Synthesis and Ultrafast Broadband Optical Limiting Properties of a Two-Branched Twistacene
(This article belongs to the Section Materials Chemistry)
Abstract
:1. Introduction
2. Results and Discussion
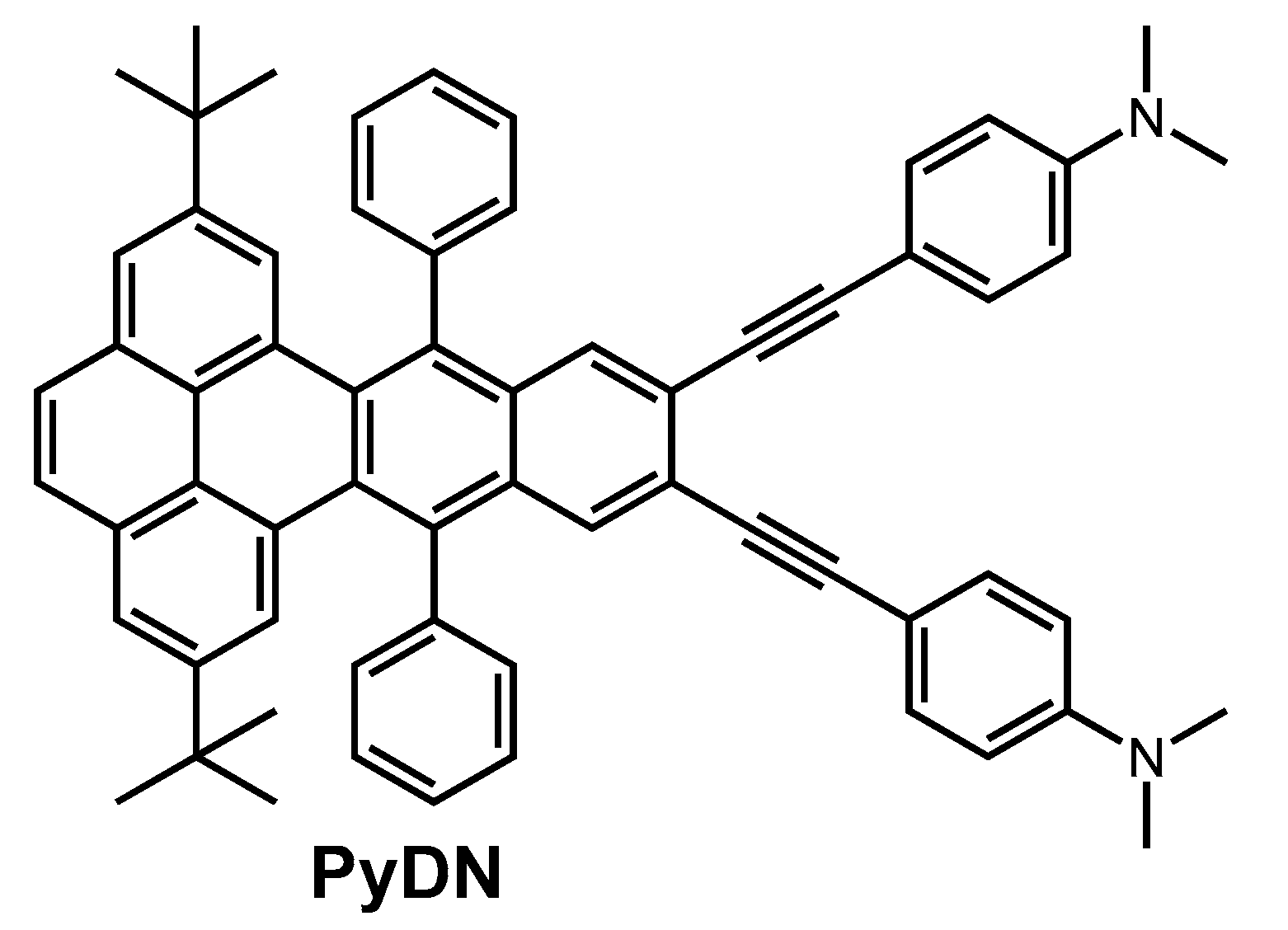
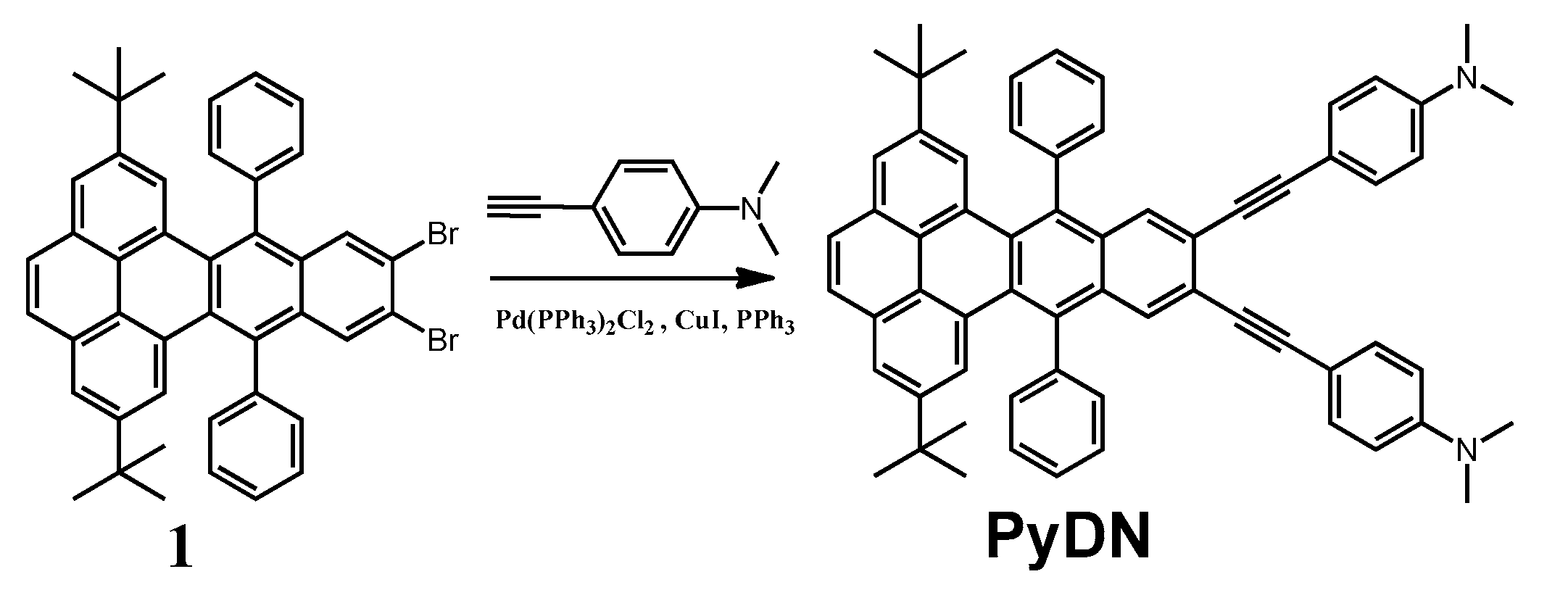
2.1. Molecular Design and Synthesis
2.2. Electronic Structure Analysis
2.3. UV-Vis Absorption and Emission
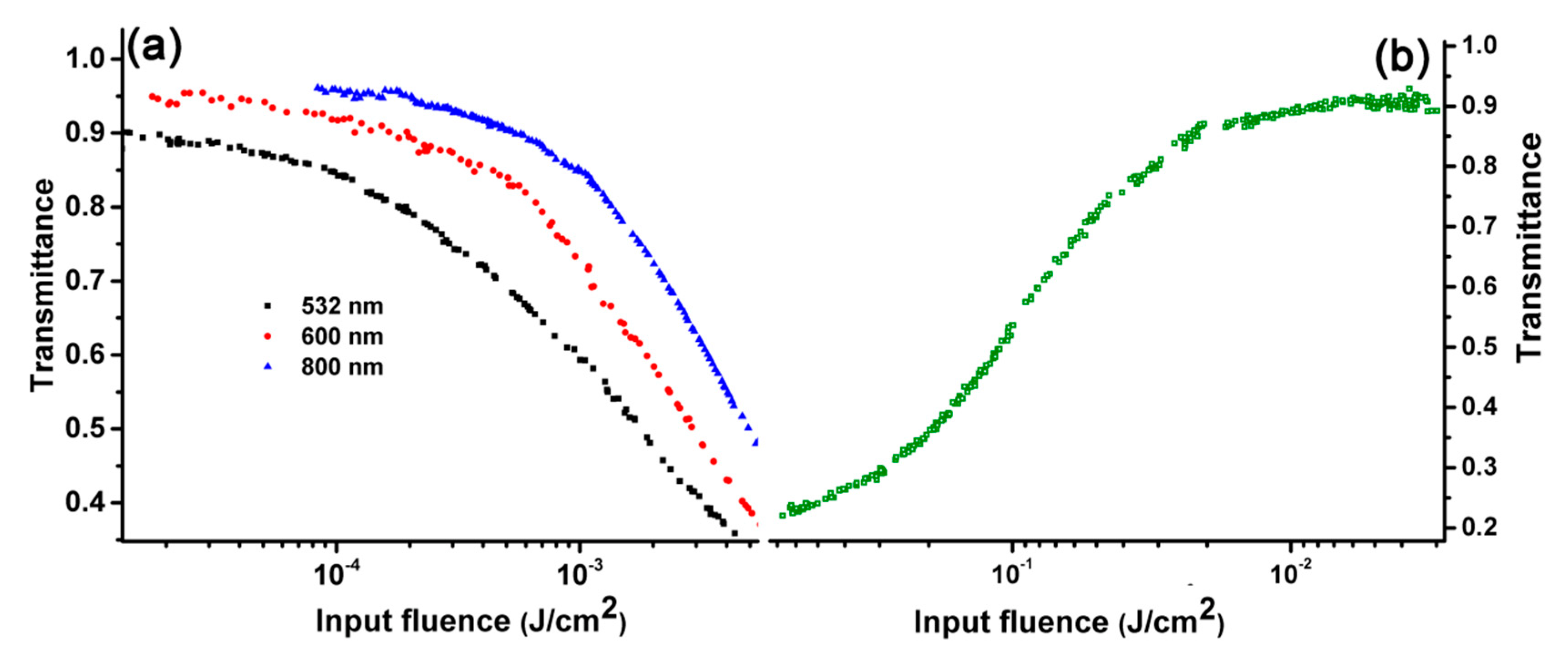
2.4. Open Aperture Z-scan and Transient Absorption Spectrum Experiments
2.5. Two-Photon-Excited Fluorescence (TPEF) Experiment
2.6. Ultrafast Optical Limiting
3. Materials and Methods
3.1. General Method
3.2. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mihailov, S.J.; Hnatovsky, C.; Abdukerim, N.; Walker, R.B.; Lu, P.; Xu, Y.; Bao, X.; Ding, H.; De Silva, M.; Coulas, D.; et al. Ultrafast Laser Processing of Optical Fibers for Sensing Applications. Sensors 2021, 21, 1447. [Google Scholar] [CrossRef]
- Lei, S.; Zhao, X.; Yu, X.; Hu, A.; Vukelic, S.; Jun, M.B.G.; Joe, H.-E.; Yao, Y.L.; Shin, Y.C. Ultrafast Laser Applications in Manufacturing Processes: A State-of-the-Art Review. J. Manuf. Sci. Eng. 2020, 142, 031005. [Google Scholar] [CrossRef]
- Leburn, C.G. Unifying ultrafast laser imaging and spectroscopy. PhotonicViews 2021, 18, 96–99. [Google Scholar] [CrossRef]
- Lim, G.-K.; Chen, Z.-L.; Clark, J.; Goh, R.G.S.; Ng, W.-H.; Tan, H.-W.; Friend, R.H.; Ho, P.K.H.; Chua, L.-L. Giant broadband nonlinear optical absorption response in dispersed graphene single sheets. Nat. Photonics 2011, 5, 554–560. [Google Scholar] [CrossRef]
- Kumar, V. Linear and Nonlinear Optical Properties of Graphene: A Review. J. Electron. Mater. 2021, 50, 3773–3799. [Google Scholar] [CrossRef]
- Xing, F.; Wang, Y.; Wang, J.; Zhou, S.; Zhao, J.; Xie, Z. Highly dispersed antimonene oxide quantum dots and their hybrid gel glasses for broadband nonlinear optical limiting. J. Mater. Chem. C 2021, 9, 10084–10088. [Google Scholar] [CrossRef]
- Sun, X.; Hu, X.; Sun, J.; Xie, Z.; Zhou, S. Strong optical limiting properties of Ormosil gel glasses doped with silver nano-particles. N. J. Chem. 2019, 43, 6274–6278. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, J.; Yan, C.; Xie, Z.; Zhang, G.; Shao, X.; Zhang, D.; Zhou, S. Diketopyrrolopyrrole based donor–acceptor π-conjugated copolymers with near-infrared absorption for 532 and 1064 nm nonlinear optical materials. J. Mater. Chem. C 2020, 8, 12993–13000. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, B.; Hou, X.; Yan, C.; Sun, X.; Xie, Z.; Shao, X.; Zhou, S. Broadband optical limiting of a novel twisted tetrathiafulvalene incorporated donor–acceptor material and its Ormosil gel glasses. J. Mater. Chem. C 2018, 6, 8495–8501. [Google Scholar] [CrossRef]
- Dini, D.; Calvete, M.J.; Hanack, M. Nonlinear Optical Materials for the Smart Filtering of Optical Radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef]
- Tutt, L.W.; Boggess, T.F. A review of optical limiting mechanisms and devices using organics, fullerenes, semiconductors and other materials. Prog. Quantum Electron. 1993, 17, 299–338. [Google Scholar] [CrossRef]
- Spangler, C.W. Recent development in the design of organic materials for optical power limiting. J. Mater. Chem. 1999, 9, 2013–2020. [Google Scholar] [CrossRef]
- Lin, T.-C.; Li, M.-L.; Liu, C.-Y.; Tsai, M.-Y.; Lee, Y.-H.; Febriani, Y.; Lin, J.-H.; Shen, Y.-K. Synthesis and Two-Photon Properties of Multi-Branched Fluorophores Composed of Ladder-Type Conjugated Cores and Functionalized Diquinoxalinylamino Peripheries. Eur. J. Org. Chem. 2014, 28, 1615–1621. [Google Scholar] [CrossRef]
- Xiao, Z.; Shi, Y.; Sun, R.; Ge, J.; Li, Z.; Fang, Y.; Wu, X.; Yang, J.; Zhao, M.; Song, Y. Ultrafast broadband optical limiting in simple pyrene-based molecules with high transmittance from visible to infrared regions. J. Mater. Chem. C 2016, 4, 4647–4653. [Google Scholar] [CrossRef]
- Jia, J.; Wu, X.; Fang, Y.; Yang, J.; Guo, X.; Xu, Q.; Han, Y.; Song, Y. Ultrafast Broad-Band Optical Limiting in Simple Hydrazone Derivatives with a Π-Conjugated System: Effect of Two-Photon-Induced Singlet-State Absorption. J. Phys. Chem. C 2018, 122, 16234–16241. [Google Scholar] [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef]
- Duong, H.M.; Bendikov, M.; Steiger, D.; Zhang, Q.; Sonmez, G.; Yamada, J.; Wudl, F. Efficient Synthesis of a Novel, Twisted and Stable, Electroluminescent “Twistacene”. Org. Lett. 2003, 5, 4433–4436. [Google Scholar] [CrossRef]
- Jin, P.; Song, T.; Xiao, J.; Zhang, Q. Recent Progress in Using Pyrene-4,5-diketones and Pyrene-4,5,9,10-tetraketones as Building Blocks to Construct Large Acenes and Heteroacenes. Asian J. Org. Chem. 2018, 7, 2130–2146. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, J.; Fu, Q.; Feng, H.; Zhang, X.; Ren, T.; Wang, S.; Ma, D.; Wang, X.; Chen, H. Synthesis and Physical Properties of the Conjugated Dendrons Bearing Twisted Acenes Used in Solution Processing of Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2013, 5, 11136–11141. [Google Scholar] [CrossRef]
- Martinez-Abadia, M.; Antonicelli, G.; Zuccatti, E.; Atxabal, A.; Melle-Franco, M.; Hueso, L.E.; Mateo-Alonso, A. Synthesis and Properties of a Twisted and Stable Tetracyano-Substituted Tetrabenzoheptacene. Org. Lett. 2017, 19, 1718–1721. [Google Scholar] [CrossRef]
- Lv, B.; Xiao, J.; Zhou, J.; Zhang, X.; Duan, J.; Su, W.; Zhao, J. Synthesis, Crystal Analyses, Physical Properties, and Electroluminescent Behavior of Unsymmetrical Heterotwistacenes. ACS Appl. Mater. Interfaces 2016, 8, 18998–19003. [Google Scholar] [CrossRef]
- Wei, L.; Deng, X.; Yu, X.; Li, X.; Wang, W.; Zhang, C.; Xiao, J. Double π-Extended Helicene Derivatives Containing Pentagonal Rings: Synthesis, Crystal Analyses, and Photophysics. J. Org. Chem. 2021, 86, 17535–17542. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xiao, J.; Zhang, X.; Shen, X.; Liu, X.; Shen, F.; Yi, Y.; Song, Y. Effect of the mismatch structure on crystal packing, physical properties and third-order nonlinearity of unsymmetrical twistacenes. Dyes Pigm. 2016, 134, 9–18. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, J.; Sun, R.; Jin, T.; Yang, J.; Shi, G.; Wang, Y.; Zhang, X.; Song, Y. Spindle-Type Conjugated Compounds Containing Twistacene Unit: Synthesis and Ultrafast Broadband Reverse Saturable Absorption. Adv. Opt. Mater. 2017, 5, 1600712. [Google Scholar] [CrossRef]
- Song, T.; Han, Y.; Jin, P.; Li, X.; Song, Y.; Xiao, J. The enhanced two-photon absorption behavior of twistfuranacenes to phenylacetylene-functionalized twistacenes. J. Mater. Chem. C 2019, 7, 6344–6351. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Chen, Y.-F.; Hu, C.-L.; Hsu, C.-S. Two-photon absorption and optical power limiting properties in femtosecond regime of novel multi-branched chromophores based on tri-substituted olefinic scaffolds. J Mater. Chem. 2009, 19, 7075–7080. [Google Scholar] [CrossRef]
- Ceymann, H.; Rosspeintner, A.; Schreck, M.H.; Mützel, C.; Stoy, A.; Vauthey, E.; Lambert, C. Cooperative enhancement versus additivity of two-photon-absorption cross sections in linear and branched squaraine superchromophores. Phys. Chem. Chem. Phys. 2016, 18, 16404–16413. [Google Scholar] [CrossRef] [Green Version]
- Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, G.A.H.; Caricato, M.; Li, X.; Hratchian, H.P.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Sun, C.-L.; Liao, Q.; Li, T.; Li, J.; Jiang, J.-Q.; Xu, Z.-Z.; Wang, X.-D.; Shen, R.; Bai, D.-C.; Wang, Q.; et al. Rational design of small indolic squaraine dyes with large two-photon absorption cross section. Chem. Sci. 2015, 6, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Stryland, E.W.V. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Teran, N.B.; He, G.S.; Baev, A.; Shi, Y.; Swihart, M.T.; Prasad, P.N.; Marks, T.J.; Reynolds, J.R. Twisted Thiophene-Based Chromophores with Enhanced Intramolecular Charge Transfer for Cooperative Amplification of Third-Order Optical Nonlinearity. J. Am. Chem. Soc. 2016, 138, 6975–6984. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, W.; Li, G.; Huo, D.; Guo, Y.; Niu, X.; Wan, Y.; Tang, B.; Xia, A. Excited-State Symmetry-Breaking Charge Separation Dynamics in Multibranched Perylene Diimide Molecules. J. Phys. Chem. Lett. 2020, 11, 10329–10339. [Google Scholar] [CrossRef]
- Sebastian, E.; Hariharan, M. Null Exciton-Coupled Chromophoric Dimer Exhibits Symmetry-Breaking Charge Separation. J. Am. Chem. Soc. 2021, 143, 13769–13781. [Google Scholar] [CrossRef]
- Hu, W.; Prasad, P.N.; Huang, W. Manipulating the Dynamics of Dark Excited States in Organic Materials for Phototheranostics. Acc. Chem. Res. 2021, 54, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Ji, W.; Patil, P.S.; Dharmaprakash, S.M.; Wang, H.-T. Two-photon-induced excited-state absorption: Theory and experiment. Appl. Phys. Lett. 2008, 92, 091118. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Dubinina, G.G.; Price, R.S.; Abboud, K.A.; Wicks, G.; Wnuk, P.; Stepanenko, Y.; Drobizhev, M.; Rebane, A.; Schanze, K.S. Phenylene Vinylene Platinum(II) Acetylides with Prodigious Two-Photon Absorption. J. Am. Chem. Soc. 2012, 134, 19346–19349. [Google Scholar] [CrossRef]
- Jiang, X.-F.; Polavarapu, L.; Neo, S.T.; Venkatesan, T.; Xu, Q.-H. Graphene Oxides as Tunable Broadband Nonlinear Optical Materials for Femtosecond Laser Pulses. J. Phys. Chem. Lett. 2012, 3, 785–790. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, Y.; Wang, H.; Qi, X.; He, J.; Xiao, S. Donor-Acceptor Type Reduced Graphene-Oxide and a Tin-Selenide Nanohybrid With Broad and Ultrafast Optical Limiting Properties. Front. Phys. 2020, 8, 298. [Google Scholar] [CrossRef]
- Husain, A.; Ganesan, A.; Sebastian, M.; Makhseed, S. Large ultrafast nonlinear optical response and excellent optical limiting behaviour in pyrene-conjugated zinc(II) phthalocyanines at a near-infrared wavelength. Dyes Pigm. 2021, 184, 108787. [Google Scholar] [CrossRef]





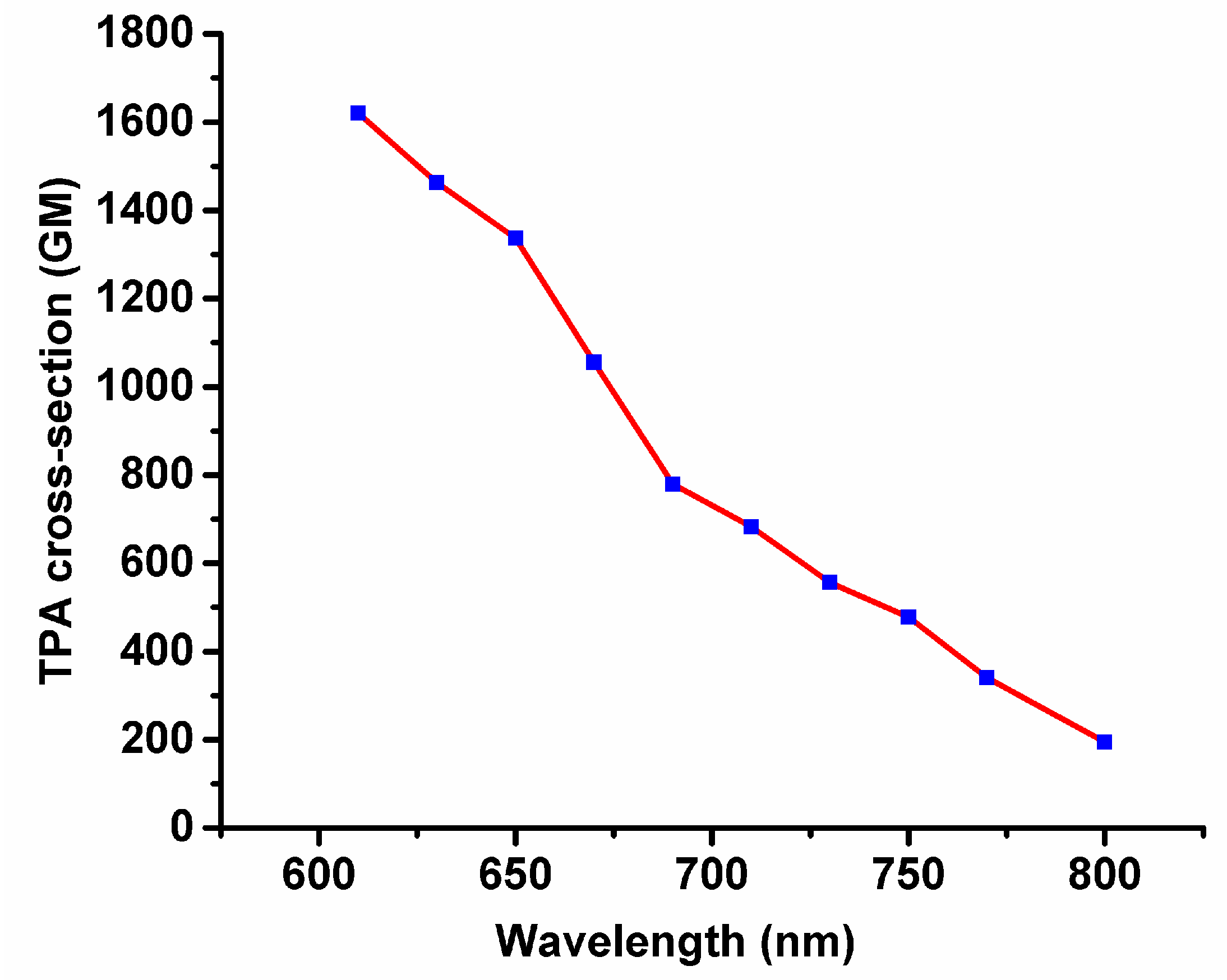
| 532 nm | 600 nm | 700 nm | 800 nm | |
|---|---|---|---|---|
| β [10−2 cmGW−1] | 9.57 | 5.00 | 1.41 | 0.54 |
| σTPA [GM] | 2476 | 1147 | 277 | 93 |
| γ [10−4 cm3GW−2] | 6.50 | 2.98 | 2.13 | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Xiao, J.; Wu, X.; Wang, Y.; Zhang, X.; Song, Y. Synthesis and Ultrafast Broadband Optical Limiting Properties of a Two-Branched Twistacene. Molecules 2022, 27, 3564. https://doi.org/10.3390/molecules27113564
Han Y, Xiao J, Wu X, Wang Y, Zhang X, Song Y. Synthesis and Ultrafast Broadband Optical Limiting Properties of a Two-Branched Twistacene. Molecules. 2022; 27(11):3564. https://doi.org/10.3390/molecules27113564
Chicago/Turabian StyleHan, Yanbing, Jinchong Xiao, Xingzhi Wu, Yuxiao Wang, Xueru Zhang, and Yinglin Song. 2022. "Synthesis and Ultrafast Broadband Optical Limiting Properties of a Two-Branched Twistacene" Molecules 27, no. 11: 3564. https://doi.org/10.3390/molecules27113564
APA StyleHan, Y., Xiao, J., Wu, X., Wang, Y., Zhang, X., & Song, Y. (2022). Synthesis and Ultrafast Broadband Optical Limiting Properties of a Two-Branched Twistacene. Molecules, 27(11), 3564. https://doi.org/10.3390/molecules27113564





