Effects of Saignée and Bentonite Treatment on Phenolic Compounds of Marquette Red Wines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Chemical Properties
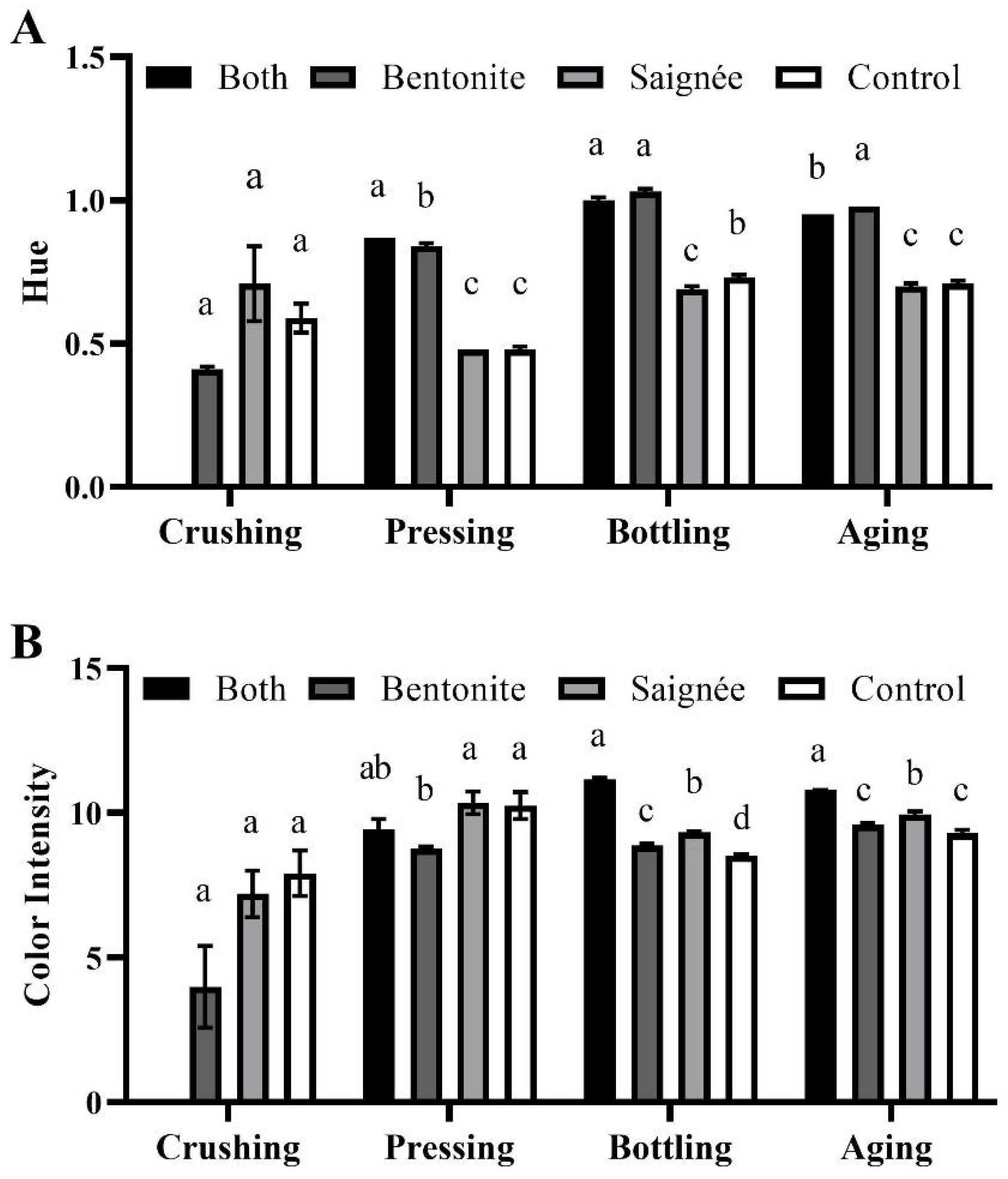
2.2. Wine Color
2.3. Monomeric Phenolic Compounds
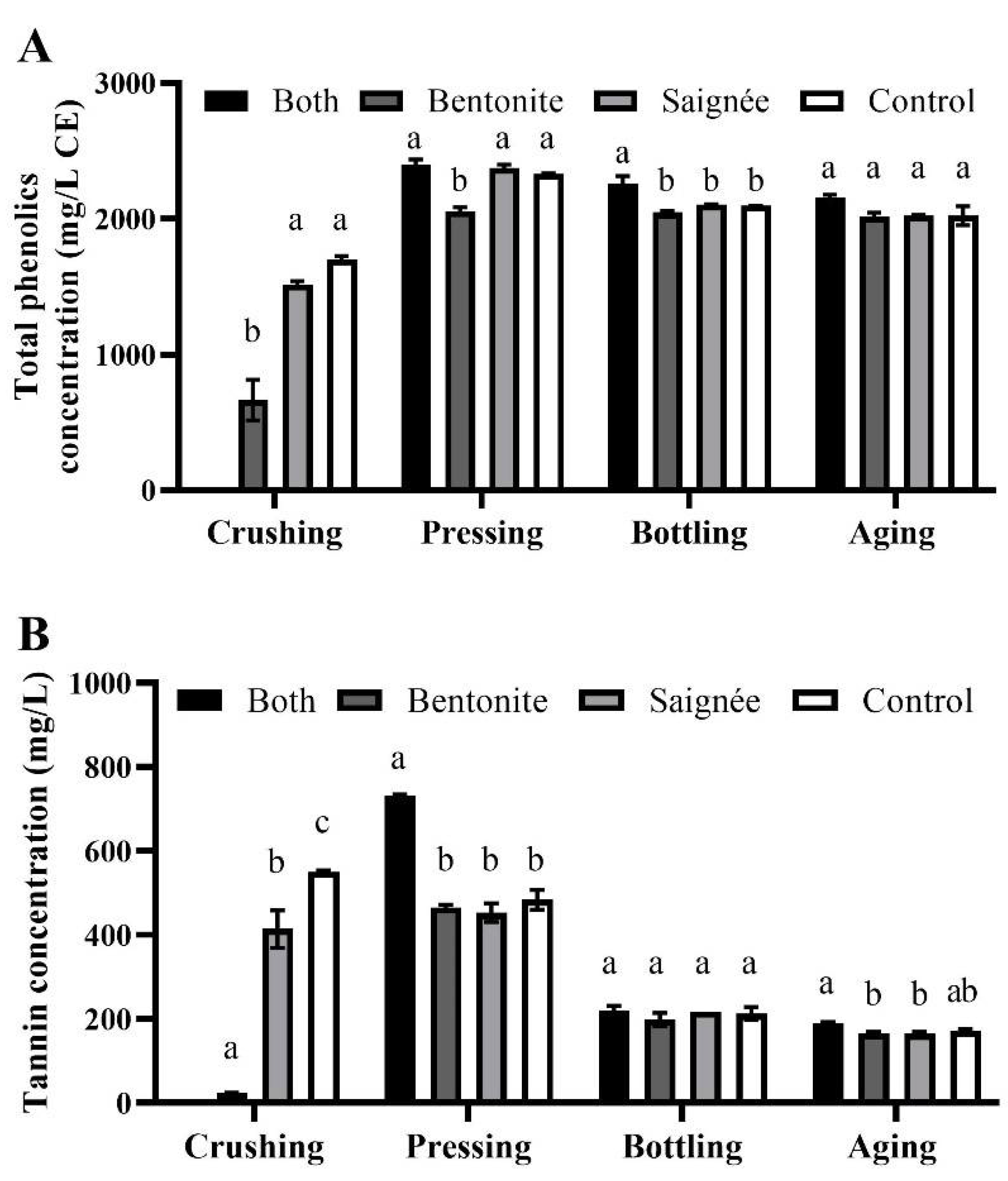
2.4. Iron-Reactive Phenolic Compounds and Tannins Content
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Winemaking Protocol
- Control: 41.6 L of must was held on skins for three days prior to yeast inoculation.
- Saignée: 41.6 L of must was held on skins for three days and 3.8 L of juice (approximate 9% v/v run-off) was initially removed.
- Bentonite: Must was firstly pressed to relative dryness, and 1.32 g/L sodium-calcium-bentonite fining agent (FermoBent® PORE-TEC, Germany) was added into the juice. After three days, the juice was racked off the bentonite mud and fully returned (300 L) into macrobin with skins.
- Saignée plus bentonite (both): Application of the exact same protocol as the bentonite treatment, with 40 L of wine retained (13.7% v/v run-off) and returned into macrobin with skins after racking off the bentonite mud.
3.3. Basic Checmial Properties
3.4. Color Measurements
3.5. Monomeric Pheonlics Content
3.6. Total Iron-Rective Phenolics and Tannin Content
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and Polymeric Procyanidins from Grape Seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Kuhl, T.L.; Waterhouse, A.L. Friction Forces of Saliva and Red Wine on Hydrophobic and Hydrophilic Surfaces. Food Res. Int. 2019, 116, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manns, D.C.; Coquard Lenerz, C.T.M.; Mansfield, A.K. Impact of Processing Parameters on the Phenolic Profile of Wines Produced from Hybrid Red Grapes Maréchal Foch, Corot Noir, and Marquette. J. Food Sci. 2013, 78, C696–C702. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of Ethanol Concentration on the Extraction of Color and Phenolic Compounds from the Skin and Seeds of Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Norton, E.L. Chemistry and Reactivity of Tannins in Vitis Spp.: A Review. Molecules 2020, 25, 2110. [Google Scholar] [CrossRef]
- Sparrow, A.M.; Holt, H.E.; Pearson, W.; Dambergs, R.G.; Close, D.C. Accentuated Cut Edges (ACE): Effects of Skin Fragmentation on the Composition and Sensory Attributes of Pinot Noir Wines. Am. J. Enol. Vitic. 2016, 67, 169–178. [Google Scholar] [CrossRef]
- Kang, W.; Bindon, K.A.; Wang, X.; Muhlack, R.A.; Smith, P.A.; Niimi, J.; Bastian, S.E.P. Chemical and Sensory Impacts of Accentuated Cut Edges (ACE) Grape Must Polyphenol Extraction Technique on Shiraz Wines. Foods 2020, 9, 1027. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Jiménez-Martínez, M.D.; Jurado, R.; Iniesta, J.A.; Terrades, S.; Andrés, A.; Gómez-Plaza, E. Application of High-Power Ultrasounds during Red Wine Vinification. Int. J. Food Sci. Technol. 2017, 52, 1314–1323. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound Assisted Extraction of Phenolic Compounds from Grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Morel-Salmi, C.; Souquet, J.-M.; Bes, M.; Cheynier, V. Effect of Flash Release Treatment on Phenolic Extraction and Wine Composition. J. Agric. Food Chem. 2006, 54, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Neves, N.; de Araújo Pantoja, L.; dos Santos, A.S. Thermovinification of Grapes from the Cabernet Sauvignon and Pinot Noir Varieties Using Immobilized Yeasts. Eur. Food Res. Technol. 2014, 238, 79–84. [Google Scholar] [CrossRef]
- Cheng, Y.; Savits, J.R.; Watrelot, A.A. Effect of the Application Time of Accentuated Cut Edges (ACE) on Marquette Wine Phenolic Compounds. Molecules 2022, 27, 542. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Bouska, L. Optimization of the Ultrasound-Assisted Extraction of Polyphenols from Aronia and Grapes. Food Chem. 2022, 386, 132703. [Google Scholar] [CrossRef] [PubMed]
- Springer, L.F.; Sacks, G.L. Protein-Precipitable Tannin in Wines from Vitis Vinifera and Interspecific Hybrid Grapes (Vitis Ssp.): Differences in Concentration, Extractability, and Cell Wall Binding. J. Agric. Food Chem. 2014, 62, 7515–7523. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, P.; Marcotte, C.; Angers, P.; Pedneault, K. Pomace Limits Tannin Retention in Frontenac Wines. Food Chem. 2019, 277, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Springer, L.F.; Chen, L.A.; Stahlecker, A.C.; Cousins, P.; Sacks, G.L. Relationship of Soluble Grape-Derived Proteins to Condensed Tannin Extractability during Red Wine Fermentation. J. Agric. Food Chem. 2016, 64, 8191–8199. [Google Scholar] [CrossRef]
- Baron, R.; Mayen, M.; Merida, J.; Medina, M. Comparative Study of Browning and Flavan-3-Ols during the Storage of White Sherry Wines Treated with Different Fining Agents. J. Sci. Food Agric. 2000, 80, 226–230. [Google Scholar] [CrossRef]
- Main, G.L.; Morris, J.R. Color of Riesling and Vidal Wines as Affected by Bentonite, Cufex (R), and Sulfur Dioxide Juice Treatments. Am. J. Enol. Vitic. 1991, 42, 354–357. [Google Scholar]
- González-Neves, G.; Favre, G.; Gil, G. Effect of Fining on the Colour and Pigment Composition of Young Red Wines. Food Chem. 2014, 157, 385–392. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez, A. Color and Phenolic Compounds of a Young Red Wine. Influence of Wine-Making Techniques, Storage Temperature, and Length of Storage Time. J. Agric. Food Chem. 2000, 48, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Singleton, V.L. Effects on Red Wine Quality of Removing Juice before Fermentation to Simulate Variation in Berry Size. Am. J. Enol. Vitic. 1972, 23, 106–113. [Google Scholar]
- Wu, Y.; Xing, K.; Zhang, X.; Wang, H.; Wang, F.; Wang, Y.; Li, J. Effect of Pre-Fermentation Saignée Treatment on Phenolic Compound Profile in Wine Made of Cabernet Sauvignon. J. Food Biochem. 2017, 41, e12380. [Google Scholar] [CrossRef]
- Casassa, L.F.; Larsen, R.C.; Harbertson, J.F. Effects of Vineyard and Winemaking Practices Impacting Berry Size on Evolution of Phenolics during Winemaking. Am. J. Enol. Vitic. 2016, 67, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Teng, B.; Petrie, P.R.; Espinase Nandorfy, D.; Smith, P.; Bindon, K. Pre-Fermentation Water Addition to High-Sugar Shiraz Must: Effects on Wine Composition and Sensory Properties. Foods 2020, 9, 1193. [Google Scholar] [CrossRef]
- Gawel, R.; Iland, P.G.; Leske, P.A.; Dunn, C.G. Compositional and Sensory Differences in Syrah Wines Following Juice Run-off Prior to Fermentation. J. Wine Res. 2001, 12, 5–18. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Wine-Making of High Coloured Wines: Extended Pomace Contact and Run-off of Juice Prior to Fermentation. Food Sci. Technol. Int. 2004, 10, 287–295. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.S.; Harwood, E.D.; Weller, K.M.; Ross, C.F. Chemical and Sensory Effects of Saignée, Water Addition, and Extended Maceration on High Brix Must. Am. J. Enol. Vitic. 2009, 4, 450–460. [Google Scholar]
- Burtch, C.E.; Mansfield, A.K.; Manns, D.C. Reaction Kinetics of Monomeric Anthocyanin Conversion to Polymeric Pigments and their Significance to Color in Interspecific Hybrid Wines. J. Agric. Food Chem. 2017, 65, 6379–6386. [Google Scholar] [CrossRef]
- Stankovic, S.; Jovic, S.; Zivkovic, J.; Pavlovic, R. Influence of Age on Red Wine Colour during Fining with Bentonite and Gelatin. Int. J. Food Prop. 2012, 15, 326–335. [Google Scholar] [CrossRef]
- Balík, J.; Kyseláková, M.; Veverka, J.; Tříska, J.; Vrchotová, N.; Totušek, J.; Lefnerová, D. The Effect of Clarification on Colour, Concentration of Anthocyanins and Polyphenols in Red Wine. Acta Hortic. 2007, 754, 563–568. [Google Scholar] [CrossRef]
- He, S.; Hider, R.; Zhao, J.; Tian, B. Effect of Bentonite Fining on Proteins and Phenolic Composition of Chardonnay and Sauvignon Blanc Wines. S. Afr. J. Enol. Vitic. 2020, 41, 113–120. [Google Scholar] [CrossRef]
- Watrelot, A.A. Tannin Content in Vitis Species Red Wines Quantified Using Three Analytical Methods. Molecules 2021, 26, 4923. [Google Scholar] [CrossRef] [PubMed]
- Vernhet, A.; Dupre, K.; Boulange-Petermann, L.; Cheynier, V.; Pellerin, P.; Moutounet, M. Composition of Tartrate Precipitates Deposited on Stainless Steel Tanks during the Cold Stabilization of Wines. Part II. Red Wines. Am. J. Enol. Vitic. 1999, 50, 398–403. [Google Scholar]
- Ritchey, J.G.; Waterhouse, A.L. A Standard Red Wine: Monomeric Phenolic Analysis of Commercial Cabernet Sauvignon Wines. Am. J. Enol. Vitic. 1999, 50, 91–100. [Google Scholar]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC Analysis of Diverse Grape and Wine Phenolics Using Direct Injection and Multidetection by DAD and Fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Badet-Murat, M.L.; Waterhouse, A.L. Oak Barrel Tannin and Toasting Temperature: Effects on Red Wine Condensed Tannin Chemistry. LWT-Food Sci. Technol. 2018, 91, 330–338. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of Polymeric Pigments in Grape Berry Extracts and Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar]
- Heredia, T.M.; Adams, D.O.; Fields, K.C.; Held, P.G.; Harbertson, J.F. Evaluation of a Comprehensive Red Wine Phenolics Assay Using a Microplate Reader. Am. J. Enol. Vitic. 2006, 4, 497–502. [Google Scholar]
| Time Point | Treatment | pH | TA 3 (g/L) | Ethanol (vol%) | Tartaric Acid (g/L) | Malic Acid (g/L) |
|---|---|---|---|---|---|---|
| Crushing | Bentonite | 3.19 ± 0.06 b 1 | 10.03 ± 0.13 b | - | 2.92 ± 0.36 a | 10.52 ± 0.29 b |
| Saignée | 3.38 ± 0.00 a | 12.84 ± 0.13 a | - | 2.38 ± 0.01 a | 12.54 ± 0.24 a | |
| Control | 3.37 ± 0.01 a | 14.20 ± 0.60 a | - | 2.46 ± 0.10 a | 12.38 ± 0.07 a | |
| Pressing | Both 2 | 3.80 ± 0.01 a | 10.50 ± 0.00 ab | - | - | - |
| Bentonite | 3.76 ± 0.00 ab | 10.13 ± 0.26 b | - | - | - | |
| Saignée | 3.73 ± 0.04 ab | 10.79 ± 0.13 a | - | - | - | |
| Control | 3.69 ± 0.01 b | 10.13 ± 0.00 b | - | - | - | |
| Bottling | Both | 3.93 ± 0.01 a | 7.13 ± 0.00 b | 13.30 ± 0.39 a | 4.17 ± 0.10 a | 5.37 ± 0.14 ab |
| Bentonite | 3.91 ± 0.01 a | 7.22 ± 0.13 b | 13.18 ± 0.13 a | 4.03 ± 0.01 a | 4.69 ± 0.58 b | |
| Saignée | 3.86 ± 0.00 b | 7.50 ± 0.00 a | 13.95 ± 0.39 a | 3.68 ± 0.04 b | 6.34 ± 0.18 a | |
| Control | 3.85 ± 0.01 b | 7.13 ± 0.00 b | 14.09 ± 0.19 a | 4.06 ± 0.01 a | 6.44 ± 0.06 a | |
| Aging | Both | 3.74 ± 0.01 a | 7.83 ± 0.07 a | 14.11 ± 0.02 b | 2.85 ± 0.00 a | 2.75 ± 0.06 a |
| Bentonite | 3.70 ± 0.01 a | 7.80 ± 0.03 a | 13.89 ± 0.01 c | 2.63 ± 0.03 c | 2.42 ± 0.16 ab | |
| Saignée | 3.63 ± 0.01 b | 7.73 ± 0.07 a | 14.47 ± 0.01 a | 2.79 ± 0.00 b | 2.30 ± 0.02 b | |
| Control | 3.64 ± 0.02 b | 7.59 ± 0.13 a | 14.49 ± 0.00 a | 2.81 ± 0.01 ab | 2.28 ± 0.04 b |
| Time Point | Treatment | Unknown 1 | Delphinidin-3-G | Peonidin-3,5-DG | Malvidin-3,5-DG | Unknown 2 | Unknown 3 | Malvidin-3-G | Unknown 4 | Malvidin-3-(6”-acetylglucoside) | Total Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressing | Both 2 | 78.06 ± 0.15 b 1 | 32.02 ± 0.41 b | 25.30 ± 0.04 b | 138.23 ± 4.90 a | 25.81 ± 1.34 b | 2.83 ± 0.03 a | 17.20 ± 0.47 b | 6.93 ± 0.36 b | 5.83 ± 0.10 c | 332.21 ± 6.06 c |
| Bentonite | 66.89 ± 0.40 c | 29.96 ± 2.91 b | 24.45 ± 0.51 b | 123.90 ± 0.42 b | 24.36 ± 0.55 b | 3.00 ± 0.21 a | 16.98 ± 0.56 b | 6.33 ± 0.45 b | 5.55 ± 0.25 c | 301.42 ± 3.49 d | |
| Saignée | 105.10 ± 1.78 a | 60.73 ± 0.72 a | 30.21 ± 1.65 a | 145.68 ± 1.89 a | 46.79 ± 1.70 a | 4.53 ± 1.19 a | 25.56 ± 0.11 a | 14.01 ± 0.50 a | 14.05 ± 0.03 a | 446.66 ± 4.73 a | |
| Control | 102.27 ± 0.21 a | 57.33 ± 3.53 a | 27.84 ± 0.03 ab | 139.62 ± 1.38 a | 42.10 ± 1.64 a | 4.27 ± 0.04 a | 24.92 ± 0.45 a | 13.43 ± 0.11 a | 12.92 ± 0.20 b | 424.69 ± 4.77 b | |
| Bottling | Both | 68.79 ± 4.11 b | 29.79 ± 1.36 b | 13.63 ± 1.59 a | 105.55 ± 5.02 a | 18.65 ± 0.83 b | 1.38 ± 0.20 a | 12.92 ± 0.89 b | 5.68 ± 0.40 b | 5.42 ± 0.28 b | 261.81 ± 11.51 b |
| Bentonite | 50.40 ± 0.58 c | 22.24 ± 0.00 c | 13.24 ± 0.10 a | 79.82 ± 3.36 b | 14.48 ± 0.08 b | 1.25 ± 0.00 a | 10.40 ± 0.17 b | 4.43 ± 0.06 b | 4.20 ± 0.07 b | 200.47 ± 3.92 c | |
| Saignée | 82.34 ± 2.21 a | 44.42 ± 0.57 a | 13.44 ± 2.93 a | 96.95 ± 4.65 a | 29.60 ± 0.31 a | 1.61 ± 0.61 a | 19.32 ± 0.40 a | 10.94 ± 0.26 a | 10.25 ± 0.73 a | 308.87 ± 5.66 a | |
| Control | 75.91 ± 0.47 ab | 43.12 ± 3.38 a | 15.60 ± 0.30 a | 97.41 ± 0.87 a | 29.48 ± 2.08 a | 1.37 ± 0.10 a | 17.74 ± 1.29 a | 10.29 ± 1.00 a | 10.17 ± 0.95 a | 301.09 ± 8.90 a | |
| Aging | Both | 56.04 ± 1.67 b | 21.19 ± 0.51 b | 9.39 ± 0.76 b | 76.70 ± 2.82 a | 14.05 ± 0.37 b | 0.73 ± 0.06 bc | 8.51 ± 0.16 b | 4.01 ± 0.04 b | 4.15 ± 0.13 c | 194.78 ± 6.18 b |
| Bentonite | 43.07 ± 0.17 c | 15.79 ± 0.31 c | 8.92 ± 0.05 b | 61.15 ± 2.17 b | 11.19 ± 0.12 c | 0.71 ± 0.02 c | 7.15 ± 0.13 c | 2.88 ± 0.13 c | 2.73 ± 0.20 d | 153.60 ± 2.65 c | |
| Saignée | 69.09 ± 1.91 a | 31.77 ± 0.97 a | 13.05 ± 1.14 a | 81.04 ± 0.45 a | 22.53 ± 0.16 a | 0.85 ± 0.00 ab | 13.18 ± 0.25 a | 7.98 ± 0.18 a | 8.25 ± 0.20 a | 247.74 ± 3.86 a | |
| Control | 64.66 ± 0.13 a | 30.01 ± 0.05 a | 11.86 ± 0.47 ab | 76.51 ± 0.09 a | 21.72 ± 0.51 a | 0.90 ± 0.03 a | 12.41 ± 0.34 a | 7.79 ± 0.28 a | 7.37 ± 0.02 b | 233.25 ± 1.60 a |
| Time Point | Treatment | Gallic Acid | (+)-Catechin | (−)-Epicatechin | Caftaric Acid | Quercetin-3-O-glucoside | Myricetin | Quercetin | Total Non-Anthocyanin Phenolics |
|---|---|---|---|---|---|---|---|---|---|
| Pressing | Both 2 | 85.07 ± 0.03 a 1 | 87.24 ± 0.76 a | 29.17 ± 0.38 b | 19.82 ± 0.01 b | 13.95 ± 0.61 b | 0.96 ± 0.05 b | 2.18 ± 0.12 b | 238.38 ± 0.36 b |
| Bentonite | 78.59 ± 0.19 b | 88.59 ± 0.23 a | 27.96 ± 0.15 b | 23.06 ± 0.61 b | 12.65 ± 0.64 b | 0.81 ± 0.05 b | 2.15 ± 0.05 b | 233.80 ± 1.63 b | |
| Saignée | 66.64 ± 1.28 d | 102.92 ± 8.16 a | 33.90 ± 1.26 a | 58.89 ± 1.60 a | 18.78 ± 0.27 a | 1.44 ± 0.05 a | 3.13 ± 0.06 a | 285.71 ± 12.02 a | |
| Control | 70.08 ± 0.34 c | 98.62 ± 0.06 a | 34.28 ± 0.03 a | 61.37 ± 0.11 a | 17.28 ± 0.38 a | 1.50 ± 0.22 a | 3.18 ± 0.08 a | 286.32 ± 0.20 a | |
| Bottling | Both | 134.90 ± 6.90 a | 103.71 ± 8.83 a | 39.34 ± 0.90 a | 27.26 ± 1.27 b | 15.47 ± 0.51 a | 3.27 ± 0.09 a | 4.64 ± 0.06 a | 328.58 ± 18.57 a |
| Bentonite | 117.70 ± 2.94 b | 99.59 ± 2.10 a | 37.54 ± 1.76 a | 27.71 ± 0.61 b | 11.82 ± 0.11 b | 2.17 ± 0.00 b | 3.42 ± 0.09 b | 299.95 ± 7.21 ab | |
| Saignée | 80.98 ± 0.08 c | 92.73 ± 1.00 a | 32.70 ± 0.29 b | 58.81 ± 1.14 a | 17.35 ± 0.34 a | 3.40 ± 0.01 a | 4.62 ± 0.09 a | 290.59 ± 0.21 ab | |
| Control | 80.50 ± 1.31 c | 90.52 ± 1.05 a | 33.01 ± 0.14 b | 57.19 ± 1.04 a | 15.75 ± 1.03 a | 3.59 ± 0.44 a | 4.78 ± 0.46 a | 285.33 ± 2.57 b | |
| Aging | Both | 158.66 ± 0.00 a | 112.94 ± 5.85 a | 51.87 ± 0.63 a | 27.33 ± 0.01 b | 13.24 ± 0.04 c | 5.18 ± 0.20 b | 6.03 ± 0.12 a | 375.25 ± 6.61 a |
| Bentonite | 150.64 ± 0.79 b | 119.43 ± 2.67 a | 52.22 ± 0.10 a | 29.75 ± 0.05 b | 10.43 ± 0.04 d | 3.66 ± 0.03 c | 4.68 ± 0.02 b | 370.79 ± 1.64 a | |
| Saignée | 94.05 ± 1.21 c | 117.41 ± 7.45 a | 43.66 ± 0.41 b | 62.67 ± 0.30 a | 15.48 ± 0.05 a | 5.67 ± 0.05 a | 6.39 ± 0.19 a | 345.34 ± 5.82 b | |
| Control | 96.15 ± 0.82 c | 115.41 ± 4.26 a | 43.43 ± 0.76 b | 61.59 ± 1.32 a | 14.37 ± 0.50 b | 5.97 ± 0.11 a | 6.14 ± 0.45 a | 342.80 ± 4.06 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Watrelot, A.A. Effects of Saignée and Bentonite Treatment on Phenolic Compounds of Marquette Red Wines. Molecules 2022, 27, 3482. https://doi.org/10.3390/molecules27113482
Cheng Y, Watrelot AA. Effects of Saignée and Bentonite Treatment on Phenolic Compounds of Marquette Red Wines. Molecules. 2022; 27(11):3482. https://doi.org/10.3390/molecules27113482
Chicago/Turabian StyleCheng, Yiliang, and Aude A. Watrelot. 2022. "Effects of Saignée and Bentonite Treatment on Phenolic Compounds of Marquette Red Wines" Molecules 27, no. 11: 3482. https://doi.org/10.3390/molecules27113482
APA StyleCheng, Y., & Watrelot, A. A. (2022). Effects of Saignée and Bentonite Treatment on Phenolic Compounds of Marquette Red Wines. Molecules, 27(11), 3482. https://doi.org/10.3390/molecules27113482






