Standardization: Geneva, Switzerland, 1985.
- Ribeiro, H.M.; Allegro, M.; Marto, J.; Pedras, B.; Oliveira, N.G.; Paiva, A.; Barreiros, S.; Gonçalves, L.M.D.; Simões, P.M.C. Converting Spent Coffee Grounds into Bioactive Extracts with Potential Skin Antiaging and Lightening Effects ACS Sustainable. Chem. Eng. 2018, 6, 6289–6295. [Google Scholar]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.; Petrovski, Ž.; et al. Highlighting the Biological Potential of the Brown Seaweed Fucus spiralis for Skin Applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef]
- Marques, P.; Marto, J.; Gonçalves, L.M.; Pacheco, R.; Fitas, M.; Pinto, P.; Serralheiro, M.L.M.; Ribeiro, H.M. Cynara scolymus L.: A promising Mediterranean extract for topical anti-aging prevention. Ind. Crops Prod. 2017, 109, 699–706. [Google Scholar]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and Characterization of a New Quercus Suber-Based Pickering Emulsion for Topical Application. Pharmaceuticals 2019, 11, 131. [Google Scholar] [CrossRef] [Green Version]
| EOs Main Components (≥5%) | RI | Samples | |||||
|---|---|---|---|---|---|---|---|
| Eucalyptus globulus | Eg_OE_1_G | Eg_OE_2_B | Eg_OE_3_O | Eg_OE_4_E | Eg_OE_5_P | Eg_OE_6_S | |
| α-Pinene | 930 | 13.2 | 13.3 | 11.0 | 21.8 | 14.7 | 13.8 |
| 1,8-Cineole | 1005 | 65.2 | 63.2 | 59.5 | 53.9 | 58.2 | 49.4 |
| Limonene | 1009 | 8.2 | 17.2 | 13.7 | 16.6 | 12.5 | 18.0 |
| α-Terpenyl acetate | 1334 | 2.2 | t | 5.4 | 0.2 | 0.8 | 0.9 |
| Pinus pinaster | Pp_OE_1_G | Pp_OE_2_P | Pp_OE_3_S | ||||
| α-Pinene | 930 | 27.0 | 44.6 | 36.5 | |||
| β-Pinene | 963 | 28.0 | 23.0 | 18.8 | |||
| β-Myrcene | 975 | 11.0 | 5.0 | 5.9 | |||
| δ-3-Carene | 1000 | 6.6 | 2.1 | 1.8 | |||
| Limonene | 1009 | 4.5 | 3.9 | 3.3 | |||
| β-Caryophyllene | 1414 | 4.5 | 5.0 | 8.7 | |||
| Germacrene-D | 1474 | 6.3 | 1.7 | 5.6 | |||
| Pinus pinea | Ppi_OE_1_B | ||||||
| α-Pinene | 930 | 7.6 | |||||
| Limonene | 1009 | 72.8 | |||||
| Cryptomeria japonica | Cj_OE_1_M | ||||||
| α-Pinene | 930 | 26.1 | |||||
| Sabinene | 958 | 18.1 | |||||
| Phyllocladene | 2006 | 13.8 |
| HdVs Main Components (≥5%) | RI | Samples | |||
|---|---|---|---|---|---|
| Eucalyptus globulus | Eg_Hd_1_G | Eg_Hd_2_O | Eg_Hd_3_E | Eg_Hd_4_P | |
| 1,8-Cineole | 1005 | 80.2 | 55.5 | 53.5 | 4.5 |
| Limonene | 1009 | 7.3 | 14.1 | 6.7 | 1.5 |
| trans-Pinocarveol | 1106 | 4.9 | 0.4 | 8.0 | 36.6 |
| cis-p-2-Menthen-1-ol | 1114 | t | t | t | 4.6 |
| Myrtenal | 1153 | t | t | 5.5 | |
| α-Terpineol | 1159 | 2.7 | 17.2 | 24.7 | 5.3 |
| Myrtenol | 1168 | t | t | 12.0 | |
| cis-Carveol | 1202 | 1.1 | 0.1 | 2.8 | 8.6 |
| Pinus pinaster | Pp_Hd_1_G | Pp_Hd_2_P | |||
| 1,8-Cineole | 1005 | 5.0 | t | ||
| cis-p-2-Menthen-1-ol | 1114 | t | 14.0 | ||
| neo-Isopulegol | 1116 | 14.0 | |||
| Terpinen-4-ol | 1148 | 7.5 | |||
| p-Cymen-8-ol | 1148 | 7.5 | t | ||
| α-Terpineol | 1159 | 43.8 | 38.1 | ||
| Verbenone | 1164 | 17.9 | 28.7 | ||
| Perilla alcohol | 1274 | 6.6 | t | ||
| Thymol | 1275 | 6.6 | t | ||
| Cryptomeria japonica | Cj_Hd_1_M | ||||
| 1,8-Cineole | 1005 | 6.3 | |||
| Terpinen-4-ol | 1148 | 56.2 | |||
| α-Terpineol | 1159 | 4.6 | |||
| Phyllocladene | 2006 | 4.8 |
| Essential Oils | DPPH (IC50, mg/mL) | ORAC (µmol TE/g) | Reduction of ROS * (%) |
|---|---|---|---|
| Eg_OE_1_G | 197.6 ± 20.4 | 113245.9 ± 15003.8 | 40.0 ± 0.9 |
| Eg_OE_2_B | 647.3 ± 5.7 | 53669.2 ± 8659.3 | 49.3 ± 0.8 |
| Eg_OE_3_O | 151.8 ± 0.0 | 171891.9 ± 25388.4 | −15.7 ± 1.5 |
| Eg_OE_4_E | WA | 113884.2 ± 14067.0 | 27.2 ± 0.8 |
| Eg_OE_5_P | WA | 86174.9 ± 9813.9 | 50.0 ± 0.0 |
| Eg_OE_6_S | 246.7 ± 24.5 | 160532.2 ± 16659.3 | 6.8 ± 1.2 |
| Pp_OE_1_G | 55.2 ± 0.9 | 161208.7 ± 24896.4 | 34.3 ± 3.7 |
| Pp_OE_2_P | WA | 355575.7 ± 30254.3 | 29.5 ± 0.5 |
| Pp_OE_3_S | WA | 565450.6 ± 70377.8 | 21.7 ± 1.9 |
| Ppi_OE_1_B | 195.7 ± 22.9 | 165063.9 ± 20907.1 | −3.3 ± 1.2 |
| Cj_OE_1_M | 23.1 ± 0.2 | 224877.9 ± 25680.9 | 83.5 ± 2.8 |
| Hydrolates | |||
| Eg_Hd_1_G | WA | 84.1 ± 10.0 | 81.0 ± 2.3 |
| Eg_Hd_2_O | WA | 1129.7 ± 100.6 | 46.8 ± 5.0 |
| Eg_Hd_3_E | WA | 454.6 ± 39.7 | - |
| Eg_Hd_4_P | WA | 238.5 ± 24.5 | 79.2 ± 2.0 |
| Pp_Hd_1_G | WA | 212.2 ± 16.9 | 84.8 ± 1.3 |
| Pp_Hd_2_P | WA | 295.1 ± 44.4 | 80.3 ± 1.9 |
| Cj_Hd_1_M | WA | 131.1 ± 10.8 | 92.8 ± 1.3 |
| Ascorbic Acid | 0.04 ± 1.1 | - | 95.3 ± 0.5 |
| Minimum Inhibitory Concentrations (MICs) (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| EOs Samples | Staphylococcus aureus ATCC 6538 | Bacillus subtilis ATCC 6633 | Pseudomonas aeruginosa ATCC 9027 | Escherichia coli ATCC 8739 | Candida albicans ATCC 10231 | Aspergillus brasiliensis ATCC 16404 |
| Eg_OE_1_G | 125 | 31.25 | 500 | 15.62 | 7.81 | >500 |
| Eg_OE_2_B | 125 | 31.25 | 500 | 3.90 | 3.90 | >500 |
| Eg_OE_3_O | 62.5 | 15.62 | 31.25 | 15.62 | 31.25 | >500 |
| Eg_OE_4_E | 125 | 15.62 | 500 | 62.5 | 31.25 | >500 |
| Eg_OE_5_P | 62.5 | 1.95 | 500 | 3.90 | 3.90 | >500 |
| Eg_OE_6_S | 62.5 | 15.62 | 500 | 15.62 | 7.81 | >500 |
| Pp_OE_1_G | >500 | >500 | >500 | >500 | >500 | >500 |
| Pp_OE_2_P | 31.25 | 15.62 | 500 | 15.62 | 62.5 | >500 |
| Pp_OE_3_S | >500 | 15.62 | >500 | 125 | 125 | >500 |
| Ppi_OE_1_B | 62.5 | 7.81 | >500 | 125 | 15.62 | >500 |
| Cj_OE_1_M | >500 | >500 | >500 | >500 | >500 | >500 |
| Sociodemographic Characteristics | All Samples (n = 100) n (%) |
|---|---|
| Age range (years) | |
| <18 | 6 (6%) |
| 18–30 | 22 (22%) |
| 31–40 | 13 (13%) |
| 41–50 | 30 (30%) |
| 51–60 | 20 (20%) |
| >60 | 9 (9%) |
| Gender | |
| Female | 67 (67%) |
| Male | 33 (33%) |
| Education | |
| Primary education | 13 (13%) |
| Secondary education | 26 (26%) |
| Higher education | 61 (61%) |
| Region | |
| Countryside | 70 (70%) |
| City | 30 (30%) |
| Section 1. Emulsions’ Odour. Odoriferous Characterization of Emulsions | N (%) | ||||
|---|---|---|---|---|---|
| Evaluation of odours | Cj_OE_1_M | Ppi_OE_1_B | Eg_OE_1_G | Pp_OE_1_G | Pp_OE_2_P |
| 1. Without odour | 3 (3%) | 1 (1%) | 0 (0%) | 15 (15%) | 13 (13%) |
| 2. Slightly perceptible | 37 (37%) | 8 (8%) | 4 (4%) | 40 (40%) | 28 (28%) |
| 3. Perceptible | 46 (46%) | 38 (38%) | 15 (15%) | 28 (28%) | 42 (42%) |
| 4. Very perceptible | 11 (11%) | 34 (34%) | 33 (33%) | 14 (14%) | 11 (11%) |
| 5. Intense odour | 3 (3%) | 19 (19%) | 48 (48%) | 3 (3%) | 6 (6%) |
| Classification of odours | Cj_OE_1_M | Ppi_OE_1_B | Eg_OE_1_G | Pp_OE_1_G | Pp_OE_2_P |
| 1. Very unpleasant | 0 (0%) | 9 (9%) | 36 (36%) | 4 (4%) | 5 (5%) |
| 2. Unpleasant | 22 (22%) | 25 (25%) | 27 (27%) | 41 (41%) | 44 (44%) |
| 3. Pleasant and hot odour | 15 (15%) | 12 (12%) | 15 (15%) | 10 (10%) | 13 (13%) |
| 4. Pleasant and fresh odour | 60 (60%) | 53 (53%) | 22 (22%) | 30 (30%) | 27 (27%) |
| Ranking in order of preference | Cj_OE_1_M | Ppi_OE_1_B | Eg_OE_1_G | Pp_OE_1_G | Pp_OE_2_P |
| 1. Hateful Odour | 3 (3%) | 8 (8%) | 32 (32%) | 9 (9%) | 11 (11%) |
| 2. Unpleasant Odour | 17 (17%) | 18 (18%) | 23 (23%) | 41 (41%) | 43 (43%) |
| 3. Neither pleasant nor unpleasant | 33 (33%) | 31 (31%) | 13 (13%) | 30 (30%) | 22 (22%) |
| 4. Pleasant Odour | 28 (28%) | 24 (24%) | 20 (20%) | 18 (18%) | 14 (14%) |
| 5. Favourite Odour | 19 (19%) | 19 (19%) | 12 (12%) | 2 (2%) | 10 (10%) |
| Do you think that emulsions belong to the same plant species? | |||||
| Positive answers | 41 (41%) | ||||
| Uncertainly answers | 34 (34%) | ||||
| Negative answers | 25 (25%) |
| Section 1. Emulsions’ Odour. Feelings of Well-Being Caused by the Emulsions’ Odour | N (%) |
|---|---|
| Emulsions that caused feelings of well-being | |
| Ppi_OE_1_B and Eg_OE_1_G | 2 (2%) |
| Cj_OE_1_M, Pp_OE_1_G and Pp_OE_2_P | 3 (3%) |
| Cj_OE_1_M | 20 (20%) |
| Cj_OE_1_M and Pp_OE_1_G | 1 (1%) |
| Eg_OE_1_G and Pp_OE_2_P | 2 (2%) |
| Cj_OE_1_M and Pp_OE_2_P | 2 (2%) |
| Cj_OE_1_M and Ppi_OE_1_B | 5 (5%) |
| Pp_OE_2_P | 6 (6%) |
| Ppi_OE_1_B | 11 (11%) |
| Cj_OE_1_M, Ppi_OE_1_B and Eg_OE_1_G | 5 (5%) |
| Eg_OE_1_G | 17 (17%) |
| Cj_OE_1_M, Eg_OE_1_G, Pp_OE_2_P and Pp_OE_1_G | 1 (1%) |
| Cj_OE_1_M and Eg_OE_1_G | 1 (1%) |
| Pp_OE_1_G and Pp_OE_2_P | 2 (2%) |
| Ppi_OE_1_B and Pp_OE_2_P | 1 (1%) |
| Cj_OE_1_M, Ppi_OE_1_B and Pp_OE_1_G | 2 (2%) |
| Cj_OE_1_M, Ppi_OE_1_B, Pp_OE_1_G and Pp_OE_2_P | 1 (1%) |
| Pp_OE_1_G | 1 (1%) |
| None | 17 (17%) |
| Feelings of well-being | |
| Refreshing | 23 (23%) |
| Decongestant | 15 (15%) |
| Decongestant, Stimulating and Refreshing | 2 (2%) |
| Decongestant and Refreshing | 4 (4%) |
| Relaxing, Decongestant and Refreshing | 7 (7%) |
| Relaxing | 18 (18%) |
| Relaxing and Stimulating | 1 (1%) |
| Stimulating and Refreshing | 1 (1%) |
| Relaxing and Refreshing | 4 (4%) |
| Stimulating | 3 (3%) |
| Relaxing, Stimulating and Refreshing | 1 (1%) |
| Relaxing and Decongestant | 2 (2%) |
| Decongestant and Stimulating | 2 (2%) |
| None | 17 (17%) |
| Section 2. Applicability’s of Emulsions’ Odour | N (%) | |||||
|---|---|---|---|---|---|---|
| Purchasing a Product with Emulsions’ Odours | Perfume | Air Freshener | Massage Cream | Toothpaste | Shampoo | Candy |
| Probability of buying a product with Cj_OE_1_M odour | ||||||
| 1. Would never buy | 43 (43%) | 12 (12%) | 13 (13%) | 32 (32%) | 20 (20%) | 49 (49%) |
| 2. Unlikely | 28 (28%) | 31 (31%) | 25 (25%) | 36 (36%) | 31 (31%) | 37 (37%) |
| 3. Likely | 19 (19%) | 34 (34%) | 33 (33%) | 17 (17%) | 27 (27%) | 8 (8%) |
| 4. Quite likely | 4 (4%) | 5 (5%) | 11 (11%) | 4 (4%) | 7 (7%) | 2 (2%) |
| 5. Would buy | 6 (6%) | 18 (18%) | 18 (18%) | 11 (11%) | 15 (15%) | 4 (4%) |
| Probability of buying a product with Ppi_OE_1_B odour | ||||||
| 1. Would never buy | 52 (52%) | 22 (22%) | 16 (16%) | 26 (26%) | 31 (31%) | 43 (43%) |
| 2. Unlikely | 29 (29%) | 26 (26%) | 29 (29%) | 27 (27%) | 25 (25%) | 27 (27%) |
| 3. Likely | 11 (11%) | 29 (29%) | 34 (34%) | 27 (27%) | 23 (23%) | 19 (19%) |
| 4. Quite likely | 3 (3%) | 11 (11%) | 6 (6%) | 9 (9%) | 7 (7%) | 4 (4%) |
| 5. Would buy | 5 (5%) | 12 (12%) | 15 (15%) | 11 (11%) | 14 (14%) | 7 (7%) |
| Probability of buying a product with Eg_OE_1_G odour | ||||||
| 1. Would never buy | 63 (63%) | 36 (36%) | 38 (38%) | 44 (44%) | 37 (37%) | 53 (53%) |
| 2. Unlikely | 20 (20%) | 22 (22%) | 25 (25%) | 30 (30%) | 28 (28%) | 20 (20%) |
| 3. Likely | 9 (9%) | 20 (20%) | 17 (17%) | 13 (13%) | 17 (17%) | 8 (8%) |
| 4. Quite likely | 4 (4%) | 5 (5%) | 7 (7%) | 7 (7%) | 7 (7%) | 5 (5%) |
| 5. Would buy | 4 (4%) | 17 (17%) | 13 (13%) | 6 (6%) | 11 (11%) | 14 (14%) |
| Probability of buying a product with Pp_OE_2_P odour | ||||||
| 1. Would never buy | 50 (50%) | 25 (25%) | 18 (18%) | 39 (39%) | 34 (34%) | 47 (47%) |
| 2. Unlikely | 34 (34%) | 35 (35%) | 38 (38%) | 37 (37%) | 35 (35%) | 35 (35%) |
| 3. Likely | 12 (12%) | 28 (28%) | 29 (29%) | 19 (19%) | 22 (22%) | 13 (13%) |
| 4. Quite likely | 3 (3%) | 8 (8%) | 11 (11%) | 5 (5%) | 6 (6%) | 2 (2%) |
| 5. Would buy | 1 (1%) | 4 (4%) | 4 (4%) | 0 (0%) | 3 (3%) | 3 (3%) |
| Probability of buying a product with Pp_OE_1_G odour | ||||||
| 1. Would never buy | 54 (54%) | 32 (32%) | 27 (27%) | 42 (42%) | 38 (38%) | 54 (54%) |
| 2. Unlikely | 32 (32%) | 40 (40%) | 33 (33%) | 40 (40%) | 34 (34%) | 39 (39%) |
| 3. Likely | 10 (10%) | 19 (19%) | 28 (28%) | 13 (13%) | 18 (18%) | 6 (6%) |
| 4. Quite likely | 1 (1%) | 3 (3%) | 6 (6%) | 2 (2%) | 5 (5%) | 1 (1%) |
| 5. Would buy | 3 (3%) | 6 (6%) | 6 (6%) | 3 (3%) | 5 (5%) | 0 (0%) |
| Section 2. Applicability of Emulsions’ Odour. Other Applicability of Emulsions’ Odour | N (%) |
|---|---|
| Aromatherapy and bath bombs | 1 (1%) |
| Soaps | 2 (2%) |
| Cleaning products | 10 (10%) |
| Massage oils | 1 (1%) |
| Repellents | 2 (2%) |
| Nasal spray | 1 (1%) |
| Incense and cleaning products | 1 (1%) |
| Shower gel | 1 (1%) |
| Ointment medications (analgesics) | 3 (3%) |
| Wood Furniture Cleaning Products | 1 (1%) |
| Hand and face cream | 1 (1%) |
| Disinfectant | 1 (1%) |
| Body and hand cream | 1 (1%) |
| Deodorant | 2 (2%) |
| Candles and Soaps | 1 (1%) |
| Shaving cream | 1 (1%) |
| Nasal decongestant | 2 (2%) |
| Car air freshener and cleaning products | 1 (1%) |
| None | 9 (9%) |
| Plant Species | EOs Code * | Hds Code |
|---|---|---|
| Eucalyptus globulus | Eg_OE_1_G | Eg_Hd_1_G |
| Eg_OE_2_B | - | |
| Eg_OE_3_O | Eg_Hd_2_O | |
| Eg_OE_4_E | Eg_Hd_3_E | |
| Eg_OE_5_P | Eg_Hd_4_P | |
| Eg_OE_6_S | - | |
| Pinus pinaster | Pp_OE_1_G | Pp_Hd_1_G |
| Pp_OE_2_P | Pp_Hd_2_P | |
| Pp_OE_3_S | - | |
| Pinus pinea | Ppi_OE_1_B | - |
| Cryptomeria japonica | Cj_OE_1_M | Cj_Hd_1_M |
| INCI * | Trade Name | Function | (%, w/w) |
|---|---|---|---|
| Phase A | |||
| Ceteareth-11 | Eumulgin B1® | Non-ionic O/W emulsifier | 1.5 |
| Ceteareth-20 | Eumulgin | Non-ionic O/W emulsifier | 1.5 |
| Cetyl alcohol | Cetyl alcohol | Thickener | 2.0 |
| Paraffinum liquidum | Mineral oil | Emollient | 2.5 |
| Decyl oleate | Tegosoft DO® | 4.5 | |
| Phase B | |||
| Glycerin | Glycerin | Humectant | 5.0 |
| Aqua | Purified water | Solvent | 82.0 |
| Phase C | |||
| Parfum | Essential Oil | Fragrance | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruas, A.; Graça, A.; Marto, J.; Gonçalves, L.; Oliveira, A.; da Silva, A.N.; Pimentel, M.; Moura, A.M.; Serra, A.T.; Figueiredo, A.C.; et al. Chemical Characterization and Bioactivity of Commercial Essential Oils and Hydrolates Obtained from Portuguese Forest Logging and Thinning. Molecules 2022, 27, 3572. https://doi.org/10.3390/molecules27113572
Ruas A, Graça A, Marto J, Gonçalves L, Oliveira A, da Silva AN, Pimentel M, Moura AM, Serra AT, Figueiredo AC, et al. Chemical Characterization and Bioactivity of Commercial Essential Oils and Hydrolates Obtained from Portuguese Forest Logging and Thinning. Molecules. 2022; 27(11):3572. https://doi.org/10.3390/molecules27113572
Chicago/Turabian StyleRuas, Ana, Angelica Graça, Joana Marto, Lídia Gonçalves, Ana Oliveira, Alexandra Nogueira da Silva, Madalena Pimentel, Artur Mendes Moura, Ana Teresa Serra, Ana Cristina Figueiredo, and et al. 2022. "Chemical Characterization and Bioactivity of Commercial Essential Oils and Hydrolates Obtained from Portuguese Forest Logging and Thinning" Molecules 27, no. 11: 3572. https://doi.org/10.3390/molecules27113572
APA StyleRuas, A., Graça, A., Marto, J., Gonçalves, L., Oliveira, A., da Silva, A. N., Pimentel, M., Moura, A. M., Serra, A. T., Figueiredo, A. C., & Ribeiro, H. M. (2022). Chemical Characterization and Bioactivity of Commercial Essential Oils and Hydrolates Obtained from Portuguese Forest Logging and Thinning. Molecules, 27(11), 3572. https://doi.org/10.3390/molecules27113572